An In Situ Evaluation of Different CAM Plants as Plant Microbial Fuel Cells for Energy Recovery in the Atacama Desert
Abstract
:1. Introduction
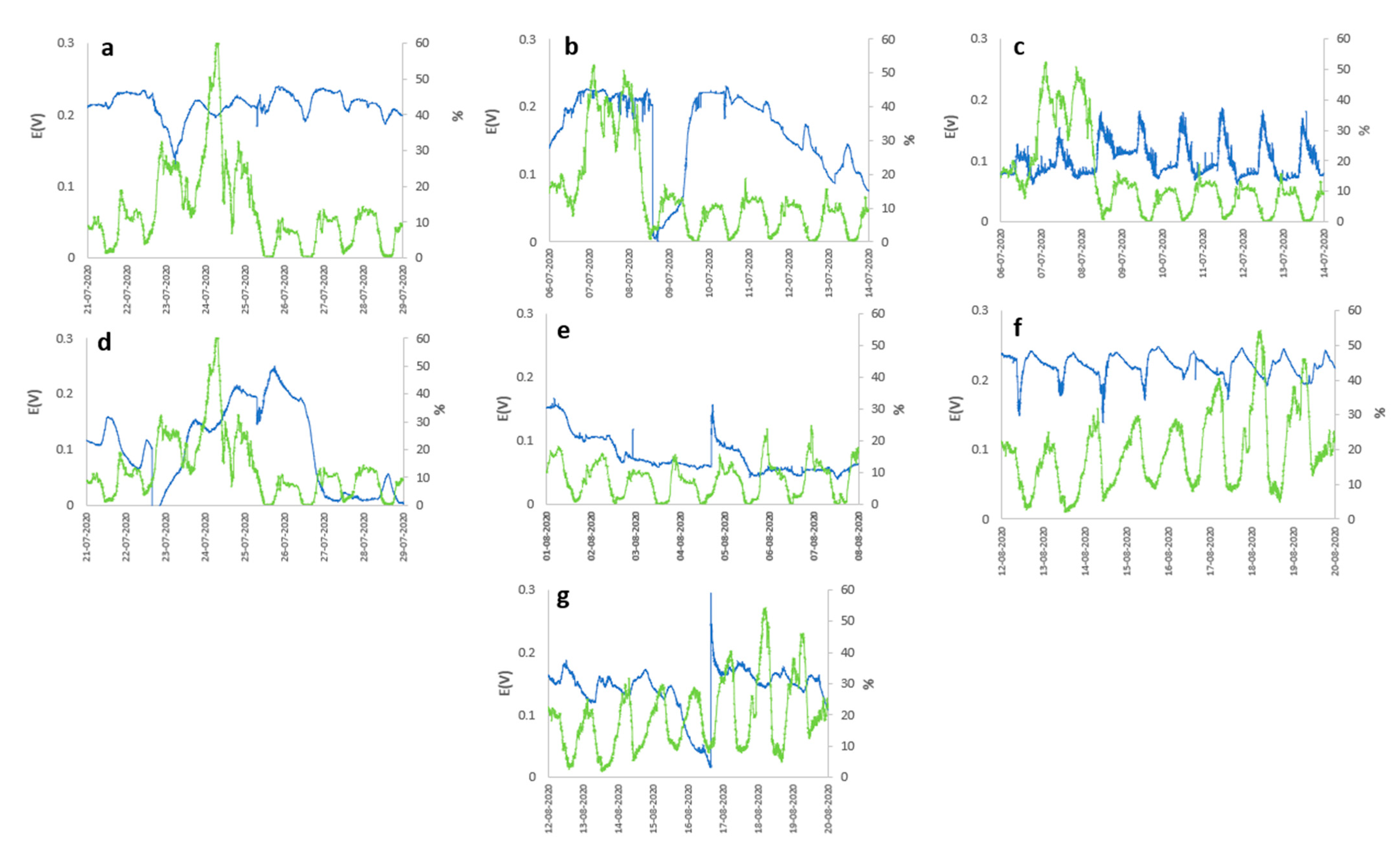
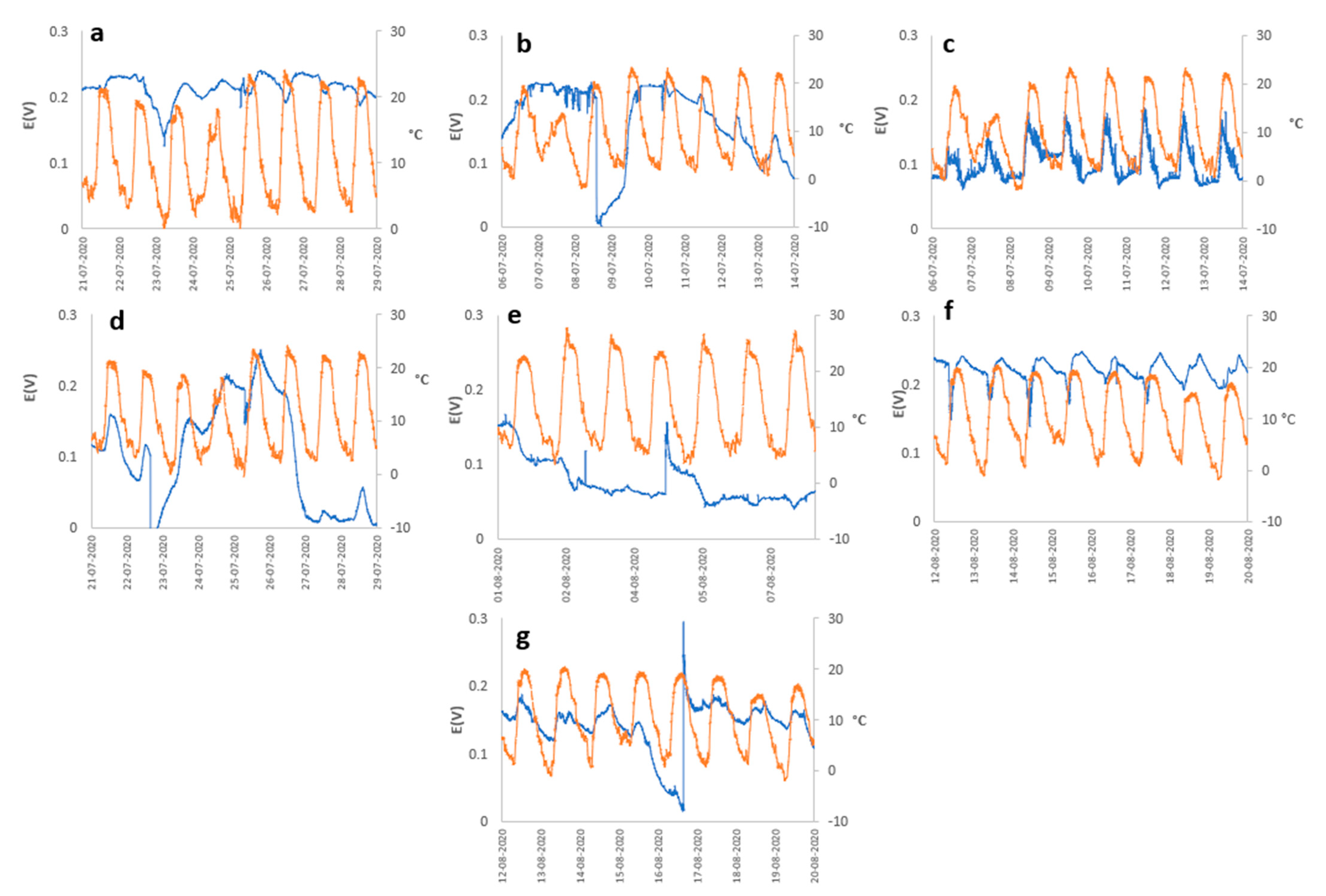
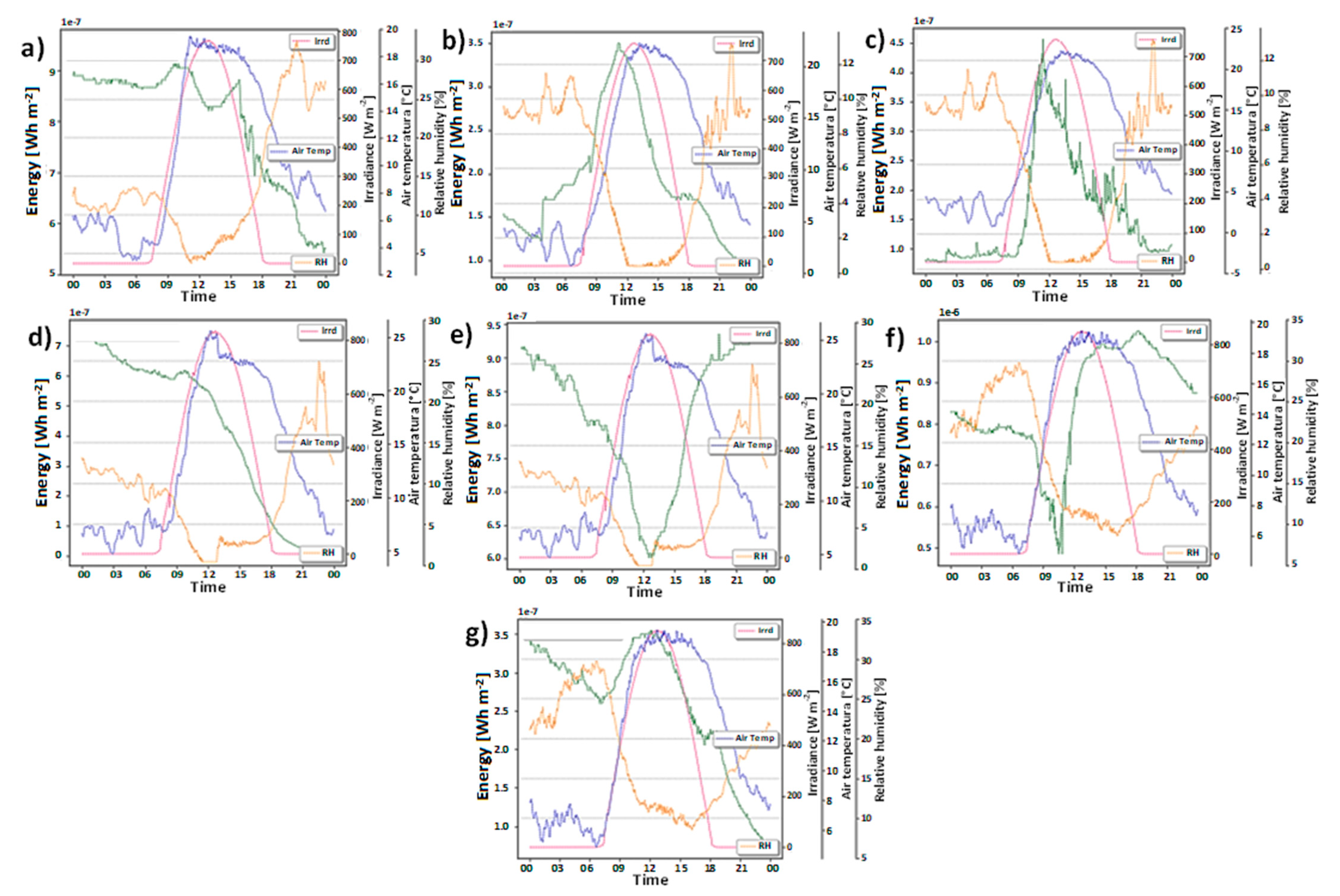
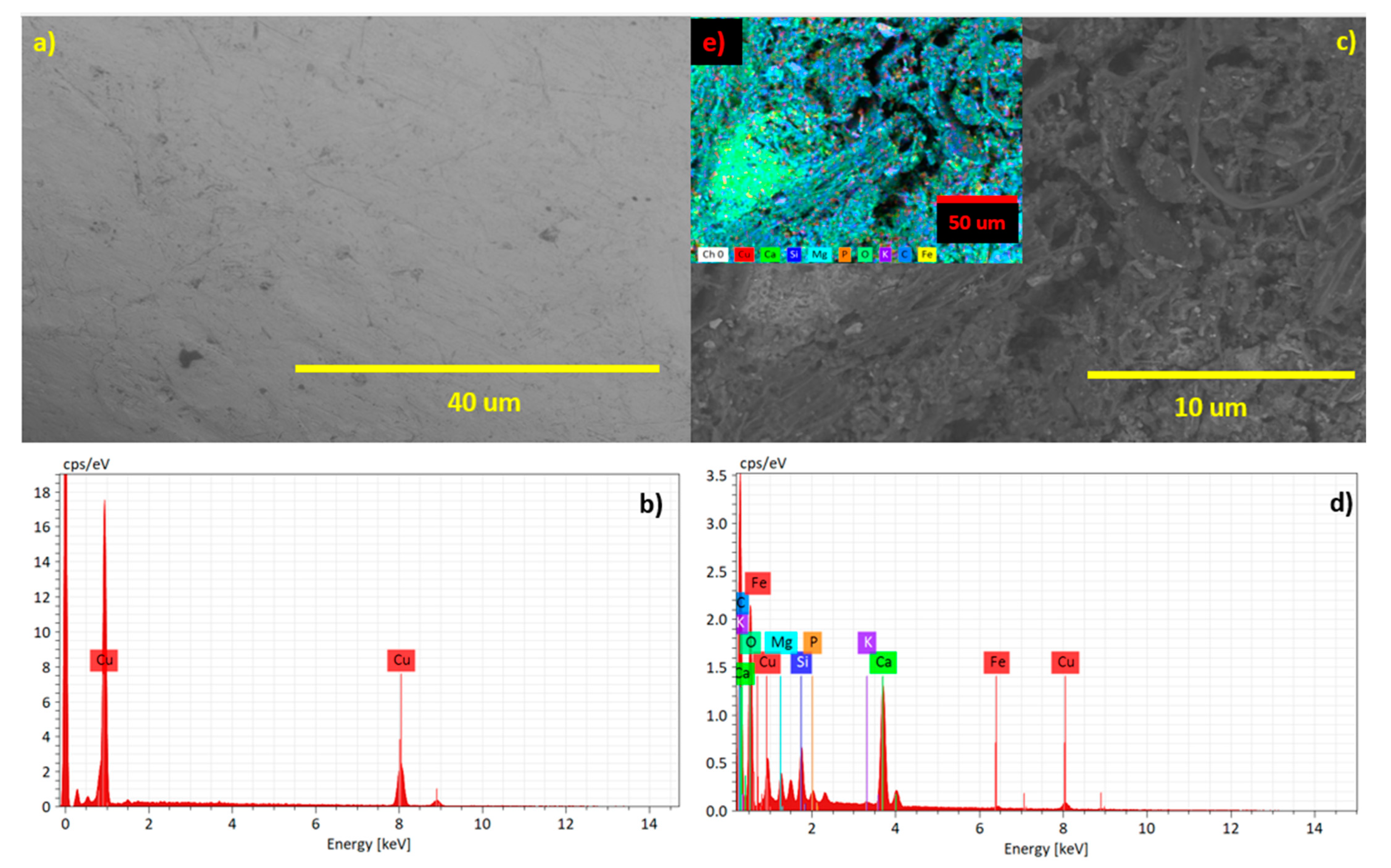
2. Results and Discussion
3. Materials and Methods
3.1. Open Circuit Potential (OCP) Monitoring
3.2. Electrodes Surface Characterization
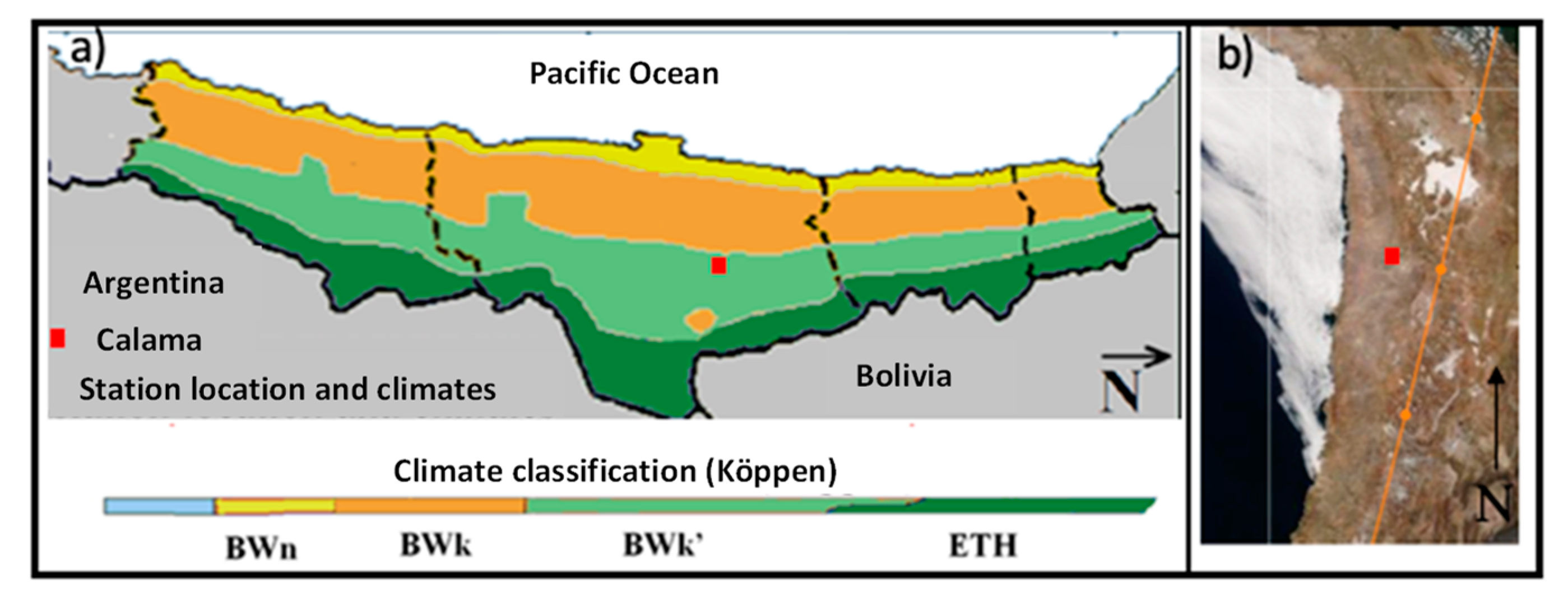
3.3. Meteorology Monitoring
3.4. Experimental Data Analysis
- (a)
- The current generated during the experiments was calculated using Ohm’s law, according to the following expressiowhere is the current generated from the plants, is the potential generated for the plants of the experimental measurements, and R is the impedance resistance of the equipment, respectively.
- (b)
- The power generated by the plants is calculated according to Equation (9)where is the power generated from the plants.
- (c)
- Finally, accumulated energy recovery from the plant was obtained by calculating the area under the curve using the trapezoid method according to the following expression:where E is the energy generated for each plant during the experiment, n is the number of samples, is the power calculated using Equation (9) for the n sample, and t is the time [45,46].
3.5. CAM Species Plants as PMFC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parrado, C.; Girard, A.; Simon, F.; Fuentealba, E. 2050 LCOE (Levelized Cost of Energy) projection for a hybrid PV (photovoltaic)-CSP (concentrated solar power) plant in the Atacama Desert, Chile. Energy 2016, 94, 422–430. [Google Scholar] [CrossRef]
- Kiehl, J.T.; Trenberth, K.E. Earth’s Annual Global Mean Energy Budget. Bull. Am. Meteorol. Soc. 1997, 78, 197–208. [Google Scholar] [CrossRef]
- Kim, D.; Ramanathan, V. Solar radiation budget and radiative forcing due to aerosols and clouds. J. Geophys. Res. Atmos. 2008, 113, D02203. [Google Scholar] [CrossRef]
- Nitisoravut, R.; Regmi, R. Plant microbial fuel cells: A promising biosystems engineering. Renew. Sustain. Energy Rev. 2017, 76, 81–89. [Google Scholar] [CrossRef]
- De Schamphelaire, L.; Bossche, L.V.D.; Dang, H.S.; Höfte, M.; Boon, N.; Rabaey, K.; Verstraete, W. Microbial Fuel Cells Generating Electricity from Rhizodeposits of Rice Plants. Environ. Sci. Technol. 2008, 42, 3053–3058. [Google Scholar] [CrossRef] [PubMed]
- Raman, K.; Lan, J.C.-W. Performance and kinetic study of photo microbial fuel cells (PMFCs) with different electrode distances. Appl. Energy 2012, 100, 100–105. [Google Scholar] [CrossRef]
