Impact of Chitosan-Based Foliar Application on the Phytochemical Content and the Antioxidant Activity in Hemp (Cannabis sativa L.) Inflorescences
Abstract
:1. Introduction
2. Results
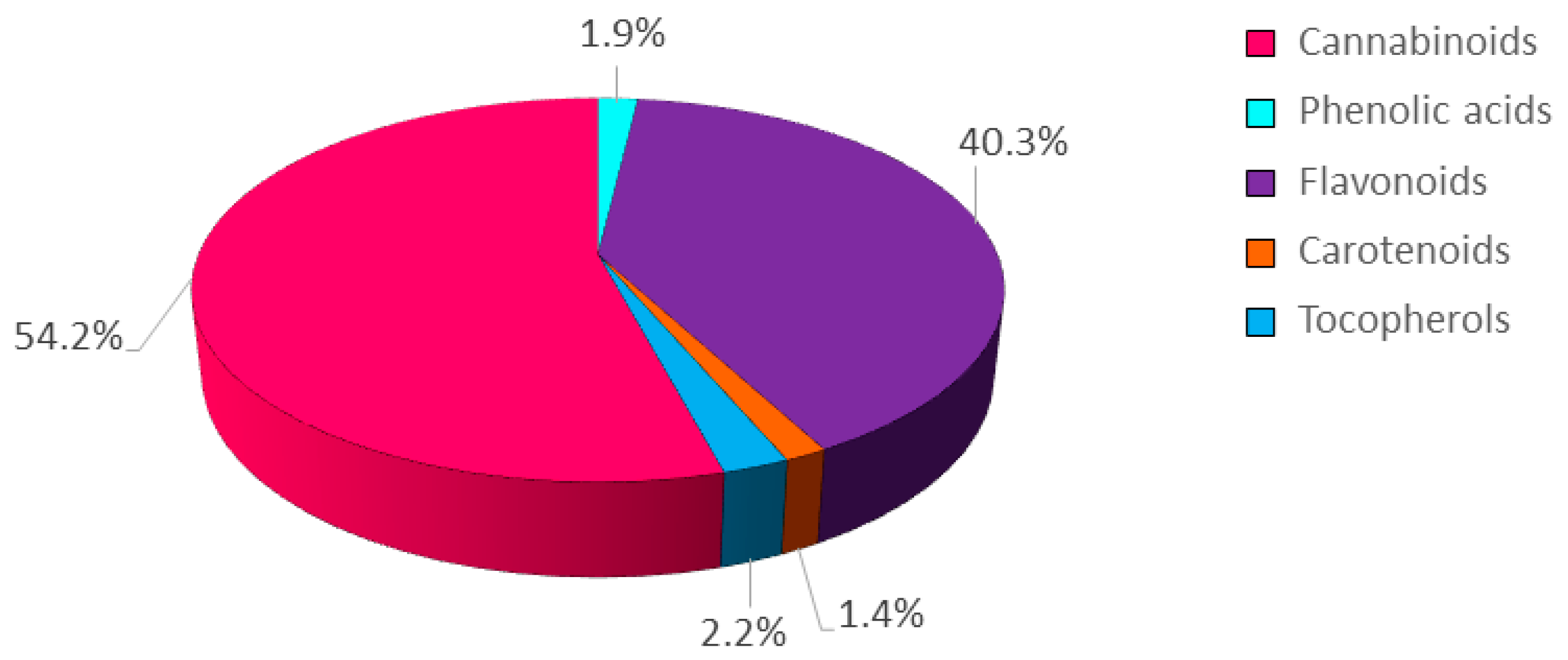
2.1. Characterization of the Phytochemical Profile and the Antioxidant Activity of the Hemp Inflorescences
2.2. Correlation Analysis of the Traits Examined in the Hemp Inflorescences
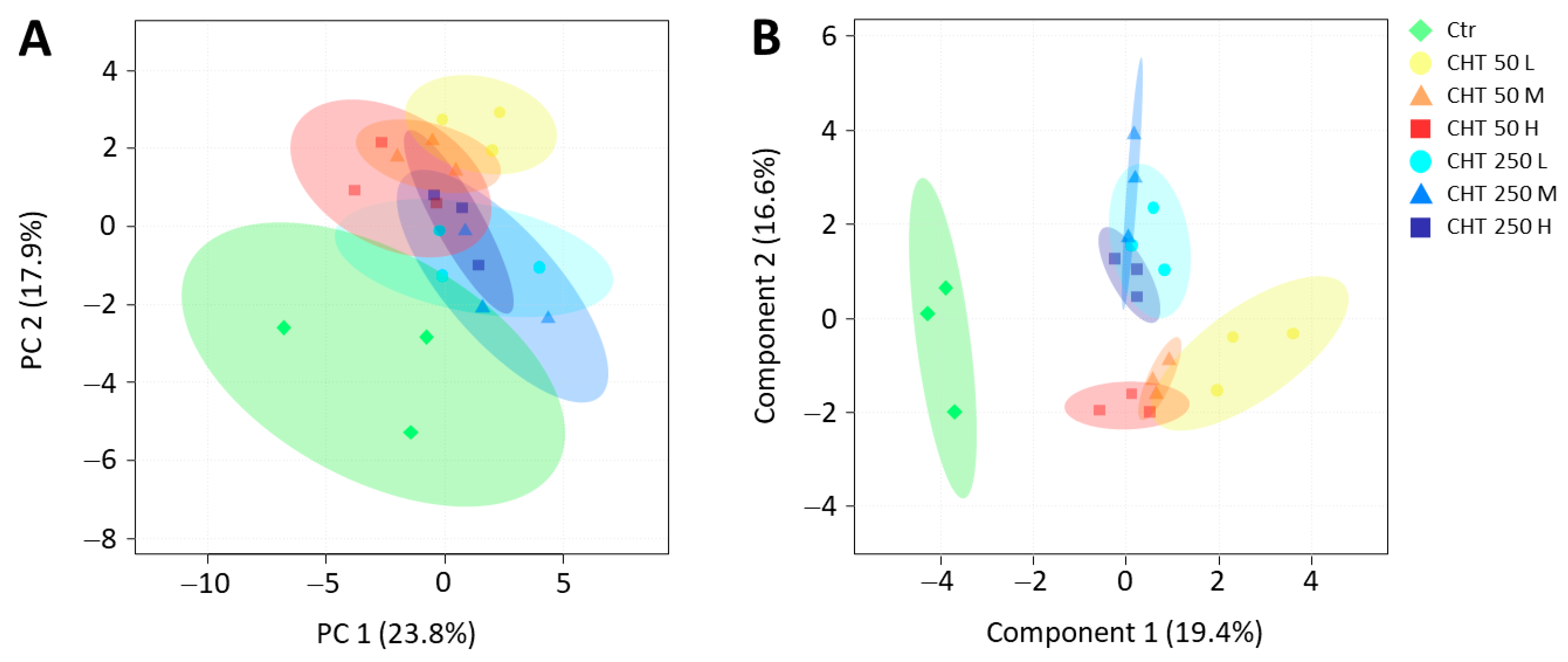
2.3. Variations in the Phytochemical Content and the Antioxidant Activity in Hemp Inflorescences Due to CHT Treatments
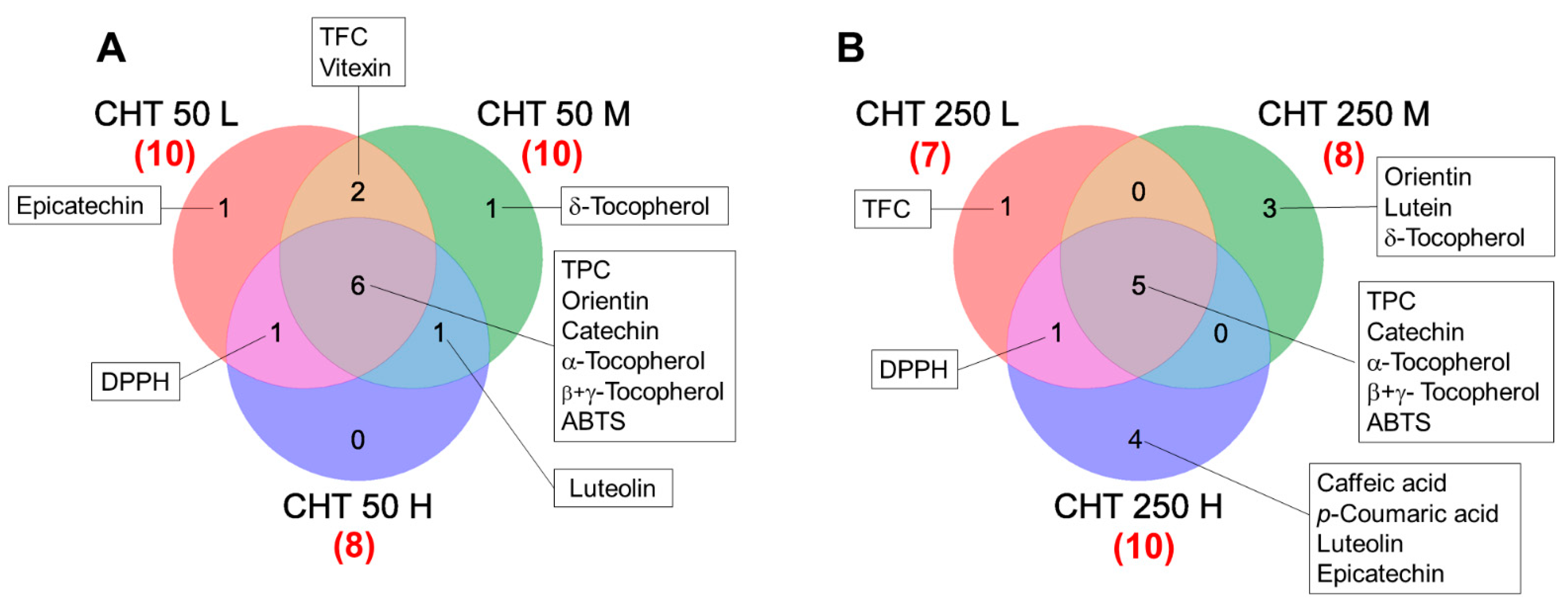
2.4. Identification of Candidate Biomarkers for CHT Treatments
3. Discussion
3.1. Phytochemicals and Antioxidant Activity in the Inflorescences of the Hemp cv. Codimono
3.2. Effect of CHT-Based Foliar Treatments on Phytochemical Content and Antioxidant Activity in the Inflorescences from the Hemp cv. Codimono
4. Materials and Methods
4.1. Preparation of the Chitosan Solutions
4.2. Plant Material and Growing Conditions
4.3. Spectrophotometric Measurements
4.3.1. Determination of TPC and TFC
4.3.2. Determination of the Total Antioxidant Activity
4.4. HPLC Measurements
4.4.1. Determination of Phenolic Compounds
4.4.2. Determination of Carotenoids
4.4.3. Determination of Tocopherols
4.4.4. Determination of Cannabinoids
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandra, S.; Lata, H.; ElSohly, M.A. Cannabis sativa L.-Botany and Biotechnology; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–474. [Google Scholar]
- Crocq, M.A. History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef]
- Ash, A.L. Hemp–Production and Utilization. Econ. Bot. 1948, 2, 159–169. [Google Scholar] [CrossRef]
- Chouvy, P.-A. Cannabis cultivation in the world: Heritages, trends and challenges. EchoGéo 2019, 48, 50. [Google Scholar] [CrossRef]
- Giupponi, L.; Leoni, V.; Carrer, M.; Ceciliani, G.; Sala, S.; Panseri, S.; Pavlovic, R.; Giorgi, A. Overview on Italian hemp production chain, related productive and commercial activities and legislative framework. Ital. J. Agron. 2020, 15, 1552. [Google Scholar] [CrossRef]
- Guerra, R.O.; do Carmo Neto, J.R.; da Silva, P.E.F.; Borges, Y.L.L.; Franco, P.I.R.; Machado, J.R.; da Silva, M.V. Cannabis sativa: A look at protozoa, helminths, insect vectors, and pests. Fitoterapia 2023, 166, 105467. [Google Scholar] [CrossRef]
