Unraveling the Potential of Organic Oregano and Tarragon Essential Oils: Profiling Composition, FT-IR and Bioactivities
Abstract
:1. Introduction
- Describing the volatile profile of the two EOs;
- Identifying distinctive FT-IR bands;
- Determining the antibacterial activity;
- Screening cytotoxicity on cell lines.
2. Results
2.1. GC-MS Volatile Profile
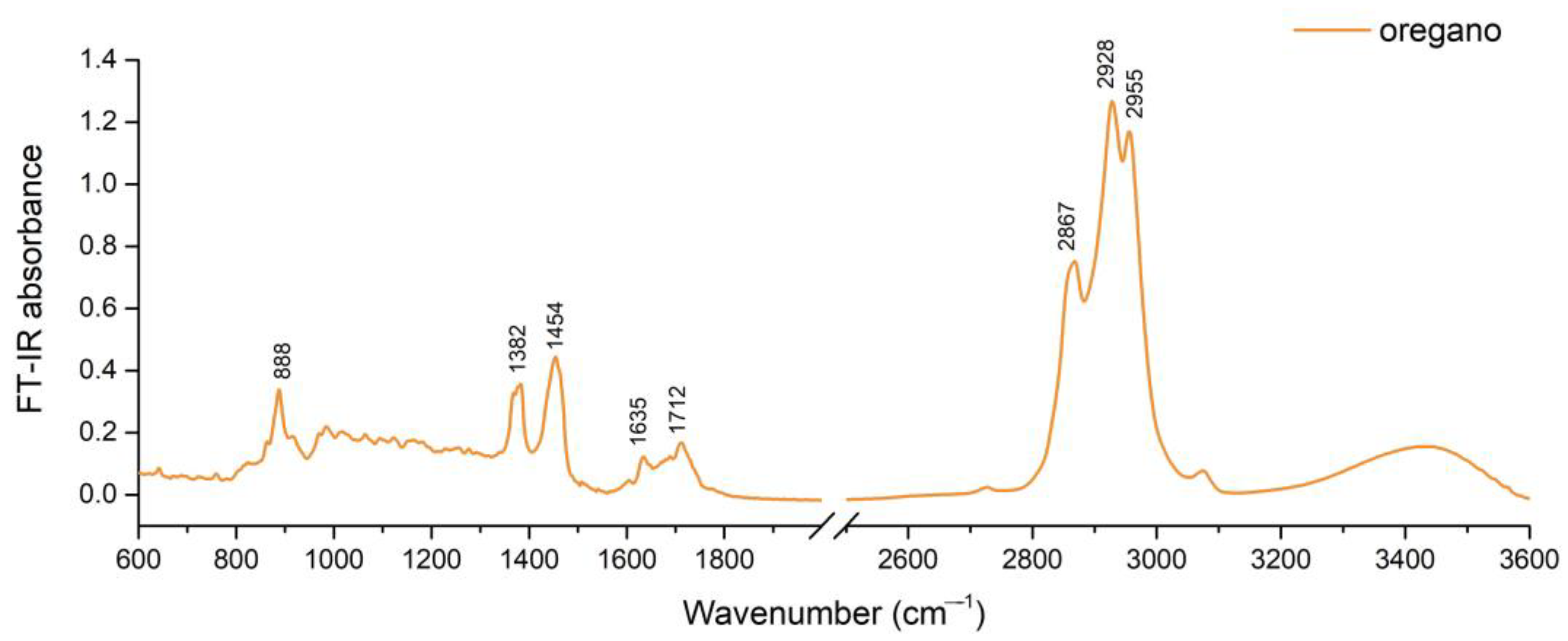
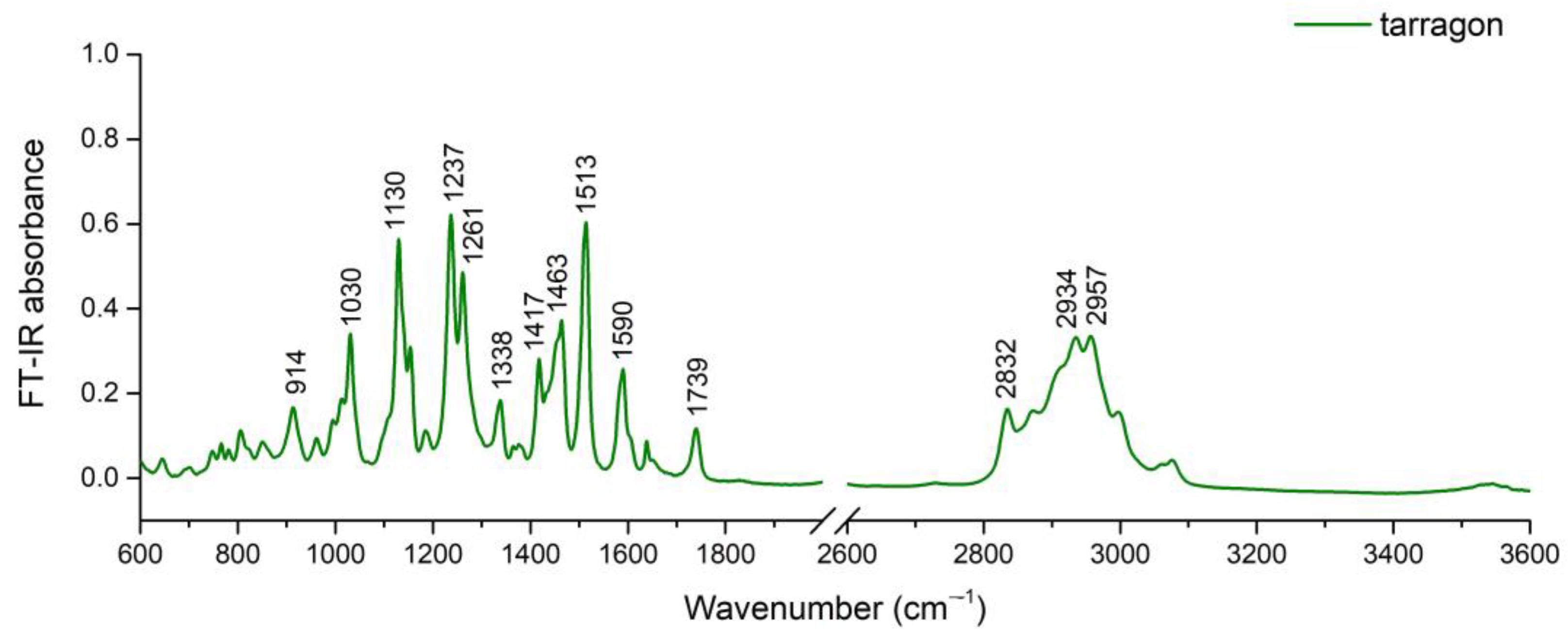
2.2. FT-IR Spectra
2.3. Antibacterial Potential
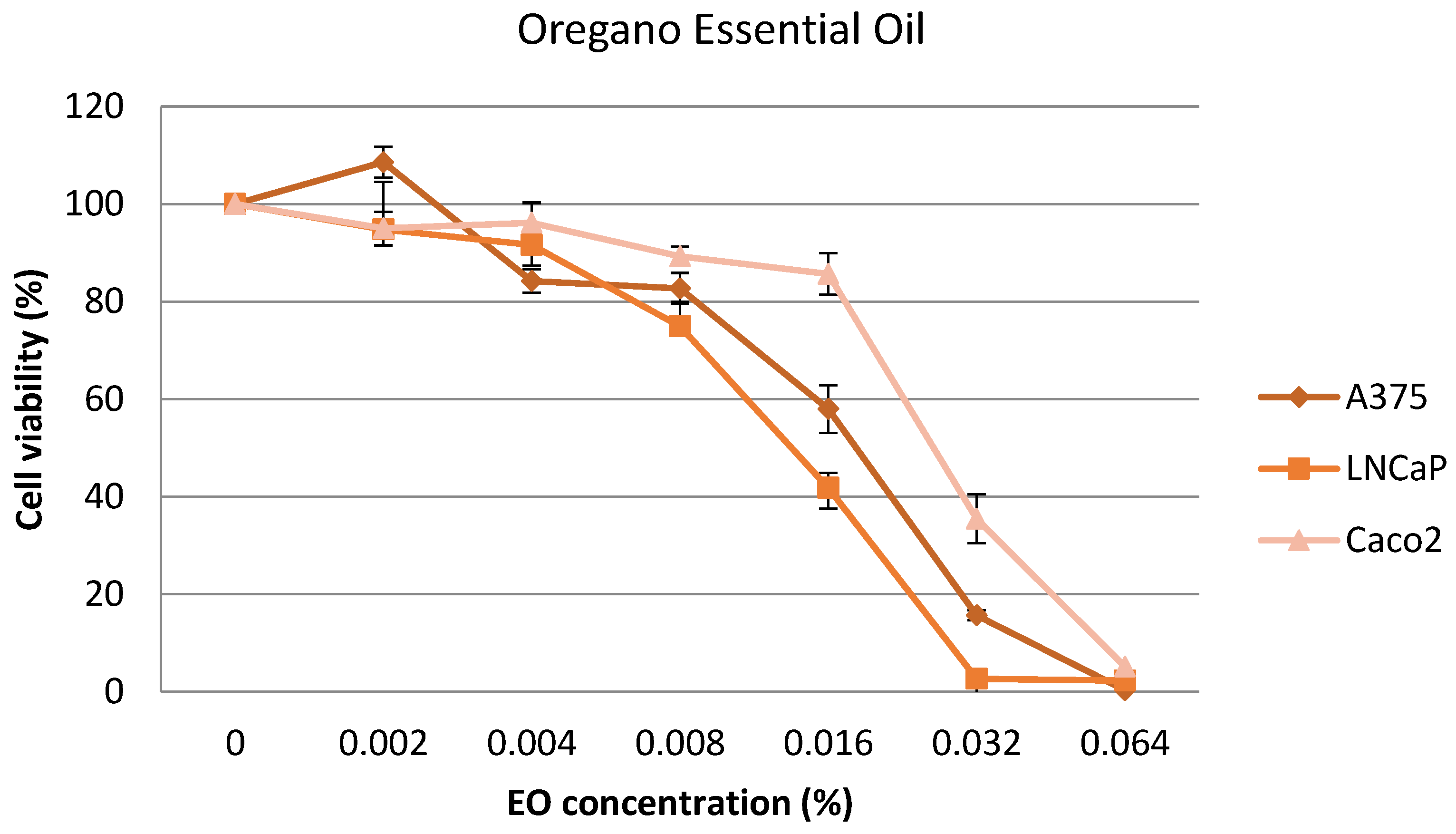
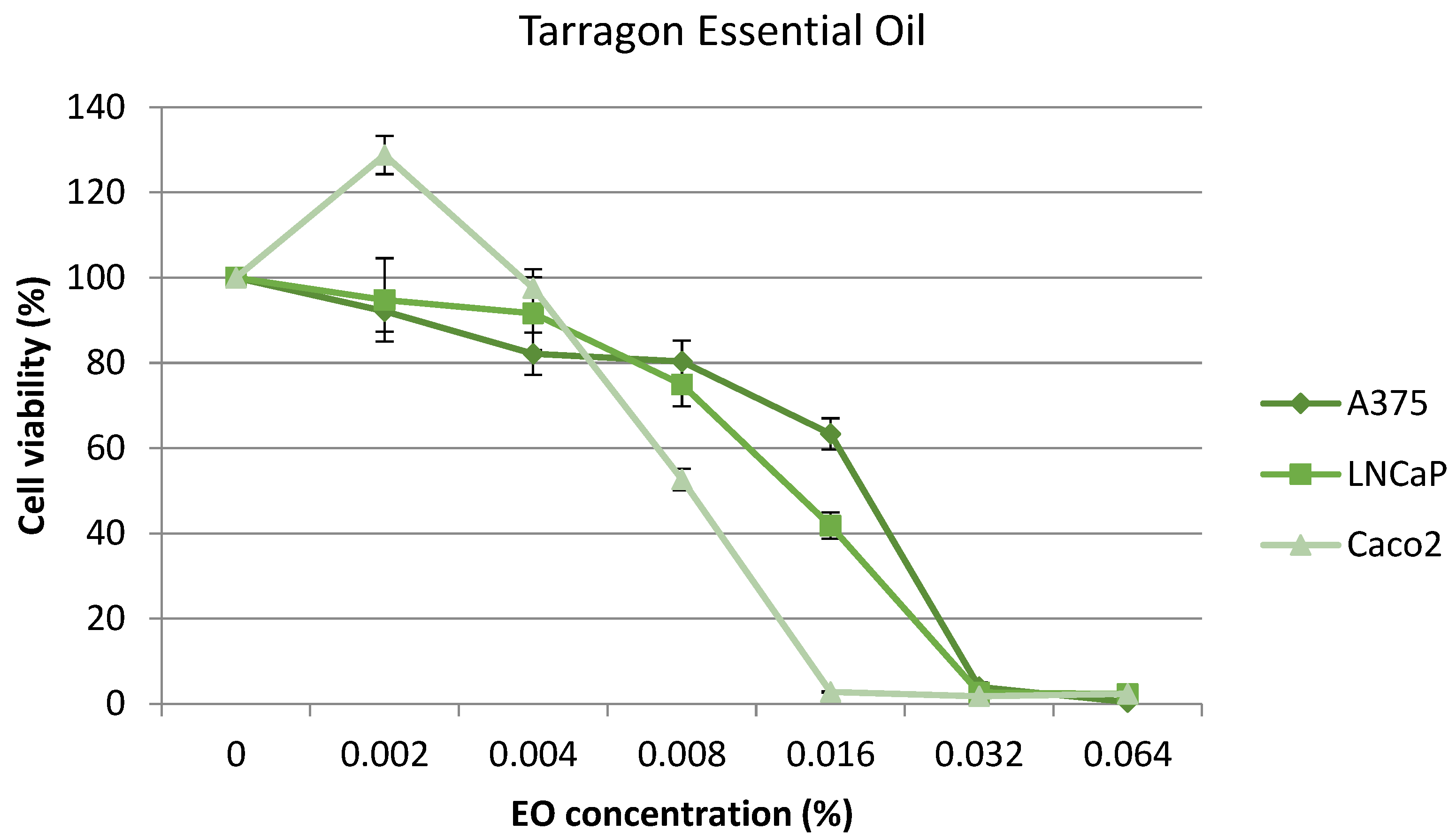
2.4. Cytotoxic Effect on Cell Lines
3. Discussion
4. Materials and Methods
4.1. Herb Cultivation and Essential Oil Extraction
4.2. GC-MS Volatile Profiling
4.3. FT-IR Analysis
4.4. Antibacterial Activity
4.4.1. Preparation of Microbial Strains
4.4.2. Assessment of Minimum Inhibitory Concentration (MIC)
4.4.3. Assessment of the Minimum Bactericidal Concentration (MBC)
4.5. Cytotoxic Effect on Cell Lines
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Opara, E.I.; Chohan, M. Culinary Herbs and Spices: Their Bioactive Properties, the Contribution of Polyphenols and the Challenges in Deducing Their True Health Benefits. Int. J. Mol. Sci. 2014, 15, 19183–19202. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Dima, S. Essential Oils in Foods: Extraction, Stabilization, and Toxicity. Curr. Opin. Food Sci. 2015, 5, 29–35. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E. Essential Oils from Herbs and Spices as Natural Antioxidants: Diversity of Promising Food Applications in the Past Decade. Food Rev. Int. 2022, 38, 403–433. [Google Scholar] [CrossRef]
- Pisseri, F.; Bertoli, A.; Pistelli, L. Essential Oils in Medicine: Principles of Therapy. Parassitologia 2008, 50, 89–91. [Google Scholar] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A Status Review on the Medicinal Properties of Essential Oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- El-Shemy, H. Potential of Essential Oils; IntechOpen: London, UK, 2018; ISBN 978-1-78923-779-5. [Google Scholar]
