Indonesian Euphorbiaceae: Ethnobotanical Survey, In Vitro Antibacterial, Antitumour Screening and Phytochemical Analysis of Euphorbia atoto
Abstract
1. Introduction
2. Results and Discussion
2.1. Ethnobotanical Data
2.1.1. Illness Categories
2.1.2. Ethnobotanical Uses of the Selected Species
| Species | Part Used | Location | Traditional Uses | References |
|---|---|---|---|---|
| M. tanarius (L.) Müll.Arg. | Leaf | Aceh | High cholesterol | [8] |
| West Java | Itchy, skin diseases in babies, wounds, gastric ulcers | [8] | ||
| Gorontalo | Itchy | [8] | ||
| North Sulawesi | Leucorrhoea, diarrhoea, sores, wounds, stomach ache | [8] | ||
| Banten | Gastric ulcer | [33] | ||
| Southeast Sulawesi | Internal organ treatments | [11] | ||
| West Timor | Malaria | [8] | ||
| North Maluku | Fertility, eye diseases | [8,11] | ||
| North Sumatera | Muscle injury | [10] | ||
| Bark | Papua | Eye diseases, skin diseases, malaria, headache, snake bite treatment, lymphoid problems | [11] | |
| Maluku | Bloody diarrhoea, postpartum care, mouth ulcer | [8,9] | ||
| West Java | Haemorrhoids | [8] | ||
| East Nusa Tenggara | Bone injury, postpartum haemorrhage | [11] | ||
| Fruits | Papua | Malaria | [11] | |
| Root | North Maluku | Cough | [10] | |
| Exudate/sap | Southeast Maluku | Boils | [11] | |
| North Maluku | Skin diseases | [10] | ||
| Queensland | Sores, wound healing | [12] | ||
| M. mollissimus (Geiseler) Airy Shaw. | Leaf | Eastern Indonesia * | Urinary tract diseases | [34] |
| East Nusa Tenggara | Flatulence | [11] | ||
