Dual Benefits of Hydrogel Remediation of Cadmium-Contaminated Water or Soil and Promotion of Vegetable Growth under Cadmium Stress
Abstract
:1. Introduction
2. Results
2.1. Swelling Degree of Hydrogel
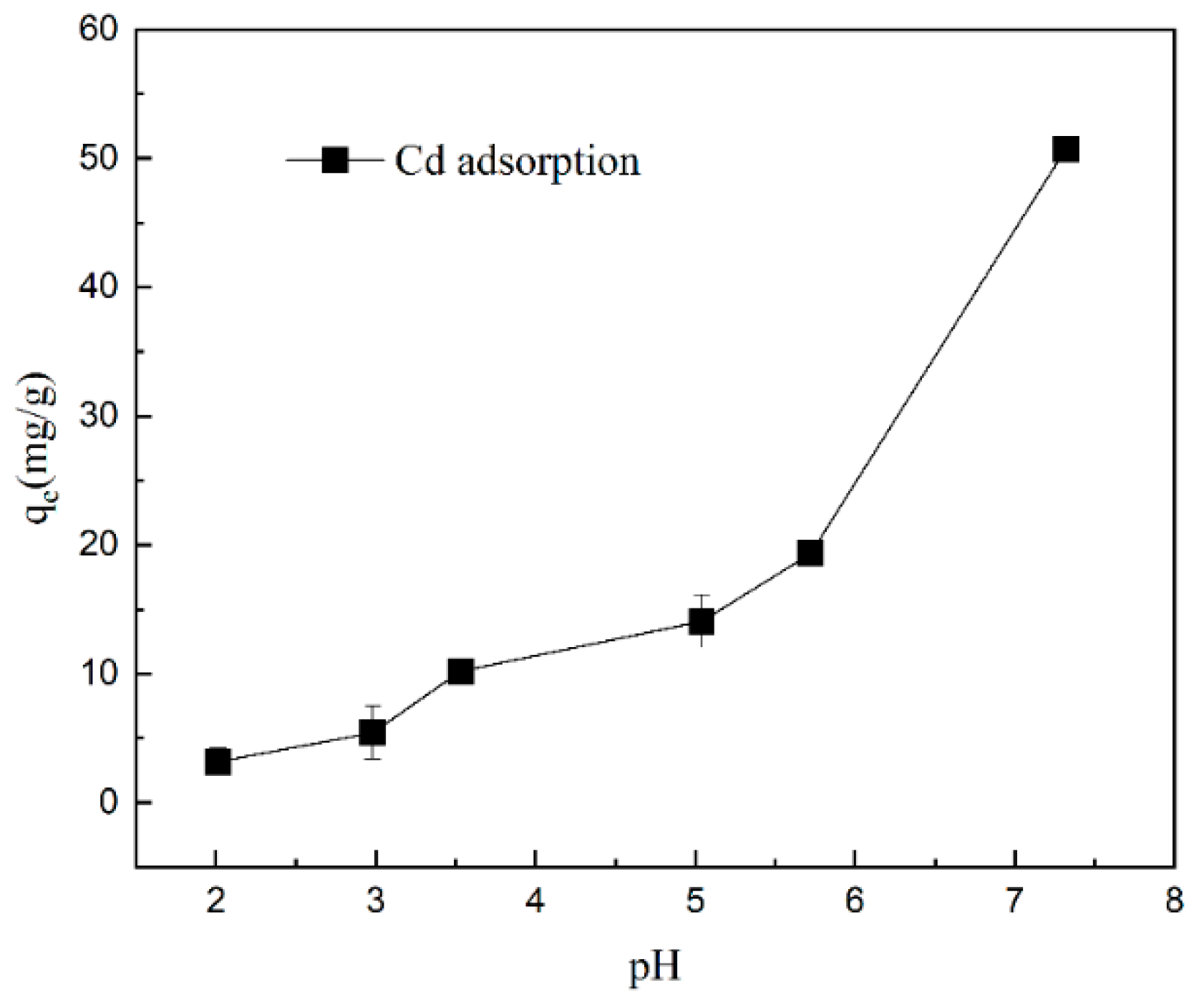
2.2. Effect of pH on Gel Adsorption
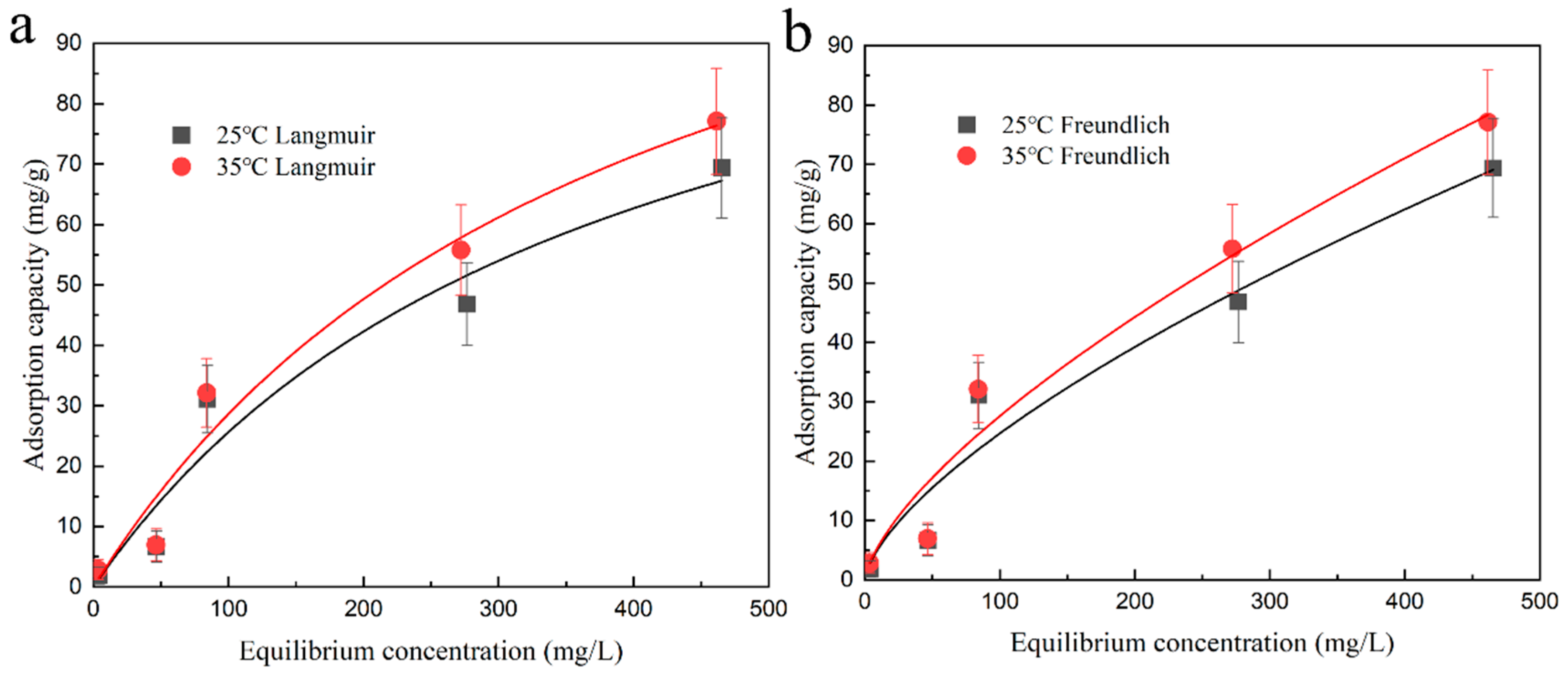
2.3. Isothermal Adsorption
2.4. Adsorption Thermodynamic
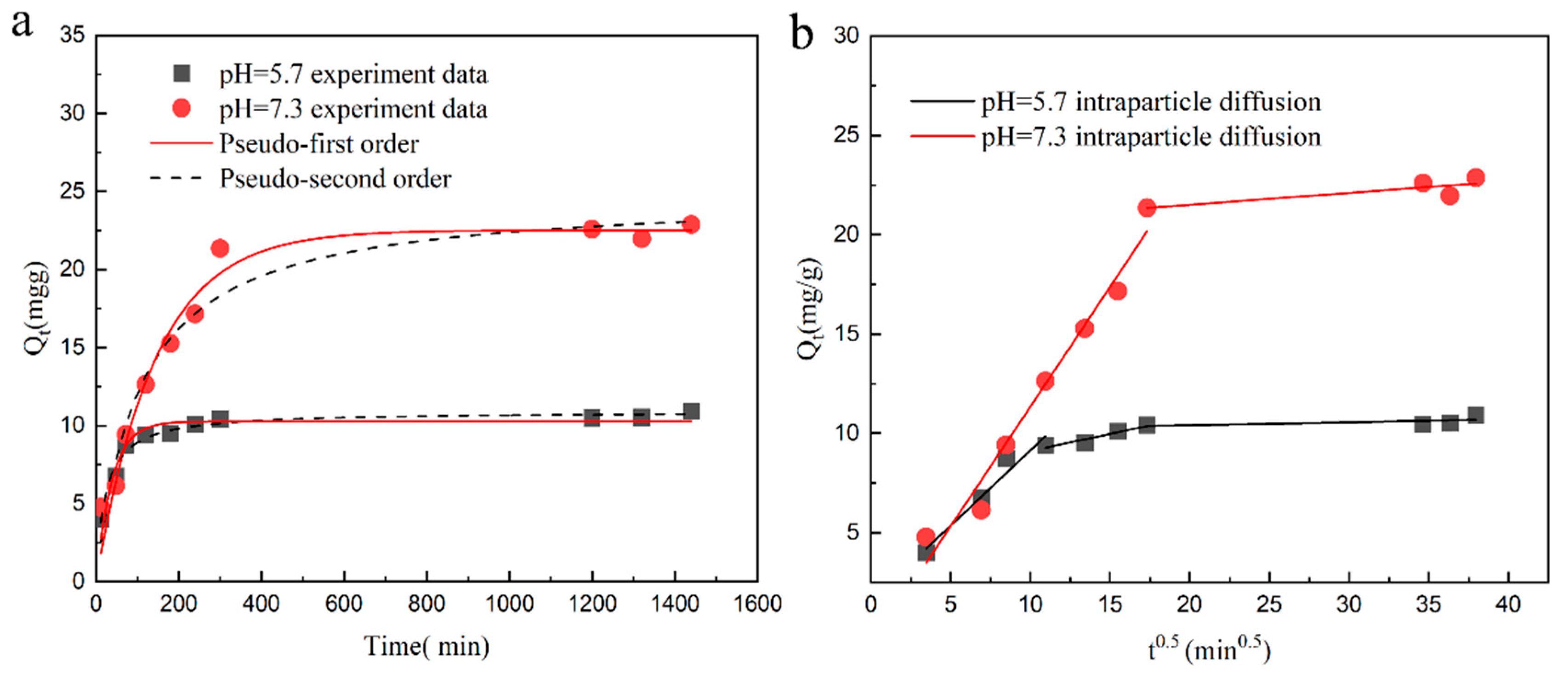
2.5. Adsorption Kinetics
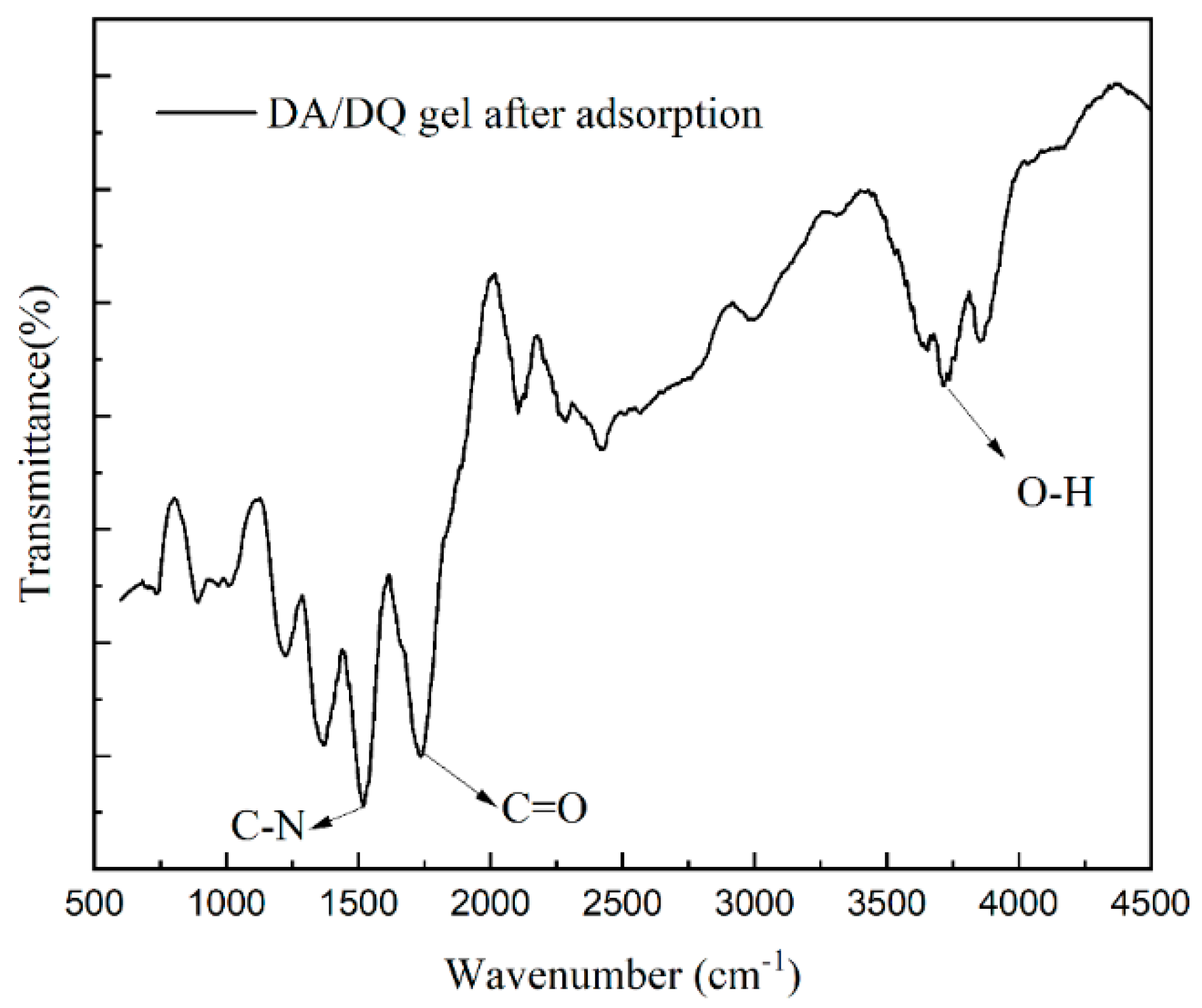
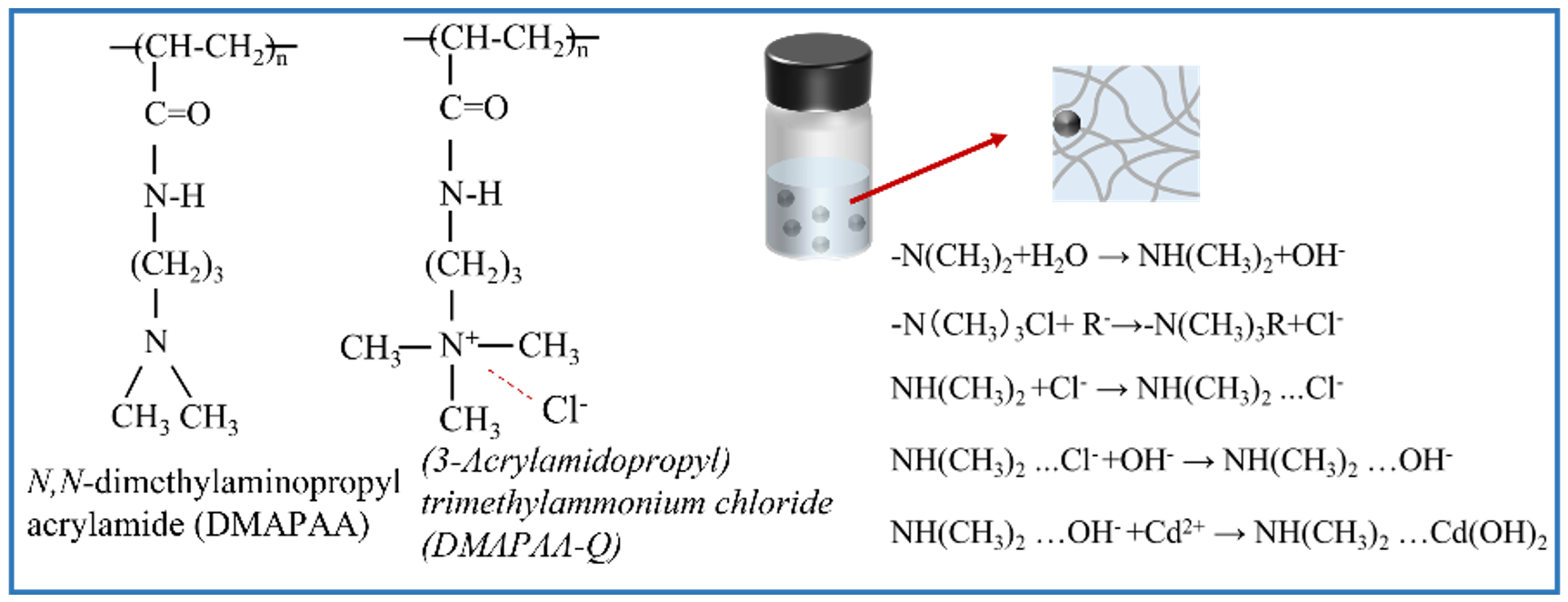
2.6. Characterization of Hydrogel Structure
2.7. Soil Experiment
2.7.1. Physical Picture of Vegetable Growth
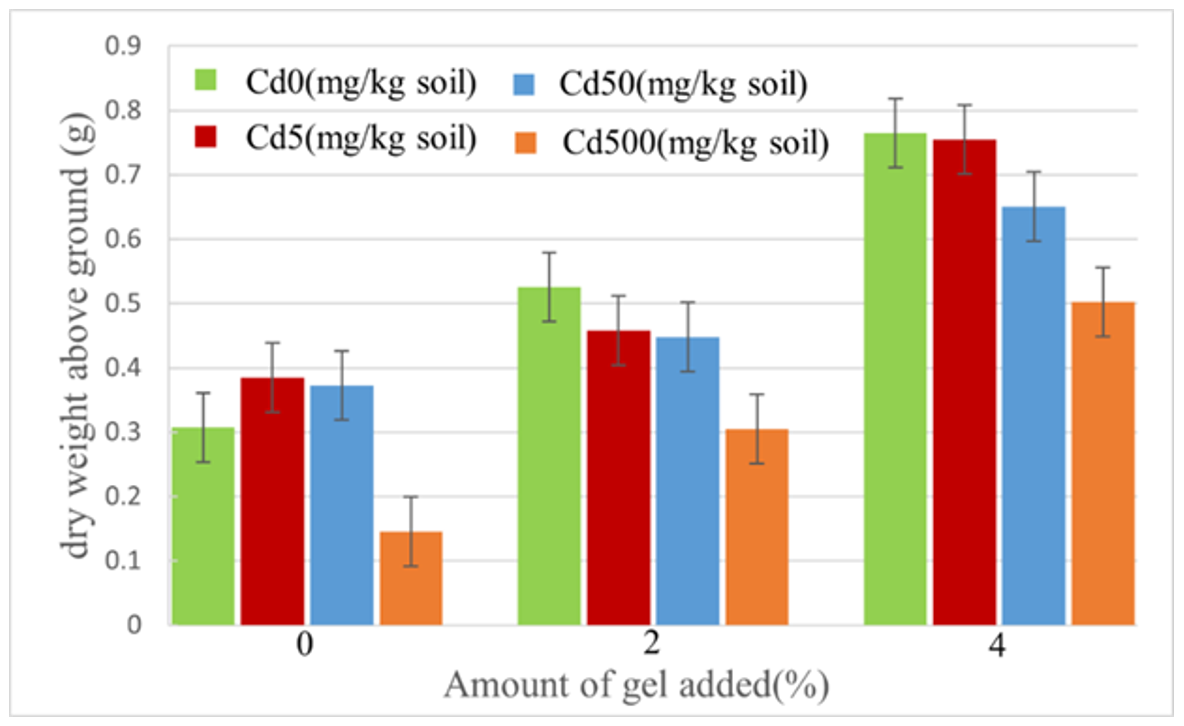
2.7.2. Shoot Dry Weight
2.7.3. Cadmium Uptake in Swiss Chard
2.7.4. Other Elements
3. Discussion
4. Experimental Methods
4.1. Materials
4.2. Synthesis of Hydrogel
4.3. Swelling Properties of Hydrogels
4.3.1. Swelling Degree of Hydrogel in Water Solutions
4.3.2. Soil Water Holding Rate
4.4. Adsorption Experiment
4.4.1. Adsorption Thermodynamic Experiments with Different pH Values
4.4.2. pH Effect Experiment
4.4.3. Adsorption Kinetic Experiment
4.4.4. Adsorption Thermodynamics at Different Temperatures
4.4.5. Adsorption Model
4.5. Experimental Design
4.5.1. Dry Weight of Swiss Chard
4.5.2. Plant Digestion
4.6. Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diarra, I.; Prasad, S. The Current State of Heavy Metal Pollution in Pacific Island Countries: A review. Appl. Spectrosc. Rev. 2020, 56, 27–51. [Google Scholar] [CrossRef]