- Angelova, V.; Ivanova, R.; Delibaltova, V.; Ivanov, K. Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp). Ind. Crops Prod. 2004, 19, 197–205. [Google Scholar] [CrossRef]
- Ferrarini, A.; Fracasso, A.; Spini, G.; Fornasier, F.; Taskin, E.; Fontanella, M.C.; Beone, G.M.; Amaducci, S.; Puglisi, E. Bioaugmented phytoremediation of metal-contaminated soils and sediments by hemp and giant reed. Front. Microbiol. 2021, 12, 645893. [Google Scholar] [CrossRef]
- Väisänena, T.; Batello, P.; Lappalainen, R.; Tomppo, L. Modification of hemp fibers (Cannabis sativa L.) for composite applications. Ind. Crops Prod. 2018, 111, 422–429. [Google Scholar] [CrossRef]
- Modi, A.A.; Shahid, R.; Saeed, M.U.; Younas, T. Hemp is the future of plastics. E3S Web Conf. 2018, 51, 03002. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Kumar, V.; Rani, A.; Jain, R. Cannabis sativa: A plant suitable for phytoremediation and bioenergy production. In Phytoremediation Potential of Bioenergy Plants; Bauddh, K., Singh, B., Korstad, J., Eds.; Springer: Singapore, 2007; pp. 269–285. [Google Scholar]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A review of hemp as food and nutritional supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef]
- Frassinetti, S.; Moccia, E.; Caltavuturo, L.; Gabriele, M.; Longo, V.; Bellani, L.; Giorgi, G.; Giorgetti, L. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018, 262, 56–66. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. The origin and biomedical relevance of cannabigerol. Int. J. Mol. Sci. 2022, 23, 7929. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural antioxidants from plant extracts in skincare cosmetics: Recent applications, challenges and perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Pavlovic, R.; Panseri, S.; Giupponi, L.; Leoni, V.; Citti, C.; Cattaneo, C.; Cavaletto, M.; Giorgi, A. Phytochemical and ecological analysis of two varieties of hemp (Cannabis sativa L.) grown in a mountain environment of Italian Alps. Front. Plant Sci. 2019, 10, 1265. [Google Scholar] [CrossRef]
- Giupponi, L.; Leoni, V.; Pavlovic, R.; Giorgi, A. Influence of altitude on phytochemical composition of hemp inflorescence: A metabolomic approach. Molecules 2020, 25, 1381. [Google Scholar] [CrossRef]