| Bark | East Nusa Tenggara | Bone injury | [11] | |
| H. giganteus Zoll. & Moritzi | Leaf | North Sumatra | Itchy, fever | [35] |
| Seeds | Fever | [36] | ||
| M. rufidulus (Miq.) Müll.Arg. | Leaf | West Kalimantan | Bone injury | [37] |
| S. indica (Willd.) Esser. | Fruits | Thailand | Constipation | [38] |
| Leaf | North Sumatra | Measles | [39] | |
| E. atoto G.Forst. | Leaf | Jambi | Postpartum care | [11] |
| Nicobar Islands | Ulcer, sores, old wound healing | [40] | ||
| Rheumatism, lumbago | [40] | |||
| Bark | Jambi | Headache | [11] | |
| Whole plants | Samoa | Wound healing, abscess | [41] | |
| E. hypericifolia L. | Leaf | South Africa | Gonorrhoea | [42] |
| Juice | Uganda | Snake bite treatment, scorpion bite treatment | [43] |
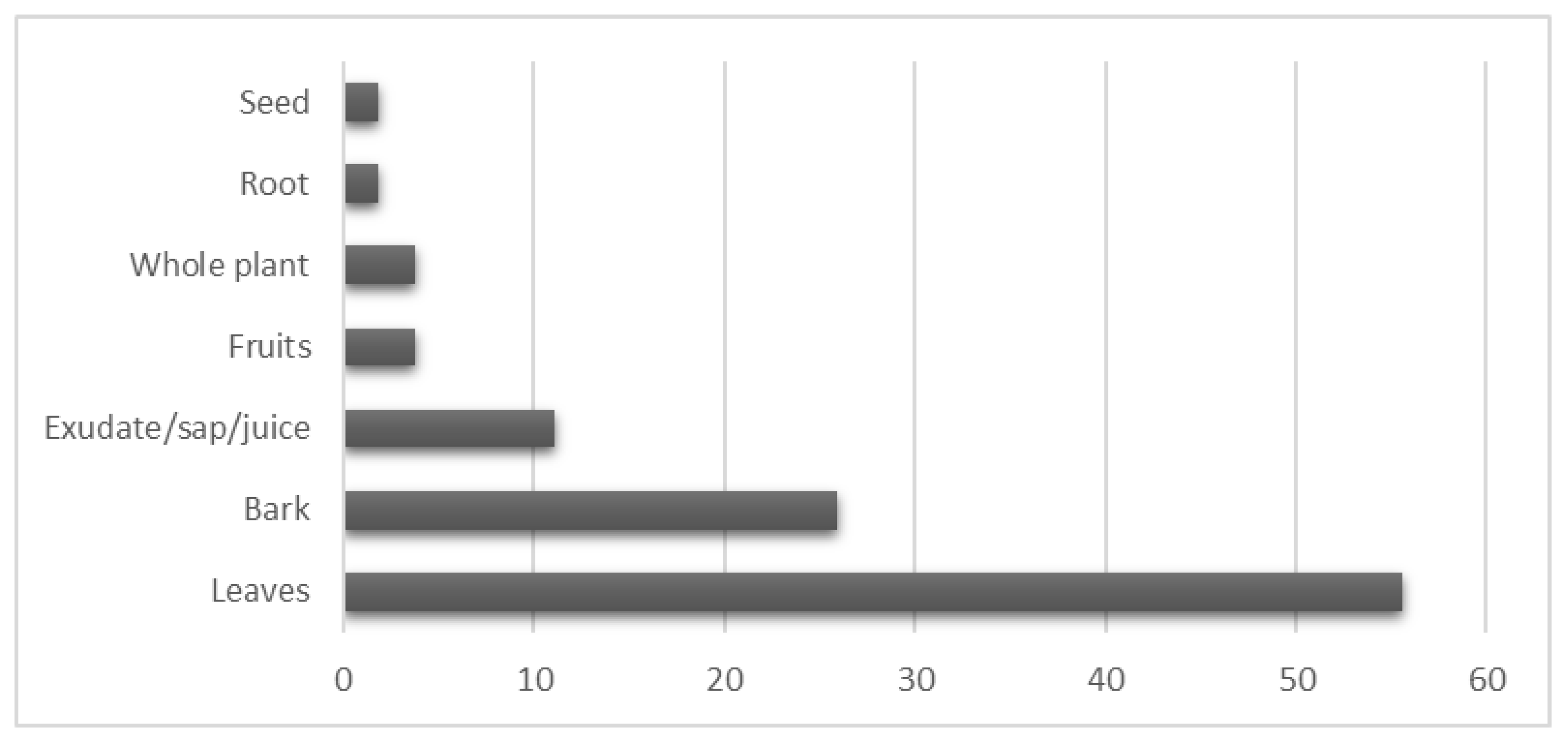
2.1.3. Plant Parts Used
2.2. Antimicrobial Activity Screening
2.2.1. Screening by the Disk Diffusion Method
2.2.2. Determination of MICs by the Microdilution Method
2.3. Antitumour Activity
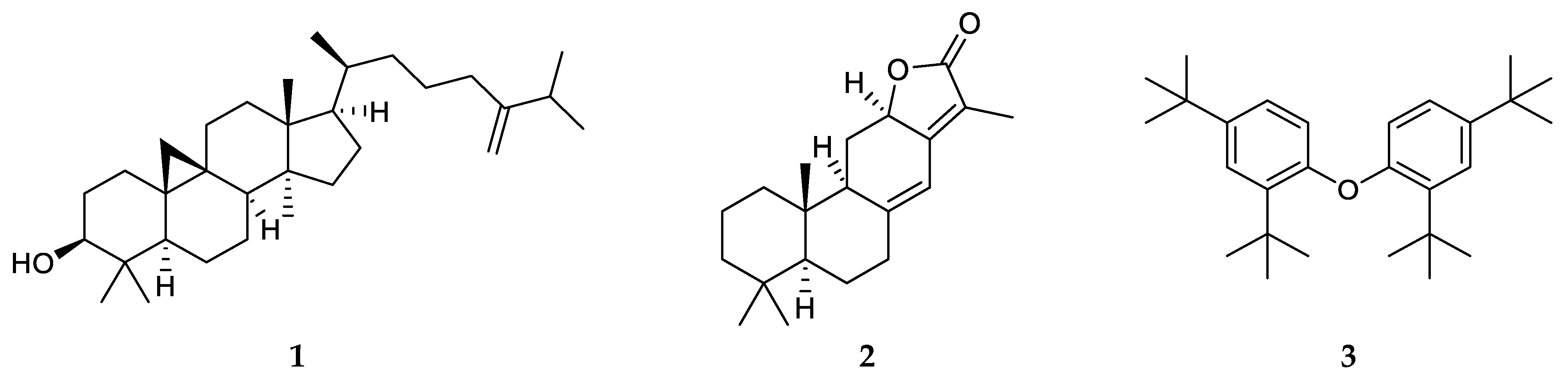
2.4. Isolation and Identification of Compounds from E. atoto
2.5. Investigation of the Antitumour Effects of 1
3. Materials and Methods
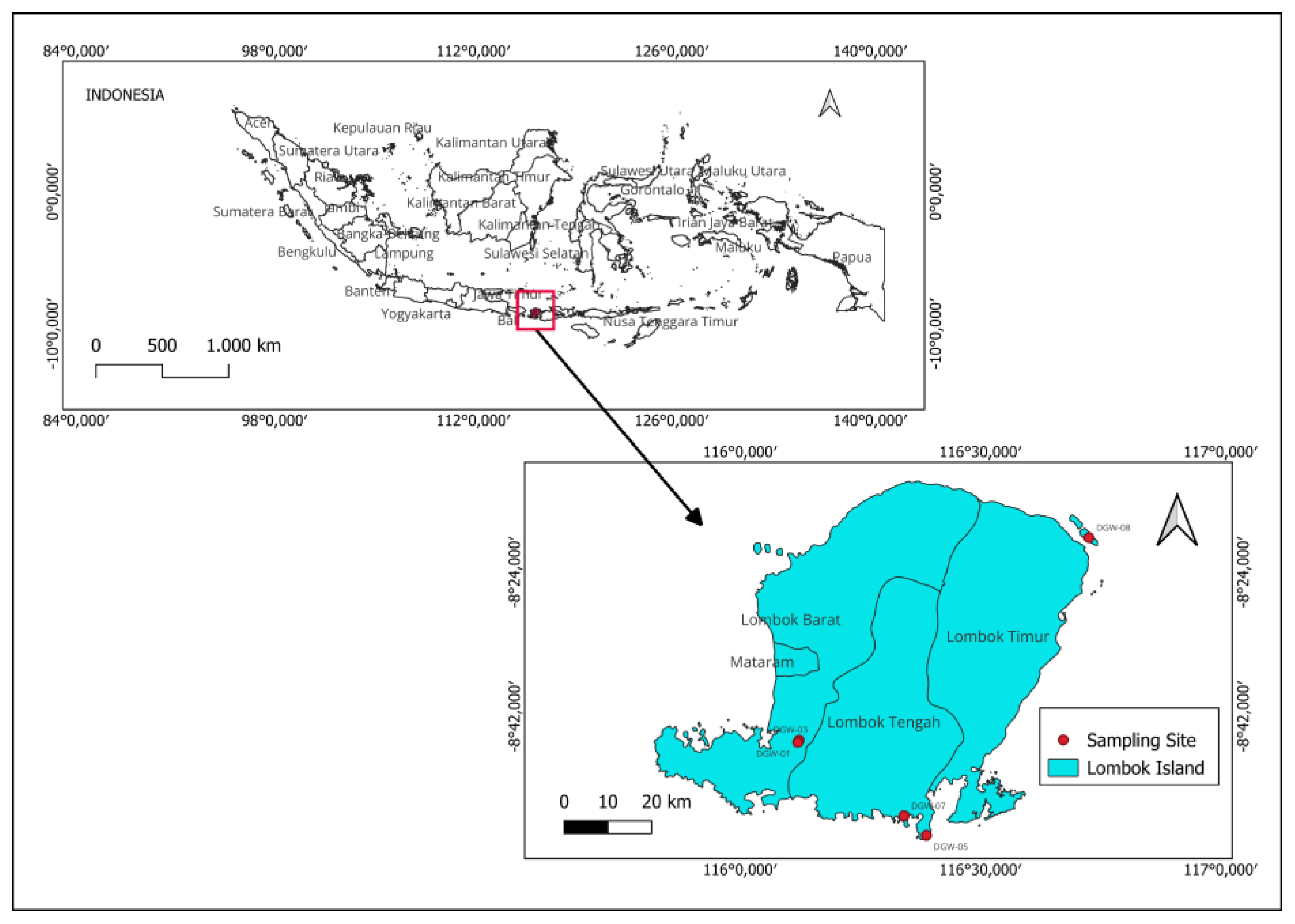
3.1. Plant Materials
3.2. Ethnobotanical Data and Analysis
3.3. General Experiment Procedures
3.4. Preparation of Plant Extracts and Fractions
3.5. Isolation of Compounds from E. atoto
3.6. Bacterial and Fungal Strains and Culture Conditions for Antimicrobial Assays
3.7. Determination of Antibacterial Activity Using the Disk Diffusion Method