- Barsova, N.; Yakimenko, O.; Tolpeshta, I.; Motuzova, G. Current State and Dynamics of Heavy Metal Soil Pollution in Russian Federation—A review. Environ. Pollut. 2019, 249, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Arunakumara, K.K.I.U.; Walpola, B.C.; Yoon, M.-H. Current Status of Heavy Metal Contamination in Asia’s Rice Lands. Rev. Environ. Sci. Bio/Technol. 2013, 12, 355–377. [Google Scholar] [CrossRef]
- Wang, L.; Cui, X.; Cheng, H.; Chen, F.; Wang, J.; Zhao, X.; Lin, C.; Pu, X. A Review of Soil Cadmium Contamination in China Including a Health Risk Assessment. Environ. Sci. Pollut. Res. Int. 2015, 22, 16441–16452. [Google Scholar] [CrossRef]
- Qi, M.; Wu, Y.; Zhang, S.; Li, G.; An, T. Pollution Profiles, Source Identification and Health Risk Assessment of Heavy Metals in Soil near a Non-Ferrous Metal Smelting Plant. Int. J. Environ. Res. Public Health 2023, 20, 1004. [Google Scholar] [CrossRef]
- Ma, H.; Mi, M.; Wang, C.; Wu, X.; Zhen, Z. The Concentrations, Sources, Ecological, and Human Health Risk Assessment of Heavy Metals in Roadside Soils of Six Cities in Shanxi Province, China. Environ. Toxicol. Chem. 2023, 42, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, B.; Zhou, Z. Pollution Assessment and Source Apportionment of Heavy Metals in Soil From Lead—Zinc Mining Areas of South China. J. Environ. Chem. Eng. 2023, 11, 109320. [Google Scholar] [CrossRef]