- Hall, H.N.; Wilkinson, D.J.; Le Bon, M. Oregano Essential Oil Improves Piglet Health and Performance through Maternal Feeding and Is Associated with Changes in the Gut Microbiota. Anim. Microbiome 2021, 3, 2. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Alekseeva, M.; Zagorcheva, T.; Atanassov, I.; Rusanov, K. Origanum vulgare L.—A Review on Genetic Diversity, Cultivation, Biological Activities and Perspectives for Molecular Breeding. Bulg. J. Agric. Sci. 2020, 26, 1183–1197. [Google Scholar]
- Shafiee-Hajiabad, M.; Hardt, M.; Honermeier, B. Comparative Investigation about the Trichome Morphology of Common Oregano (Origanum vulgare L. subsp. vulgare) and Greek Oregano (Origanum vulgare L. subsp. hirtum). J. Appl. Res. Med. Aromat. Plants 2014, 1, 50–58. [Google Scholar] [CrossRef]
- Skoufogianni, E.; Solomou, A.D.; Danalatos, N.G. Ecology, Cultivation and Utilization of the Aromatic Greek Oregano (Origanum vulgare L.): A Review. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 545–552. [Google Scholar] [CrossRef]
- Ekiert, H.; Świątkowska, J.; Knut, E.; Klin, P.; Rzepiela, A.; Tomczyk, M.; Szopa, A. Artemisia dracunculus (Tarragon): A Review of Its Traditional Uses, Phytochemistry and Pharmacology. Front. Pharmacol. 2021, 12, 653993. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Karimzadeh, G.; Naghavi, M.R.; Naghdi Badi, H.; Rashidi Monfared, S. Expression of Artemisinin Biosynthesis and Trichome Formation Genes in Five Artemisia Species. Ind. Crops Prod. 2018, 112, 130–140. [Google Scholar] [CrossRef]
- Fildan, A.P.; Pet, I.; Stoin, D.; Bujanca, G.; Lukinich-Gruia, A.T.; Jianu, C.; Jianu, A.M.; Radulescu, M.; Tofolean, D.E. Artemisia Dracunculus Essential Oil Chemical Composition and Antioxidant Properties. Rev. Chim. 2019, 70, 59–62. [Google Scholar] [CrossRef]
- Kamaraj, C.; Balasubramani, G.; Siva, C.; Raja, M.; Balasubramanian, V.; Raja, R.K.; Tamilselvan, S.; Benelli, G.; Perumal, P. Ag Nanoparticles Synthesized Using β-Caryophyllene Isolated from Murraya Koenigii: Antimalarial (Plasmodium Falciparum 3D7) and Anticancer Activity (A549 and HeLa Cell Lines). J. Clust. Sci. 2017, 28, 1667–1684. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Vârban, D.; Zăhan, M.; Pop, C.R.; Socaci, S.; Ștefan, R.; Crișan, I.; Bota, L.E.; Miclea, I.; Muscă, A.S.; Deac, A.M.; et al. Physicochemical Characterization and Prospecting Biological Activity of Some Authentic Transylvanian Essential Oils: Lavender, Sage and Basil. Metabolites 2022, 12, 962. [Google Scholar] [CrossRef]