- Wetser, K.; Sudirjo, E.; Buisman, C.J.; Strik, D.P. Electricity generation by a plant microbial fuel cell with an integrated oxygen reducing biocathode. Appl. Energy 2015, 137, 151–157. [Google Scholar] [CrossRef]
- Timmers, R.A.; Rothballer, M.; Strik, D.P.B.T.B.; Engel, M.; Schulz, S.; Schloter, M.; Hartmann, A.; Hamelers, B.; Buisman, C. Microbial community structure elucidates performance of Glyceria maxima plant microbial fuel cell. Appl. Microbiol. Biotechnol. 2012, 94, 537–548. [Google Scholar] [CrossRef]
- Shaikh, R.; Rizvi, A.; Quraishi, M.; Pandit, S.; Mathuriya, A.S.; Gupta, P.K.; Singh, J.; Prasad, R. Bioelectricity production using plant-microbial fuel cell: Present state of art. S. Afr. J. Bot. 2021, 140, 393–408. [Google Scholar] [CrossRef]
- Kouzuma, A.; Kaku, N.; Watanabe, K. Microbial electricity generation in rice paddy fields: Recent advances and perspectives in rhizosphere microbial fuel cells. Appl. Microbiol. Biotechnol. 2014, 98, 9521–9526. [Google Scholar] [CrossRef]
- Moqsud, M.A.; Hannan, M.A.; Omine, K. Assessment of factors influencing bioelectricity generation in paddy plant microbial fuel cells. Glob. Adv. Res. J. Agric. Sci. 2015, 4, 840–850. [Google Scholar]
- Strik, D.P.; Timmers, R.A.; Helder, M.; Steinbusch, K.J.; Hamelers, H.V.; Buisman, C.J. Microbial solar cells: Applying photosynthetic and electrochemically active organisms. Trends Biotechnol. 2011, 29, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Helder, M.; Strik, D.P.; Hamelers, H.V.M.; Buisman, C.J.N. The flat-plate plant-microbial fuel cell: The effect of a new design on internal resistances. Biotechnol. Biofuels 2012, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Helder, M.; Strik, D.P.; Timmers, R.A.; Raes, S.M.; Hamelers, H.V.; Buisman, C.J. Resilience of roof-top Plant-Microbial Fuel Cells during Dutch winter. Biomass Bioenergy 2013, 51, 1–7. [Google Scholar] [CrossRef]
- Brunelli, D.; Tosato, P.; Rossi, M. Flora Health Wireless Monitoring with Plant-Microbial Fuel Cell. Procedia Eng. 2016, 168, 1646–1650. [Google Scholar] [CrossRef]
- Brunelli, D.; Tosato, P.; Rossi, M. Flora Monitoring with a Plant-Microbial Fuel Cell. In Applications in Electronics Pervading Industry, Environment and Society; Springer: Cham, Switzerland, 2017; Volume 429, pp. 41–48. [Google Scholar] [CrossRef]
- Brunelli, D.; Tosato, P.; Rossi, M. Microbial fuel cell as a biosensor and a power source for flora health monitoring. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar] [CrossRef]