- Azhar, U.; Ahmad, H.; Shafqat, H.; Babar, M.; Shahzad Munir, H.M.; Sagir, M.; Arif, M.; Hassan, A.; Rachmadona, N.; Rajendran, S.; et al. Remediation Techniques for Elimination of Heavy Metal Pollutants from Soil: A Review. Environ. Res. 2022, 214, 113918. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A Comparison of Technologies for Remediation of Heavy Metal Contaminated Soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Aparicio, J.D.; Raimondo, E.E.; Saez, J.M.; Costa-Gutierrez, S.B.; Álvarez, A.; Benimeli, C.S.; Polti, M.A. The Current Approach to Soil Remediation: A Review of Physicochemical and Biological Technologies, and the Potential of Their Strategic Combination. J. Environ. Chem. Eng. 2022, 10, 107141. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent Advances in Conventional and Contemporary Methods for Remediation of Heavy Metal-Contaminated Soils. 3 Biotech 2018, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on Remediation Technologies of Soil Contaminated by Heavy Metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Navarro, A.; Cardellach, E.; Cañadas, I.; Rodríguez, J. Solar Thermal Vitrification of Mining Contaminated Soils. Int. J. Miner. Process 2013, 119, 65–74. [Google Scholar] [CrossRef]
- Khan, F.I.; Husain, T.; Hejazi, R. An Overview and Analysis of Site Remediation Technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Hung, Y.T.; Shammas, N.K. Physicochemical Treatment Processes; Handbook of Environmental Engineering Series; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3. [Google Scholar]
