The Role of Stress Modifier Biostimulants on Adaptive Strategy of Oregano Plant for Increasing Productivity under Water Shortage
Abstract
:1. Introduction
2. Results
2.1. Total Dry Weight (TDW), Essential Oil Yield (EOY), Relative Water Content (RWC), Chlorophyll a and b (Chl a and Chl b) Content
2.1.1. TDW
2.1.2. EOY
2.1.3. RWC
2.1.4. Chl a and Chl b
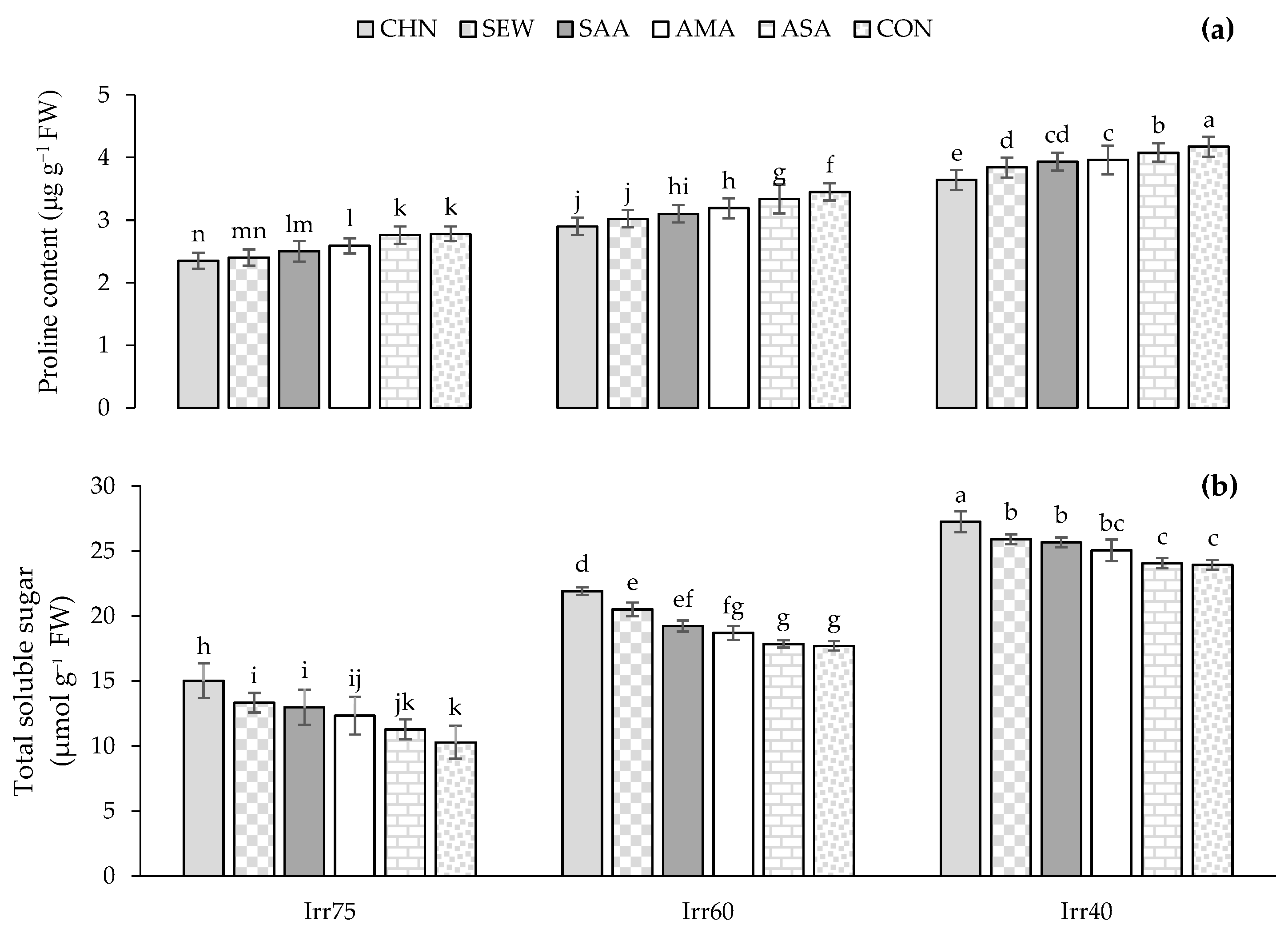
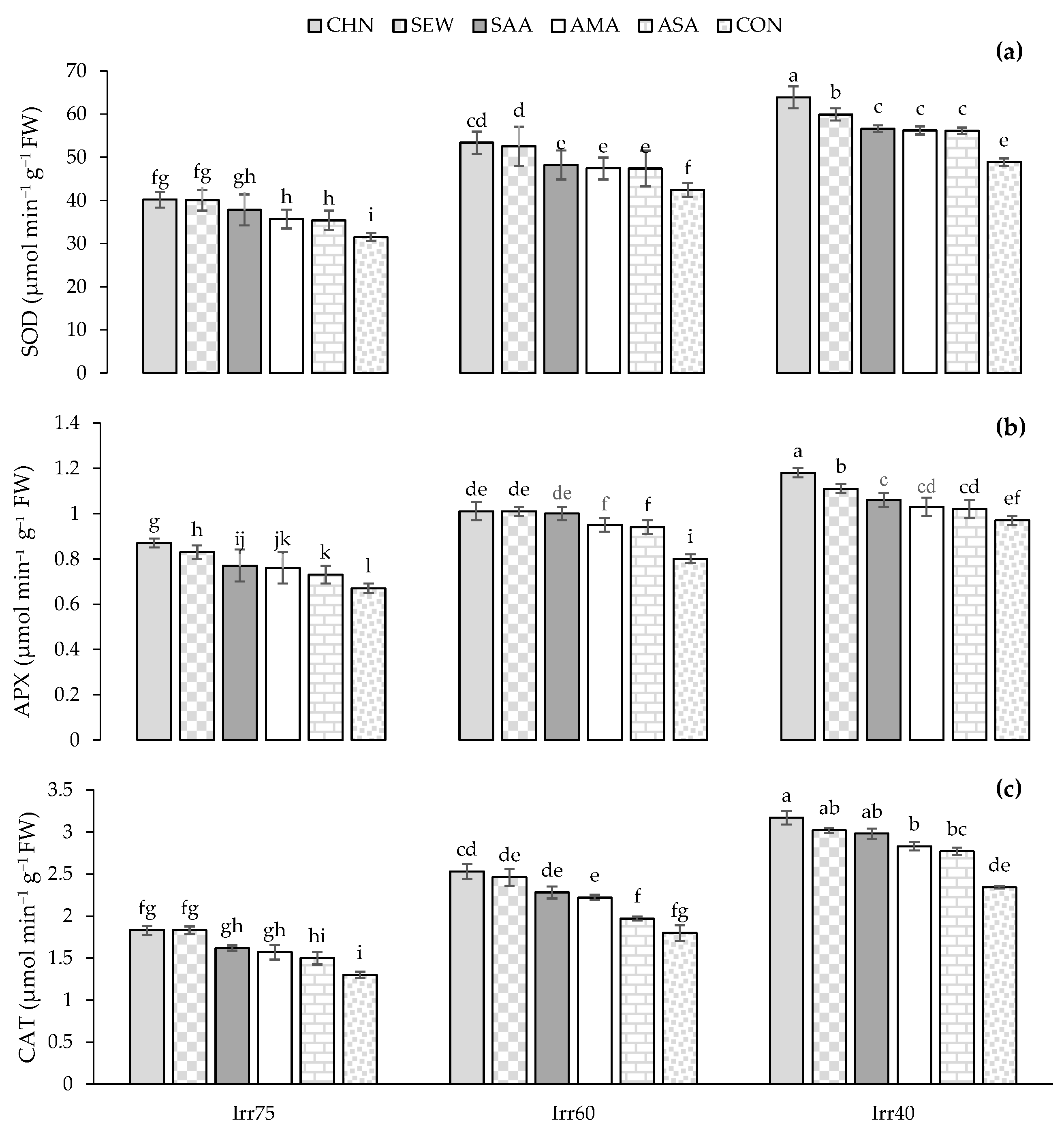
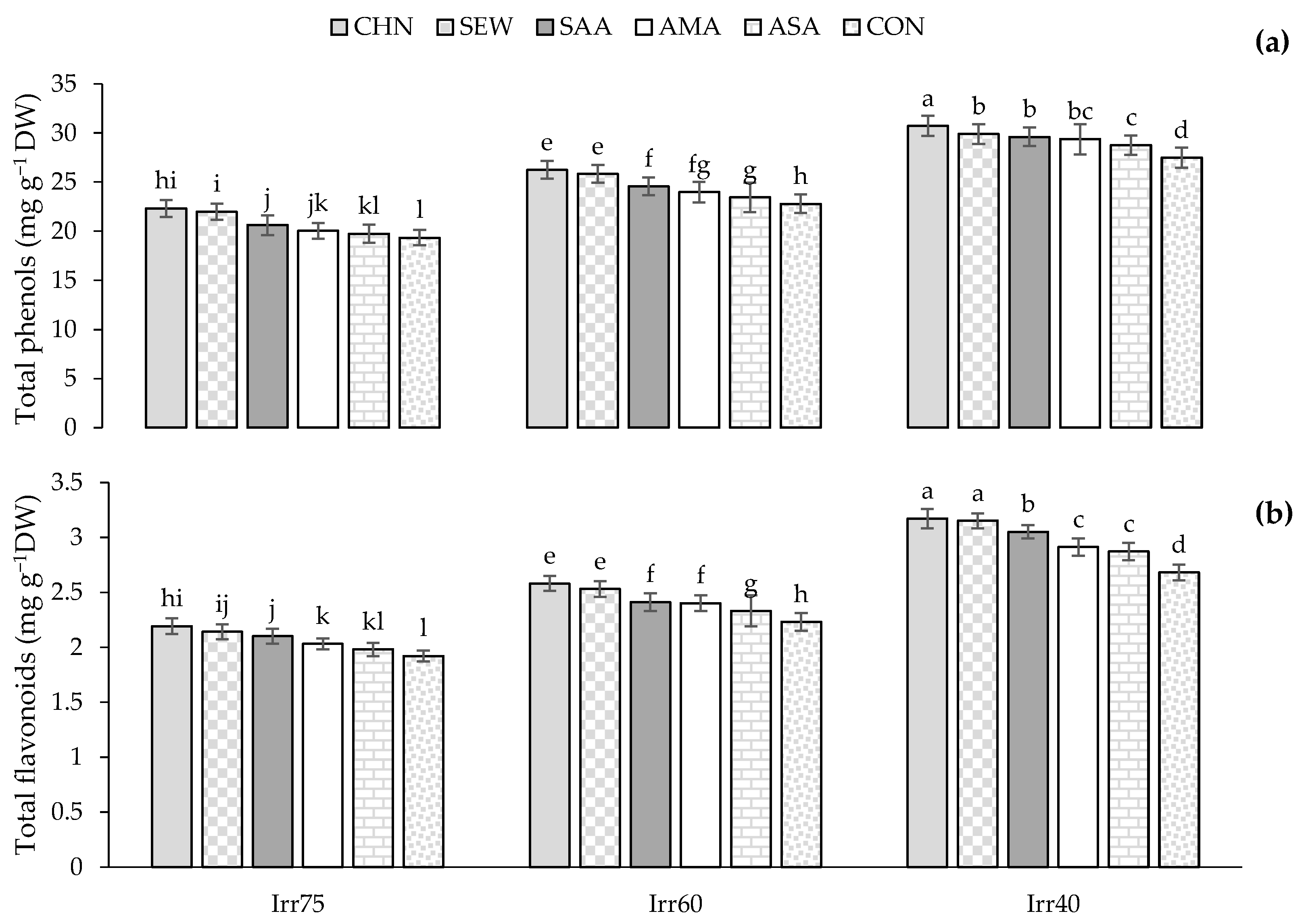
2.2. Total Soluble Sugar (TSS) and Proline Content (Pro), Antioxidant Enzymes Activity, Total Phenols (TPC), Flavonoids (TFC) Content, Nutrients of N, K, and P
2.2.1. TSS and Pro Content
2.2.2. CAT, SOD, and APX Activities
2.2.3. TPC and TFC
2.2.4. Nutrients of N, K, and P
3. Discussion
4. Materials and Methods
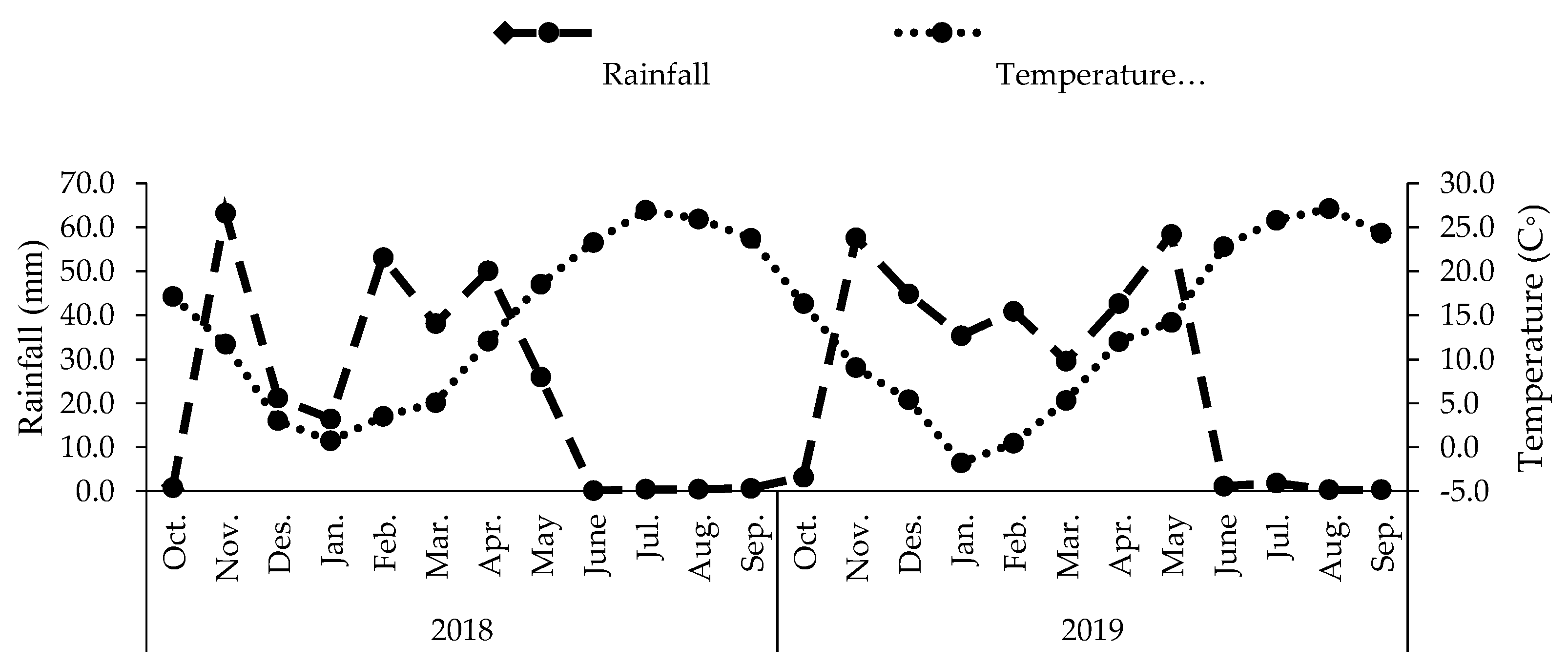
4.1. Experimental Design
4.2. Plant Material and Stress Modulators Application
4.3. Measurements
4.3.1. Total Dry Weight (TDW) Determination
4.3.2. Essential Oil Yield (EOY)
4.3.3. Relative Water Content (RWC)
4.3.4. Chl a and Chl b Content
4.3.5. Proline Content
4.3.6. Total Soluble Sugars (TSS) Determination
4.3.7. Enzyme Extractions and Assays
4.3.8. Total Polyphenols and Flavonoids (TPC and TFC)
4.3.9. Nutrients of N, P and K
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aytaç, Z.; Gülbandılar, A.; Kürkçüoğlu, M. Humic Acid Improves Plant Yield, Antimicrobial Activity and Essential Oil Composition of Oregano (Origanum vulgare L. subsp. hirtum (Link.) Ietswaart). Agronomy 2022, 12, 2086. [Google Scholar] [CrossRef]
- Hancioglu, N.E.; Kurunc, A.; Tontul, I.; Topuz, A. Growth, water use, yield and quality parameters in oregano affected by reduced irrigation regimes. J. Sci. Food Agric. 2021, 101, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, M.S.; Argüello, J.A.; Bima, P.I. Genotype-dependent architectural and physiological responses regulate the strategies of two oregano cultivars to water excess and deficiency regimes. Ind. Crops Prod. 2021, 161, 113206. [Google Scholar] [CrossRef]
- Gerami, F.; Moghaddam, P.R.; Ghorbani, R.; Hassani, A. Effects of irrigation intervals and organic manure on morphological traits, essential oil content and yield of oregano (Origanum vulgare L.). An. Acad. Bras. Cienc. 2016, 88, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ali, S.; Manghwar, H.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. Melatonin function and crosstalk with other phytohormones under normal and stressful conditions. Genes 2022, 13, 1699. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, A.; Huang, H.; Zheng, M.; Zaman, W.; Li, D.; Saqib, S.; Zhao, H.; Lü, S. Molecular cloning and functional analysis of GmLACS2-3 reveals its involvement in cutin and suberin biosynthesis along with abiotic stress tolerance. Int. J. Mol. Sci. 2021, 22, 9175. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Mohammadi, M.M.; Siavash Moghaddam, S.; Heydarzadeh, S.; Gitari, H. Effects of Stress Modifier Biostimulants on Vegetative Growth, Nutrients, and Antioxidants Contents of Garden Thyme (Thymus vulgaris L.) Under Water Deficit Conditions. J. Plant Growth Regul. 