Non-Volatile Terpenoids and Lipophilic Flavonoids from Achillea erba-rotta Subsp. moschata (Wulfen) I. Richardson
Abstract
1. Introduction
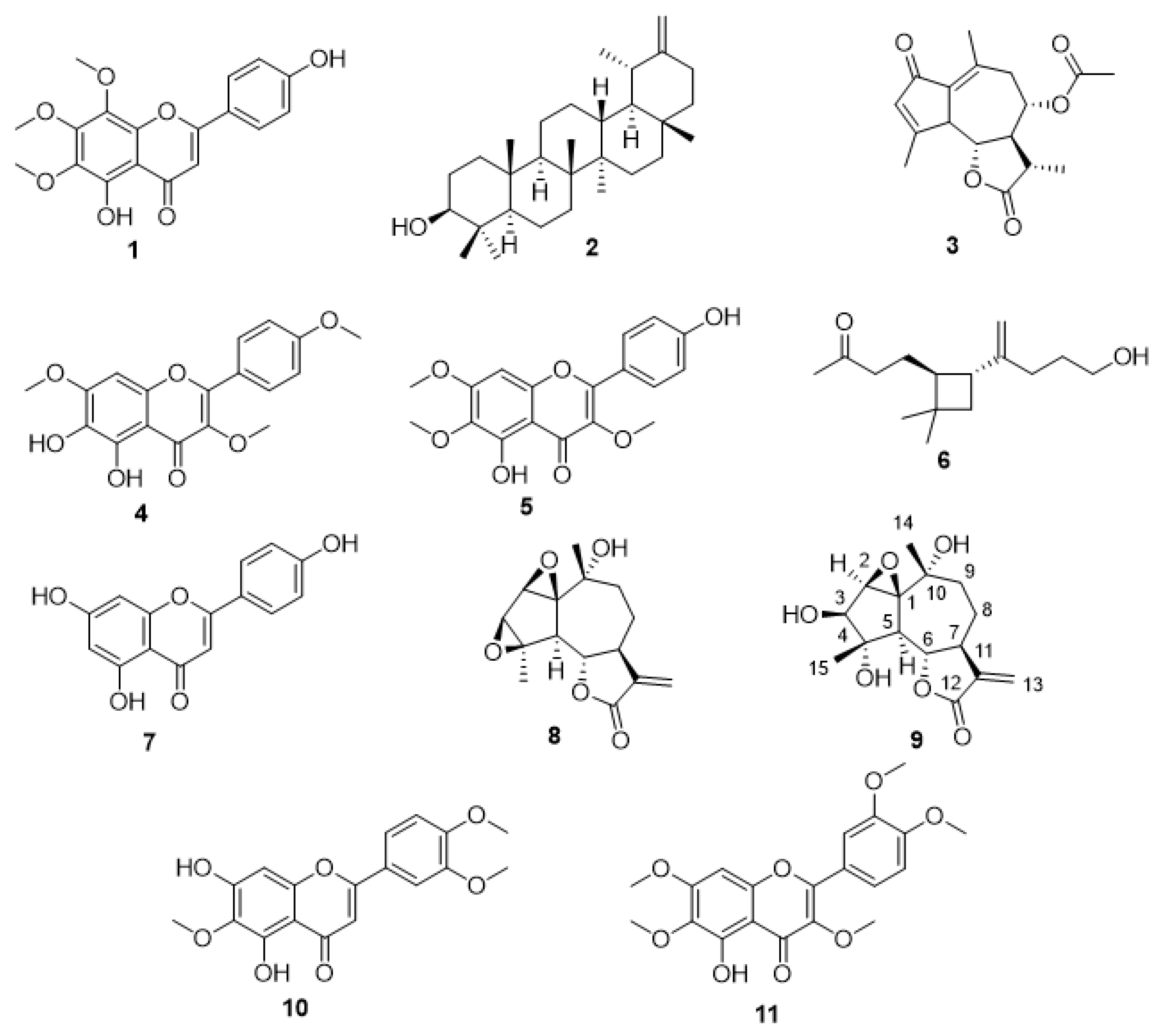
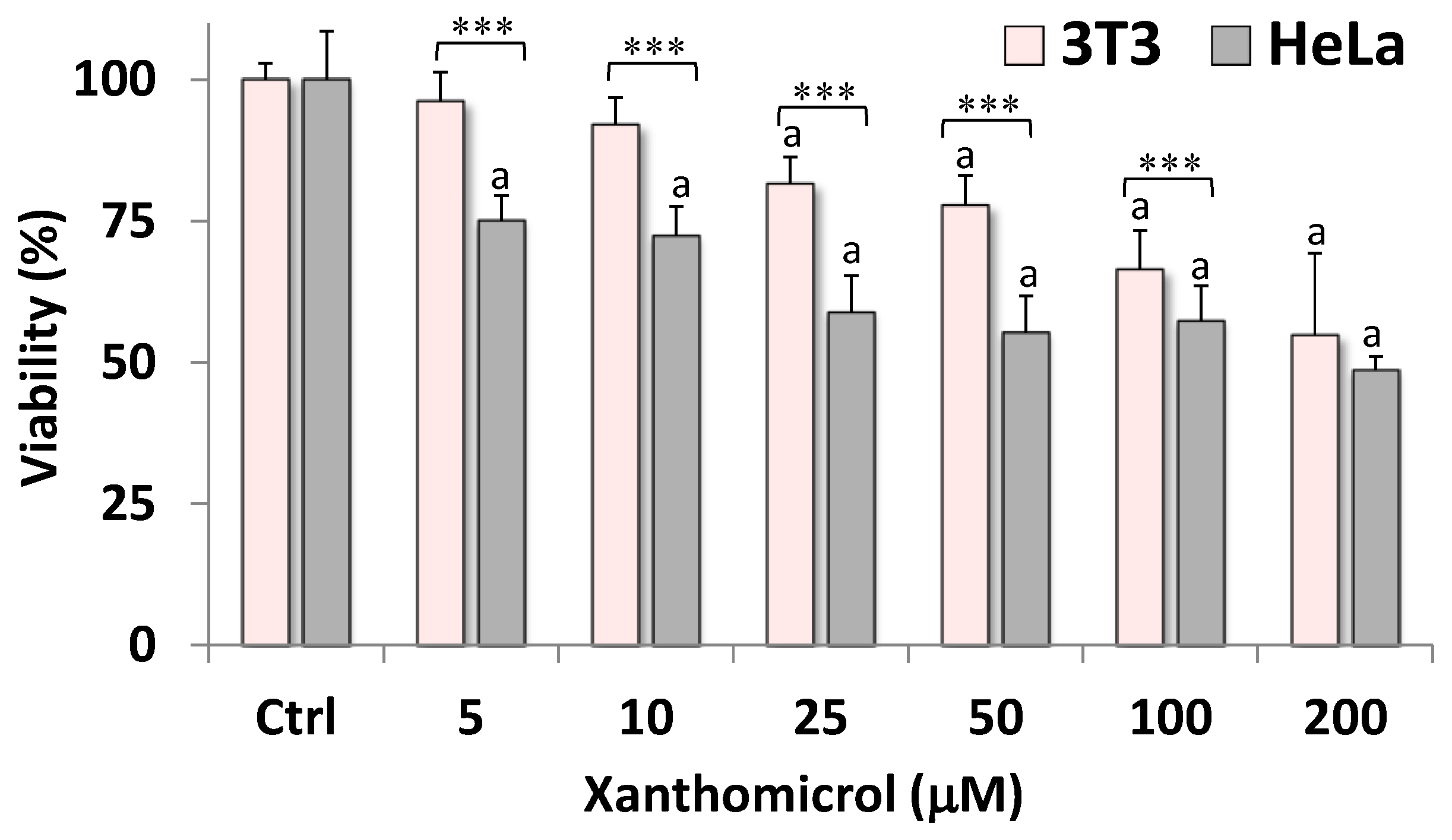
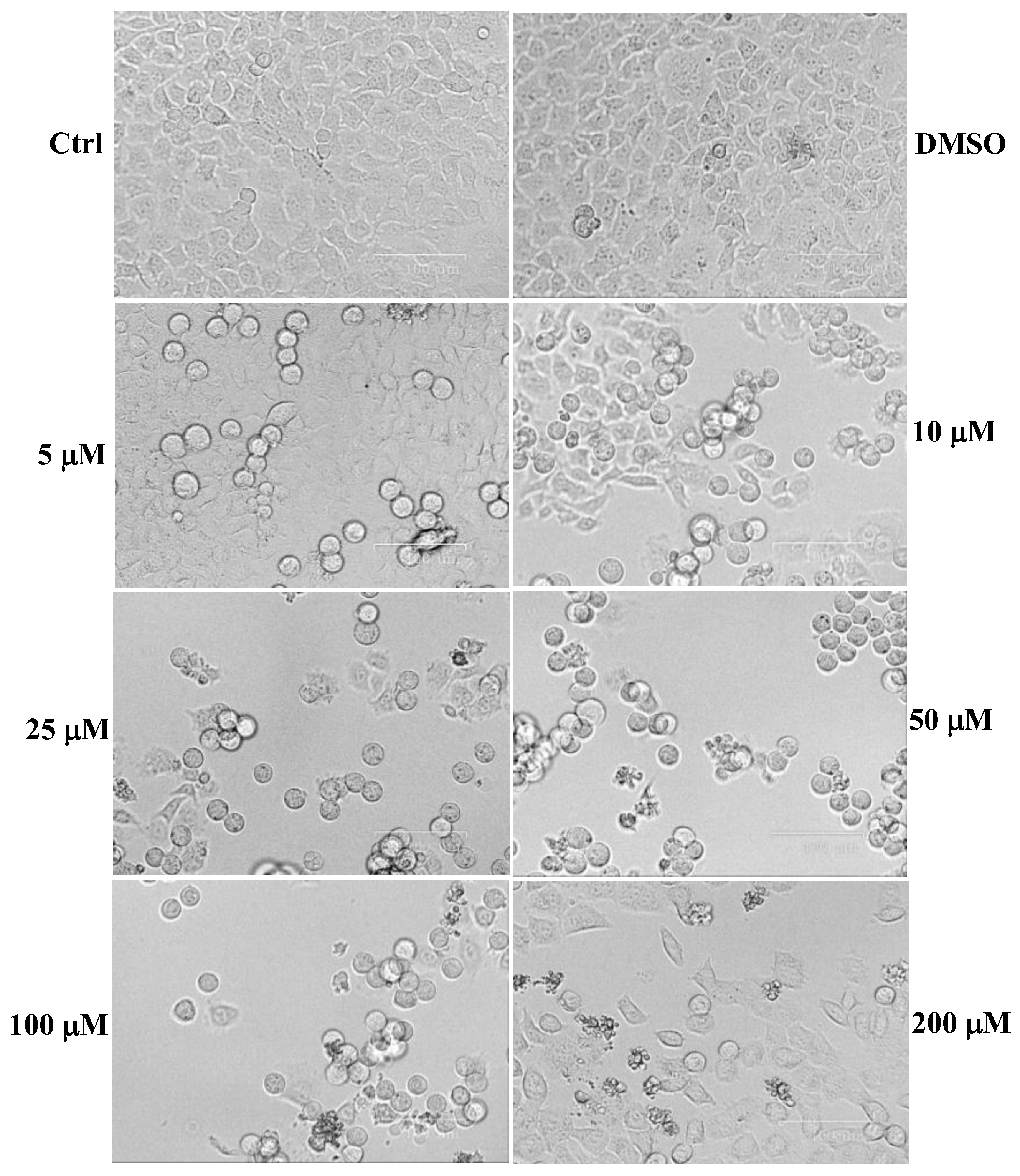
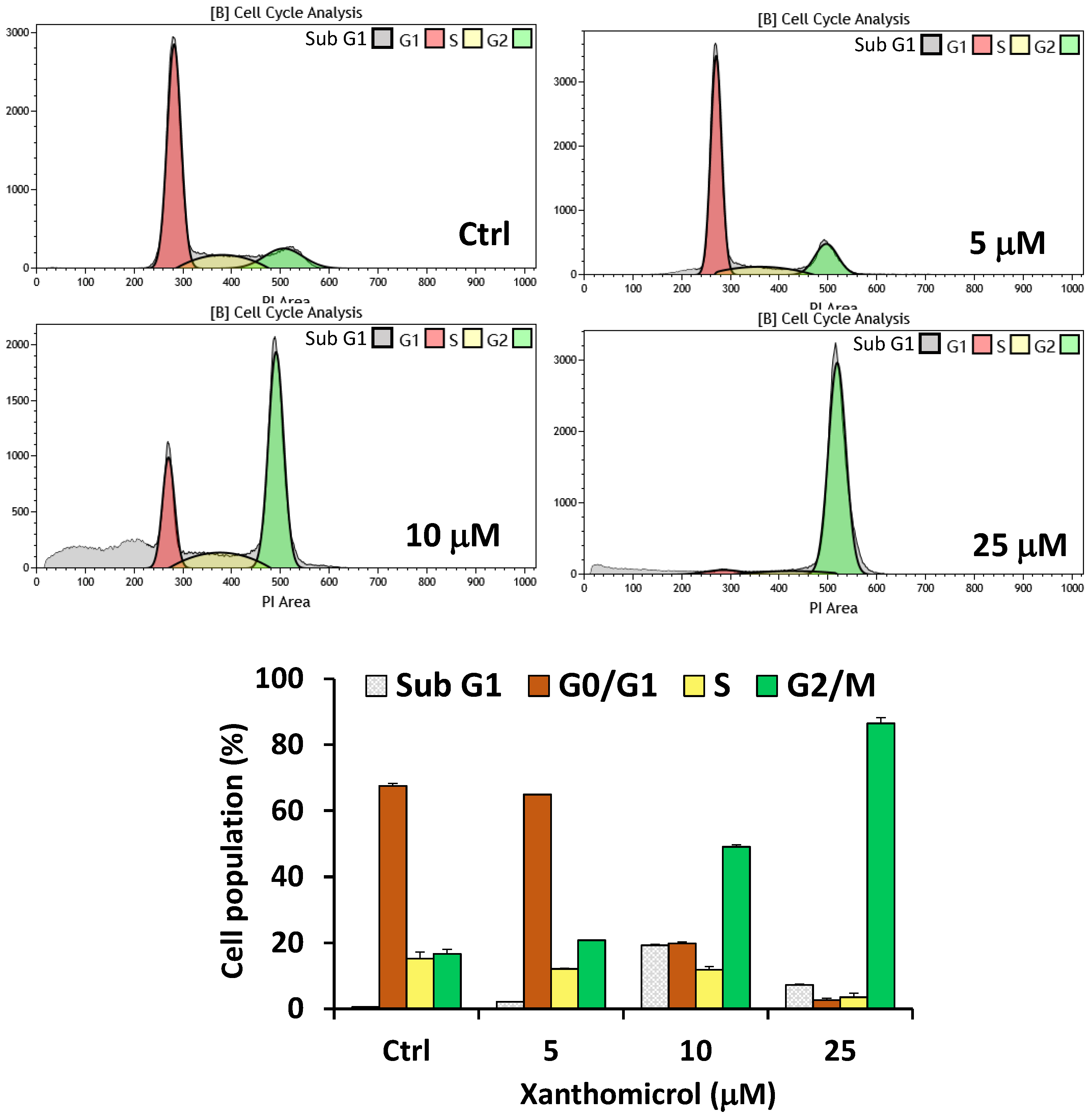
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Cytotoxic Activity
4.5. Cell Cycle
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, I.B.K. Achillea L. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1976; Volume 4, pp. 160–161. [Google Scholar]
- Pignatti, S.; Guarino, S.; La Rosa, M. Achillea moschata . In La Flora d’Italia & Flora Digitale, 2nd ed.; Edagricole: Bologna, Italy, 2018; Volume 3, p. 836. [Google Scholar]
- Argentieri, M.P.; Madeo, M.; Avato, P.; Iriti, M.; Vitalini, S. Polyphenol content and bioactivity of Achillea moschata from the Italian and Swiss Alps. Z. Für Nat. C 2020, 75, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Grande, S.; Visioli, F.; Agradi, E.; Fico, G.; Tomè, F. Antioxidant activity of wild plants collected in Valsesia, an alpine region of Northern Italy. Phytother. Res. 2006, 20, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Madeo, M.; Tava, A.; Iriti, M.; Vallone, L.; Avato, P.; Cocuzza, C.E.; Simonetti, P.; Argentieri, M.P. Chemical profile, antioxidant and antibacterial activities of Achillea moschata Wulfen, an endemic species from the Alps. Molecules 2016, 21, 830. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Garzoli, S.; Sisto, F.; Pezzani, R.; Argentieri, M.P.; Scarafoni, A.; Ciappellano, S.; Zorzan, M.; Capraro, J.; Collazuol, D.; et al. Digestive and gastroprotective effects of Achillea erba-rotta subsp. moschata (Wulfen) I.Richardson (syn. A. moschata Wulfen) (Asteraceae): From traditional uses to preclinical studies. J. Ethnopharmacol. 2022, 298, 115670. [Google Scholar] [CrossRef]
- Apel, L.; Lorenz, P.; Urban, S.; Sauer, S.; Spring, O.; Stintzing, F.C.; Kammerer, D.R. Phytochemical characterization of different yarrow species (Achillea sp.) and investigations into their antimicrobial activity. Z. Für Nat. C 2021, 76, 55–65. [Google Scholar] [CrossRef]
- Nano, G.M.; Appendino, G.; Bicchi, C.; Frattini, C. Constituents of Achillea erba-rotta. Riv. Ital. E.P.P.O.S. 1981, 63, 139–140. [Google Scholar]
- Ryoo, S.B.; Oh, H.K.; Sung, A.Y.; Moon, S.H.; Choe, E.K.; Oh, T.Y.; Park, K.J. The Effects of Eupatilin (Stillen®) on Motility of Human Lower Gastrointestinal Tracts. Korean J. Physiol. Pharmacol. 2014, 18, 383–390. [Google Scholar] [CrossRef]