- Rozas, E.E.; Mendes, M.A.; Nascimento, C.A.; Espinosa, D.C.; Oliveira, R.; Oliveira, G.; Custodio, M.R. Bioleaching of Electronic Waste Using Bacteria Isolated from the Marine Sponge Hymeniacidon Heliophila (Porifera). J. Hazard. Mater. 2017, 329, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Wawrzkiewicz, M.; Hubicki, Z. Anion Exchange Resins as Effective Sorbents for Removal of Acid, Reactive, and Direct Dyes from Textile Wastewaters. In Ion Exchange-Studies and Applications; IntechOpen: London, UK, 2015; pp. 37–72. [Google Scholar]
- Saber-Samandari, S.; Saber-Samandari, S.; Gazi, M. Cellulose-Graft-Polyacrylamide/Hydroxyapatite Composite Hydrogel with Possible Application in Removal of Cu (II) ions. React. Funct. Polym. 2013, 73, 1523–1530. [Google Scholar] [CrossRef]
- Li, P.; Zhou, M.; Liu, H.; Lei, H.; Jian, B.; Liu, R.; Li, X.; Wang, Y.; Zhou, B. Preparation of Green Magnetic Hydrogel from Soybean Residue Cellulose for Effective and Rapid Removal of Copper Ions from Wastewater. J. Environ. Chem. Eng. 2022, 10, 108213. [Google Scholar] [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef]
- Hashem, A.; Aniagor, C.O.; Afifi, M.A.-F.; Abou-Okeil, A.; Samaha, S.H. Synthesis of Super-Absorbent Poly(AN)-g-Starch Composite Hydrogel and Its Modelling for Aqueous Sorption of Cadmium ions. Korean J. Chem. Eng. 2021, 38, 2157–2170. [Google Scholar] [CrossRef]