3.8. Determination of the Minimum Inhibitory Concentration (MIC)
3.9. Cell Line Cultures
3.10. Antiproliferative Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elfahmi; Woerdenbag, H.J.; Kayser, O. Jamu: Indonesian traditional herbal medicine towards rational phytopharmacological use. J. Herb. Med. 2014, 4, 51–73. [Google Scholar] [CrossRef]
- Budiarti, M.; Maruzy, A.; Mujahid, R.; Sari, A.N.; Jokopriyambodo, W.; Widayat, T.; Wahyono, S. The use of antimalarial plants as traditional treatment in Papua Island, Indonesia. Heliyon 2020, 6, e05562. [Google Scholar] [CrossRef]
- Heinrich, M. Ethnobotany and its role in drug development. Phytother. Res. 2000, 14, 479–488. [Google Scholar] [CrossRef]
- Hammadi, R.; Kúsz, N.; Dávid, C.Z.; Mwangi, P.W.; Berkecz, R.; Szemerédi, N.; Spengler, G.; Hohmann, J.; Vasas, A. Polyoxypregnane ester derivatives and lignans from Euphorbia gossypina var. coccinea pax. Plants 2022, 11, 1299. [Google Scholar] [CrossRef] [PubMed]
- Wirasisya, D.G.; Hohmann, J. An Overview of the traditional use, phytochemistry, and biological activity of the genus Homalanthus. Fitoterapia 2023, 166, 105466. [Google Scholar] [CrossRef]
- Staub, P.O.; Geck, M.S.; Weckerle, C.S.; Casu, L.; Leonti, M. Classifying diseases and remedies in ethnomedicine and ethnopharmacology. J. Ethnopharmacol. 2015, 174, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Plant of the World Online. Macaranga tanarius (L.) Müll.Arg. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:351381-1 (accessed on 19 July 2023).