- Berechet, M.D.; Stelescu, M.D.; Manaila, E.; Craciun, G. Chemical Composition of the Essential Oil of Artemisia absinthium from Romania. REV CHIM 2015, 66, 1814–1818. [Google Scholar]
- Osanloo, M.; Firooziyan, S.; Abdollahi, A.; Hatami, S.; Nematollahi, A.; Elahi, N.; Zarenezhad, E. Nanoemulsion and Nanogel Containing Artemisia dracunculus Essential Oil; Larvicidal Effect and Antibacterial Activity. BMC Res. Notes 2022, 15, 276. [Google Scholar] [CrossRef]
- Otibi, F.A.; Rizwana, H. Chemical Composition, FTIR Studies, Morphological Alterations, and Antifungal Activity of Leaf Extracts of Artemisia sieberi from Saudi Arabia. Int. J. Agric. Biol. 2019, 21, 1241–1248. [Google Scholar]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental Chemistry of Essential Oils and Volatile Organic Compounds, Methods of Analysis and Authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Kemprai, P.; Protim Mahanta, B.; Sut, D.; Barman, R.; Banik, D.; Lal, M.; Proteem Saikia, S.; Haldar, S. Review on Safrole: Identity Shift of the ‘Candy Shop’ Aroma to a Carcinogen and Deforester. Flavour Fragr. J. 2020, 35, 5–23. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Methyl Eugenol: Its Occurrence, Distribution, and Role in Nature, Especially in Relation to Insect Behavior and Pollination. J. Insect Sci. 2012, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Khodakov, G.V.; Kotikov, I.V.; Pankovetskii, V.N. Component Composition of Essential Oil from Artemisia abrotanum and A. dracunculus. Chem. Nat. Compd. 2009, 45, 905–908. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Yang, X.-N.; Zhu, X.; Xiao, X.-R.; Yang, X.-W.; Qin, H.-B.; Gonzalez, F.J.; Li, F. Role of Metabolic Activation in Elemicin-Induced Cellular Toxicity. J. Agric. Food Chem. 2019, 67, 8243–8252. [Google Scholar] [CrossRef]