2022, 41, 2059–2072. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Jalilian, J.; Pirzad, A.; Jamei, R. Impact of bio-fertilizers under supplementary irrigation and rain-fed conditions on some physiological responses and forage quality of smooth vetch (Vicia dasycarpa). J. Agric. Sci. 2023, 29, 777–787. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Jalilian, J.; Pirzad, A.; Petrussa, E.; Jamei, R. Fodder value and physiological aspects of rainfed smooth vetch affected by biofertilizers and supplementary irrigation in an agri-silviculture system. Agrofor. Syst. 2022, 95, 221–232. [Google Scholar] [CrossRef]
- Rahimzadeh, S.; Pirzad, A. Arbuscular mycorrhizal fungi and Pseudomonas in reduce drought stress damage in flax (Linum usitatissimum L.): A field study. Mycorrhiza 2017, 27, 537–552. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, K.; Fan, Y.; Fang, H.; Nie, Y.; Wu, X.L. The bacterial MtrAB Two-Component System regulates the cell wall homeostasis responding to environmental alkaline stress. Microbiol. Spectrum. 2022, 10, e02311-22. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Shah, S.; Ullah, S.; Mansour, A.T.; Shalaby, T.A. Impacts of ascorbic acid and alpha-tocopherol on Chickpea (Cicer arietinum L.) grown in water deficit regimes for sustainable production. Sustainability 2022, 14, 8861. [Google Scholar] [CrossRef]
- Dawood, M.F.; Zaid, A.; Latef, A.A.H.A. Salicylic acid spraying-induced resilience strategies against the damaging impacts of drought and/or salinity stress in two varieties of Vicia faba L. seedlings. J. Plant Growth Regul. 2022, 41, 1919–1942. [Google Scholar] [CrossRef]
- Teixeira, W.F.; Soares, L.H.; Fagan, E.B.; da Costa Mello, S.; Reichardt, K.; Dourado-Neto, D. Amino acids as stress reducers in soybean plant growth under different water-deficit conditions. J. Plant Growth Regul. 2019, 39, 905–919. [Google Scholar] [CrossRef]
- El Refaey, A.; Mohamed, Y.I.; El-Shazly, S.M.; Abd El Salam, A.A. Effect of salicylic and ascorbic acids foliar application on Picual olive trees growth under water stress condition. Egypt. J. Soil Sci. 2022, 62, 1–16. [Google Scholar] [CrossRef]
- Naz, S.; Mushtaq, A.; Ali, S.; Muhammad, H.M.; Saddiq, B.; Ahmad, R.; Zulfiqar, F.; Hayat, F.; Tiwari, R.K.; Lal, M.K.; et al. Foliar application of ascorbic acid enhances growth and yield of lettuce (Lactuca sativa) under saline conditions by improving antioxidant defence mechanism. Funct. Plant Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- González-Villagra, J.; Reyes-Díaz, M.M.; Tighe-Neira, R.; Inostroza-Blancheteau, C.; Escobar, A.L.; Bravo, L.A. Salicylic acid improves antioxidant defense system and photosynthetic performance in aristotelia chilensis plants subjected to moderate drought stress. Plants 2022, 11, 639. [Google Scholar] [CrossRef]
- Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Beltrão, J.G.T.; Mastinu, A. Physiological and qualitative response of Cucurbita pepo L. to salicylic acid under controlled water stress conditions. Horticulturae 2022, 8, 79. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Arena, C.; Vitale, E.; Rahimi, A.; Mirzapour, M.; Nasar, J.; Kisaka, O.; Sow, S.; Ranjan, S.; Gitari, H. Impact of different fertilizer sources under supplemental irrigation and rainfed conditions on eco-physiological responses and yield characteristics of dragon’s head (Lallemantia iberica). Plants 2023, 12, 1693. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Zhang, G.; Lu, J.; Ren, T.; Cong, R.; Lu, Z.; Zhang, Y.; Liao, S.; Li, X. Potassium deficiency limits water deficit tolerance of rice by reducing leaf water potential and stomatal area. Agric. Water Manag. 2022, 271, 107744. [Google Scholar] [CrossRef]
- Rahimi, A.; Siavash Moghaddam, S.; Ghiyasi, M.; Heydarzadeh, S.; Ghazizadeh, K.; Popović-Djordjević, J. The Influence of chemical, organic and biological fertilizers on agrobiological and antioxidant properties of Syrian cephalaria (Cephalaria Syriaca L.). Agriculture 2019, 9, 122. [Google Scholar] [CrossRef]
- Yildiztekin, M.; Tuna, A.L.; Kaya, C. Physiological effects of the brown seaweed (Ascophyllum nodosum) and humic substances on plant growth, enzyme activities of certain pepper plants grown under salt stress. Acta Biol. Hung. 2018, 69, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Shafie, F.; Bayat, H.; Aminifard, M.H.; Daghighi, S. Biostimulant effects of seaweed extract and amino acids on growth, antioxidants, and nutrient content of yarrow (Achillea millefolium L.) in the field and greenhouse conditions. Commun. Soil. Sci. Plant Anal. 2021, 52, 964–975. [Google Scholar] [CrossRef]
- Alizadeh, A.; Moghaddam, M.; Asgharzade, A.; Sourestani, M.M. Phytochemical and physiological response of Satureja hortensis L. to different irrigation regimes and chitosan application. Ind. Crops Prod. 2020, 158, 112990. [Google Scholar] [CrossRef]
- Razavizadeh, R.; Mosayebi, Z.; Forghani, A.H. The Role of Chitosan in Improvisation the Drought Stress in Carum copticum through Biochemical and Essential Oil Components. Russ. J. Plant Physiol. 2022, 69, 156. [Google Scholar] [CrossRef]
- Danesh-Shahraki, H.; Pirbalouti, A.G.; Rajabzadeh, F.; Kachouei, M.A. Water Deficit Stress Mitigation by the Foliar Spraying of Salicylic Acid and Proline on the Volatile Oils and Growth Features of Hyssop (Hyssopus officinalis L.). J. Essent. Oil Bear. Plants. 2023, 26, 115–129. [Google Scholar] [CrossRef]
- Pirzad, A.; Mohammadzadeh, S. Water use efficiency of three mycorrhizal Lamiaceae species (Lavandula officinalis, Rosmarinus officinalis and Thymus vulgaris). Agric. Water Manag. 2018, 204, 1–10. [Google Scholar] [CrossRef]
- Wenneck, G.S.; Saath, R.; Rezende, R.; de Souza Terassi, D.; Moro, A.L. The management of water replacement in oregano cultivation changes the content and composition of the oil extracted from the leaves. Sci. Hortic. 2023, 309, 111627. [Google Scholar] [CrossRef]
- Virga, G.; Sabatino, L.; Licata, M.; Tuttolomondo, T.; Leto, C.; La Bella, S. Effects of irrigation with different sources of water on growth, yield and essential oil compounds in oregano. Plants 2020, 9, 1618. [Google Scholar] [CrossRef] [PubMed]