- Zhang, P.; Zou, K.; Yuan, L.; Liu, J.; Liu, B.; Qing, T.-P.; Feng, B. A Biomass Resource Strategy for Alginate-Polyvinyl Alcohol Double Network Hydrogels and Their Adsorption to Heavy Metals. Sep. Purif. Technol. 2022, 301, 122050. [Google Scholar] [CrossRef]
- Hu, Z.H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated Cellulose Nanocrystal/Sodium Alginate Hydrogel Beads for Adsorption of Pb(II) from Aqueous Solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Zhang, C.; Li, W.; Chen, C.; Zhang, Z.; Chen, H.; Wang, J.; Li, Y.; Zhang, Y. Nano-FeS Incorporated into Stable Lignin Hydrogel: A Novel Strategy for Cadmium Removal from Soil. Environ. Pollut. 2020, 264, 114739. [Google Scholar] [CrossRef]
- Zhou, T.; Zhao, M.; Zhao, X.; Guo, Y.; Zhao, Y. Simultaneous Remediation and Fertility Improvement of Heavy Metals Contaminated Soil by a Novel Composite Hydrogel Synthesized from Food Waste. Chemosphere 2021, 275, 129984. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, J.; Prasher, S.O.; ElSayed, E.; Patel, R.M.; Nzediegwu, C.; Mawof, A. Effect of Hydrogel Based Soil Amendments on Heavy Metal Uptake by Spinach Grown with Wastewater Irrigation. J. Clean. Prod. 2021, 311, 127644. [Google Scholar] [CrossRef]
- Hou, X.; Li, Y.; Pan, Y.; Jin, Y.; Xiao, H. Controlled Release of Agrochemicals and Heavy Metal Ion Capture Dual-Functional Redox-Responsive Hydrogel for Soil Remediation. Chem. Commun. Camb. 2018, 54, 13714–13717. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Zhou, N.; Cao, X.; Du, X.; Wang, H.; Wang, M.; Liu, S.; Nguyen, K.; Schmidt-Rohr, K.; Xu, Q.; Liang, G.; et al. Hyper-Crosslinkers Lead to Temperature- and pH-Responsive Polymeric Nanogels with Unusual Volume Change. Angew. Chem. Int. Ed. Engl. 2017, 56, 2623–2627. [Google Scholar] [CrossRef]
