Abstract
Cannabis (Cannabis sativa L.) is an outstanding source of bioactive natural products, with more than 150 different phytocannabinoids isolated throughout the decades; however, studies of their bioactivity have historically concentrated on the so-called “big four” [∆9-THC (1a), CBD (2a), CBG (3a) and CBC (4a)]. Among the remaining products, which have traditionally been referred to as “minor cannabinoids”, cannabinol (CBN, 5a) stands out for its important repercussions and implications on the global scientific landscape. Throughout this review, we will describe why CBN (5a) deserves a prominent place within the so-called “cannabinome”, providing an overview on its history, the syntheses developed, and its bioactivity, highlighting its promising pharmacological potential and the significant impact that the study of its chemistry had on the development of new synthetic methodologies.
1. Introduction
There is extensive historical evidence that cannabis (Cannabis sativa L.) has been used for different purposes, among them industrial [1], ornamental [2], and pharmaceutical (e.g., treating rheumatic pain, constipation, gout, and gynecological disorders) [3] applications. Nowadays, the intake of marijuana is permitted in many countries of the world for the treatment of different pathologies [4], including nausea caused by chemotherapy, anorexia in patients suffering from AIDS, and pain management [5,6].
The biological activity of cannabis is strictly related to phytocannabinoids, the hallmark secondary metabolites of this remarkable plant [7]. This class of meroterpenoids is characterized by great chemical diversity among its constituents; however, a general phytocannabinoid structure is easily identifiable due to its characteristic hybrid nature; since this class derives from the merging of polyketides and amevalonate biosynthetic pathway, a resorcinyl core decorated with p-oriented isoprenyl residues and an alkyl side chain is easily identifiable [8].
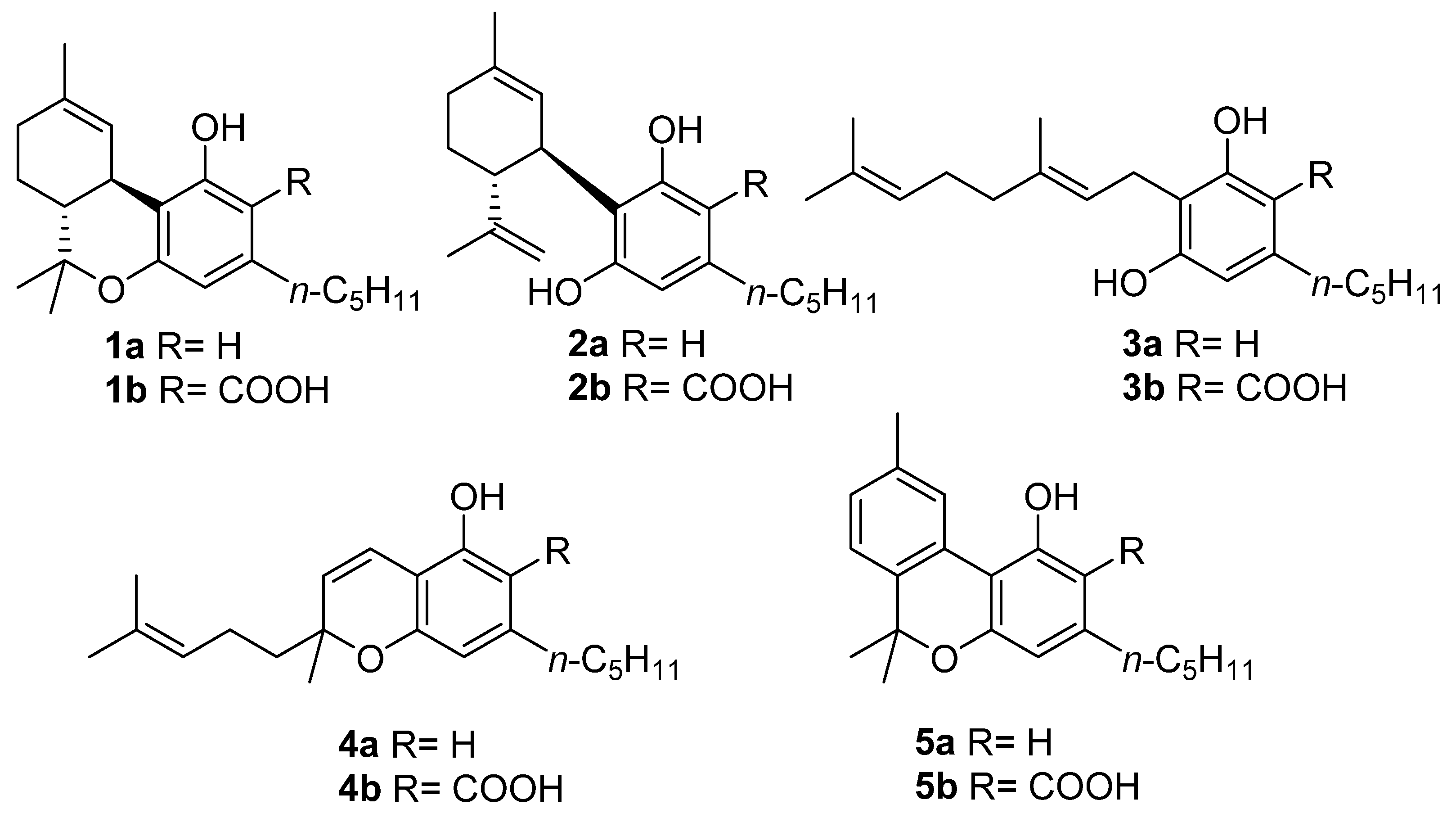
Despite this impressive variety, early studies in this field focused almost exclusively on the narcotic principle of marijuana, ∆9-tetrahydrocannabinol (∆9-THC, 1a, Figure 1), eventually expanding to the other related compounds, which are cannabidiol (CBD, 2a, Figure 1), cannabigerol (CBG, 3a, Figure 1), and cannabichromene (CBC, 4a, Figure 1), that together form a group of compounds often referred to as “the major cannabinoids” or “big four”[9]. As our understanding of the biological mechanisms underlying ∆9-THC narcotic properties developed, the endocannabinoid system (ECS) was discovered [10], and its complexity, homeostatic role, and potential for drug discovery prompted a reconsideration of the other three major cannabinoids derived from cannabis. In addition to these studies, CBD (2a) was developed into a standardized extract (Sativex) and as an active pharmaceutical ingredient (API) (Epidiolex). The latter is the drug of choice for the treatment of certain rare genetic forms of epilepsy, while Sativex (a combination of ∆9-THC and CBD) is used to treat spasticity associated with multiple sclerosis [5]. Likewise, CBG (3a) and CBC (4a) found their way into the literature as well [11,12].
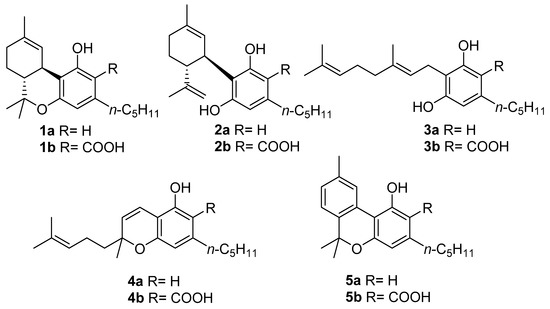
Figure 1.
The structures of the “big four” phytocannabinoids [∆9-THC (1a), CBD (2a), CBG (3a) and CBC (4a)] and their acidic precursors (1b–4b), CBN (5a) and its acidic form CBNA (5b).
Most of the studies on phytocannabinoids, both chemical and biological, have focused on the “big four” due to their high extraction yield from vegetable sources or easy accessibility through total synthesis [11,12,13,14,15,16,17]. However, cannabis plants are also capable of producing more than 150 other compounds referred to as “minor cannabinoids” [18], which have significant structural differences and specific biological properties [19,20]; among these compounds, one stands out in particular, namely cannabinol (CBN, 5a, Figure 1).
CBN (5a) is one of the most famous phytocannabinoids in C. sativa, and although several phytocannabinoids have been identified in different plants and fungi, CBN (5a) has only been identified in cannabis. In the cannabinoid family, CBN (5a) is unique in several ways, the main one being its origin: whereas the acidic precursors of major cannabinoids (1b, 2b,4b, Figure 1) are generated by the result of different cyclizations of the terpenyl moiety of cannabigerolic acid (CBGA, 3b, in turn obtained from the condensation of olivetolic acid with geranylpyrophosphate) mediated by particular cyclases [21], a biosynthetic pathway for cannabinolic acid (CBNA, 5b, Figure 1), and so of CBN (5a) itself, has not been identified; the latter is a degradation artifact of ∆9-THC (1a) which, as result of air oxidation, undergoes aromatization at the level of the menthyl moiety [22]. However, small amounts of CBNA (5b) have been found in some hemp samples [23], suggesting that, in particular conditions, oxidative degradation may also occur to tetrahydrocannabinolic acid (THCA, 1b, Figure 1), the acidic precursor of ∆9-THC (1a), as well as before decarboxylation.
CBN (5a) was probably considered a “minor” phytocannabioid owing to its unfortunate and confusing discovery: it was the first phytocannabinoid to be isolated from hashish in the late 19th century, but its structure was not fully solved until 1940 due to some issues related to both nomenclature misunderstandings and the nature of the plant material used for the extraction (see Section 2). This confusing situation—associated with its limited availability and the discovery of more interesting bioactive phytocannabinoids—severely hindered its characterization from a biological and pharmacological standpoint.
Therefore, our purpose is to give this forgotten and mistreated phytocannabinoid due prominence and attention, focusing on its well-known synthetic pathways and highlighting the urgent need to fill the gaps in its biological field.
2. History
Marijuana is perhaps one of the oldest plants grown by mankind, so much that we can say that Cannabis sativa L. and humanity share a close and intertwined history [24]. From the perspective of time, CBN (5a) probably plays the most important role of all the cannabinoids: due to its exceptional stability and direct chemical relationship with the psychoactive constituent Δ9-THC (1a), the natural product has been assumed to be the most relevant marker [25] for the identification of narcotic cannabis in archaeological plant samples.
CBN’s (5a) extraordinary stability has been demonstrated by the discovery of plant material (seeds) dating back to 750 BC, found in a tomb in the Xinjiang-Uighur autonomous region (China), still containing high levels of the molecule [26].
Also attributed to CBN (5a) are the great uncertainty and ambiguity that plagued the first studies on the psychotropic properties of C. sativa: a significant limitation of the initial researches was related to the poor quality of the plant material investigated, which was mostly imported, directly or through Egypt, from India [27]. As a result of the long journey between the collection site and the European laboratories, usually lasting months, combined with uncontrolled and careless storage conditions, a sharp reduction in Δ9-THC (1a) levels in favor of CBN (5a) was achieved, leading to a relative falsification of the biological observations [28].