- Helder, M.; Strik, D.; Hamelers, H.; Kuijken, R.; Buisman, C. New plant-growth medium for increased power output of the Plant-Microbial Fuel Cell. Bioresour. Technol. 2012, 104, 417–423. [Google Scholar] [CrossRef]
- Wang, C.; Guo, L.; Li, Y.; Wang, Z. Systematic Comparison of C3 and C4 Plants Based on Metabolic Network Analysis. BMC Syst. Biol. 2012, 6 (Suppl. 2), S9. [Google Scholar] [CrossRef]
- Maddalwar, S.; Nayak, K.K.; Kumar, M.; Singh, L. Plant microbial fuel cell: Opportunities, challenges, and prospects. Bioresour. Technol. 2021, 341, 125772. [Google Scholar] [CrossRef]
- Timmers, R.A.; Strik, D.P.; Hamelers, H.V.; Buisman, C.J. Characterization of the internal resistance of a plant microbial fuel cell. Electrochim. Acta 2012, 72, 165–171. [Google Scholar] [CrossRef]
- Lin, C.-C.; Wei, C.-H.; Chen, C.-I.; Shieh, C.-J.; Liu, Y.-C. Characteristics of the photosynthesis microbial fuel cell with a Spirulina platensis biofilm. Bioresour. Technol. 2013, 135, 640–643. [Google Scholar] [CrossRef]
- Srikanth, S.; Pavani, T.; Sarma, P.; Mohan, S.V. Synergistic interaction of biocatalyst with bio-anode as a function of electrode materials. Int. J. Hydrogen Energy 2011, 36, 2271–2280. [Google Scholar] [CrossRef]
- Baudler, A.; Langner, M.; Rohr, C.; Greiner, A.; Schröder, U. Metal-Polymer Hybrid Architectures as Novel Anode Platform for Microbial Electrochemical Technologies. ChemSusChem 2017, 10, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Liu, P.; Liang, P.; Hao, W.; Li, M.; Huang, X. Dual-signal-biosensor based on luminescent bacteria biofilm for real-time online alert of Cu(II) shock. Biosens. Bioelectron. 2019, 142, 111500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, G.; Wen, J.; Xu, Y.; Sun, J.; Ning, X.-A.; Lu, X.; Wang, Y.; Yang, Z.; Yuan, Y. Electrochemical and microbial community responses of electrochemically active biofilms to copper ions in bioelectrochemical systems. Chemosphere 2018, 196, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Baudler, A.; Schmidt, I.; Langner, M.; Greiner, A.; Schröder, U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 2048–2055. [Google Scholar] [CrossRef]
- Asif, M.; Aziz, A.; Ashraf, G.; Iftikhar, T.; Sun, Y.; Xiao, F.; Liu, H. Unveiling microbiologically influenced corrosion engineering to transfigure damages into benefits: A textile sensor for H2O2 detection in clinical cancer tissues. Chem. Eng. J. 2022, 427, 131398. [Google Scholar] [CrossRef]