- Węglarz, Z.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Bączek, K. The Quality of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare) Cultivated in the Temperate Climate of Central Europe. Foods 2020, 9, 1671. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Dardioti, A.; Krigas, N.; Lanaras, T. Autumn Essential Oils of Greek Oregano. Phytochemistry 1997, 44, 883–886. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential Oil Diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef]
- Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Milenković, A.; Stanojević, J.; Cvetković, D. The Yield, Chemical Composition, and Antioxidant Activities of Essential Oils from Different Plant Parts of the Wild and Cultivated Oregano (Origanum vulgare L.). Horticulturae 2022, 8, 1042. [Google Scholar] [CrossRef]
- Jianu, C.; Lukinich-Gruia, A.T.; Rădulescu, M.; Mioc, M.; Mioc, A.; Șoica, C.; Constantin, A.T.; David, I.; Bujancă, G.; Radu, R.G. Essential Oil of Origanum vulgare var. Aureum L. from Western Romania: Chemical Analysis, In Vitro and In Silico Screening of Its Antioxidant Activity. Appl. Sci. 2023, 13, 5076. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Salami, S.A.; Nazeri, V.; Maggi, F.; Craker, L. Essential Oil Profile of Oregano (Origanum vulgare L.) Populations Grown under Similar Soil and Climate Conditions. Ind. Crops Prod. 2018, 119, 183–190. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Arnold, N.A.; Menichini, F.; Senatore, F. Composition, Antibacterial, Antioxidant and Antiproliferative Activities of Essential Oils from Three Origanum Species Growing Wild in Lebanon and Greece. Nat. Prod. Res. 2016, 30, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Benbrahim, C.; Barka, M.S.; Basile, A.; Maresca, V.; Flamini, G.; Sorbo, S.; Carraturo, F.; Notariale, R.; Piscopo, M.; Khadir, A.; et al. Chemical Composition and Biological Activities of Oregano and Lavender Essential Oils. Appl. Sci. 2021, 11, 5688. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical Composition and Bioactivity of Different Oregano (Origanum vulgare) Extracts and Essential Oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Alinkina, E.S.; Vorobyova, A.K.; Misharina, T.A.; Fatkullina, L.D.; Burlakova, E.B.; Khokhlov, A.N. Cytogerontological Studies of Biological Activity of Oregano Essential Oil. Mosc. Univ. Biol. Sci. Bull. 2012, 67, 52–57. [Google Scholar] [CrossRef]