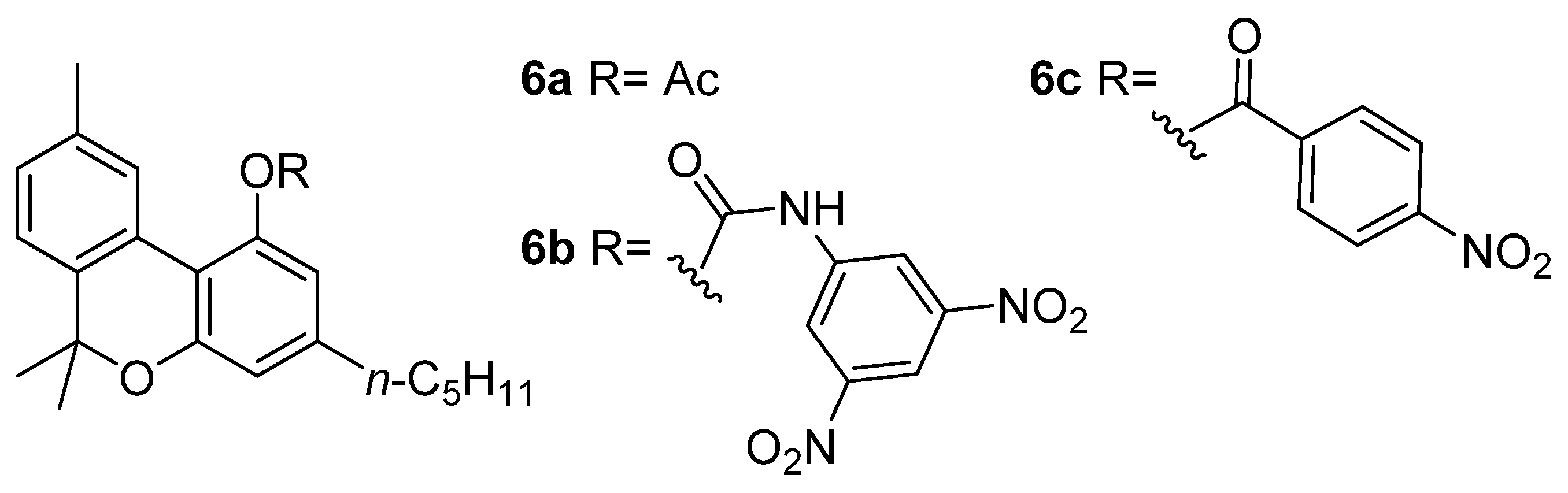
Wood coined the term “cannabinol” at the end of the 19th century in order to describe the “red oil”, a dense resin containing both CBN (5a) and other major phytocannabinoids as well [29]; these compounds are brownish and colorless oils or white solids, whereas the ruby red color likely resulted from quinoid structures [30] forming during the oil purification. Obtaining this resin required a difficult and complex preparation optimized by Wood himself, Spivey, and Easterfield at Cambridge University, and entailed the distillation of ethanolic or ethereal Cannabis extract at reduced pressure (2 mm) and collecting the vapors at a temperature of between 100 and 220 °C (corresponding to a bath temperature of 170–300 °C) [29]. Initially considered to be a pure compound, “red oil” was acetylated by Easterfield resulting in a crystalline compound with an optically inactive nature (6a, Figure 2). Its natural phenol (5a) was then referred to as “cannabinol”, taking the name used previously for the narcotic red oil and making CBN (5a) the first phytocannabinoid isolated [31].
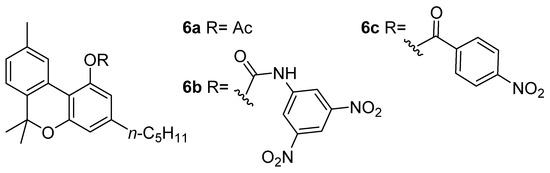
Figure 2.
The structures of CBN adducts used for its isolation and characterization: CBN-acetate (6a), CBN-3,5-dinitrophenylurethane (6b) and CBN-4-nitrophenyl ester (6c).
A tragic fate marked by CBN (5a) awaited the trio of scientists, despite their initial promising results: Spivey died in an explosion during the nitration of the “red oil” [32], while Wood became severely ill after voluntarily testing its effects [33]. Aside from this, Easterfield is also reported to have died during this research, due to an explosion associated with the hydrogenation of the “red oil”, though later sources report that he survived and relocated to New Zealand [34].
An additional intriguing, albeit tragic episode related to the first research on CBN (5a) involves another scientist of the same age, C. R. Marshall, who ingested approximately 100 mg of “red oil” as a means of combating boredom during the distillation of diethylzinc, a highly flammable liquid, to assess whether the hypothetic compound was narcotic. A little more than 45 min later, he was found leaning against the distillation flask in the lab, giggling and repeating “this is lovely” as flames spread around him due to oxygen leaks that set fire to diethylzinc. Only the prompt intervention of Marshall’s colleagues prevented a catastrophe, and he recovered quickly afterward [35]. Despite its historical significance, the “red oil” brought, in addition to the tragedies mentioned above, a great deal of confusion to the cannabis research community during its early years, mainly due to two major factors: first, the issue of the name “cannabinol” itself, which has been transferred from the distillate (narcotic) to the natural product (inactive) [31]; the second reason is related to the fact that the plant material from which the oil was distilled often exhibited a fluctuating phytochemical profile resulting from different methods of storage under ambiguous conditions, a profile further influenced by the dramatic conditions of the resin distillation [27].
A “full stop” to the confusion that hovered over CBN (5a) was posed in the 1930s by Cahn (famous for nomenclature and stereochemistry with the Cahn–Ingold–Prelog rules [36]): by defining red oil as “raw cannabinol” and the pure compound as “cannabinol”, he was able to elucidate almost completely the structure of the natural product through degradation studies, identifying a dibenzopyranic structure with the presence of a phenol and of a n-pentyl on the resorcinyl portion, indicating their relative positions [37].
In the following decade, two scientists worked independently on elucidating this structure, Roger Adams from Illinois State University and Alexander R. Todd (“Tod Almighty” to his students [38]) from the University of Manchester, obtaining several results that still have significant implications for the field of cannabinoids research. In his own experiments, the first obtained crystalline phenylurethane of CBN (6b) during a procedure involving 3,5-dinitrobenzoylazide treatment of “red oil” [39]; instead, the second sought to remove virtually all CBN from the mother liquor as p-nitrobenzoyl chloride ester (6c) [40], allowing him to isolate another cannabinoid, later identified as CBD (2a) [41]. Through a total synthesis of the CBN (5a) in 1940, Adams was able to establish the structure of the molecule definitively [42] (see Section 3.2).
Over the following decades, with the isolation and characterization of major phytocannabinoids, research on CBN (5a) was slowly set aside, eclipsed by the important and marked pharmacological activity of two compounds in particular, Δ9-THC (1a) and CBD (2a). The diphenyl structure of the natural compound, however, makes it an important model compound for the development of new synthetic methodologies that have led to the design of numerous total syntheses (see Section 3).
3. Synthesis
In today’s world, total synthesis has advanced dramatically thanks to powerful methodologies and sophisticated instruments; however, even for structures that may appear small and simple at first glance, the total synthesis of organic molecules still poses significant challenges for chemists, especially in terms of time efficiency, yields and, more importantly, environmentally friendly processes [43].
The need for simple and effective syntheses of CBN (5a) arises from the fact that extractions from plant sources are extremely limited for the following reasons: first, the yields are not very reproducible, since the concentration of the natural product is closely related to the state of conservation of the plant materials; in addition, CBN (5a) exhibits characteristics of polarity and solubility that are similar to those of other cannabinoids—primarily Δ9-THC (1a)—factors that make extraction a disadvantageous option. In addition to derivatization from “red oil” [29], currently new, more modern methods have been reported, primarily using long-lasting liquid extraction in a Soxhlet apparatus or pressurized liquid extraction [44], with ultrasonication of the extract reported to be an effective method for increasing the extraction yield [45].
Throughout the course of the last century, synthetic methodologies have been refined, leading to several strategies to finally synthesize CBN (5a) in excellent yields and solving the problem of obtaining this compound through extraction from the plant.
3.1. Semisynthesis
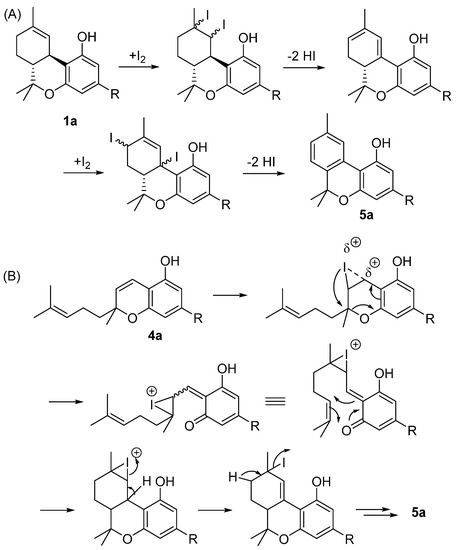
Since CBN (5a) is an oxidative degradation product of D9-THC (1a), aromatization of its natural precursor or its analogues is one of the simplest and most effective ways to finally achieve it. Different methods have been proposed for oxidizing the C ring of D9-THC (1a) and several of its regioisomers to CBN (5a): Adams first reported this reaction under relatively harsh conditions (heating with sulfur at approximately 250 °C) [42]. The reaction can be performed, in a less extreme environment, using N-bromosuccinimide and carbon tetrachloride in the presence of UV light as well, as reported by Razdan [46]. Recently, chloranyl (tetrachloro-1,4-benzoquinone) was found to selectively oxidize D9-THC (1a) while leaving other isomeric tetrahydrocannabinols unaffected [47]. Another protocol for dehydrogenation, involving selenium dioxide and trimethylsilyl polyphosphate (prepared from P4O10 and hexamethyldisiloxane), has also been described [23].