- Zhu, X.; Logan, B.E. Copper anode corrosion affects power generation in microbial fuel cells. J. Chem. Technol. Biotechnol. 2014, 89, 471–474. [Google Scholar] [CrossRef]
- Pamintuan, K.R.S.; Sanchez, K.M. Power generation in a plant-microbial fuel cell assembly with graphite and stainless steel electrodes growing Vigna Radiata. IOP Conf. Ser. Mater. Sci. Eng. 2019, 703, 012037. [Google Scholar] [CrossRef]
- Bombelli, P.; Iyer, D.M.R.; Covshoff, S.; McCormick, A.J.; Yunus, K.; Hibberd, J.M.; Fisher, A.C.; Howe, C.J. Comparison of power output by rice (Oryza sativa) and an associated weed (Echinochloa glabrescens) in vascular plant bio-photovoltaic (VP-BPV) systems. Appl. Microbiol. Biotechnol. 2012, 97, 429–438. [Google Scholar] [CrossRef]
- Lovley, D.R. Microbial fuel cells: Novel microbial physiologies and engineering approaches. Curr. Opin. Biotechnol. 2006, 17, 327–332. [Google Scholar] [CrossRef]
- Veste, M.; Herppich, W.B. Comparative ecophysiology of the leaf-succulents Augea capensis (C3) and Malephora purpureo-crocea (CAM) in the Knersvlakte, Succulent Karoo, South Africa. Flora 2021, 278, 151807. [Google Scholar] [CrossRef]
- Hartzell, S.; Bartlett, M.S.; Porporato, A. Unified representation of the C3, C4, and CAM photosynthetic pathways with the Photo3 model. Ecol. Model. 2018, 384, 173–187. [Google Scholar] [CrossRef]
- Yuan, H.; Hou, Y.; Abu-Reesh, I.M.; Chen, J.; He, Z. Oxygen reduction reaction catalysts used in microbial fuel cells for energy-efficient wastewater treatment: A review. Mater. Horizons 2016, 3, 382–401. [Google Scholar] [CrossRef]
- Wang, J.; Song, X.; Wang, Y.; Bai, J.; Li, M.; Dong, G.; Lin, F.; Lv, Y.; Yan, D. Bioenergy generation and rhizodegradation as affected by microbial community distribution in a coupled constructed wetland-microbial fuel cell system associated with three macrophytes. Sci. Total. Environ. 2017, 607–608, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Sophia, A.C.; Sreeja, S. Green energy generation from plant microbial fuel cells (PMFC) using compost and a novel clay separator. Sustain. Energy Technol. Assess. 2017, 21, 59–66. [Google Scholar] [CrossRef]
- Timmers, R.A.; Strik, D.P.; Arampatzoglou, C.; Buisman, C.J.; Hamelers, H.V. Rhizosphere anode model explains high oxygen levels during operation of a Glyceria maxima PMFC. Bioresour. Technol. 2012, 108, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Mier, A.A.; Olvera-Vargas, H.; Mejía-López, M.; Longoria, A.; Verea, L.; Sebastian, P.; Arias, D.M. A review of recent advances in electrode materials for emerging bioelectrochemical systems: From biofilm-bearing anodes to specialized cathodes. Chemosphere 2021, 283, 131138. [Google Scholar] [CrossRef]