- Poorghadir, M.; Torkashvand, A.M.; Mirjalili, S.A.; Moradi, P. Interactions of amino acids (proline and phenylalanine) and biostimulants (salicylic acid and chitosan) on the growth and essential oil components of savory (Satureja hortensis L.). Biocata. Agric. Biotechnol. 2020, 30, 101815. [Google Scholar] [CrossRef]
- Rahimi, A.; Gitari, H.; Lyons, G.; Heydarzadeh, S.; Tunçtürk, M.; Tunçtürk, R. Effects of vermicompost, compost and animal manure on vegetative growth, physiological and antioxidant activity characteristics of Thymus vulgaris L. Under water stress. Yuzuncu Yıl. Univ. J. Agric. Sci. 2023, 33, 40–53. [Google Scholar] [CrossRef]
- Yasmin, A.; Mannan, M.A.; Sarker, U.; Dola, D.B.; Higuchi, H.; Ercisli, S.; Ali, B.; Saleem, M.H.; Babalola, O.O. Foliar application of seaweed extracts enhances yield and drought tolerance of soybean. Front. Plant Sci. 2022, 13, 992880. [Google Scholar]
- Das, D.; Bisht, K.; Chauhan, A.; Gautam, S.; Jaiswal, J.P.; Salvi, P.; Lohani, P. Morpho-physiological and Biochemical responses in wheat foliar sprayed with zinc-chitosan-salicylic acid nanoparticles during drought stress. Plant Nano Biol. 2023, 4, 100034. [Google Scholar] [CrossRef]
- Ghanbari Moheb Seraj, R.; Behnamian, M.; Ahmadikhah, A.; Shariati, V.; Dezhsetan, S. Chitosan and salicylic acid regulate morpho-physiological and phytochemical parameters and improve water-deficit tolerance in milk thistle (Silybum marianum L.). Acta Physiol. Plant. 2021, 43, 101. [Google Scholar] [CrossRef]
- Davari, K.; Rokhzadi, A.; Mohammadi, K.; Pasari, B. Paclobutrazol and amino acid-based biostimulant as beneficial compounds in alleviating the drought stress effects on Safflower (Carthamus tinctorius L.). J. Soil Sci. Plant Nutr. 2021, 22, 674–690. [Google Scholar] [CrossRef]
- Amirnia, R.; Ghiyasi, M.; Moghaddam, S.S.; Rahimi, A.; Damalas, C.A.; Heydarzadeh, S. Nitrogen-fixing soil bacteria plus mycorrhizal fungi improve seed yield and quality traits of lentil (Lens culinaris Medik). J. Soil Sci. Plant Nutr. 2019, 19, 592–602. [Google Scholar] [CrossRef]
- Mohammadi, M.; Modarres-Sanavy, S.A.M.; Pirdashti, H.; Zand, B.; Tahmasebi-Sarvestani, Z. Arbuscular mycorrhizae alleviate water deficit stress and improve antioxidant response, more than nitrogen fixing bacteria or chemical fertilizer in the evening primrose. Rhizosphere 2019, 9, 76–89. [Google Scholar] [CrossRef]
- Heydari, S.; Pirzad, A. Mycorrhizal fungi and Thiobacillus co-inoculation improve the physiological indices of Lallemantia iberica under salinity stress. Curr. Microbiol. 2020, 77, 2523–2534. [Google Scholar] [CrossRef]
- Hussain, I.; Ashraf, M.Y.; Saleem, M.H.; Ashraf, M.A.; Ali, B.; Shereen, A.; Farid, G.; Ali, M.; Shirazi, M.U.; Saleem, A.; et al. Alleviating effects of salicylic acid spray on stage-based growth and antioxidative defense system in two drought-stressed rice (Oryza sativa L.) cultivars. Turk. J. Agric. For. 2023, 47, 79–99. [Google Scholar]