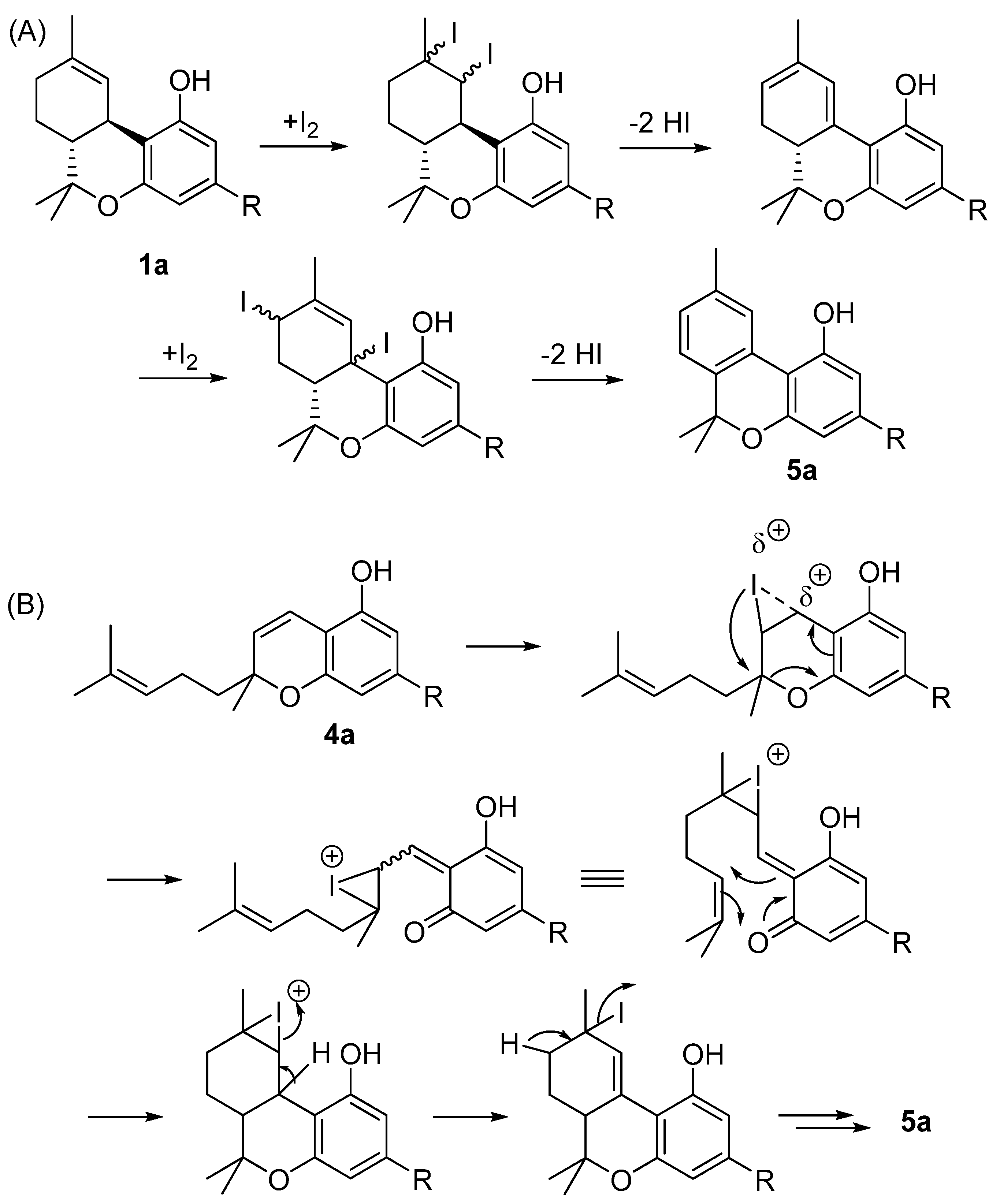
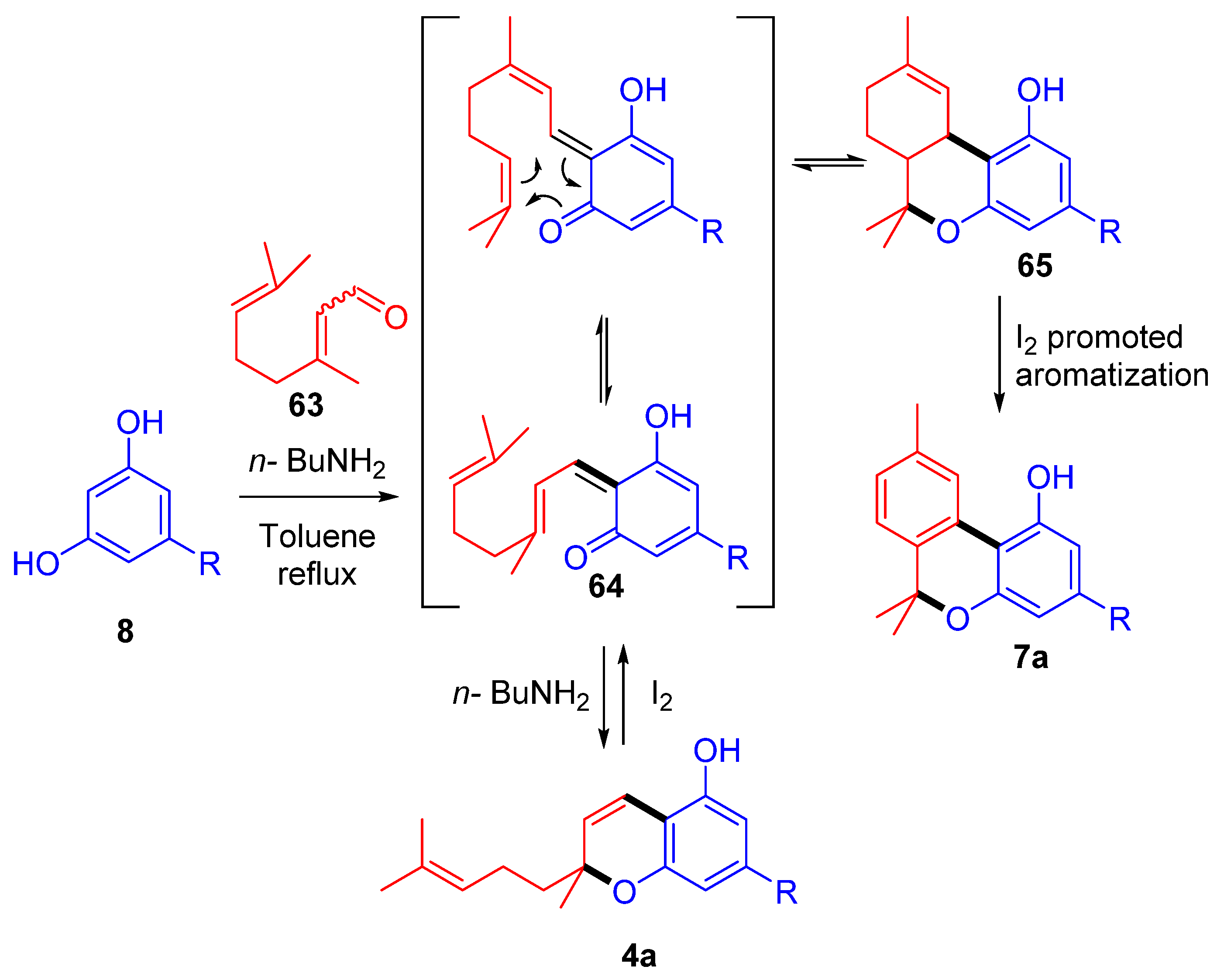
We have recently reported that the iodine treatment of D9-THC (1a) and THCA (1b) directly produces CBN (5a) through a series of iodination–dehydroiodination steps driven by the transition from menthyl to p-cymyl aromatization (Scheme 1) [48]. Likewise, CBD (2a) or CBC (4a) can be used as starting materials, yielding between 50 and 70%. As a result of the acidic environment, CBD (2a) in situ cyclization occurs to D9-THC (1a), whereas with CBC (4a), the addition of iodine to the chromene bond causes the electrocyclic opening of the heterocyclic ring, followed by a hetero Diels–Alder reaction, which leads to tetrahydrocannabinol derivatives that are then aromatized through iodine addition-hydroiodic acid elimination reactions [49].
Scheme 1.
(A) The semisynthesis of CBN (5a) through an iodine-promoted cyclization of Δ9-THC (1a); (B) semisynthesis of CBN (5a) through an iodine promoted prenylchromene-benzochromane rearrangement of CBC (4a). R= n-pentyl.
3.2. Total Synthesis through Lactonic Intermediate
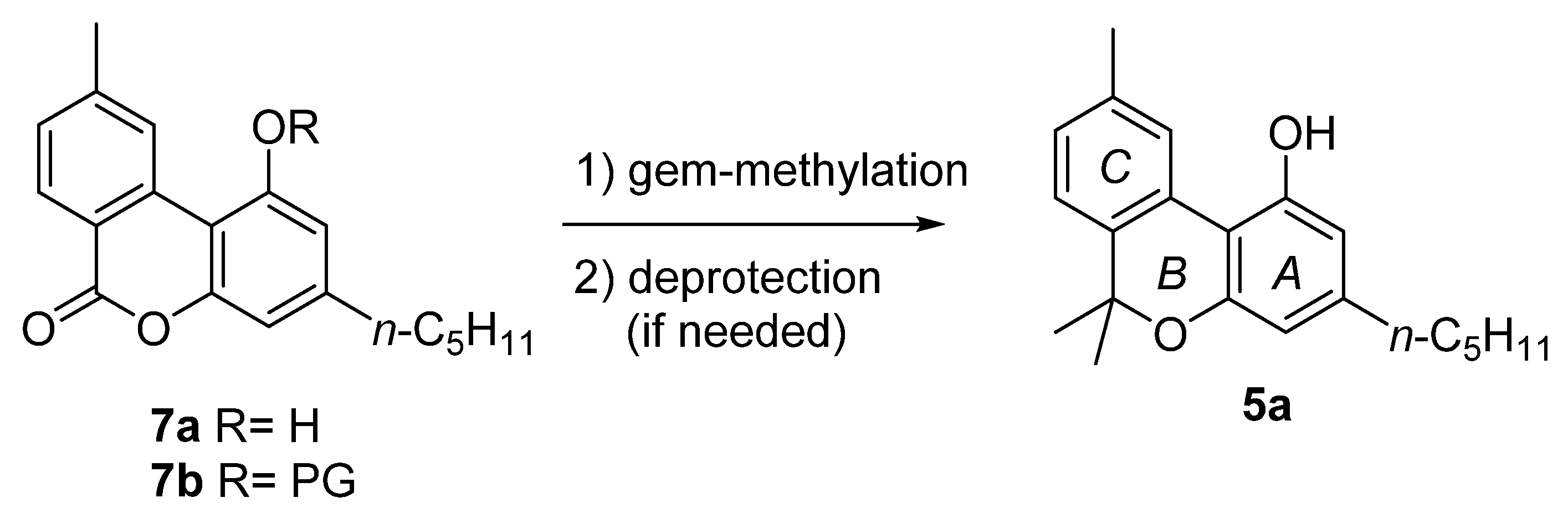
The majority of the total synthesis of CBN (5a) involves the achievement of lactone intermediate 7a or 7b (Figure 3), which is then converted into 5a by gem-methylation, and the subsequent deprotection of the phenolic hydroxyl, if needed.
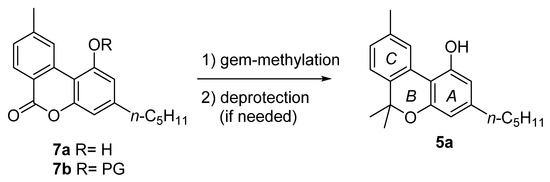
Figure 3.
The structure of lactone intermediate 7a–b.