- Mustaqim, W.A. Macaranga tanarius (L.) Müll.Arg. Euphorbiaceae. In Ethnobotany of the Mountain Regions of Southeast Asia; Franco, F.M., Ed.; Ethnobotany of Mountain Regions; Springer International Publishing: Cham, Switzerland, 2021; pp. 651–662. [Google Scholar]
- National Institute of Health Research and Development. National Report: Exploration on Local Knowledge of Ethnomedicine and Community-Based Medicinal Plants in Indonesia (Ristoja 2017); NIHRD Publisher Agency: Jakarta, Indonesia, 2017. [Google Scholar]
- National Institute of Health Research and Development. National Report: Exploration on Local Knowledge of Ethnomedicine and Community-Based Medicinal Plants in Indonesia (Ristoja 2015); NIHRD Publisher Agency: Jakarta, Indonesia, 2015. [Google Scholar]
- National Institute of Health Research and Development. National Report: Exploration on Local Knowledge of Ethnomedicine and Community-Based Medicinal Plants in Indonesia (Ristoja 2012); NIHRD Publisher Agency: Jakarta, Indonesia, 2012. [Google Scholar]
- Turpin, G.; Ritmejerytė, E.; Jamie, J.; Crayn, D.; Wangchuk, P. Aboriginal medicinal plants of Queensland: Ethnopharmacological uses, species diversity, and biodiscovery pathways. J. Ethnobiol. Ethnomed. 2022, 18, 54. [Google Scholar] [CrossRef]
- Amir Rawa, M.S.; Nurul Azman, N.A.; Mohamad, S.; Nogawa, T.; Wahab, H.A. In vitro and in silico anti-acetylcholinesterase activity from Macaranga tanarius and Syzygium jambos. Molecules 2022, 27, 2648. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Yu, Y.-H.; Ye, S.-R.; Chen, Y.-W. Antibacterial and antioxidant activity of the fruit of Macaranga tanarius, the plant origin of Taiwanese green propolis. Antioxidants 2022, 11, 1242. [Google Scholar] [CrossRef]
- Heil, M.; Fiala, B.; Maschwitz, U.; Linsenmair, K.E. On benefits of indirect defence: Short- and long-term studies of antiherbivore protection via mutualistic ants. Oecologia 2001, 126, 395–403. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Chang, C.-C.; Hsieh, C.-C.; Shih, Y.-H.; Chang, H.-C.; Lin, C.-L. Therapeutic potential of extracts from Macaranga tanarius (mte) in diabetic nephropathy. Plants 2023, 12, 656. [Google Scholar] [CrossRef]
- Péresse, T.; Jézéquel, G.; Allard, P.-M.; Pham, V.-C.; Huong, D.T.M.; Blanchard, F.; Bignon, J.; Lévaique, H.; Wolfender, J.-L.; Litaudon, M.; et al. Cytotoxic prenylated stilbenes isolated from Macaranga tanarius. J. Nat. Prod. 2017, 80, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Harinantenaina, L.; Matsunami, K.; Otsuka, H.; Shinzato, T.; Takeda, Y. Macaflavanones a−g, prenylated flavanones from the leaves of Macaranga tanarius. J. Nat. Prod. 2008, 71, 1872–1876. [Google Scholar] [CrossRef]
- Kumazawa, S.; Murase, M.; Momose, N.; Fukumoto, S. Analysis of antioxidant prenylflavonoids in different parts of Macaranga tanarius, the plant origin of Okinawan propolis. Asian Pac. J. Trop. Med. 2014, 7, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-G.; Khadke, S.K.; Yamano, A.; Woo, J.-T.; Lee, J. Antimicrobial and antibiofilm activities of prenylated flavanones from Macaranga tanarius. Phytomedicine 2019, 63, 153033. [Google Scholar] [CrossRef]
- Phommart, S.; Sutthivaiyakit, P.; Chimnoi, N.; Ruchirawat, S.; Sutthivaiyakit, S. Constituents of the leaves of Macaranga tanarius. J. Nat. Prod. 2005, 68, 927–930. [Google Scholar] [CrossRef]
- Tseng, M.-H.; Chou, C.-H.; Chen, Y.-M.; Kuo, Y.-H. Allelopathic prenylflavanones from the fallen leaves of Macaranga tanarius. J. Nat. Prod. 2001, 64, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Plant of the World Online. Euphorbia atoto G.Forst. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:345695-1 (accessed on 18 July 2023).