- Loutfy, N.; Azooz, M.; Abou Alhamd, M.F. Exogenously-applied salicylic acid and ascorbic acid modulate some physiological traits and antioxidative defense system in Zea mays L. seedlings under drought stress. Egypt. J. Bot. 2020, 60, 313–324. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Kumar, P.; Upadhyay, S.K. Seed priming and foliar application of chitosan ameliorate drought stress responses in mungbean genotypes through modulation of morpho-physiological attributes and increased antioxidative defense mechanism. J. Plant Growth Regul. 2022, 42, 6137–6154. [Google Scholar] [CrossRef]
- Aazami, M.A.; Maleki, M.; Rasouli, F.; Gohari, G. Protective effects of chitosan based salicylic acid nanocomposite (CS-SA NCs) in grape (Vitis vinifera cv.‘Sultana’) under salinity stress. Sci. Rep. 2023, 13, 883. [Google Scholar] [CrossRef]
- Akhtar, G.; Faried, H.N.; Razzaq, K.; Ullah, S.; Wattoo, F.M.; Shehzad, M.A.; Sajjad, Y.; Ahsan, M.; Javed, T.; Dessoky, E.S.; et al. Chitosan-induced physiological and biochemical regulations confer drought tolerance in pot marigold (Calendula officinalis L.). Agronomy 2022, 12, 474. [Google Scholar] [CrossRef]
- Moolphuerk, N.; Lawson, T.; Pattanagul, W. Chitosan mitigates the adverse effects and improves photosynthetic activity in rice (Oryza sativa L.) seedlings under drought condition. J. Crop Improv. 2022, 36, 638–655. [Google Scholar] [CrossRef]
- Hembade, V.L.; Yashveer, S.; Taunk, J.; Sangwan, S.; Tokas, J.; Singh, V.; Redhu, N.S.; Grewal, S.; Malhotra, S.; Kumar, M. Chitosan-Salicylic acid and Zinc sulphate nano-formulations defend against yellow rust in wheat by activating pathogenesis-related genes and enzymes. Plant Physiol. Biochem. 2022, 192, 129–140. [Google Scholar] [CrossRef]
- Mohammadzadeh, S.; Pirzad, A. Biochemical responses of mycorrhizal-inoculated Lamiaceae (Lavender, Rosemary and Thyme) plants to drought: A field study. Soil Sci. Plant Nutr. 2020, 67, 41–49. [Google Scholar] [CrossRef]
- Vosoughi, N.; Gomarian, M.; Pirbalouti, A.G.; Khaghani, S.; Malekpoor, F. Essential oil composition and total phenolic, flavonoid contents, and antioxidant activity of sage (Salvia officinalis L.) extract under chitosan application and irrigation frequencies. Ind. Crops Prod. 2018, 117, 366–374. [Google Scholar] [CrossRef]
- Gaafar, A.A.; Ali, S.I.; El-Shawadfy, M.A.; Salama, Z.A.; Sękara, A.; Ulrichs, C.; Abdelhamid, M.T. Ascorbic acid induces the increase of secondary metabolites, antioxidant activity, growth, and productivity of the common bean under water stress conditions. Plants 2020, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Shohani, F.; Fazeli, A.; Sarghein, S.H. The effect of silicon application and salicylic acid on enzymatic and non-enzymatic reactions of Scophularia striata L. under drought stress. Sci. Hortic. 2023, 319, 112143. [Google Scholar] [CrossRef]
- Abd-Rabbu, H.S.; Wahba, H.E.; Khalid, K.A. The effects of foliar application of chitosan on the morphological and chemical characters of French lavender against water deficiency. Vegetos 2023. [Google Scholar] [CrossRef]
- Mohkami, Z.; Bidarnamani, F. The effect of chitosan and salicylic acid elicitors on morphological and phytochemical properties of Thymus daenensis Celak. Crop Sci. Res. Arid. Reg. 2023, 4, 503–517. [Google Scholar]