- Beleggia, R.; Menga, V.; Fulvio, F.; Fares, C.; Trono, D. Effect of genotype, year, and their interaction on the accumulation of bioactive compounds and the antioxidant activity in industrial hemp (Cannabis sativa L.) inflorescences. Int. J. Mol. Sci. 2023, 24, 8969. [Google Scholar] [CrossRef]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Fraschetti, C.; Filippi, A.; Di Sotto, A.; Di Giacomo, S.; Mazzoccanti, G.; Gasparrini, F.; et al. Cannabis sativa L. inflorescences from monoecious cultivars grown in central Italy: An untargeted chemical characterization from early flowering to ripening. Molecules 2020, 25, 1908. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Botta, B.; Quaglio, D.; Ghirga, F.; Balducci, S.; Cammarone, S.; Campiglia, E.; Giusti, A.M.; et al. A multimethodological characterization of Cannabis sativa L. inflorescences from seven dioecious cultivars grown in Italy: The effect of different harvesting stages. Molecules 2021, 26, 2912. [Google Scholar] [CrossRef] [PubMed]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Sobolev, A.P.; Giusti, A.M.; Vinci, G.; Cammarone, S.; Tortora, C.; Lamelza, L.; Prencipe, S.A.; et al. Industrial hemp (Cannabis sativa L.) inflorescences as novel food: The effect of different agronomical practices on chemical profile. Foods 2022, 11, 3658. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Mukta, J.A.; Sabir, A.A.; Gupta, D.R.; Mohi-Ud-Din, M.; Hasanuzzaman, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Chitosan biopolymer promotes yield and stimulates accumulation of antioxidants in strawberry fruit. PLoS ONE 2018, 13, e0203769. [Google Scholar] [CrossRef] [PubMed]
- Quitadamo, F.; De Simone, V.; Beleggia, R.; Trono, D. Chitosan-induced activation of the antioxidant defense system counteracts the adverse effects of salinity in durum wheat. Plants 2021, 10, 1365. [Google Scholar] [CrossRef]
- Singh, R.K.; Soares, B.; Goufo, P.; Castro, I.; Cosme, F.; Pinto-Sintra, A.L.; Inês, A.; Oliveira, A.A.; Falco, V. Chitosan upregulates the genes of the ROS pathway and enhances the antioxidant potential of grape (Vitis vinifera L. ‘Touriga Franca’ and ‘Tinto Cão’) tissues. Antioxidants 2019, 8, 525. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Pacifico, D.; Miselli, F.; Carboni, A.; Moschella, A.; Mandolino, G. Time course of cannabinoid accumulation and chemotype development during the growth of Cannabis sativa L. Euphytica 2008, 160, 231–240. [Google Scholar] [CrossRef]