- Giordano, O.S.; Guerreiro, E.; Pestchanker, M.J.; Guzman, J.; Pastor, D.; Guardia, T. The Gastric Cytoprotective Effect of Several Sesquiterpene Lactones. J. Nat. Prod. 1990, 53, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Hinge, V.R.; Shaikh, I.M.; Chavhan, R.L.; Deshmukh, A.S.; Shelake, R.M.; Ghuge, S.A.; Dethe, A.M.; Suprasanna, P.; Kadam, U. Assessment of genetic diversity and volatile content of commercially grown banana (Musa spp.) cultivars. Sci. Rep. 2022, 12, 7979. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.; Ahmad, V.U.; Farooq, U.; Jassbi, A.R.; Raziullah, H.S. Matricarin. Acta Crystallogr. E 2002, 58, o324–o325. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Shafizadeh, F.; Campbell, J.A.; Craig, A.C.; Campana, C.F.; Craig, R.E. Canin from Artemisia cana Pursh ssp. cana. Crystal structure and identification of chrysartemin A. J. Org. Chem. 1983, 48, 125–127. [Google Scholar] [CrossRef]
- Jakupovic, J.; Boeker, R.; Grenz, M.; Paredes, L.; Bohlmann, F.; El-Din, A.S. Highly oxygenated guaianolides from Otanthus maritimus. Phytochemistry 1988, 27, 1135–1140. [Google Scholar] [CrossRef]
- Öksüz, S. Sesquiterpenoids and other constituents from Tanacetum cilicium. Phytochemistry 1990, 29, 887–890. [Google Scholar] [CrossRef]
- Gonenc, T.; Argyropoulou, C.; Erdogan, T.; Gousiadou, C.; Juergenliemk, G.; Kıvçak, B.; Skaltsa, H. Chemical constituents from Anthemis wiedemanniana Fisch. & Mey. Biochem. Syst. Ecol. 2011, 39, 51–55. [Google Scholar] [CrossRef]
- Liu, Y.L.; Mabry, T.J. Sesquiterpene lactones from Artemisia frigida. J. Nat. Prod. 1981, 44, 722–728. [Google Scholar] [CrossRef]