- Chen, M.-T.; Kuoh, C.-S.; Lee, K.-K. Studies on the constituents of Euphorbia atoto Forst.f. Taiwan Pharm. J. 1979, 31, 114–117. [Google Scholar]
- Hart, N.K.; Johns, S.R.; Lamberton, J.A. (+)-9-aza-1-methylbicyclo[3,3,1]nonan-3-one, a new alkaloid from Euphorbia atoto Forst. Aust. J. Chem. 1967, 20, 561–563. [Google Scholar] [CrossRef]
- Zhao, H.; Duan, R.-J.; Kong, C.-H.; Dai, H.-F.; Mei, W.-L.; Xu, F.-Q.; Huang, S.-Z. Two new anti-inflammatory trachylobane diterpenoids from Euphorbia atoto. J Asian Nat. Prod. Res. 2023. [Google Scholar] [CrossRef]
- Norhanom, A.W.; Yadav, M. Tumour promoter activity in Malaysian Euphorbiaceae. Br. J. Cancer 1995, 71, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Sang, J.; Li, W.; Tian, Y.; Zou, M.-F.; Tang, G.-H.; Yin, S. Structurally diverse triterpenoids with cytotoxicity from Euphorbia hypericifolia. Fitoterapia 2021, 151, 104888. [Google Scholar] [CrossRef] [PubMed]
- Azis, H.A.; Taher, M.; Susanti, D.; Zakariac, Z.A. In vitro wound healing activity of Sapium indicum willd leaf extracts. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 126–132. [Google Scholar]
- Chumkaew, P.; Karalai, C.; Ponglimanont, C.; Chantrapromma, K. Antimycobacterial activity of phorbol esters from the fruits of Sapium indicum. J. Nat. Prod. 2003, 66, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Mokmued, K.; Dechayont, B.; Phuaklee, P.; Liplung, C.; Muangpoolsawad, H.; Nuengchamnong, N.; Prommee, N. Evaluation of anti-inflammatory, cytotoxic, anti-h. pylori, antioxidant activities, and phytochemical compositions of Shirakiopsis indica (Willd.) Esser. ScienceAsia 2021, 47, 549. [Google Scholar] [CrossRef]
- Tanaka, N.; Takahashi, S.; Yoshino, Y.; Nakatani, M.; Ahmed, F.A.; Hossain, G.M.; Chen, C.-H.; Lee, K.-H.; Kashiwada, Y. Tigliane-type diterpene esters from the fruits of Shirakiopsis indica and their anti-HIV activity. J. Nat. Prod. 2022, 85, 2687–2693. [Google Scholar] [CrossRef]
- Khastini, R.O.; Wahyuni, I.; Saraswati, I. Ethnobotanical study of digestive systems disorders in Baduy ethnic, Indonesisa. BIOTROPIA-Southeast Asian J. Trop. Biol. 2021, 28. [Google Scholar]
- Nisa, U.; Astana, P.R.W.; Triyono, A.; Ardiyanto, D.; Fitriani, U.; Zulkarnain, Z.; Novianto, F.; Jannah, W.D.M. Ethnobotanical study of medicinal plants used for treating urinary tract problems in eastern Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 905, 012119. [Google Scholar] [CrossRef]
- Silalahi, M.; Nisyawati, N.; Walujo, E.B.; Mustaqim, W. Ethnomedicine of medicinal plants by Batak Phakpak subethnic in the Surung Mersada village, Phakpak Bharat district, North Sumatera. J. Ilmu Dasar 2018, 19, 77–92. [Google Scholar] [CrossRef][Green Version]
- Silalahi, M.; Nisyawati; Walujo, E.B.; Supriatna, J.; Mangunwardoyo, W. The local knowledge of medicinal plants trader and diversity of medicinal plants in the Kabanjahe traditional market, North Sumatra, Indonesia. J. Ethnopharmacol. 2015, 175, 432–443. [Google Scholar] [CrossRef]
- Mustofa, F.; Rahmawati, N.; Saryanto, S. Ethnomedicine of Medicinal plants used by traditional healers to facilitate bone injury healing in Kalimantan, Indonesia. J. Tumbuh. Obat Indones. 2021, 14, 36–54. [Google Scholar] [CrossRef]
- Maneenoon, K.; Khuniad, C.; Teanuan, Y.; Saedan, N.; Prom-in, S.; Rukleng, N.; Kongpool, W.; Pinsook, P.; Wongwiwat, W. Ethnomedicinal plants used by traditional healers in Phatthalung province, Peninsular Thailand. J. Ethnobiol. Ethnomed. 2015, 11, 43. [Google Scholar] [CrossRef]
- Susiarti, S.; Sambas, E.N. Local knowledge on medicinal plants of Batak Mandailing and Nias communities in Batang Toru, North Sumatra, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 197, 012008. [Google Scholar] [CrossRef]
- Dagar, H.S. Plant folk medicines among Nicobarese tribals of car Nicobar island, India. Econ. Bot. 1989, 43, 215–224. [Google Scholar] [CrossRef]
- Uhe, G. Medicinal Plants of Samoa: A preliminary survey of the use of plants for medicinal purposes in the Samoan islands. Econ. Bot. 1974, 28, 1–30. [Google Scholar] [CrossRef]