- Regulation (EU) 2021/2115 of the European Parliament and of the Council of 2 December 2021 Establishing Rules on Support for Strategic Plans to Be Drawn up by Member States under the Common Agricultural Policy (CAP Strategic Plans) and Financed by the European Agricultural Guarantee Fund (EAGF) and by the European Agricultural Fund for Rural Development (EAFRD) and Repealing Regulations (EU) No 1305/2013 and (EU) No 1307/2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32021R2115 (accessed on 4 September 2023).
- Jin, D.; Henry, P.; Shan, J.; Chen, J. Identification of chemotypic markers in three chemotype categories of Cannabis using secondary metabolites profiled in inflorescences, leaves, stem bark, and roots. Front. Plant Sci. 2021, 12, 699530. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of phenolic compounds in commercial Cannabis sativa L. inflorescences using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef]
- Kriese, U.; Schumann, E.; Weber, W.E.; Beyer, M.; Brühl, L.; Matthäus, B. Oil content, tocopherol composition and fatty acid patterns of the seeds of 51 Cannabis sativa L. genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Chen, T.; He, J.; Zhang, J.; Zhang, H.; Qian, P.; Hao, J.; Li, L. Analytical characterization of hempseed (seed of Cannabis sativa L.) oil from eight regions in China. J. Diet. Suppl. 2010, 7, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Galasso, I.; Russo, R.; Mapelli, S.; Ponzoni, E.; Brambilla, I.M.; Battelli, G.; Reggiani, R. Variability in seed traits in a collection of Cannabis sativa L. genotypes. Front. Plant Sci. 2016, 7, 688. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect of genotype and growing year on the nutritional, phytochemical, and antioxidant properties of industrial hemp (Cannabis sativa L.) seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, A.; Aita, S.E.; Cannazza, G.; Capriotti, A.L.; Cavaliere, C.; Citti, C.; Bosco, C.D.; Gentili, A.; Montone, C.M.; Paris, R.; et al. Evaluation of the carotenoid and fat-soluble vitamin profile of industrial hemp inflorescence by liquid chromatography coupled to mass spectrometry and photodiode-array detection. J. Chromatogr. A 2023, 1692, 463838. [Google Scholar] [CrossRef]
- Muñoz, P.; Munné-Bosch, S. Vitamin E in plants: Biosynthesis, transport, and function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef]
- André, A.; Leupin, M.; Kneubühl, M.; Pedan, V.; Chetschik, I. Evolution of the polyphenol and terpene content, antioxidant activity and plant morphology of eight different fiber-type cultivars of Cannabis sativa L. cultivated at three sowing densities. Plants 2020, 9, 1740. [Google Scholar] [CrossRef]