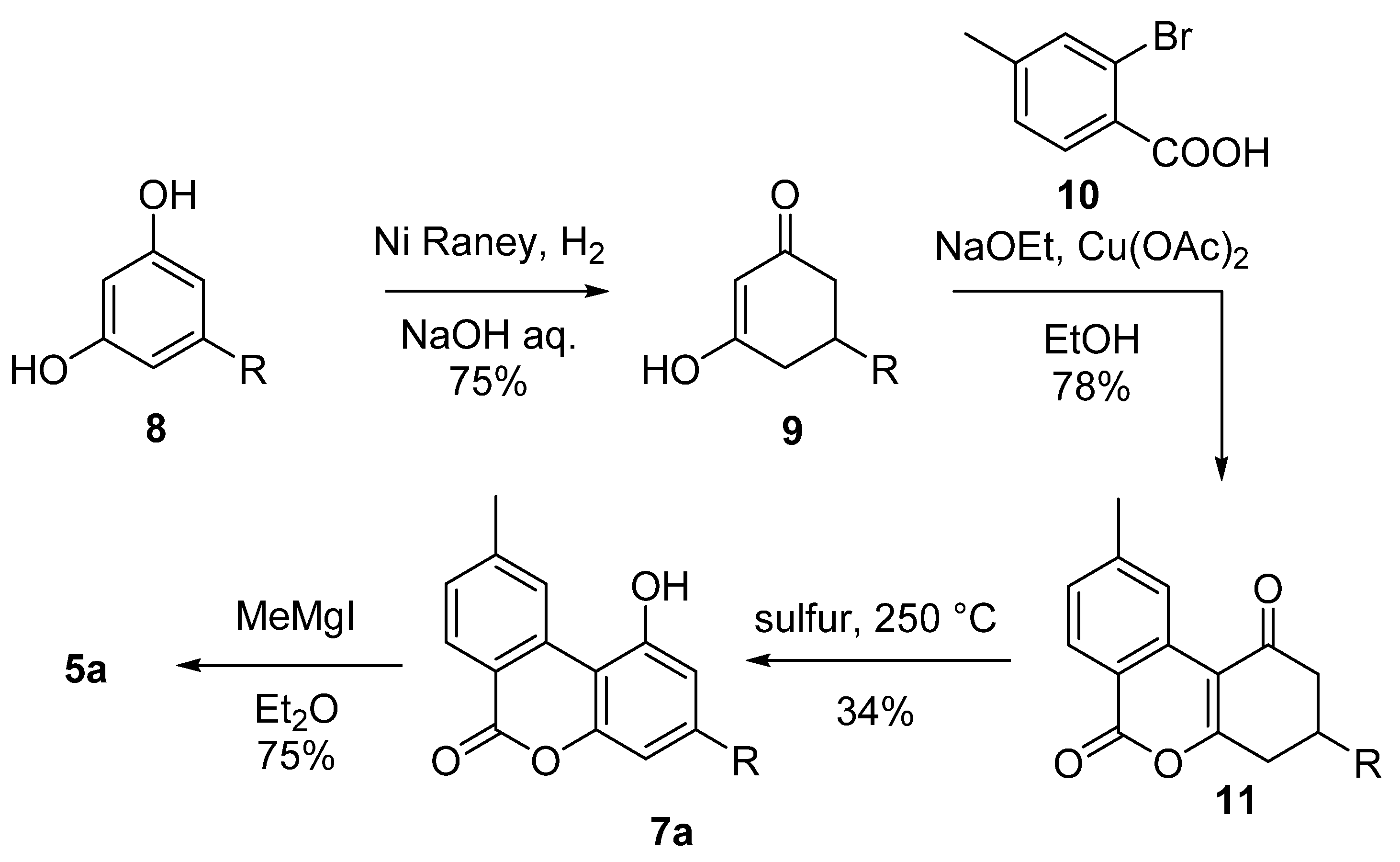
This includes the first total synthesis of CBN (5a) conducted by Adams in 1940 in order to confirm the chemical structure of the natural product (Scheme 2) [42]. In his synthesis, Adams relied on the condensation of 5-n-amyl-1,3-cyclohexanedione (9), obtained from the hydrogenation of olivetol (8) with Nickel–Raney, with methyl-2-bromobenzoic acid (10) to form the corresponding pyrone 11. The subsequent aromatization carried out with sulfur provides lactone 7a, which was gem-methylated through the use of methyl magnesium iodide to finally provide 5a with an overall moderate yield.
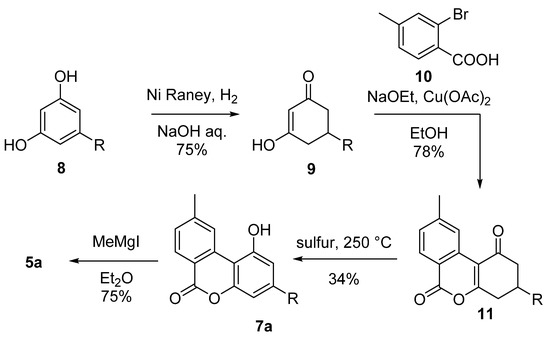
Scheme 2.
Adams’ synthesis of CBN (5a). R = n-pentyl.
Nevertheless, in the most recent synthetic strategies, the aromatization step has been set aside in favour of other techniques, which allow for a greater degree of efficiency and yield to be achieved. Depending on the method by which lactone 7a was obtained, these can be grouped into two main categories: through a biphenyl coupling and through cyclization reactions.
3.2.1. Synthesis through a Biphenyl Coupling Approach
In order to complete the tricyclic structure of CBN (5a), one of the most common approaches is to combine two aromatic portions in a coupling reaction to connect rings A and C of the natural product, then an intramolecular reaction to complete the formation of ring B; this is probably due to the extensive amount of research and development that has been undertaken in the last decades in an attempt to optimize the synthesis of the biphenyl core, one of the most common privileged structures in medicinal chemistry [50].
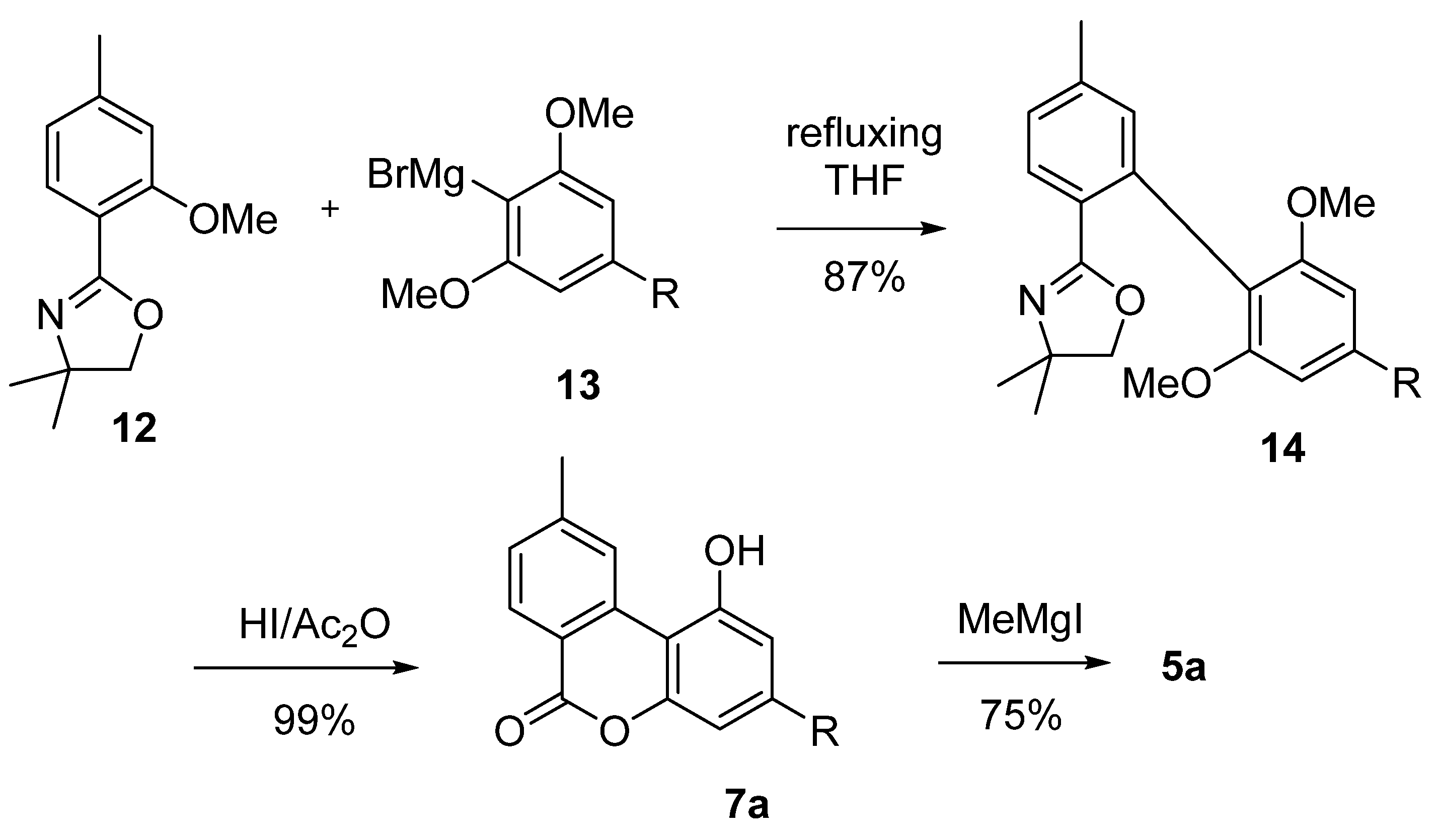
Salemink et al. exploited Grignard’s chemistry for the coupling of the two aromatic moieties of rings A and C [51]. The Grignard’s reagent derived from 2-bromo-1,3-dimethoxy-5-pentylbenzene (13) was reacted with the oxazoline derivative 12 to achieve the corresponding biphenyl 14. Next, deprotection of the phenolic hydroxyls and lactonization are obtained in a one-pot through the use of an HI/acetic anhydride mixture, which generated lactone 7a, successively methylated to 5a using MeMgI in accordance with Adams’ method [42] (Scheme 3). An optimized version of this approach by Miyano et al. is not based on the use of the oxazoline derivative 12, but on its more stable and easily available 2,6-dialkylphenolic benzoate ester analogues [52].
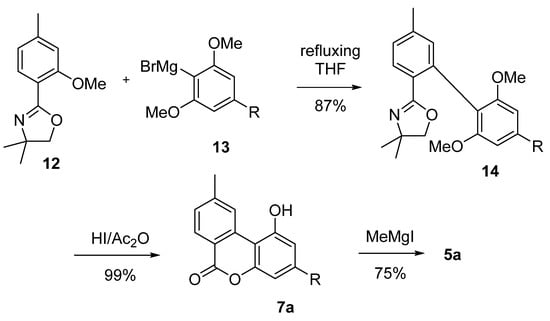
Scheme 3.
Salemink’s synthesis of CBN (5a) using a Grignard approach. R = n-pentyl.