- Arpanahi, A.A.; Feizian, M.; Mehdipourian, G.; Khojasteh, D.N. Arbuscular mycorrhizal fungi inoculation improve essential oil and physiological parameters and nutritional values of Thymus daenensis Celak and Thymus vulgaris L. under normal and drought stress conditions. Eur. J. Soil Biol. 2020, 100, 103217. [Google Scholar] [CrossRef]
- Saurabh, V.; Barman, K.; Singh, A.K. Synergistic effect of salicylic acid and chitosan on postharvest life and quality attributes of jamun (Syzygium cumini Skeels) fruit. Acta Physiol. Plant. 2019, 41, 89. [Google Scholar] [CrossRef]
- Mokhtassi-Bidgoli, A.; AghaAlikhani, M.; Nassiri-Mahallati, M.; Zand, E.; GonzalezAzndujar, J.L.; Azari, A. Agronomic performance, seed quality and nitrogen uptake of Descurainia sophia in response to different nitrogen rates and water regimes. Ind. Crop. Prod. 2013, 44, 583–592. [Google Scholar] [CrossRef]
- Clevenger, J.F. Apparatus for the determination of volatile oil. J. Am. Pharm. Assoc. 1928, 17, 345–349. [Google Scholar] [CrossRef]
- Costa, O.B.D.; Del Menezzi, C.H.S.; Benedito, L.E.C.; Resck, I.S.; Vieira, R.F.; Ribeiro Bizzo, H. Essential oil constituents and yields from leaves of Blepharocalyx salicifolius (Kunt) O. Berg and Myracrodruon urundeuva (Allemão) collected during daytime. Int. J. For. Res. 2014, 2014, 982576. [Google Scholar] [CrossRef]
- Saadat, B.; Pirzad, A.; Jalilian, J. Yield-related biochemical response of understory mycorrhizal yellow sweet clover (Melilotus officinalis L.) to drought in agrisilviculture. Arch. Agron. Soil Sci. 2021, 67, 603–1620. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Paquin, R.; Lechasseur, P. Observations sur une méthode de dosage de la proline libre dans les extraits de plantes. Can. J. Bot. 1979, 57, 1851–1854. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Tejera, G.N.A.; Olivera, M.; Iribarne, C.; Lluch, C. Partial purification and characterization of a non-specific acid phosphatase in leaves and root nodules of Phaseolus vulgaris. Plant Physiol. Biochem. 2004, 42, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts: Its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in bulgaria fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Perkin, E. Analytical Methods for Atomic Absorbtion Spectrophotometry; Perkin-Elmer Inc.: Waltham, MA, USA, 1982. [Google Scholar]
- Waling, I.; Van Vark, W.; Houba, V.J.G.; Van der Lee, J.J. Soil and Plant Analysis, a Series of Syllabi: Plant Analysis Procedures; Wageningen Agriculture University Press: Wageningen, The Netherland, 1989. [Google Scholar]
- Schuman, G.E.; Stanley, M.A.; Knudsen, D. Automated total nitrogen analysis of soil and plant samples. Soil Sci. Soc. Am. J. 1973, 37, 480–481. [Google Scholar] [CrossRef]
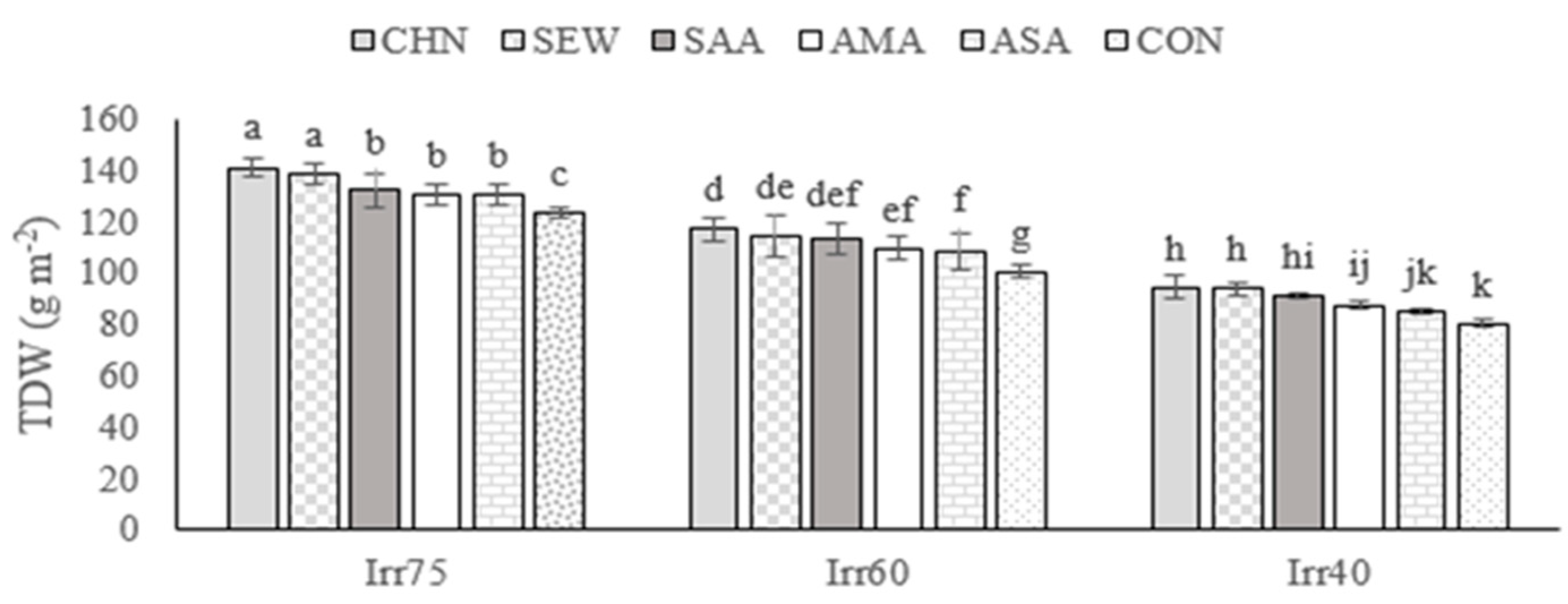
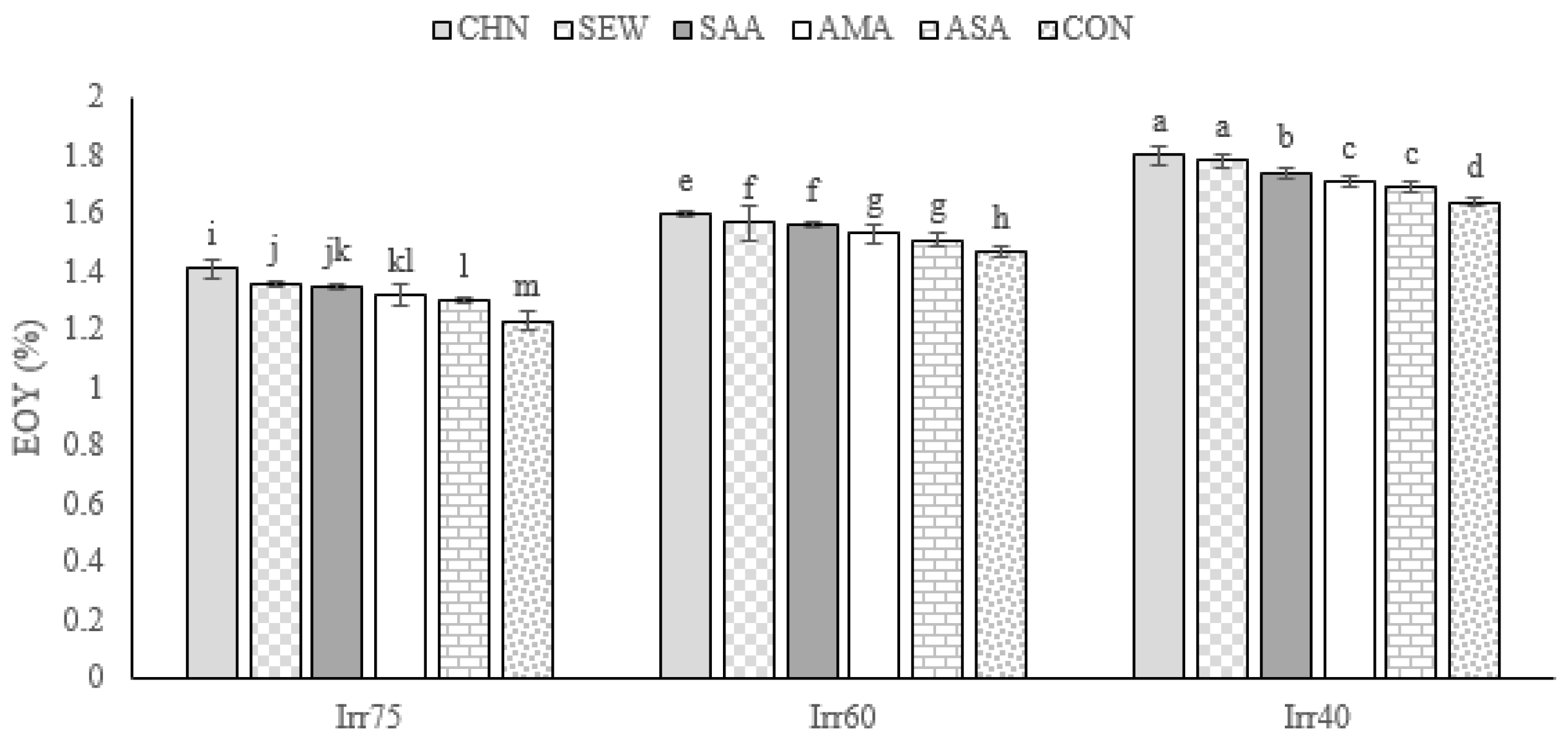
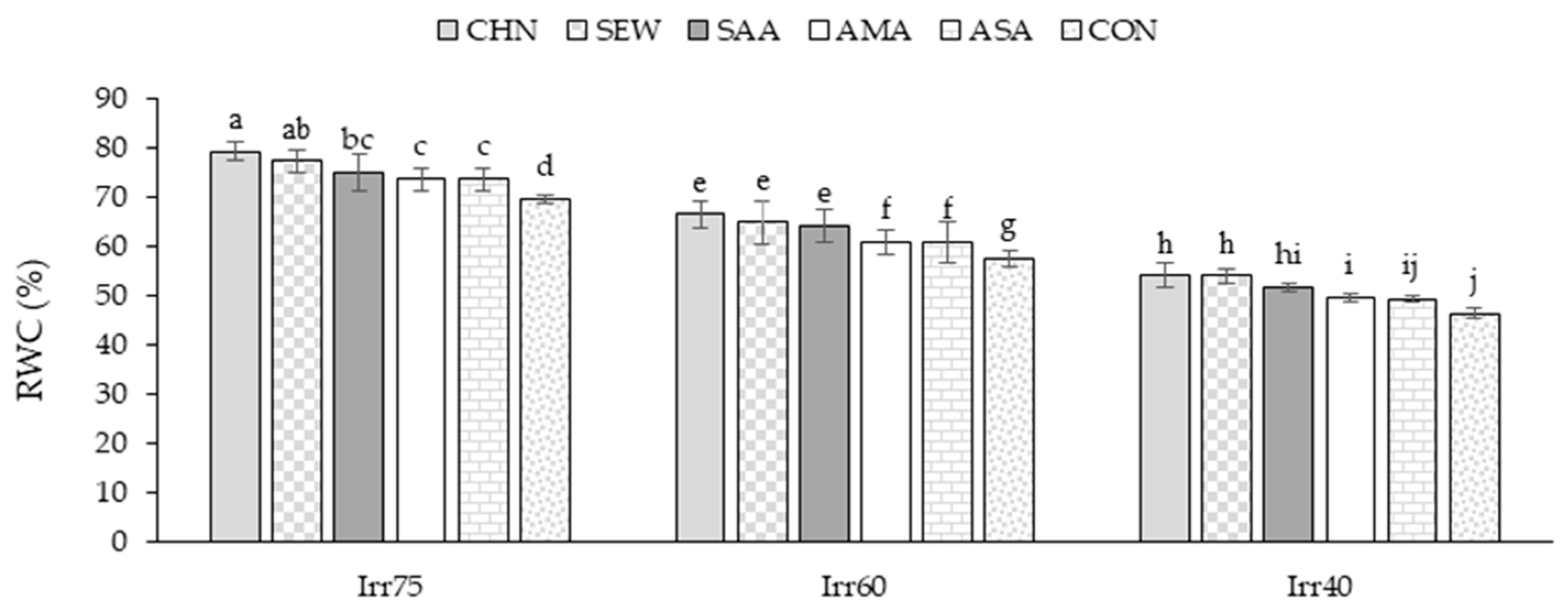
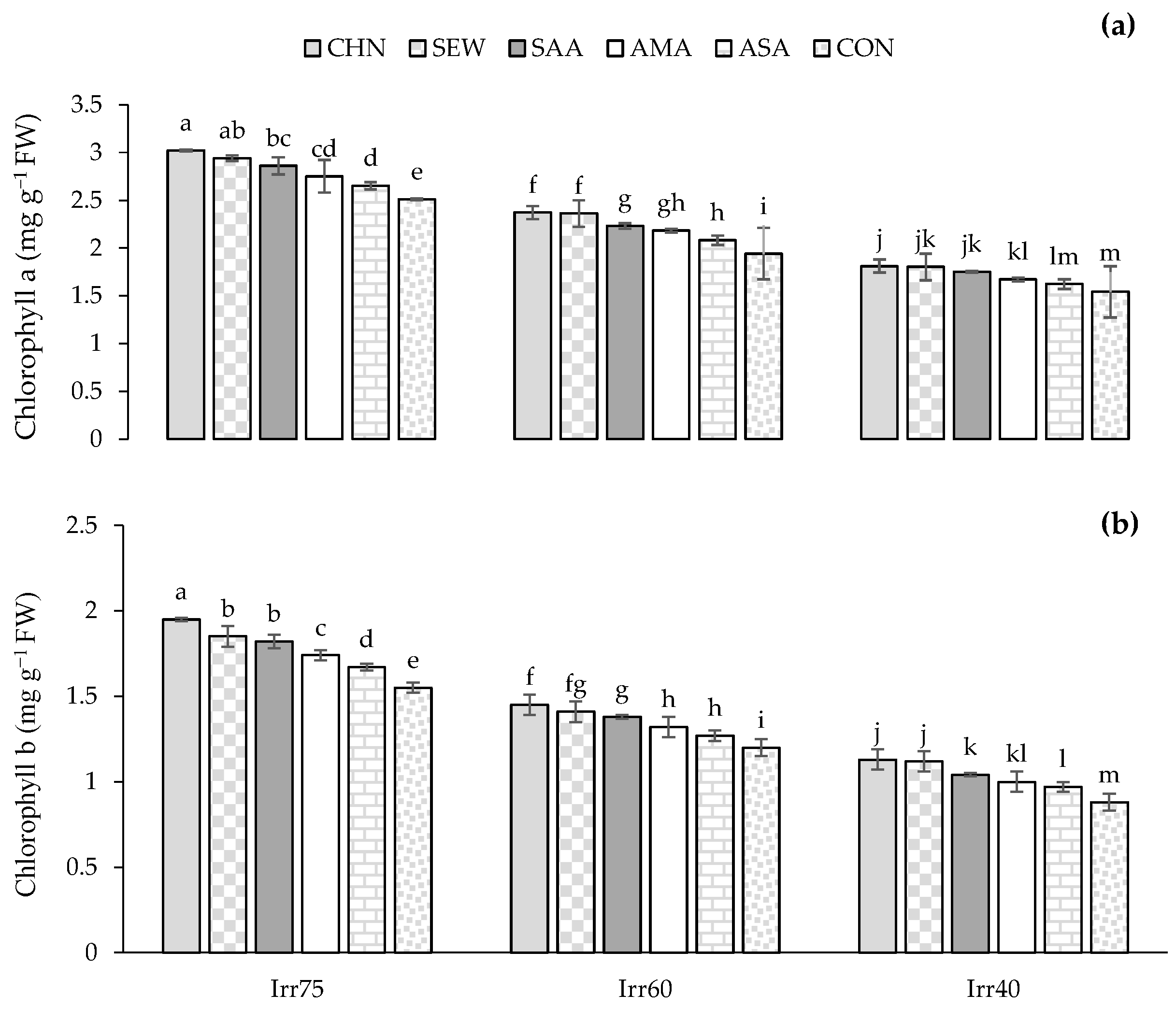

| Source of Variation | df | TDW (g m−2) | EOY (%) | RWC (%) | Chl a (mg g−1 FW) | Chl b (mg g−1 FW) |
|---|---|---|---|---|---|---|
| Year (Y) | 1 | 201.93 * | 0.0003 ns | 65.27 ** | 0.0003 ns | 0.0001 ns |
| Rep (Y) | 4 | 0.50 | 0.001 | 0.24 | 0.10 | 0.007 |