- Skibsted, L.H. Vitamin and non-vitamin antioxidants and their interaction in food. J. Food Drug Anal. 2012, 20, 355–358. [Google Scholar] [CrossRef]
- Li, H.; Tsao, R.; Deng, Z. Factors affecting the antioxidant potential and health benefits of plant foods. Can. J. Plant Sci. 2012, 92, 1101–1111. [Google Scholar] [CrossRef]
- Suwanchaikasem, P.; Nie, S.; Selby-Pham, J.; Walker, R.; Boughton, B.A.; Idnurm, A. Hormonal and proteomic analyses of southern blight disease caused by Athelia rolfsii and root chitosan priming on Cannabis sativa in an in vitro hydroponic system. Plant Direct. 2023, 7, e528. [Google Scholar] [CrossRef]
- Kim, H.-J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2005, 53, 3696–3701. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B.; Dresler, S.; Rubinowska, K.; Matraszek-Gawron, R. Eliciting effect of foliar application of chitosan lactate on the phytochemical properties of Ocimum basilicum L. and Melissa officinalis L. Food Chem. 2021, 342, 128358. [Google Scholar] [CrossRef] [PubMed]
- Salimgandomi, S.; Shabrangy, A. The effect of Chitosan on antioxidant activity and some secondary metabolites of Mentha piperita L. J. Pharm. Health Sci. 2016, 4, 135–142. [Google Scholar]
- Vosoughi, N.; Gomarian, M.; Pirbalouti, A.G.; Khaghani, S.; Malekpoor, F. Essential oil composition and total phenolic, flavonoid contents, and antioxidant activity of sage (Salvia officinalis L.) extract under chitosan application and irrigation frequencies. Ind. Crops Prod. 2018, 117, 366–374. [Google Scholar] [CrossRef]
- Yin, H.; Fretté, X.C.; Christensen, L.P.; Grevsen, K. Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Origanum vulgare ssp. hirtum). J. Agric. Food Chem. 2012, 60, 136–143. [Google Scholar] [CrossRef]
- Stasińska-Jakubas, M.; Hawrylak-Nowak, B. Protective, biostimulating, and eliciting effects of chitosan and its derivatives on crop plants. Molecules 2022, 27, 2801. [Google Scholar] [CrossRef]
- Stasińska-Jakubas, M.; Hawrylak-Nowak, B.; Wójciak, M.; Dresler, S. Comparative effects of two forms of chitosan on selected phytochemical properties of Plectranthus amboinicus (Lour.). Molecules 2023, 28, 376. [Google Scholar] [CrossRef]