- Begley, M.J.; Hewlett, M.J.; Knight, D.W. Revised structures for guaianolide α-methylenebutyro-lactones from feverfew. Phytochemistry 1989, 28, 940–943. [Google Scholar] [CrossRef]
- Jakupovic, J.; Ganzer, U.; Pritschow, P.; Lehmann, L.; Bohlmann, F.; King, R.M. Sesquiterpene lactones and other constituents from Ursinia species. Phytochemistry 1992, 31, 863–880. [Google Scholar] [CrossRef]
- Chandler, R.F.; Hooper, S.N.; Hooper, D.L.; Jamieson, W.D.; Lewis, E. Herbal remedies of the maritime Indians: Sterols and triterpenes of Tanacetum vulgare L. (Tansy). Lipids 1982, 17, 102–106. [Google Scholar] [CrossRef]
- Van Loo, P.; De Bruyn, A.; Buděšínský, M. Reinvestigation of the structural assignment of signals in the 1H and 13C NMR spectra of the flavone apigenin. Magn. Reason. Chem. 1986, 24, 879–882. [Google Scholar] [CrossRef]
- Stout, G.H.; Stout, V.F. The structure and synthesis of xanthomicrol. Tetrahedron 1961, 14, 296–303. [Google Scholar] [CrossRef]
- Williams, C.A.; Harborne, J.B.; Geiger, H.; Hoult, J.R.S. The flavonoids of Tanacetum parthenium and T. vulgare and their anti-inflammatory properties. Phytochemistry 1999, 51, 417–423. [Google Scholar] [CrossRef]
- Mossa, J.S.; Hifnawy, M.S.; Alyahya, M.A.; Hafez, M.M.; Shehata, A.A.; El-Feraly, F.S. Flavonoids and coumarins from three Saudi Arabian compositae species. Int. J. Crude Drug Res. 1988, 26, 181–184. [Google Scholar] [CrossRef]