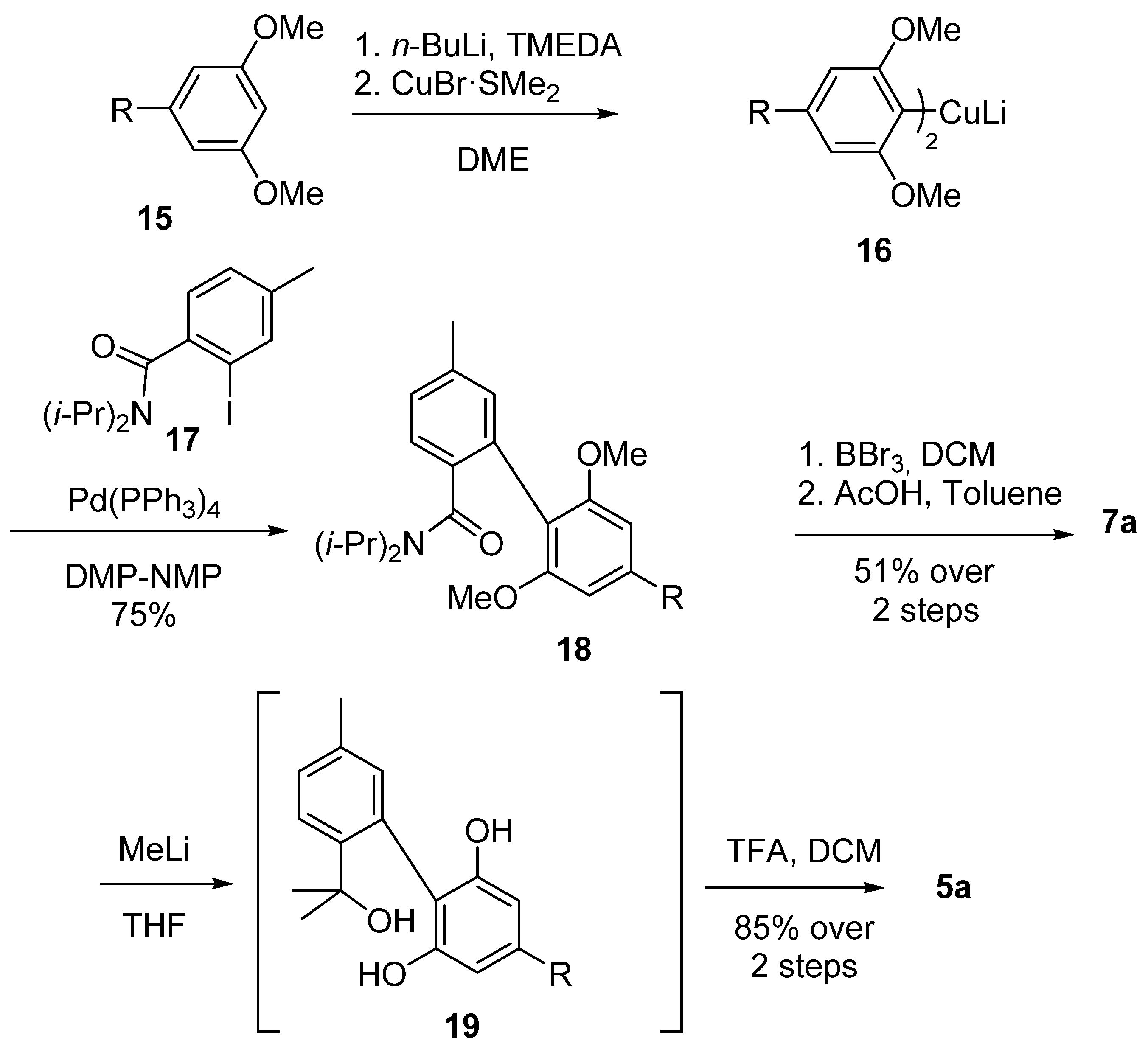
Göttlich et al. synthesized the biphenyl core through a modified Ullmann–Ziegler approach using Gilman’s cuprate 16, obtained through ortho-lithiation of bis-methylolivetol (15) with n-butyl lithium and a subsequent treatment with copper bromide-dimethylsulfide complex. Cuprate 16 then reacted with iodobenzamide 17 to provide the corresponding biphenyl 18 according to the Ullmann–Ziegler cross-coupling, and, after demethylation and subsequent acid-catalyzed cyclization, 7a was achieved with 51% yield [53]. Moreover, Göttlich modified the insertion of geminal dimethyl by using two equivalents of methyllithium to afford tertiary benzylic alcohol 19, which was then cyclized by treatment with trifluoroacetic acid to produce 5a with a yield of 85% over two steps (Scheme 4).
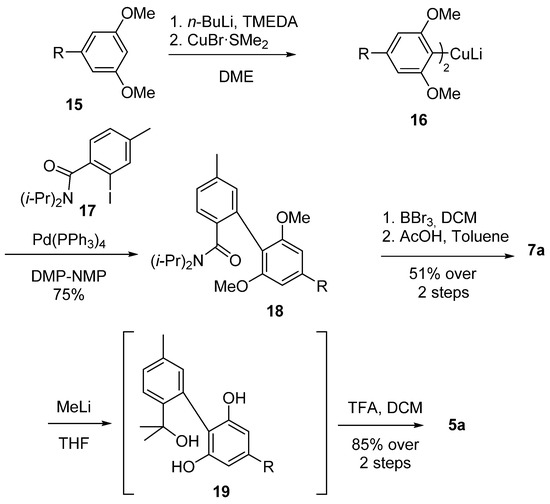
Scheme 4.
The synthesis of CBN (5a) according to Göttlich. R = n-pentyl.
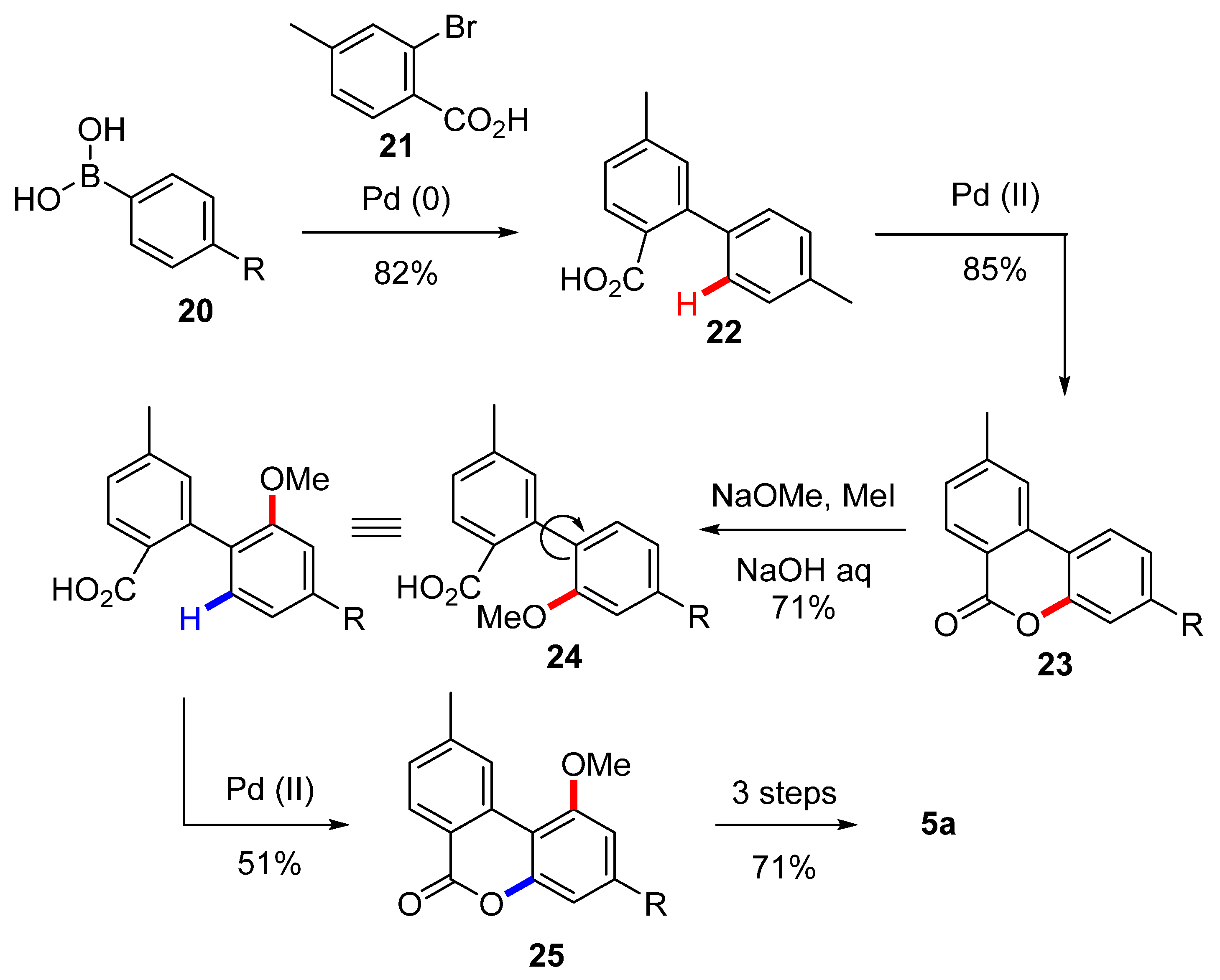
Wang et al. relied instead on a Suzuki coupling and then two subsequent Pd(II)/Pd(IV)-catalyzed carboxyl-directed C-H activation/C-O cyclization for the synthesis of the biaryl lactone precursor and the insertion of the phenolic oxygens [54] (Scheme 5). After a first cyclization of compound 22, obtained by the Suzuki coupling of boronic acid 20 and bromide 21, the corresponding lactone 23 underwent nucleophilic attack of NaOMe was then quenched with MeI. Next, hydrolysis of the ester gave the corresponding acid 24. An additional C-H activation/C-O cyclization was conducted in order to complete assembly of the molecule’s B ring, resulting in the biphenyl lactone 25, which was then deprotected and gem-methylated using the Göttlich’s protocol [53].
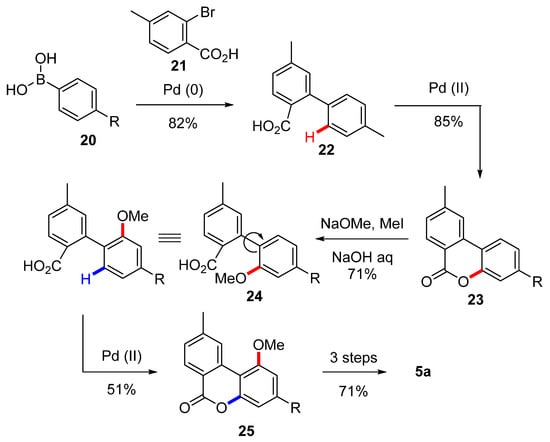
Scheme 5.
First Wang synthesis of CBN (5a). R = n-pentyl.
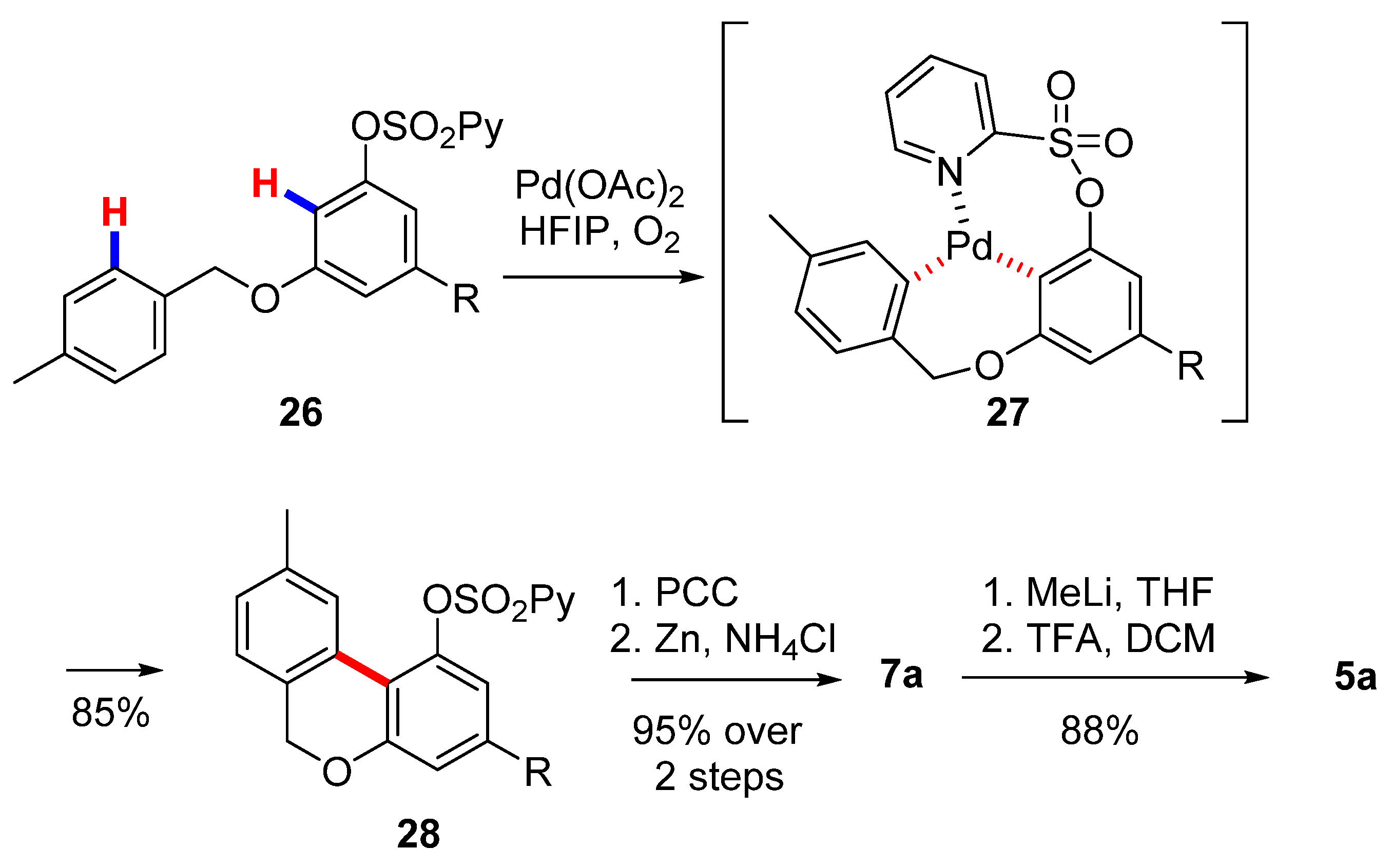
Wang’s group also exploited an intramolecular aromatic C−H/C−H coupling catalyzed by palladium to obtain the corresponding biphenyl core [55] (Scheme 6). A sulfonylpyridyl protection on the precursor 26 is required for the coordination of palladium and activation of the C-H bond (27), which resulted in excellent yields of 6H-benzo[c]chromene 28. The subsequent oxidation and deprotection of the O-(2-pyridyl)sulfonyl group allowed for obtaining lactone 7a, which was been gem-methylated by means of the protocol optimized by Göttlich [53].