- Yu, L.; Zong, Y.; Han, Y.; Zhang, X.; Zhu, Y.; Oyom, W.; Gong, D.; Prusky, D.; Bi, Y. Both chitosan and chitooligosaccharide treatments accelerate wound healing of pear fruit by activating phenylpropanoid metabolism. Int. J. Biol. Macromol. 2022, 205, 483–490. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Ma, J.H.; Xie, J.; Deng, L.L.; Yao, S.X.; Zeng, K.F. Transcriptomic and biochemical analysis of highlighted induction of phenylpropanoid pathway metabolism of citrus fruit in response to salicylic acid, Pichia membranaefaciens and oligochitosan. Postharvest Biol. Technol. 2018, 142, 81–92. [Google Scholar] [CrossRef]
- Kamalipourazad, M.; Sharifi, M.; Maivan, H.Z.; Behmanesh, M.; Chashmi, N.A. Induction of aromatic amino acids and phenylpropanoid compounds in Scrophularia striata Boiss. cell culture in response to chitosan-induced oxidative stress. Plant Physiol. Biochem. 2016, 107, 374–384. [Google Scholar] [CrossRef]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Chitosan and chitin oligomers increase phenylalanine ammonia-lyase and tyrosine ammonia-lyase activities in soybean leaves. J. Plant Physiol. 2003, 160, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Hu, X.; Zhang, W.; Rogers, W.J.; Cai, W. Hydrogen peroxide mediates defense responses induced by chitosans of different molecular weights in rice. J. Plant Physiol. 2005, 162, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Ackah, S.; Xue, S.; Osei, R.; Kweku-Amagloh, F.; Zong, Y.; Prusky, D.; Bi, Y. Chitosan treatment promotes wound healing of apple by eliciting phenylpropanoid pathway and enzymatic browning of wounds. Front. Microbiol. 2022, 13, 828914. [Google Scholar] [CrossRef]
- Picchi, V.; Calzone, A.; Gobbi, S.; Paccani, S.; Lo Scalzo, R.; Marti, A.; Faoro, F. Oxidative stress mitigation by chitosan nanoparticles in durum wheat also affects phytochemicals and technological quality of bran and semolina. Plants 2022, 11, 2021. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Harish Prashanth, K.V.; Shetty, N.P.; Giridhar, P. Elicitors, SA and MJ enhance carotenoids and tocopherol biosynthesis and expression of antioxidant related genes in Moringa oleifera Lam. leaves. Acta Physiol. Plant. 2014, 36, 2695–2704. [Google Scholar] [CrossRef]
- El Amerany, F.; Rhazi, M.; Balcke, G.; Wahbi, S.; Meddich, A.; Taourirte, M.; Hause, B. The effect of chitosan on plant physiology, wound response, and fruit quality of tomato. Polymers 2022, 14, 5006. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Cásedas, G.; Moliner, C.; Maggi, F.; Mazzara, E.; López, V. Evaluation of two different Cannabis sativa L. extracts as antioxidant and neuroprotective agents. Front. Pharmacol. 2022, 13, 1009868. [Google Scholar] [CrossRef]