- Freire, J.J.; Ahmadi, A.; McBride, C. Molecular Dynamics Simulations of the Protonated G4 Pamam Dendrimer in an Ionic Liquid System. J. Phys. Chem. B 2013, 117, 15157–15164. [Google Scholar] [CrossRef]
- Geng, W.; Zou, J.; Niu, Q.; Lin, Y.; Liu, H.; Jing, Y.; Yang, C. Recovery of Magnesium from Flue Gas Desulfurization Wastewater Using Thermomorphic Hydrophilicity Amines. Sep. Purif. Technol. 2023, 316, 123776. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-Responsive Polymers in Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymer 2017, 9, 137. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Alhaj Hamoud, Y.; Xu, X.; Wang, S.; Liu, H. A pH-Responsive/Sustained Release Nitrogen Fertilizer Hydrogel Based on Aminated Cellulose Nanofiber/Cationic Copolymer for Application in Irrigated Neutral Soils. J. Clean. Prod. 2022, 368, 133098. [Google Scholar] [CrossRef]
- Azeem, M.K.; Islam, A.; Rizwan, M.; Rasool, A.; Gul, N.; Khan, R.U.; Khan, S.M.; Rasheed, T. Sustainable and Environment Friendlier Carrageenan-Based pH-Responsive Hydrogels: Swelling Behavior and Controlled Release of Fertilizers. Colloid Polym. Sci. 2023, 301, 209–219. [Google Scholar] [CrossRef]