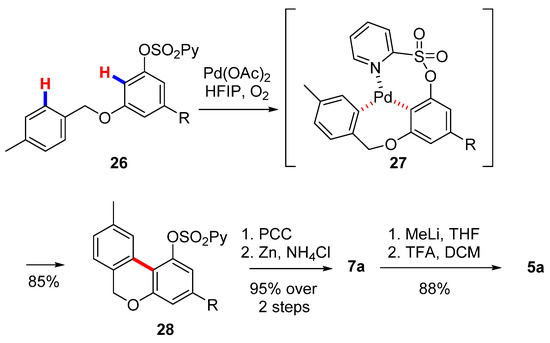
Scheme 6.
Second Wang’s synthesis of CBN (5a). R= n-pentyl.
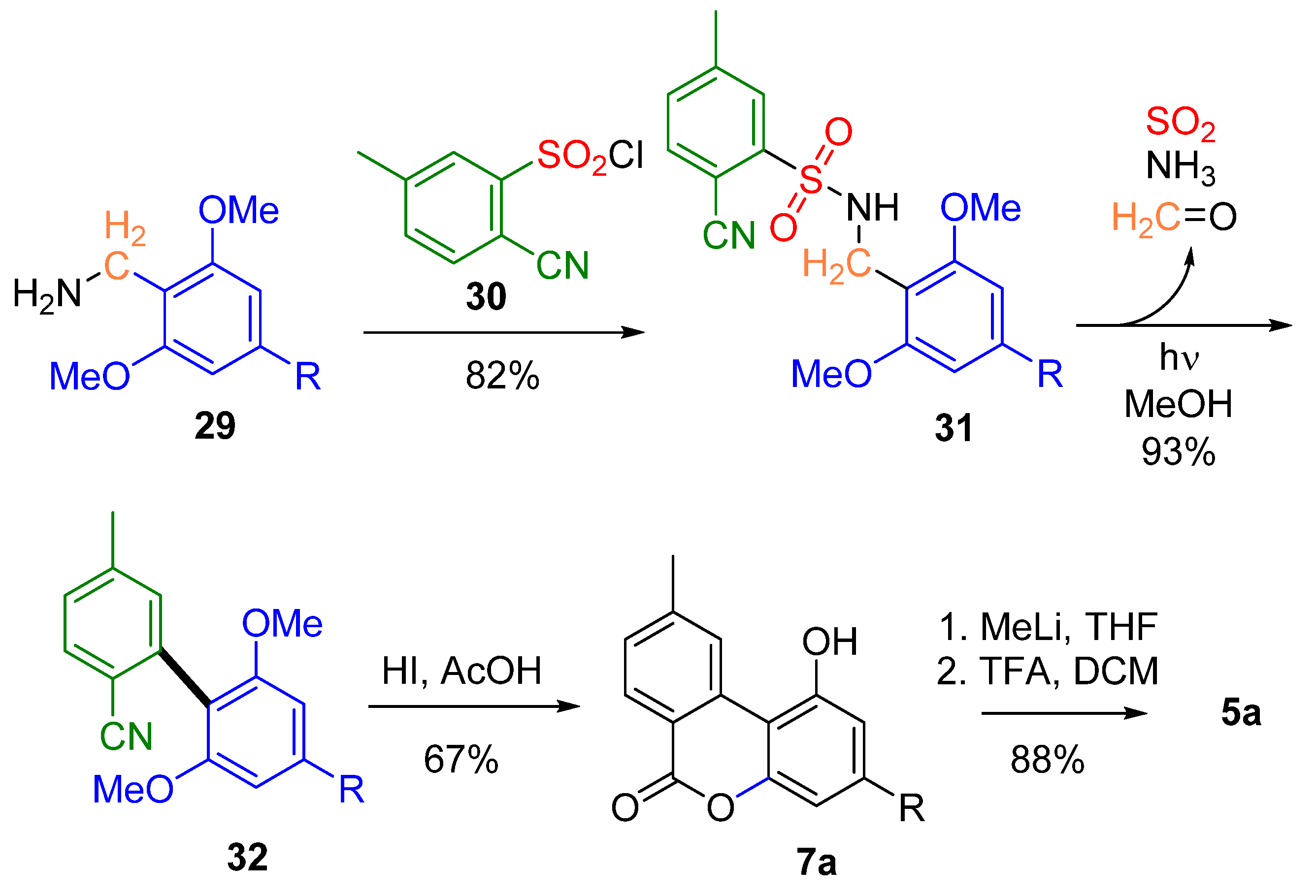
The work of Hertweck et al. was instead characterized by a totally different inspiration: bypassing the use of transition metals, where a photochemical approach was used to synthesize the biphenyl portion [56] (Scheme 7). By irradiation of sulfonamide 31, easily obtained by a nucleophilic substitution reaction involving sulfonyl chloride 30 and benzyl amine derivative 29, the biaryl nitrile 32 was obtained, which could be simultaneously demethylated, hydrolyzed and lactonized with a yield of 67% to obtain lactone 7a, readily converted into 5a as previously described [55].
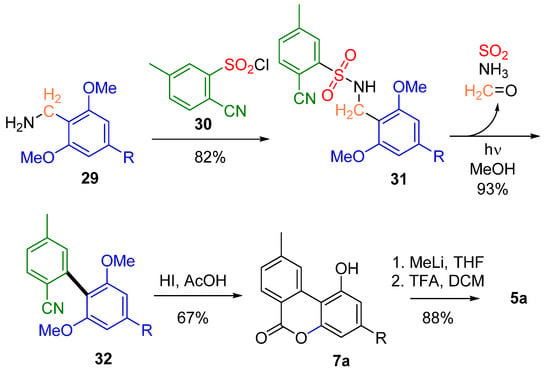
Scheme 7.
Hertweck’s metal-free CBN (5a) synthesis through a photosplicing reaction. R= n-pentyl.
3.2.2. Synthesis through a Cyclization Approach
In addition to the previously described methodologies, a second class of common approaches for the synthesis of biaryl lactone 7a takes advantage of different cyclization reactions to easily build one or more cycles of the triciclyc natural product.
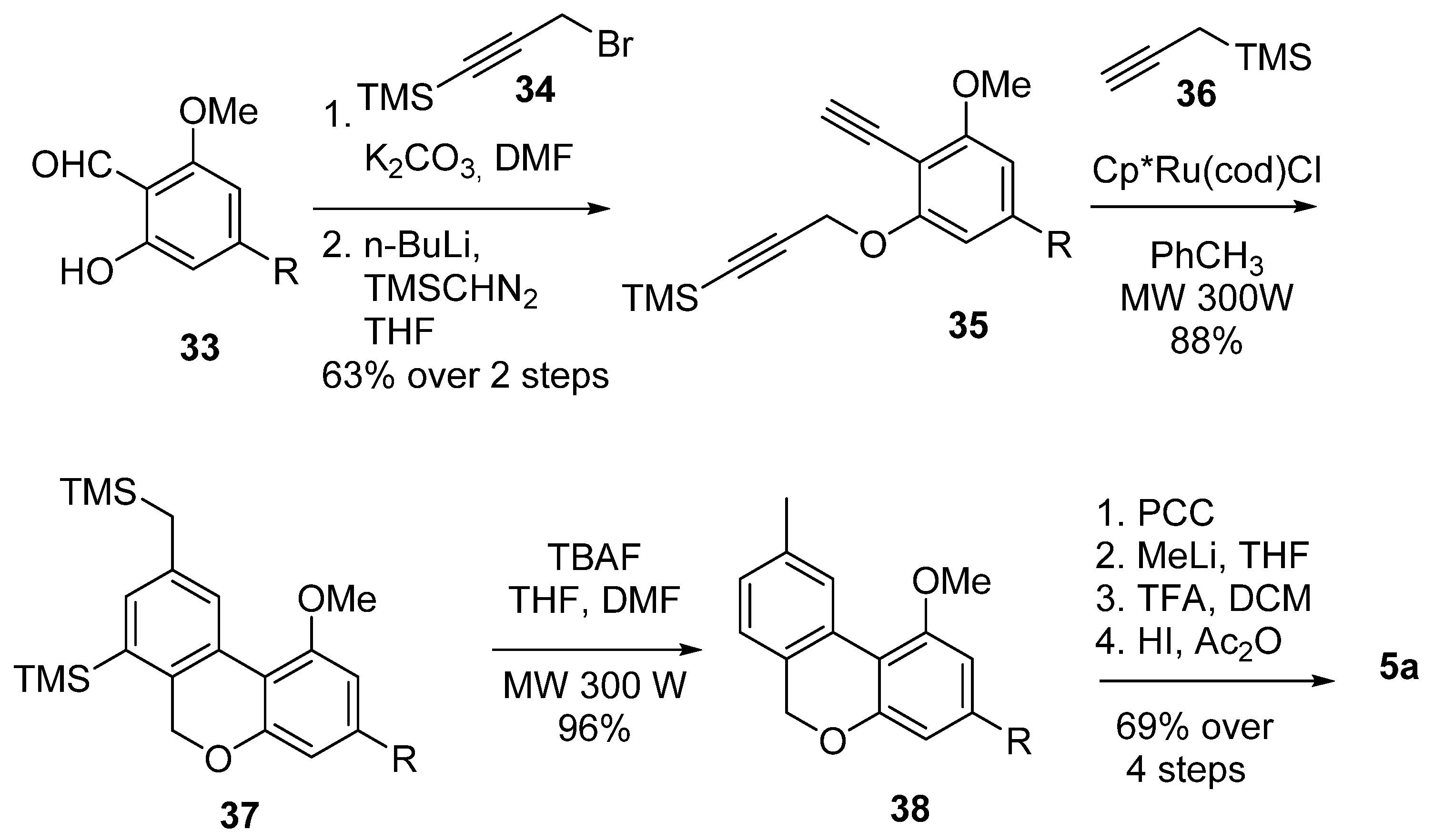
Through a regioselective [2 + 2 + 2] cyclotrimerization reaction catalysed by transition metals, Deiters et al. were able to achieve the formation of rings B and C in a single step [57] (Scheme 8): the substituted diyne 35, obtained from salicylaldehyde derivative 33 after O-alkylation with 34 and reaction with TMS-diazomethane, underwent an efficient and regioselective Cp*Ru(cod)Cl catalyzed [2 + 2 + 2] cyclotrimerization reaction with propargyltrimethylsilane (36) under microwave irradiation, providing pyran 37 with a yield of 88% as a single regioisomer. Subsequent removal of the silyl protections using TFA afforded 38, and its next oxidation, gem-methylation and deprotection finally resulted in 5a with a promising yield.