- de Almeida, P.; Blanco-Pascual, N.; Rosolen, D.; Cisilotto, J.; Creczynski-Pasa, T.; Laurindo, J. Antioxidant and Antifungal Properties of Essential Oils of Oregano (Origanum vulgare) and Mint (Mentha arvensis) against Aspergillus Flavus and Penicillium Commune for Use in Food Preservation. Food Sci. Technol. 2022, 42, e64921. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) Essential Oil Provides Anti-Inflammatory Activity and Facilitates Wound Healing in a Human Keratinocytes Cell Model. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 144, 111586. [Google Scholar] [CrossRef]
- Cheng, C.; Zou, Y.; Peng, J. Oregano Essential Oil Attenuates RAW264.7 Cells from Lipopolysaccharide-Induced Inflammatory Response through Regulating NADPH Oxidase Activation-Driven Oxidative Stress. Molecules 2018, 23, 1857. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; De Feo, V.; Camele, I. Cytotoxic Activity of Origanum vulgare L. on Hepatocellular Carcinoma Cell Line HepG2 and Evaluation of Its Biological Activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Ciobanu, C.S.; Raita, M.S.; Ghegoiu, L.; Trusca, R.; Badea, M.L.; Cimpeanu, C. Studies of the Tarragon Essential Oil Effects on the Characteristics of Doped Hydroxyapatite/Chitosan Biocomposites. Polymers 2023, 15, 1908. [Google Scholar] [CrossRef] [PubMed]
- Sahakyan, N.; Andreoletti, P.; Cherkaoui-Malki, M.; Petrosyan, M.; Trchounian, A. Artemisia dracunculus L. Essential Oil Phytochemical Components Trigger the Activity of Cellular Antioxidant Enzymes. J. Food Biochem. 2021, 45, e13691. [Google Scholar] [CrossRef] [PubMed]