- Rosa, A.; Isola, R.; Pollastro, F.; Caria, P.; Appendino, G.; Nieddu, M. The dietary flavonoid eupatilin attenuates in vitro lipid peroxidation and targets lipid profile in cancer HeLa cells. Food Funct. 2020, 11, 5179–5191. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Isola, R.; Pollastro, F.; Nieddu, M. Effect of the natural polymethoxylated flavone artemetin on lipid oxidation and its impact on cancer cell viability and lipids. Fitoterapia 2022, 156, 105102. [Google Scholar] [CrossRef] [PubMed]
- Jahaniani, F.; Ebrahimi, S.A.; Rahbar-Roshandel, N.; Mahmoudian, M. Xanthomicrol is the main cytotoxic component of Dracocephalum kotschyii and a potential anti-cancer agent. Phytochemistry 2005, 66, 1581–1592. [Google Scholar] [CrossRef]
- Moghaddam, G.; Ebrahimi, S.A.; Rahbar-Roshandel, N.; Foroumadi, A. Antiproliferative activity of flavonoids: Influence of the sequential methoxylation state of the flavonoid structure. Phytother. Res. 2012, 26, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Attari, F.; Keighobadi, F.; Abdollahi, M.; Arefian, E.; Lotfizadeh, R.; Sepehri, H.; Moridi Farimani, M. Inhibitory effect of flavonoid xanthomicrol on triple-negative breast tumor via regulation of cancer-associated microRNAs. Phytother. Res. 2021, 35, 1967–1982. [Google Scholar] [CrossRef] [PubMed]