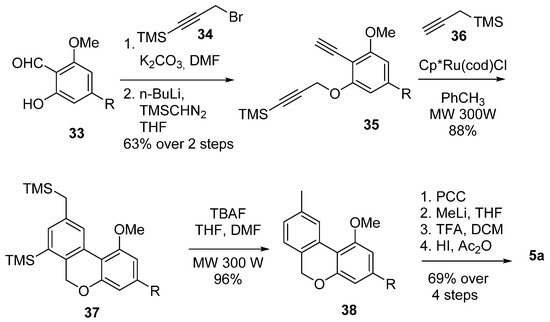
Scheme 8.
Deiters’s CBN (5a) synthesis through a regioselective transition-metal-catalyzed [2 + 2 + 2] cyclotrimerization. R = n-pentyl.
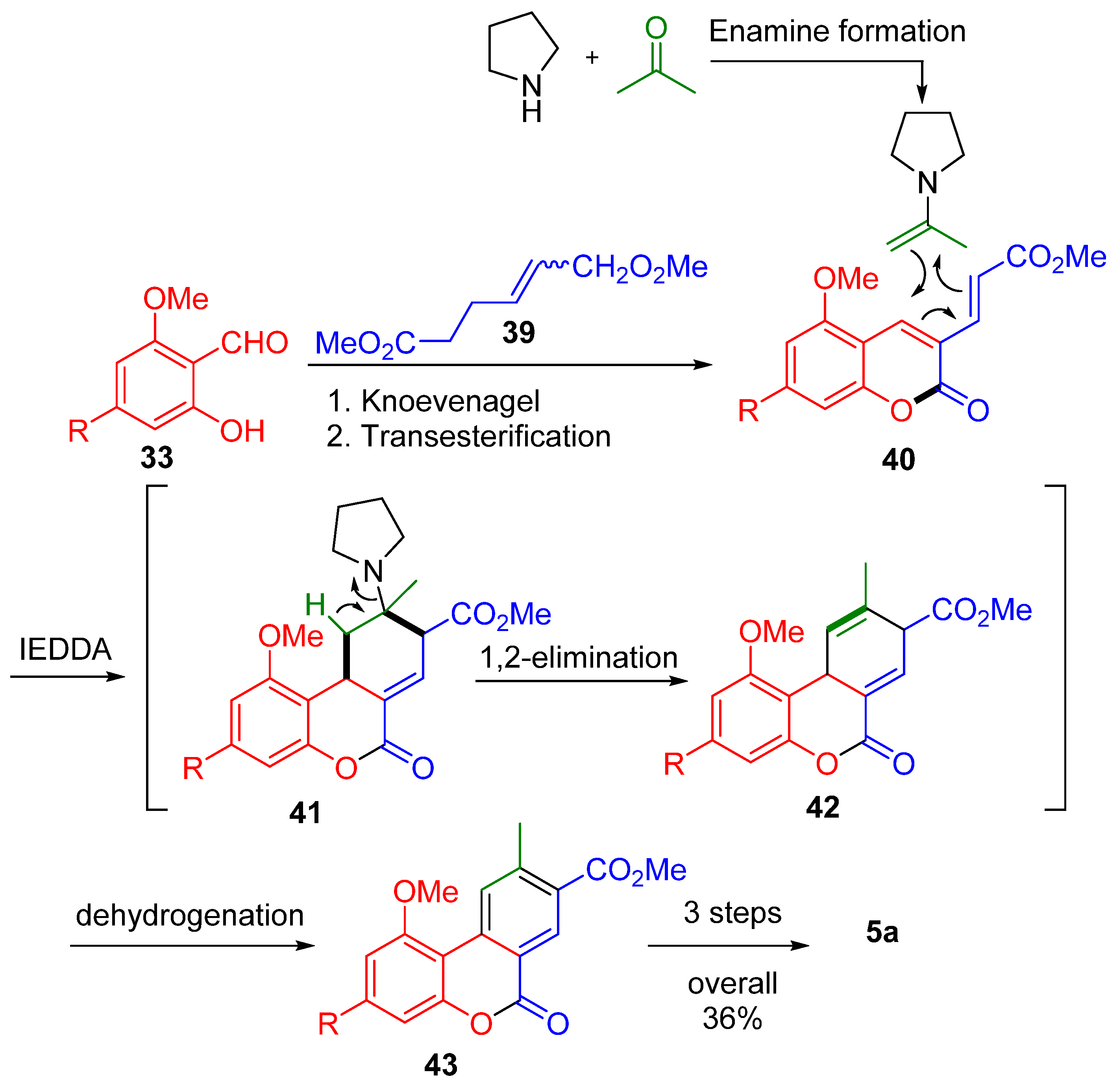
The multicomponent approach used by Bodwell et al. is also of interest, synthesizing 6H-dibenzo[b,d]pyran-6-ones via multicomponent domino reactions [58] (Scheme 9). The general transformation was comprised of six reactions: Knoevenagel condensation between salicylaldehyde derivative 33 and diester 39, followed by transesterification to lactone 40; enamine formation from acetone and pyrrolidine that then participated in an inverse electron demand Diels–Alder reaction (IEDDA) with 40, affording the corresponding tricyclic derivative 41, a 1,2-elimination to diene 42, and, next, dehydrogenation to 43. During the key inverse electron demand Diels–Alder (IEDDA) step, both diene and dienophile were generated in situ using a secondary amine.
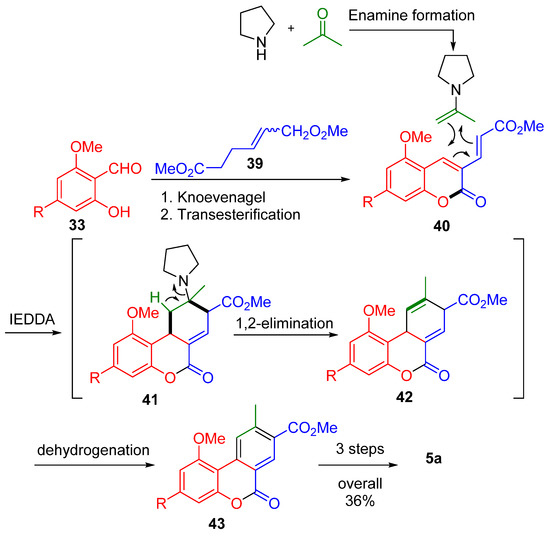
Scheme 9.
A multicomponent synthesis of CBN (5a) according to Bodwell. R = n-pentyl.
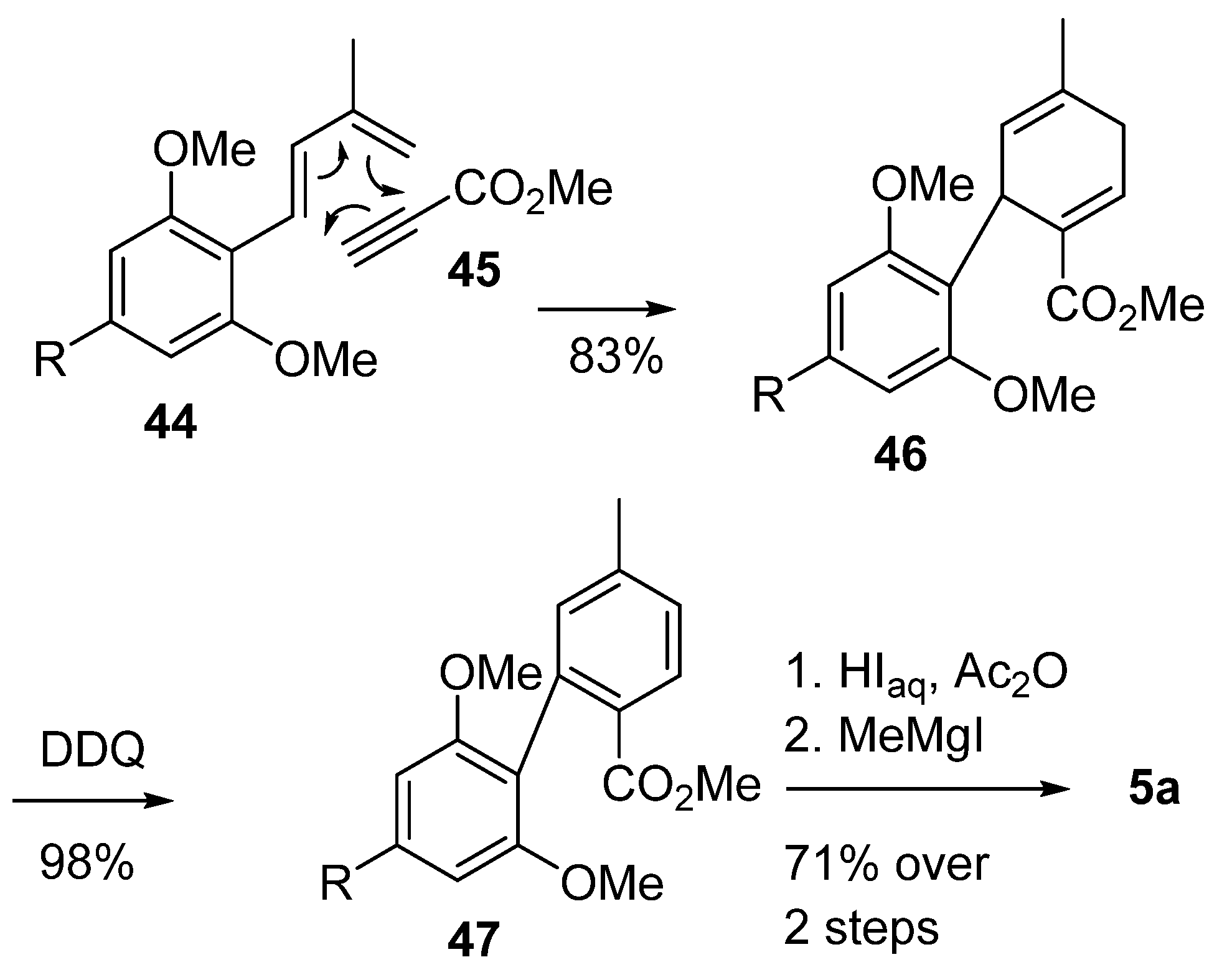
On the other hand, Minuti et al. used another Diels–Alder cyclization approach to obtain the final biphenyl 47 [59] (Scheme 10). Using olivetolic derivative 44 and methyl propiolate 45 as starting materials, phenylcyclohexadiene 46 was formed with excellent yields, and this was then aromatized with the aid of DDQ. The following steps followed what has already been reported in the literature [42].