- Sharopov, F.S.; Salimov, A.; Numonov, S.; Bakri, M.; Sangov, Z.; Habasi, M.; Aisa, H.A.; Setzer, W.N. Phytochemical Study on the Essential Oils of Tarragon (Artemisia dracunculus L.) Growing in Tajikistan and Its Comparison with the Essential Oil of the Species in the Rest of the World. Nat. Prod. Commun. 2020, 15, 1934578X2097739. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between Essential Oil Components and Antibiotics: A Review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Hudson, J. Anti-Influenza Virus Activities of Commercial Oregano Oils and Their Carriers. J. Appl. Pharm. Sci. 2012, 2, 214–218. [Google Scholar] [CrossRef]
- Lambert, R.J.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The Antibacterial and Antifungal Activity of Six Essential Oils and Their Cyto/Genotoxicity to Human HEL 12469 Cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef]
- Safinejad, K.; Mohebifar, A.; Tolouei, H.; Monfared, P.; Razmjou, A. Comparative Study on the Toxicity of Mentha piperita L. and Artemisia dracunculus L. Hydroalcoholic Extracts on Human Breast Cancer Cell Lines. Int. J. Biol. Biotechnol. 2021, 18, 253–261. [Google Scholar]
- Socaciu, M.-I.; Fogarasi, M.; Simon, E.L.; Semeniuc, C.A.; Socaci, S.A.; Podar, A.S.; Vodnar, D.C. Effects of Whey Protein Isolate-Based Film Incorporated with Tarragon Essential Oil on the Quality and Shelf-Life of Refrigerated Brook Trout. Foods 2021, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.; Kalemba, D.; Dlugaszewska, J.; Thiem, B. Chemical Composition of Essential Oils from Rare and Endangered Species—Eryngium maritimum L. and E. alpinum L. Plants 2020, 9, 417. [Google Scholar] [CrossRef]
- Perrino, E.V.; Valerio, F.; Gannouchi, A.; Trani, A.; Mezzapesa, G. Ecological and Plant Community Implication on Essential Oils Composition in Useful Wild Officinal Species: A Pilot Case Study in Apulia (Italy). Plants 2021, 10, 574. [Google Scholar] [CrossRef]
- Bodea, I.M.; Cătunescu, G.M.; Pop, C.R.; Fiț, N.I.; David, A.P.; Dudescu, M.C.; Stănilă, A.; Rotar, A.M.; Beteg, F.I. Antimicrobial Properties of Bacterial Cellulose Films Enriched with Bioactive Herbal Extracts Obtained by Microwave-Assisted Extraction. Polymers 2022, 14, 1435. [Google Scholar] [CrossRef]