- Brockhoff, A.; Behrens, M.; Massarotti, A.; Appendino, G.; Meyerhof, W. Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. J. Agric. Food Chem. 2007, 55, 6236–6243. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamone, S.; Aiello, N.; Fusani, P.; Rosa, A.; Nieddu, M.; Appendino, G.; Pollastro, F. Non-Volatile Terpenoids and Lipophilic Flavonoids from Achillea erba-rotta Subsp. moschata (Wulfen) I. Richardson. Plants 2023, 12, 402. https://doi.org/10.3390/plants12020402
Salamone S, Aiello N, Fusani P, Rosa A, Nieddu M, Appendino G, Pollastro F. Non-Volatile Terpenoids and Lipophilic Flavonoids from Achillea erba-rotta Subsp. moschata (Wulfen) I. Richardson. Plants. 2023; 12(2):402. https://doi.org/10.3390/plants12020402
Chicago/Turabian StyleSalamone, Stefano, Nicola Aiello, Pietro Fusani, Antonella Rosa, Mariella Nieddu, Giovanni Appendino, and Federica Pollastro. 2023. "Non-Volatile Terpenoids and Lipophilic Flavonoids from Achillea erba-rotta Subsp. moschata (Wulfen) I. Richardson" Plants 12, no. 2: 402. https://doi.org/10.3390/plants12020402
APA StyleSalamone, S., Aiello, N., Fusani, P., Rosa, A., Nieddu, M., Appendino, G., & Pollastro, F. (2023). Non-Volatile Terpenoids and Lipophilic Flavonoids from Achillea erba-rotta Subsp. moschata (Wulfen) I. Richardson. Plants, 12(2), 402. https://doi.org/10.3390/plants12020402