- Asowata-Ayodele, A.M.; Afolayan, A.J.; Otunola, G.A. Ethnobotanical survey of culinary herbs and spices used in the traditional medicinal system of Nkonkobe municipality, Eastern Cape, South Africa. S. Afr. J. Bot. 2016, 104, 69–75. [Google Scholar] [CrossRef]
- Okot, D.F.; Anywar, G.; Namukobe, J.; Byamukama, R. Medicinal plants species used by herbalists in the treatment of snakebite envenomation in Uganda. Trop. Med. Health 2020, 48, 44. [Google Scholar] [CrossRef] [PubMed]
- Jima, T.T.; Megersa, M. Ethnobotanical Study of Medicinal plants used to treat human diseases in Berbere District, Bale Zone of Oromia regional state, South East Ethiopia. J. Evid.-Based Complement. Altern. Med. 2018, 2018, 8602945. [Google Scholar] [CrossRef]
- Muthu, C.; Ayyanar, M.; Raja, N.; Ignacimuthu, S. Medicinal plants used by traditional healers in Kancheepuram district of Tamil Nadu, India. J. Ethnobiol. Ethnomed. 2006, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Jadid, N.; Kurniawan, E.; Himayani, C.E.S.; Andriyani; Prasetyowati, I.; Purwani, K.I.; Muslihatin, W.; Hidayati, D.; Tjahjaningrum, I.T.D. An ethnobotanical study of medicinal plants used by the tengger tribe in Ngadisari village, Indonesia. PLoS ONE 2020, 15, e0235886. [Google Scholar] [CrossRef]
- Su, P.-W.; Yang, C.-H.; Yang, J.-F.; Su, P.-Y.; Chuang, L.-Y. Antibacterial activities and antibacterial mechanism of Polygonum cuspidatum extracts against nosocomial drug-resistant pathogens. Molecules 2015, 20, 11119–11130. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.C.; Lihan, S.; Bunya, S.R.; Leong, S.S. In vitro antimicrobial efficacy of Cassia alata (linn.) leaves, stem, and root extracts against cellulitis causative agent Staphylococcus aureus. BMC Complement. Med. Ther. 2023, 23, 85. [Google Scholar]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef]
- Hall, M.D.; Handley, M.D.; Gottesman, M.M. Is resistance useless? Multidrug resistance and collateral sensitivity. Trends Pharmacol. Sci. 2009, 30, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Shamsabadipour, S.; Ghanadian, M.; Saeedi, H.; Rahimnejad, M.R.; Mohammadi-Kamalabadi, M.; Ayatollahi, S.M.; Salimzadeh, L. Triterpenes and steroids from Euphorbia denticulata lam. with anti-herpes symplex virus activity. Iran. J. Pharm. Res. 2013, 12, 759–767. [Google Scholar]
- Benjamaa, R.; Moujanni, A.; Kaushik, N.; Choi, E.H.; Essamadi, A.K.; Kaushik, N.K. Euphorbia species latex: A comprehensive review on phytochemistry and biological activities. Front. Plant Sci. 2022, 13, 1008881. [Google Scholar] [CrossRef]
- Crespi-Perellino, N.; Garofano, L.; Arlandini, E.; Pinciroli, V.; Minghetti, A.; Vincieri, F.F.; Danieli, B. Identification of new diterpenoids from Euphorbia calyptrata cell cultures. J. Nat. Prod. 1996, 59, 773–776. [Google Scholar] [CrossRef]
- Sun, B.-N.; Shen, H.-D.; Wu, H.-X.; Cui, F.; Yao, L.-X.; Cheng, Z.-Q.; Diao, Y. Chemical constituents of Onchidium struma from Chongming Island. Shanghai. Nat. Prod. Res. Dev. 2014, 26, 987–989. [Google Scholar]
- Jing, S.; Qu, Z.; Zhao, C.; Li, X.; Guo, L.; Liu, Z.; Zheng, Y.; Gao, W. Dihydroisocoumarins and dihydroisoflavones from the rhizomes of Dioscorea collettii with cytotoxic activity and structural revision of 2,2′-oxybis(1,4-di-tert-butylbenzene). Molecules 2021, 26, 5381. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural sources and bioactivities of 2,4-di-tert-butylphenol and its analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef]
- Wang, A.; Fraga, R.P.A.; Hörmann, E.; Pfaltz, A. Iridium-catalyzed asymmetric hydrogenation of unfunctionalized, trialkyl-substituted olefins. Chem. Asian J. 2011, 6, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Erwin, W.R.P.; Safitri, R.D.; Marliana, E.; Kusuma, I.W. Isolation and characterization of stigmasterol and β-sitosterol from wood bark extract of Baccaurea macrocarpa Miq. Mull. Arg. Rasayan J. Chem. 2020, 13, 2552–2558. [Google Scholar] [CrossRef]
- World Flora Online WFO: Published on the Internet. Available online: http://www.worldfloraonline.org/ (accessed on 17 May 2023).