- Akman, D.; Cirik, K.; Ozdemir, S.; Ozkaya, B.; Cinar, O. Bioelectricity generation in continuously-fed microbial fuel cell: Effects of anode electrode material and hydraulic retention time. Bioresour. Technol. 2013, 149, 459–464. [Google Scholar] [CrossRef]
- Torres, C.I.; Marcus, A.K.; Parameswaran, P.; Rittmann, B.E. Kinetic Experiments for Evaluating the Nernst−Monod Model for Anode-Respiring Bacteria (ARB) in a Biofilm Anode. Environ. Sci. Technol. 2008, 42, 6593–6597. [Google Scholar] [CrossRef]
- Watts, D.; Valdés, M.F.; Jara, D.; Watson, A. Potential residential PV development in Chile: The effect of Net Metering and Net Billing schemes for grid-connected PV systems. Renew. Sustain. Energy Rev. 2015, 41, 1037–1051. [Google Scholar] [CrossRef]
- Herrera, C.; Godfrey, L.; Urrutia, J.; Custodio, E.; Jordan, T.; Jódar, J.; Delgado, K.; Barrenechea, F. Recharge and residence times of groundwater in hyper arid areas: The confined aquifer of Calama, Loa River Basin, Atacama Desert, Chile. Sci. Total. Environ. 2021, 752, 141847. [Google Scholar] [CrossRef]
- Marzo, A.; Polo, J.; Wilbert, S.; Gueymard, C.A.; Jessen, W.; Ferrada, P.; Alonso-Montesinos, J.; Ballestrín, J. Sunbelt spectra comparison with standard ASTM G173: The Chilean case. AIP Conf. Proc. 2018, 2033, 190010. [Google Scholar] [CrossRef]
- Trigo-Gonzalez, M.; Cortés, M.; Alonso-Montesinos, J.; Martínez-Durbán, M.; Ferrada, P.; Rabanal, J.; Portillo, C.; López, G.; Batlles, F. Development and comparison of PV production estimation models for mc-Si technologies in Chile and Spain. J. Clean. Prod. 2021, 281, 125360. [Google Scholar] [CrossRef]
- Trigo-González, M.; Batlles, F.; Alonso-Montesinos, J.; Ferrada, P.; del Sagrado, J.; Martínez-Durbán, M.; Cortés, M.; Portillo, C.; Marzo, A. Hourly PV production estimation by means of an exportable multiple linear regression model. Renew. Energy 2019, 135, 303–312. [Google Scholar] [CrossRef]
- Ezzat, S.M.; El Sayed, A.M.; Salama, M.M. Use of Random Amplified Polymorphic DNA (RAPD) Technique to Study the Genetic Diversity of Eight Aloe Species. Planta Medica 2016, 82, 1381–1386. [Google Scholar] [CrossRef]
- Pegu, A.J.; Sharma, M.A. Review on Aloe Vera. IJTSRD 2019, 3, 35–40. [Google Scholar] [CrossRef]
- Kavamura, V.N.; Taketani, R.G.; Lançoni, M.D.; Andreote, F.D.; Mendes, R.; de Melo, I.S. Water Regime Influences Bulk Soil and Rhizosphere of Cereus jamacaru Bacterial Communities in the Brazilian Caatinga Biome. PLoS ONE 2013, 8, e73606. [Google Scholar] [CrossRef]