- Ravikumar, T.; Murata, H.; Koepsel, R.R.; Russell, A.J. Surface-Active Antifungal Polyquaternary Amine. Biomacromolecules 2006, 7, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Le Roux, S.M.; Millward, G.E. Adsorption of Cadmium to Iron and Manganese Oxides during Estuarine Mixing. Mar. Chem. 2008, 108, 77–84. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of Silicon on Heavy Metal Uptake at the Soil-Plant Interphase: A Review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, U.; Haider, F.U.; Maqsood, M.F.; Mohy-Ud-Din, W.; Shabaan, M.; Ahmad, M.; Kaleem, M.; Ishfaq, M.; Aslam, Z.; Shahzad, B. Recent Advances in Microbial-Assisted Remediation of Cadmium-Contaminated Soil. Plants 2023, 12, 3147. [Google Scholar] [CrossRef]
- Nassaj-Bokharaei, S.; Motesharezedeh, B.; Etesami, H.; Motamedi, E. Effect of Hydrogel Composite Reinforced with Natural Char Nanoparticles on Improvement of Soil Biological Properties and the Growth of Water Deficit-Stressed Tomato Plant. Ecotoxicol. Environ. Saf. 2021, 223, 112576. [Google Scholar] [CrossRef]






| Isotherm Model | Parameter | Initial pH | |
|---|---|---|---|
| 5.7 | 7.3 | ||
| Langmuir | qmax (mg/g) | 23.7 | 121 |
| KL (L/mg) | 0.0098 | 0.0027 | |
| R2 | 0.8537 | 0.9526 | |
| Freundlich | KF (mg/g) | 1.55 | 1.13 |
| n | 2.397 | 1.492 | |
| R2 | 0.8164 | 0.9515 | |
| T/K | |||
|---|---|---|---|
| 298.15 | −3.082 | 4.677 | 26.025 |
| 308.15 | −3.342 | 4.677 | 26.025 |
| Isotherm Model | Parameter | Initial pH | |
|---|---|---|---|
| 5.7 | 7.3 | ||
| Pseudo first order | k1 (min−1) | 0.007 | 0.007 |
| qe (mg/g) | 22.52 | 32.87 | |
| R2 | 0.967 | 0.979 | |