- Ghazal, T.S.A.; Schelz, Z.; Vidács, L.; Szemerédi, N.; Veres, K.; Spengler, G.; Hohmann, J. Antimicrobial, multidrug resistance reversal and biofilm formation inhibitory effect of Origanum majorana extracts, essential oil and monoterpenes. Plants 2022, 11, 1432. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Rodríguez-Tudela, J.L.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Denning, D.; Donnelly, J.P.; Dupont, B.; Fegeler, W.; Moore, C.; et al. Method for the determination of minimum inhibitory concentration (mic) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 2003, 9, i–viii. [Google Scholar] [CrossRef]
| Treatment Categories | No. Indications or Symptoms |
|---|---|
| Skin | 17 |
| General symptoms | 10 |
| Digestive system | 9 |
| Musculoskeletal system | 6 |
| Genital system | 3 |
| Pregnancy and childbearing | 3 |
| Eye | 2 |
| Neurological system | 2 |
| Respiratory system | 1 |
| Blood, blood-forming organs and immune system | 1 |
| Endocrine, metabolic and nutritional system | 1 |
| Urinary system | 1 |
| Total | 56 |
| Species | Fr. a | Bacteria | Fungi | |||||
|---|---|---|---|---|---|---|---|---|
| S. aureus ATCC 29213 | MRSA ATCC 43300 | S. epidermidis ATCC 12228 | B. subtilis ATCC 6633 | M. catarrhalis ATCC 25238 | C. parapsilosis ATCC 22019 | N. glabrata ATCC 2001 | ||
| Euphorbia atoto | E | 12.5 | 250 | |||||
| MW | >500 | 500 | ||||||
| Euphorbia hypericifolia | C | >500 | ||||||
| E | 500 | 250 | ||||||
| MW | 500 | |||||||
| Homalanthus giganteus | E | 250 | 250 | 25 | 12.5 | >500 | 200 | |
| MW | 500 | |||||||
| Macaranga tanarius | C | 15.6 | 500 | |||||
| E | 500 | 250 | 100 | 12.5 | 50 | |||
| Mallotus mollissimus | C | 500 | ||||||
| E | 100 | |||||||
| Mallotus rufidulus | C | 25 | 250 | |||||
| E | 250 | |||||||
| Shirakiopsis indica | H | >500 | ||||||
| C | >500 | |||||||
| E | 500 | 250 | ||||||
| MW | 25 | 500 | 250 | |||||
| Ciprofloxacin | 0.01 | 0.21 | 0.1 | 0.02 | 0.02 | |||
| Ampicillin | 0.26 | 4.14 | 1.04 | 0.01 | 0.003 | |||
| Nystatin | 0.3125 | 0.3125 | ||||||
| DMSO (%) | >2% | >2% | >2% | >2% | >2% | >2% | >2% | >2% |
| Plant Name | IC50 Value (µg/mL) a | ||
|---|---|---|---|
| Fraction | Colo 205 | Colo 320 | |
| Euphorbia atoto | n-Hex | 0.24 ± 0.06 | 55.02 ± 0.91 |
| CHCl3 | 0.23 ± 0.04 | 53.17 ± 0.49 | |
| EtOAc | N | N | |
| Aq-MeOH | - | - | |
| Euphorbia hypericifolia | n-Hex | 55.94 ± 0.64 | 72.05 ± 0.82 |
| CHCl3 | N | 36.54 ± 0.64 | |
| EtOAc | N | N | |
| Aq-MeOH | N | N | |
| Homalanthus giganteus | n-Hex | 0.92 ± 0.15 | 31.92 ± 2.69 |
| CHCl3 | 14.42 ± 1.30 | 26.18 ± 1.77 | |
| EtOAc | N | N | |
| Aq-MeOH | N | N | |
| Macaranga tanarius | n-Hex | 56.58 ± 1.14 | 66.57 ± 2.01 |
| CHCl3 | 23.02 ± 0.86 | 8.46 ± 0.36 | |
| EtOAc | N | N | |
| Aq-MeOH | N | N | |
| Mallotus mollissimus | n-Hex | 37.29 ± 1.13 | 84.40 ± 1.22 |
| CHCl3 | 57.18 ± 0.41 | 62.03 ± 0.42 | |
| EtOAc | N | N | |
| Aq-MeOH | N | N | |
| Mallotus rufidulus | n-Hex | 23.18 ± 2.22 | 57.35 ± 0.58 |
| CHCl3 | 0.56 ± 0.07 | 7.10 ± 0.60 | |
| EtOAc | N | N | |
| Aq-MeOH | N | N | |
| Shirakiopsis indica | n-Hex | 2.60 ± 0.35 | 55.53 ± 1.54 |
| CHCl3 | 1.04 ± 0.01 | 11.93 ± 0.82 | |
| EtOAc | N | N | |
| Aq-MeOH | N | N | |
| Doxorubicin b | 1.45 ± 0.19 | 1.76 ± 0.09 | |
| Cell Strain | Compound 1 | Doxorubicin | Cisplatin | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Colo 205 | 30.68 | 0.70 | 0.33 | 0.009 | 24.09 | 1.47 |