- Dutra, J.C.V.; Ferreira, J.M.; Pereira, P.R.C.; de Oliveira, J.B.-H.; Gervásio, S.V.; Xavier, M.B.; da Mota, M.M.; da Luz, A.C.; Pretti, I.R.; França, H.S.; et al. Cereus jamacaru D.C. Hydroalcoholic Extract Promotes Anti-Cytotoxic and Antitumor Activity. Pharmaceuticals 2018, 11, 130. [Google Scholar] [CrossRef]
- Alencar, N.L.; Innecco, R.; Gomes-Filho, E.; Gallão, M.I.; Alvarez-Pizarro, J.C.; Prisco, J.T.; Oliveira, A.B.D. Seed reserve composition and mobilization during germination and early seedling establishment of Cereus jamacaru D.C. ssp. jamacaru (Cactaceae). An. Da Acad. Bras. De Ciências 2012, 84, 823–832. [Google Scholar] [CrossRef]
- Meiado, M.V.; de Albuquerque, L.S.C.; Rocha, E.A.; Rojas-Aréchiga, M.; Leal, I.R. Seed germination responses of Cereus jamacaru DC. ssp. jamacaru (Cactaceae) to environmental factors. Plant Species Biol. 2010, 25, 120–128. [Google Scholar] [CrossRef]
- Bezerra, J.D.P.; Santos, M.G.S.; Barbosa, R.N.; Svedese, V.M.; Lima, D.M.M.; Fernandes, M.J.S.; Gomes, B.S.; Paiva, L.M.; Almeida-Cortez, J.S.; Souza-Motta, C.M. Fungal endophytes from cactus Cereus jamacaru in Brazilian tropical dry forest: A first study. Symbiosis 2013, 60, 53–63. [Google Scholar] [CrossRef]
- Al-Robai, S.A.; Ahmed, A.; Mohamed, H.; Alzahrani, F. Austrocylindropuntia subulata (Muehlenpf.) Backeb. (Cactaceae—Opuntioideae): An invasive new cactus record to the flora of Saudi Arabia. BioInvasions Rec. 2022, 11, 360–366. [Google Scholar] [CrossRef]
- El Bouaidi, W.; Libralato, G.; Tazart, Z.; Enaime, G.; Douma, M.; Ounas, A.; Yaacoubi, A.; Lofrano, G.; Carotenuto, M.; Saviano, L.; et al. Nature-based coagulants for drinking water treatment: An ecotoxicological overview. Water Environ. Res. 2022, 94, e10782. [Google Scholar] [CrossRef]
- Basavand, E.; Khodaygan, P.; Babaeizad, V.; Rahimian, H.; Mirhosseini, H.A. Soft rot disease caused by Klebsiella aerogenes on Austrocylindropuntia subulata in Iran. Indian Phytopathol. 2020, 73, 371–372. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; Mellado-Mojica, E.; León-Martínez, F.M.; Dzul-Cauich, J.G.; López, M.G.; García-Vieyra, M.I. Fructans (agavins) from Agave angustifolia and Agave potatorum as fat replacement in yogurt: Effects on physicochemical, rheological, and sensory properties. LWT 2021, 140, 110846. [Google Scholar] [CrossRef]
- Rangel-Landa, S.; Casas, A.; Dávila, P. Facilitation of Agave potatorum: An ecological approach for assisted population recovery. For. Ecol. Manag. 2015, 347, 57–74. [Google Scholar] [CrossRef]