- McFarland, J. Nephelometer: An Instrument for Estimating the Number of Bacteria in Suspensions Used for Calculating the Opsonic Index and for Vaccines. J. Am. Med. Assoc. 1907, XLIX, 1176–1178. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial Activity and Interactions of Plant Essential Oil Combinations against Gram-Positive and Gram-Negative Bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef]
- Cerezo, A.B.; Cătunescu, G.M.; González, M.M.-P.; Hornedo-Ortega, R.; Pop, C.R.; Rusu, C.C.; Chirilă, F.; Rotar, A.M.; Garcia-Parrilla, M.C.; Troncoso, A.M. Anthocyanins in Blueberries Grown in Hot Climate Exert Strong Antioxidant Activity and May Be Effective against Urinary Tract Bacteria. Antioxidants 2020, 9, 478. [Google Scholar] [CrossRef]
| Class | Compound | Oregano | Tarragon |
|---|---|---|---|
| bicyclic monoterpene | Sabinene | 6.32 | 9.44 |
| acyclic monoterpene | trans-β-Ocimene | 2.85 | 2.21 |
| acyclic monoterpene | Linalool | 1.85 | 0.38 |
| acyclic monoterpenoids | (4E,6Z)-allo-Ocimene | 5.18 | 2.40 |
| acyclic monoterpenoids | Linalool acetate | 3.26 | - |
| acyclic monoterpenoid | 4-Terpineol | 2.35 | 2.72 |
| acyclic monoterpenoid | α-Terpineol | 0.4 | 1.13 |
| monoterpene | β-Citronellol | - | 4.74 |
| monoterpene | Geranyl acetate | - | 3.18 |
| monoterpene | D-Carvone | - | 1.11 |
| phenylpropanoid | Methyl eugenol | - | 29.19 |
| phenylpropanoid | Isoelemicin | - | 16.33 |
| phenylpropanoid | Elemicin | - | 11.43 |
| phenylpropanoid | Methylisoeugenol | - | 2.85 |
| phenylpropanoid | p-Allyl Anisole/Chavicol methyl ester | - | 1.13 |
| sesquiterpene | α-Bergamotene | - | 1.17 |
| sesquiterpene | β-Caryophyllene | 26.89 | 2.09 |
| sesquiterpene | α Bisabolene | 1.31 | 0.39 |
| sesquiterpene | β-Cubebene | 6.95 | - |
| sesquiterpene | β-Bourbonene | 5.40 | - |
| sesquiterpene | Germacrene | 4.05 | - |
| sesquiterpene | α-Farnesene | 2.95 | - |
| sesquiterpene | γ-Muurolene | 2.86 | - |
| sesquiterpene | δ Cadinene | 2.57 | - |
| sesquiterpene | γ-Cadinene | 1.63 | - |
| sesquiterpene | γ-Elemene | 1.51 | - |
| sesquiterpene | (E,E)-α-Farnesene | 1.48 | - |
| monocyclic sesquiterpene | α-Caryophyllene | 2.74 | - |
| sesquiterpene alcohol | -Spathulenol | 2.39 | 0.82 |
| sesquiterpenoid oxide | Caryophyllene oxide | 2.42 | 0.44 |
| sesquiterpenoid | α-Cadinol | 1.71 | 0.20 |