| HTB-26 | 24.83 | 2.46 | 0.37 | 0.021 | 26.64 | 1.82 |
| MCF-7 | >100 | - | 0.60 | 0.045 | 20.61 | 1.26 |
| MRC-5 | 15.67 | 0.59 | 0.50 | 0.033 | 2.79 | 0.17 |
| Voucher No | Scientific Name | Author Name | Place of Collection | Plant Part |
|---|---|---|---|---|
| DGW-07 | Euphorbia atoto | G.Forst. | Gerupuk bay, Central Lombok | Aerial parts |
| DGW-08 | Euphorbia hypericifolia | L. | Sulat, East Lombok | Aerial parts |
| DGW-03 | Homalanthus giganteus | Zoll. & Moritzi | Mareje, West Lombok | Leaves |
| DGW-01 | Macaranga tanarius | Müll.Arg. | Mareje, West Lombok | Leaves |
| DGW-02 | Mallotus mollissimus | (Geiseler) Airy Shaw | Mareje, West Lombok | Leaves |
| DGW-04 | Mallotus rufidulus | Müll.Arg. | Mareje, West Lombok | Leaves |
| DGW-05 | Shirakiopsis indica | (Willd.) Esser | Pujut, Central Lombok | Leaves |
| Plants (Dry Weight) | Part | MeOH (L) | Yield g (%) | ||||
|---|---|---|---|---|---|---|---|
| MeOH ext. | n-Hex fr. | CHCl3 fr. | EtOAc fr. | Aq-MeOH fr. | |||
| M. tanarius (60.06 g) | Leaves | 2.4 | 15.45 | 1.36 | 3.60 | 2.38 g | 6.16 g |
| (25.72) | (8.80) | (23.30) | (15.40) | (39.87) | |||
| M. mollissimus (69.15 g) | Leaves | 3.0 | 17.75 | 4.27 | 0.72 | 0.07 | 11.60 |
| (25.67) | (24.06) | (4.06) | (0.39) | (65.35) | |||
| H. giganteus (116.4 g) | Leaves | 3.0 | 51.39 | 6.37 | 1.84 | 2.22 | 19.7 |
| (44.14) | (12.39) | (3.58) | (4.32) | (38.33) | |||
| M. rufidulus (54.12 g) | Leaves | 2.8 | 21.15 | 2.65 | 0.46 | 0.25 | 6.29 |
| (39.08) | (12.53) | (2.17) | (1.18) | (29.74) | |||
| S. indica (117.61 g) | Leaves | 4.0 | 77.98 | 12.70 | 0.56 | 8.00 | 31.62 |
| (66.30) | (16.28) | (0.72) | (10.26) | (40.54) | |||
| E. atoto (234.70 g) | Aerial parts | 4.1 | 83.55 | 8.12 | 0.78 | 0.08 | 47.80 |
| (35.59) | (9.71) | (0.93) | (0.09) | (57.21) | |||
| E. hypericifolia (14.42 g) | Aerial parts | 0.8 | 3.34 | 0.32 | 0.20 | 1.06 | 1.10 |
| (24.20) | (9.58) | (5.99) | (31.73) | (32.93) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirasisya, D.G.; Kincses, A.; Vidács, L.; Szemerédi, N.; Spengler, G.; Barta, A.; Mertha, I.G.; Hohmann, J. Indonesian Euphorbiaceae: Ethnobotanical Survey, In Vitro Antibacterial, Antitumour Screening and Phytochemical Analysis of Euphorbia atoto. Plants 2023, 12, 3836. https://doi.org/10.3390/plants12223836
Wirasisya DG, Kincses A, Vidács L, Szemerédi N, Spengler G, Barta A, Mertha IG, Hohmann J. Indonesian Euphorbiaceae: Ethnobotanical Survey, In Vitro Antibacterial, Antitumour Screening and Phytochemical Analysis of Euphorbia atoto. Plants. 2023; 12(22):3836. https://doi.org/10.3390/plants12223836
Chicago/Turabian StyleWirasisya, Dyke Gita, Annamária Kincses, Lívia Vidács, Nikoletta Szemerédi, Gabriella Spengler, Anita Barta, I Gde Mertha, and Judit Hohmann. 2023. "Indonesian Euphorbiaceae: Ethnobotanical Survey, In Vitro Antibacterial, Antitumour Screening and Phytochemical Analysis of Euphorbia atoto" Plants 12, no. 22: 3836. https://doi.org/10.3390/plants12223836
APA StyleWirasisya, D. G., Kincses, A., Vidács, L., Szemerédi, N., Spengler, G., Barta, A., Mertha, I. G., & Hohmann, J. (2023). Indonesian Euphorbiaceae: Ethnobotanical Survey, In Vitro Antibacterial, Antitumour Screening and Phytochemical Analysis of Euphorbia atoto. Plants, 12(22), 3836. https://doi.org/10.3390/plants12223836