- Prvulović, D.; Gvozdenac, S.; Latković, D.; Peić Tukuljac, M.; Sikora, V.; Kiprovski, B.; Mišan, A.; Chrysargyris, A.; Tzortzakis, N.; Ovuka, J. Phytotoxic and insecticidal activity of industrial hemp (Cannabis sativa L.) Extracts against Plodia interpunctella Hübner—A potential sunflower grain protectant. Agronomy 2023, 13, 2456. [Google Scholar] [CrossRef]
- Sharma, D.C.; Rani, S.; Kashyap, N.P. Oviposition deterrence and ovicidal properties of some plant extracts against potato tuber moth Phthorimaea operculella (Zell.). Pestic. Res. J. 1997, 9, 241–246. [Google Scholar]
- Liang, J.; Aachary, A.A.; Thiyam-Holländer, U. Hemp seed oil: Minor components and oil quality. Lipid Technol. 2015, 27, 231–233. [Google Scholar] [CrossRef]
- Tura, M.; Ansorena, D.; Astiasarán, I.; Mandrioli, M.; Toschi, T.G. Evaluation of hemp seed oils stability under accelerated storage test. Antioxidants 2022, 11, 490. [Google Scholar] [CrossRef]
- Gamna, F.; Spriano, S. Vitamin E: A review of its application and methods of detection when combined with implant biomaterials. Materials 2021, 14, 3691. [Google Scholar] [CrossRef]
- Lam, K.Y.; Ling, A.P.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A review on medicinal properties of orientin. Adv. Pharmacol. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef] [PubMed]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Zagórska-Dziok, M.; Kleczkowska, P.; Olędzka, E.; Figat, R.; Sobczak, M. Poly(chitosan-esterether-urethane) hydrogels as highly controlled genistein release systems. Int. J. Mol. Sci. 2021, 22, 3339. [Google Scholar] [CrossRef]
- Cantele, C.; Bertolino, M.; Bakro, F.; Giordano, M.; Jędryczka, M.; Cardenia, V. Antioxidant effects of hemp (Cannabis sativa L.) inflorescence extract in stripped linseed oil. Antioxidants 2020, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, S.; Mokher, J.; Laiko, I.; Burbulis, N.; Kyrychenko, H.; Dudukova, S. Phenological growth stages of hemp (Cannabis sativa L.): Codification and description according to the BBCH scale. Žemès Ūkio Moksl. 2017, 24, 31–36. [Google Scholar] [CrossRef]