| Samples | Escherichia coli ATCC 25922 | Salmonella enteritidis ATCC 13076 | Staphylococcus aureus ATCC 6538P | Listeria monocytogenes ATCC 19114 | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (μL/mL) | MBC (μL/mL) | MIC (μL/mL) | MBC (μL/mL) | MIC (μL/mL) | MBC (μL/mL) | MIC (μL/mL) | MBC (μL/mL) | |
| Oregano | 0.27 ± 0.00 b | 1.17 ± 0.00 b | 0.095 ± 0.73 b | 0.27 ± 0.00 b | 0.42 ± 0.73 b | 0.56 ± 0.00 b | 0.87 ± 0.73 b | 2.45 ± 0.00 b |
| Tarragon | 2.45 ± 0.00 a | 10.80 ± 0.00 a | 1.17 ± 0.00 a | 5.14 ± 0.00 a | 5.14 ± 0.00 a | 10.80 ± 0.00 a | 2.45 ± 0.00 a | 10.80 ± 0.00 a |
| Gentamicin (μg/mL) | 0.24 ± 0.00 | 0.24 ± 0.00 | 0.50 ± 0.73 | 0.50 ± 0.73 | 0.05 ± 0.73 | 0.05 ± 0.73 | 0.50 ± 0.00 | 0.50 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vârban, D.; Zăhan, M.; Crișan, I.; Pop, C.R.; Gál, E.; Ștefan, R.; Rotar, A.M.; Muscă, A.S.; Meseșan, Ș.D.; Horga, V.; et al. Unraveling the Potential of Organic Oregano and Tarragon Essential Oils: Profiling Composition, FT-IR and Bioactivities. Plants 2023, 12, 4017. https://doi.org/10.3390/plants12234017
Vârban D, Zăhan M, Crișan I, Pop CR, Gál E, Ștefan R, Rotar AM, Muscă AS, Meseșan ȘD, Horga V, et al. Unraveling the Potential of Organic Oregano and Tarragon Essential Oils: Profiling Composition, FT-IR and Bioactivities. Plants. 2023; 12(23):4017. https://doi.org/10.3390/plants12234017
Chicago/Turabian StyleVârban, Dan, Marius Zăhan, Ioana Crișan, Carmen Rodica Pop, Emese Gál, Răzvan Ștefan, Ancuța Mihaela Rotar, Adriana Sebastiana Muscă, Ștefania Dana Meseșan, Vasile Horga, and et al. 2023. "Unraveling the Potential of Organic Oregano and Tarragon Essential Oils: Profiling Composition, FT-IR and Bioactivities" Plants 12, no. 23: 4017. https://doi.org/10.3390/plants12234017
APA StyleVârban, D., Zăhan, M., Crișan, I., Pop, C. R., Gál, E., Ștefan, R., Rotar, A. M., Muscă, A. S., Meseșan, Ș. D., Horga, V., Ladoși, I., Olar, L., Stoie, A., & Vârban, R. (2023). Unraveling the Potential of Organic Oregano and Tarragon Essential Oils: Profiling Composition, FT-IR and Bioactivities. Plants, 12(23), 4017. https://doi.org/10.3390/plants12234017