| (Irr) | 2 | 10,483.58 ** | 0.43 ** | 3128.56 ** | 8.85 ** | 4.58 ** |
| Y × Irr | 2 | 6.94 ns | 0.0009 ns | 1.93 ns | 0.0000009 ns | 0.00003 ns |
| (Str) | 5 | 2119.89 ** | 0.31 ** | 619.68 ** | 0.82 ** | 0.19 ** |
| Y × Str | 5 | 2.28 ns | 0.0002 ns | 0.84 ns | 0.00002 ns | 0.00005 ns |
| Irr × Str | 10 | 644.55 ** | 0.07 ** | 184.34 ** | 0.19 ** | 0.08 ** |
| Y × Irr × Str | 10 | 2.81 ns | 0.0004 ns | 0.45 ns | 0.00004 ns | 0.00003 ns |
| Error | 68 | 21.17 | 0.006 | 6.83 | 0.01 | 0.002 |
| CV (%) | 4.15 | 1.64 | 4.16 | 4.82 | 3.54 | |
| Y | ||||||
| 2018 | 109.35 ± 19.81 b | 1.54 ± 0.17 a | 61.92 ± 10.73 b | 2.22 ± 0.49 a | 1.37 ± 0.30 a | |
| 2019 | 112.08 ± 18.79 a | 1.53 ± 0.18 a | 63.48 ± 10.26 a | 2.23 ± 0.51 a | 1.38 ± 0.32 a | |
| Irr | ||||||
| Irr75 | 132.79 ± 7.05 a | 1.33 ± 0.06 c | 72.66 ± 7.05 a | 2.71 ± 0.15 a | 1.76 ± 0.11 a | |
| Irr60 | 109.14 ± 6.68 b | 1.54 ± 0.05 b | 61.25 ± 8.60 b | 2.25 ± 0.29 b | 1.30 ± 0.16 b | |
| Irr40 | 90.21 ± 6.62 c | 1.73 ± 0.06 a | 54.19 ± 5.75 c | 1.72 ± 0.32 c | 1.06 ± 0.14 c | |
| Str | ||||||
| CHN | 115.94 ± 18.90 a | 1.57 ± 0.18 a | 71.17 ± 8.74 a | 2.49 ± 0.60 a | 1.53 ± 0.36 a | |
| SEW | 113.28 ± 15.63 ab | 1.55 ± 0.21 ab | 68.02 ± 5.69 b | 2.39 ± 0.49 b | 1.41 ± 0.33 b | |
| SAA | 112.99 ± 20.06 ab | 1.55 ± 0.12 b | 63.18 ± 8.13 c | 2.28 ± 0.22 c | 1.41 ± 0.24 b | |
| AMA | 112.05 ± 17.19 b | 1.54 ± 0.18 b | 59.58 ± 8.10 d | 2.20 ± 0.33 d | 1.35 ± 0.23 c | |
| ASA | 108.61 ± 21.71 c | 1.53 ± 0.15 c | 57.41 ± 6.02 e | 2.11 ± 0.53 e | 1.31 ± 0.37 d | |
| CON | 101.42 ± 16.68 d | 1.44 ± 0.19 d | 56.86 ± 4.77 e | 1.89 ± 0.44 f | 1.23 ± 0.32 e |
| Source of Variation | df | Pro (μg g−1 FW) | TSS (μmol g−1 FW) | CAT (μmol min−1 g−1 FW) | SOD (μmol min−1 g−1 FW) | APX (μmol min−1 g−1 FW) | TPC (mg g−1 DW) | TFC (mg g−1 DW) | N (%) | P (%) | K (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year (Y) | 1 | 1.60 ** | 3.93 ns | 0.74 ** | 61.59 ** | 0.03 ** | 67.97 ** | 0.31 ** | 0.002 ns | 0.00002 ns | 0.00001 ns |
| Rep (Y) | 4 | 0.02 | 0.48 | 0.01 | 0.24 | 0.002 | 1.14 | 0.004 | 0.005 | 0.0003 | 0.001 |
| (Irr) | 2 | 14.31 ** | 1471.36 ** | 6.64 ** | 2459.59 ** | 0.57 ** | 572.20 ** | 6.30 ** | 3.38 ** | 0.12 ** | 1.28 ** |
| Y × Irr | 2 | 0.005 ns | 0.34 ns | 0.30 ** | 2.47 ns | 0.001 ns | 0.22 ns | 0.005 ns | 0.0006 ns | 0.000003 ns | 0.0004 ns |
| (Str) | 5 | 1.16 ** | 14.81 ** | 2.25 ** | 452.3 ** | 0.07 ** | 45.95 ** | 0.63 ** | 0.25 ** | 0.02 ** | 0.10 ** |
| Y × Str | 5 | 0.00003 ns | 0.01 ns | 0.05 ns | 0.82 ns | 0.0002 ns | 0.0001 ns | 0.0002 ns | 0.001 ns | 0.000005 ns | 0.0001 ns |
| Irr × Str | 10 | 0.28 ** | 13.57 ** | 0.92 ** | 175.94 ** | 0.05 ** | 10.99 ** | 0.11 ** | 0.13 ** | 0.008 ** | 0.03 ** |
| Y × Irr × Str | 10 | 0.00001 ns | 0.009 ns | 0.06 ns | 0.32 ns | 0.0001 ns | 0.00004 ns | 0.0001 ns | 0.0008 ns | 0.000008 ns | 0.0002 ns |
| Error | 68 | 0.006 | 1.27 | 0.04 | 6.83 | 0.0008 | 0.29 | 0.002 | 0.002 | 0.0001 | 0.0006 |
| CV (%) | 2.56 | 6.61 | 9.52 | 5.51 | 3.18 | 2.17 | 2.03 | 1.81 | 3.83 | 1.82 | |
| Y | |||||||||||
| 2018 | 3.34 ± 2.13 a | 16.87 ± 5.49 a | 2.37 ± 2.13 a | 48.17 ± 9.66 a | 0.95 ± 0.14 a | 25.61 ± 3.83 a | 2.54 ± 0.41 a | 2.65 ± 0.30 a | 0.26 ± 0.05 a | 1.39 ± 0.18 a | |