- Kim, K.H.; Tsa, R.; Yang, R.; Cui, W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Ferioli, F.; Giambanelli, E.; Pasini, F.; D’Antuomo, F. Comparison of some kale populations for their phytochemical content: Phenolics, glucosinolates, carotenoids, and chorophylls. J. Sci. Food Agric. 2013, 93, 3478–3489. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.D.; Bladenopoulos, K.; Papageorgiou, M. Determination of tocopherol and tocotrienol content of Greek barley varieties under conventional and organic cultivation techniques using validated reverse phase high-performance liquid chromatography method. J. Sci. Food Agric. 2012, 92, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Deutsches Arzneibuch 2018 (DAB 2018). Monographien Cannabisblüten-Cannabis flos; Deutscher Apotheker Verlag: Gerlingen, Germany, 2018. [Google Scholar]
- Tolomeo, F.; Russo, F.; Kaczorova, D.; Vandelli, M.A.; Biagini, G.; Laganà, A.; Capriotti, A.L.; Paris, R.; Fulvio, F.; Carbone, L.; et al. Cis-Δ9-tetrahydrocannabinolic acid occurrence in Cannabis sativa L. J. Pharm. Biomed. Anal. 2022, 219, 114958. [Google Scholar] [CrossRef] [PubMed]
- MetaboAnalyst 5.0. Available online: https://www.metaboanalyst.ca/ (accessed on 10 September 2023).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beleggia, R.; Iannucci, A.; Menga, V.; Quitadamo, F.; Suriano, S.; Citti, C.; Pecchioni, N.; Trono, D. Impact of Chitosan-Based Foliar Application on the Phytochemical Content and the Antioxidant Activity in Hemp (Cannabis sativa L.) Inflorescences. Plants 2023, 12, 3692. https://doi.org/10.3390/plants12213692
Beleggia R, Iannucci A, Menga V, Quitadamo F, Suriano S, Citti C, Pecchioni N, Trono D. Impact of Chitosan-Based Foliar Application on the Phytochemical Content and the Antioxidant Activity in Hemp (Cannabis sativa L.) Inflorescences. Plants. 2023; 12(21):3692. https://doi.org/10.3390/plants12213692
Chicago/Turabian StyleBeleggia, Romina, Anna Iannucci, Valeria Menga, Filippo Quitadamo, Serafino Suriano, Cinzia Citti, Nicola Pecchioni, and Daniela Trono. 2023. "Impact of Chitosan-Based Foliar Application on the Phytochemical Content and the Antioxidant Activity in Hemp (Cannabis sativa L.) Inflorescences" Plants 12, no. 21: 3692. https://doi.org/10.3390/plants12213692
APA StyleBeleggia, R., Iannucci, A., Menga, V., Quitadamo, F., Suriano, S., Citti, C., Pecchioni, N., & Trono, D. (2023). Impact of Chitosan-Based Foliar Application on the Phytochemical Content and the Antioxidant Activity in Hemp (Cannabis sativa L.) Inflorescences. Plants, 12(21), 3692. https://doi.org/10.3390/plants12213692