| Pseudo second order | K2 (g/mg/min) | 3.818 | 2.539 |
| qe (mg/g) | 24.76 | 36.37 | |
| R2 | 0.951 | 0.939 | |
| Materials | Component Type | Molar Weight (g/mol) | Concentration (mol/m3) | Mass (g) |
|---|---|---|---|---|
| DMAPAA | Monomer | 105.22 | 500 | 1.315 |
| DMAPAA-Q | Monomer | 206.71 | 500 | 2.584 |
| MBAA | Linker | 154.17 | 50 | 0.193 |
| TEMED | Accelerator | 116.21 | 20 | 0.058 |
| APS | Initiator | 228.19 | 20 | 0.114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Gotoh, T.; Nakai, S.; Ueda, A. Dual Benefits of Hydrogel Remediation of Cadmium-Contaminated Water or Soil and Promotion of Vegetable Growth under Cadmium Stress. Plants 2023, 12, 4115. https://doi.org/10.3390/plants12244115
Huang J, Gotoh T, Nakai S, Ueda A. Dual Benefits of Hydrogel Remediation of Cadmium-Contaminated Water or Soil and Promotion of Vegetable Growth under Cadmium Stress. Plants. 2023; 12(24):4115. https://doi.org/10.3390/plants12244115
Chicago/Turabian StyleHuang, Jin, Takehiko Gotoh, Satoshi Nakai, and Akihiro Ueda. 2023. "Dual Benefits of Hydrogel Remediation of Cadmium-Contaminated Water or Soil and Promotion of Vegetable Growth under Cadmium Stress" Plants 12, no. 24: 4115. https://doi.org/10.3390/plants12244115
APA StyleHuang, J., Gotoh, T., Nakai, S., & Ueda, A. (2023). Dual Benefits of Hydrogel Remediation of Cadmium-Contaminated Water or Soil and Promotion of Vegetable Growth under Cadmium Stress. Plants, 12(24), 4115. https://doi.org/10.3390/plants12244115