| 2019 | 3.10 ± 0.59 b | 17.25 ± 5.57 a | 2.20 ± 2.15 b | 46.66 ± 9.18 b | 0.91 ± 0.13 b | 24.02 ± 3.72 b | 2.43 ± 0.40 b | 2.68 ± 0.28 a | 0.28 ± 0.06 a | 1.42 ± 0.19 a | |
| Irr | |||||||||||
| Irr75 | 2.58 ± 0.38 c | 10.54 ± 1.89 c | 1.89 ± 0.46 c | 39.81 ± 6.85 c | 0.81 ± 0.12 c | 20.75 ± 2.38 c | 2.09 ± 0.27 c | 2.97 ± 0.17 a | 0.34 ± 0.04 a | 1.58 ± 0.09 a | |
| Irr60 | 3.23 ± 0.32 b | 17.32 ± 1.57 b | 2.21 ± 0.47 b | 46.21 ± 7.49 b | 0.91 ± 0.10 b | 24.98 ± 2.02 b | 2.43 ± 0.21 b | 2.62 ± 0.19 b | 0.27 ± 0.05 b | 1.41 ± 0.09 b | |
| Irr40 | 3.84 ± 0.25 a | 23.32 ± 1.62 a | 2.74 ± 0.55 a | 56.21 ± 5.23 a | 1.06 ± 0.07 a | 28.72 ± 1.62 a | 2.92 ± 0.15 a | 2.36 ± 0.13 c | 0.22 ± 0.04 c | 1.20 ± 0.10 c | |
| Str | |||||||||||
| CHN | 2.82 ± 0.56 e | 18.50 ± 5.29 a | 2.81 ± 0.51 a | 53.88 ± 9.13 a | 1.02 ± 0.15 a | 26.44 ± 3.48 a | 2.68 ± 0.37 a | 2.83 ± 0.26 a | 0.32 ± 0.05 a | 1.51 ± 0.15 a | |
| SEW | 3.05 ± 0.57 d | 17.51 ± 4.96 b | 2.63 ± 0.19 b | 52.30 ± 4.88 a | 1.00 ± 0.10 b | 26.04 ± 3.44 b | 2.64 ± 0.41 b | 2.72 ± 0.23 b | 0.31 ± 0.03 b | 1.45 ± 0.12 b | |
| SAA | 3.17 ± 0.61 c | 17.09 ± 5.80 b | 2.18 ± 0.67 c | 48.88 ± 12.33 b | 0.93 ± 0.20 c | 25.82 ± 3.97 b | 2.59 ± 0.44 c | 2.67 ± 0.26 c | 0.27 ± 0.09 c | 1.39 ± 0.17 c | |
| AMA | 3.38 ± 0.59 b | 17.03 ± 4.71 b | 2.15 ± 0.81 c | 44.46 ± 11.43 c | 0.88 ± 0.17 d | 24.45 ± 3.84 c | 2.46 ± 0.33 d | 2.63 ± 0.30 d | 0.26 ± 0.08 d | 1.35 ± 0.17 d | |
| ASA | 3.43 ± 0.38 ab | 16.20 ± 5.77 c | 1.99 ± 0.37 d | 42.79 ± 4.53 cd | 0.88 ± 0.05 d | 23.88 ± 2.40 d | 2.32 ± 0.20 e | 2.53 ± 0.18 e | 0.24 ± 0.04 e | 1.34 ± 0.13 e | |
| CON | 3.47 ± 0.70 a | 16.02 ± 6.69 c | 1.94 ± 0.33 d | 42.16 ± 3.81 d | 0.87 ± 0.04 d | 22.24 ± 4.37 e | 2.21 ± 0.44 f | 2.50 ± 0.42 e | 0.23 ± 0.04 e | 1.31 ± 0.26 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdali, R.; Rahimi, A.; Siavash Moghaddam, S.; Heydarzadeh, S.; Arena, C.; Vitale, E.; Zamanian, M. The Role of Stress Modifier Biostimulants on Adaptive Strategy of Oregano Plant for Increasing Productivity under Water Shortage. Plants 2023, 12, 4117. https://doi.org/10.3390/plants12244117
Abdali R, Rahimi A, Siavash Moghaddam S, Heydarzadeh S, Arena C, Vitale E, Zamanian M. The Role of Stress Modifier Biostimulants on Adaptive Strategy of Oregano Plant for Increasing Productivity under Water Shortage. Plants. 2023; 12(24):4117. https://doi.org/10.3390/plants12244117
Chicago/Turabian StyleAbdali, Reza, Amir Rahimi, Sina Siavash Moghaddam, Saeid Heydarzadeh, Carmen Arena, Ermenegilda Vitale, and Mohammad Zamanian. 2023. "The Role of Stress Modifier Biostimulants on Adaptive Strategy of Oregano Plant for Increasing Productivity under Water Shortage" Plants 12, no. 24: 4117. https://doi.org/10.3390/plants12244117
APA StyleAbdali, R., Rahimi, A., Siavash Moghaddam, S., Heydarzadeh, S., Arena, C., Vitale, E., & Zamanian, M. (2023). The Role of Stress Modifier Biostimulants on Adaptive Strategy of Oregano Plant for Increasing Productivity under Water Shortage. Plants, 12(24), 4117. https://doi.org/10.3390/plants12244117