- Soto-Castro, D.; Pérez-Herrera, A.; García-Sánchez, E.; Santiago-García, P.A. Identification and Quantification of Bioactive Compounds in Agave potatorum Zucc. Leaves at Different Stages of Development and a Preliminary Biological Assay. Waste Biomass Valorization 2021, 12, 4537–4547. [Google Scholar] [CrossRef]
- Torres, I.; Casas, A.; Vega, E.; Martínez-Ramos, M.; Delgado-Lemus, A. Population Dynamics and Sustainable Management of Mescal Agaves in Central Mexico: Agave potatorum in the Tehuacán-Cuicatlán Valley. Econ. Bot. 2015, 69, 26–41. [Google Scholar] [CrossRef]
- Aguirre-Dugua, X.; Eguiarte, L. Genetic diversity, conservation and sustainable use of wild Agave cupreata and Agave potatorum extracted for mezcal production in Mexico. J. Arid. Environ. 2013, 90, 36–44. [Google Scholar] [CrossRef]
- Goldarazena, A.; Aguilar, H.; Kutuk, H.; Childers, C.C. Biology of three species of Agistemus (Acari: Stigmaeidae): Life table parameters using eggs of Panonychus citri or pollen of Malephora crocea as food. Exp. Appl. Acarol. 2004, 32, 281–291. [Google Scholar] [CrossRef]
- Molina, G.A.; Esparza, R.; López-Miranda, J.L.; Hernández-Martínez, A.R.; España-Sánchez, B.L.; Elizalde-Peña, E.A.; Estevez, M. Green synthesis of Ag nanoflowers using Kalanchoe Daigremontiana extract for enhanced photocatalytic and antibacterial activities. Colloids Surf. B Biointerfaces 2019, 180, 141–149. [Google Scholar] [CrossRef] [PubMed]
- van Maarseveen, C.; Jetter, R. Composition of the epicuticular and intracuticular wax layers on Kalanchoe daigremontiana (Hamet et Perr. de la Bathie) leaves. Phytochemistry 2009, 70, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk-Czepas, J.; Nowak, P.; Wachowicz, B.; Piechocka, J.; Głowacki, R.; Moniuszko-Szajwaj, B.; Stochmal, A. Antioxidant efficacy of Kalanchoe daigremontiana bufadienolide-rich fraction in blood plasma in vitro. Pharm. Biol. 2016, 54, 3182–3188. [Google Scholar] [CrossRef]
- Domenico, P. Effective microorganisms for germination and root growth in Kalanchoe daigremontiana. World J. Adv. Res. Rev. 2019, 3, 2581–9615. [Google Scholar]
- VON Caemmerer, S.; Griffiths, H. Stomatal responses to CO2 during a diel Crassulacean acid metabolism cycle in Kalanchoe daigremontiana and Kalanchoe pinnata. Plant Cell Environ. 2009, 32, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Supratman, U.; Fujita, T.; Akiyama, K.; Hayashi, H. Insecticidal compounds from Kalanchoe daigremontiana × tubiflora. Phytochemistry 2001, 58, 311–314. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, G.; Jia, J. Preliminary evaluation: The effects of Aloe ferox Miller and Aloe arborescens Miller on wound healing. J. Ethnopharmacol. 2008, 120, 181–189. [Google Scholar] [CrossRef]
- Guillén, F.; Díaz-Mula, H.M.; Zapata, P.J.; Valero, D.; Serrano, M.; Castillo, S.; Martínez-Romero, D. Aloe arborescens and Aloe vera gels as coatings in delaying postharvest ripening in peach and plum fruit. Postharvest Biol. Technol. 2013, 83, 54–57. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Review Expert Panel. Final Report on the Safety Assessment of Aloe Andongensis Extract, Aloe Andongensis Leaf Juice, Aloe Arborescens Leaf Extract, Aloe Arborescens Leaf Juice, Aloe Arborescens Leaf Protoplasts, Aloe Barbadensis Flower Extract, Aloe Barbadensis Leaf, Aloe Barbadensis Leaf Extract, Aloe Barbadensis Leaf Juice, Aloe Barbadensis Leaf Polysaccharides, Aloe Barbadensis Leaf Water, Aloe Ferox Leaf Extract, Aloe Ferox Leaf Juice, and Aloe Ferox Leaf Juice Extract1. Int. J. Toxicol. 2007, 26 (Suppl. 2), 1–50. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madrid, F.M.G.; Trigo, M.; Salazar-Avalos, S.; Carvajal-Funes, S.; Olivares, D.; Portillo, C.; Fuentealba, E.; Toro, N.; Carrasco, G.; Cáceres, L.; et al. An In Situ Evaluation of Different CAM Plants as Plant Microbial Fuel Cells for Energy Recovery in the Atacama Desert. Plants 2023, 12, 4016. https://doi.org/10.3390/plants12234016
Madrid FMG, Trigo M, Salazar-Avalos S, Carvajal-Funes S, Olivares D, Portillo C, Fuentealba E, Toro N, Carrasco G, Cáceres L, et al. An In Situ Evaluation of Different CAM Plants as Plant Microbial Fuel Cells for Energy Recovery in the Atacama Desert. Plants. 2023; 12(23):4016. https://doi.org/10.3390/plants12234016
Chicago/Turabian StyleMadrid, Felipe M. Galleguillos, Mauricio Trigo, Sebastián Salazar-Avalos, Sergio Carvajal-Funes, Douglas Olivares, Carlos Portillo, Edward Fuentealba, Norman Toro, Gilda Carrasco, Luis Cáceres, and et al. 2023. "An In Situ Evaluation of Different CAM Plants as Plant Microbial Fuel Cells for Energy Recovery in the Atacama Desert" Plants 12, no. 23: 4016. https://doi.org/10.3390/plants12234016
APA StyleMadrid, F. M. G., Trigo, M., Salazar-Avalos, S., Carvajal-Funes, S., Olivares, D., Portillo, C., Fuentealba, E., Toro, N., Carrasco, G., Cáceres, L., Jamett, I., & Soliz, A. (2023). An In Situ Evaluation of Different CAM Plants as Plant Microbial Fuel Cells for Energy Recovery in the Atacama Desert. Plants, 12(23), 4016. https://doi.org/10.3390/plants12234016