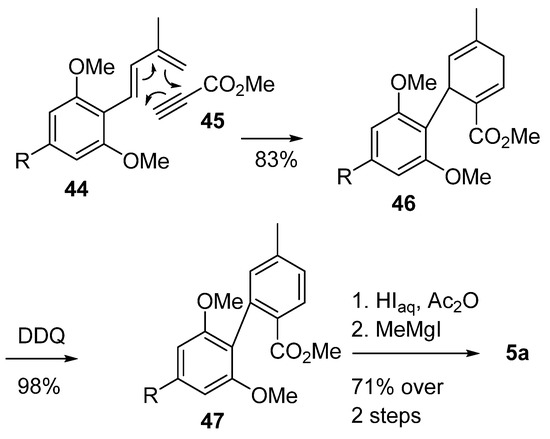
Scheme 10.
Minuti’s synthesis of CBN (5a) using a Diels–Alder approach. R = n-pentyl.
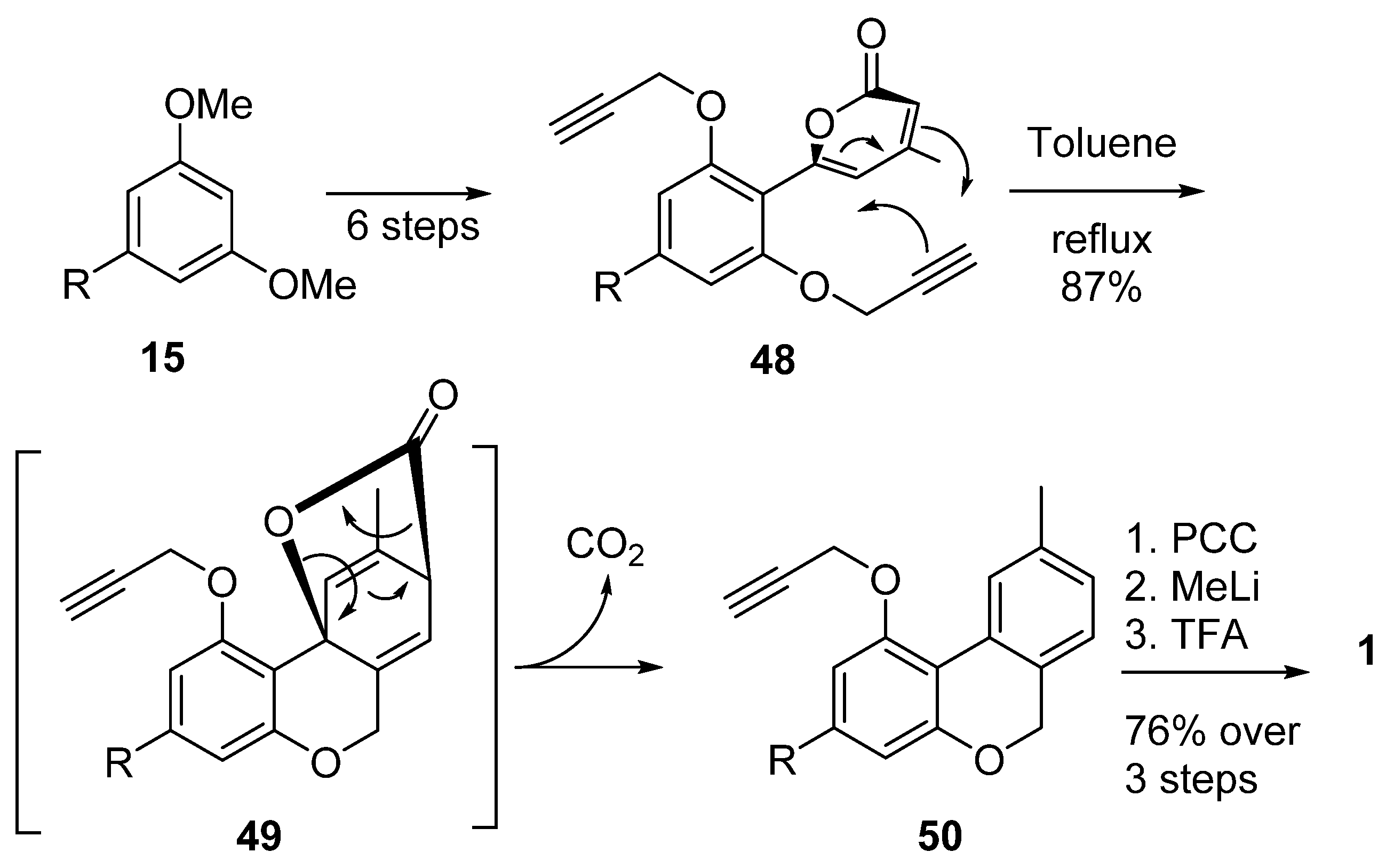
Chang et al. also used Diels–Alder chemistry, although with a different point of view: their synthesis relied upon intramolecular Diels–Alder cyclization between a pyranonic moiety and a propargyl portion (48), then a retro-hetero Diels–Alder (49) in order to obtain the 6H-benzo[c]chromene 50 with the loss of a carbon dioxide molecule [60]. After oxidation to lactone, gem-dimethylation and deprotection of the propargyl group, CBN (5a) was obtained with reasonable yields (Scheme 11).
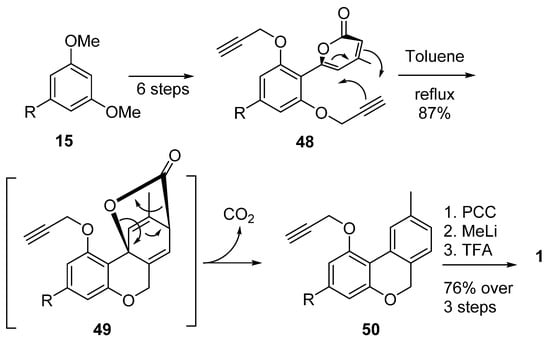
Scheme 11.
Chang’s synthesis of CBN (5a) by intramolecular Diels–Alder approach.
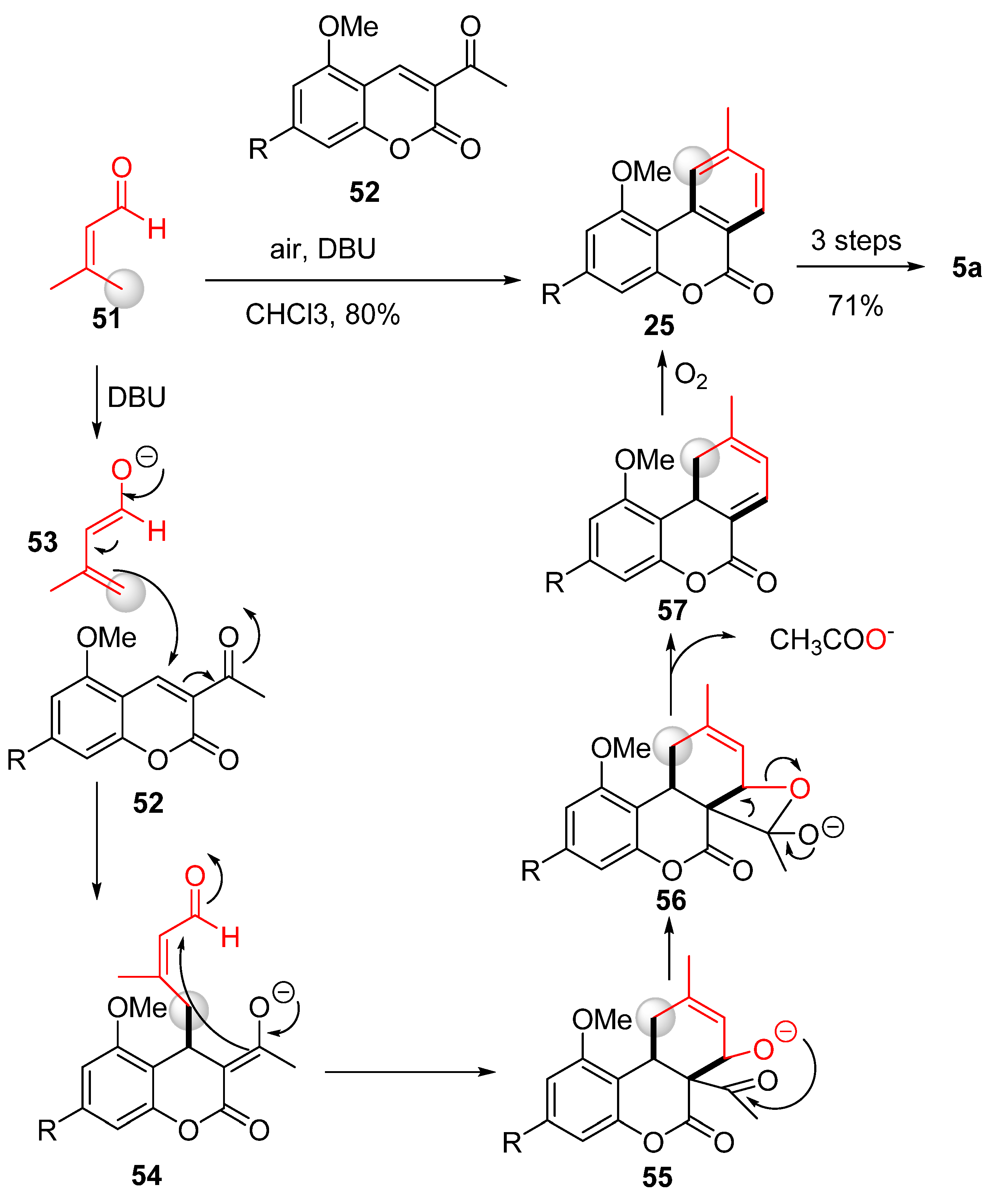
The approach taken by Chi et al. involved instead a formal [4 + 2] method to construct a new benzene ring, which enabled them to obtain different benzocoumarins, including cannabinol [61] (Scheme 12). In this process, the C ring was formed in excellent yields through the interaction between the enol form (53) of aldehyde 51 and the acetylcoumarin derivative 52. The reaction proceeded via a Michael-type addition of the enal g-carbon of intermediate 53 to coumarin 52 forming intermediate 54, which underwent an intramolecular aldol reaction to form tricyclic intermediate 55. Following intramolecular acetal formation, 56 was obtained. As a result of the elimination of an acetate from 56, compound 57 was formed; then, the reaction was completed via spontaneous oxidative aromatization (with air as an oxidant) to 25. The subsequent synthetic steps followed what has already been reported [57].
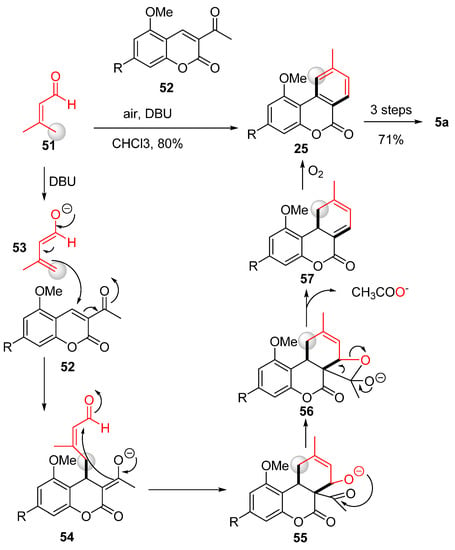
Scheme 12.
Chi’s total synthesis of CBN (5a). R = n-pentyl.
3.3. Total Synthesis through Non-Lactonic Intermediate
The development of syntheses without the lactone intermediate 7a is relatively rare; however, these syntheses are equally important and play a fundamental role in the background of the chemistry of CBN (5a).
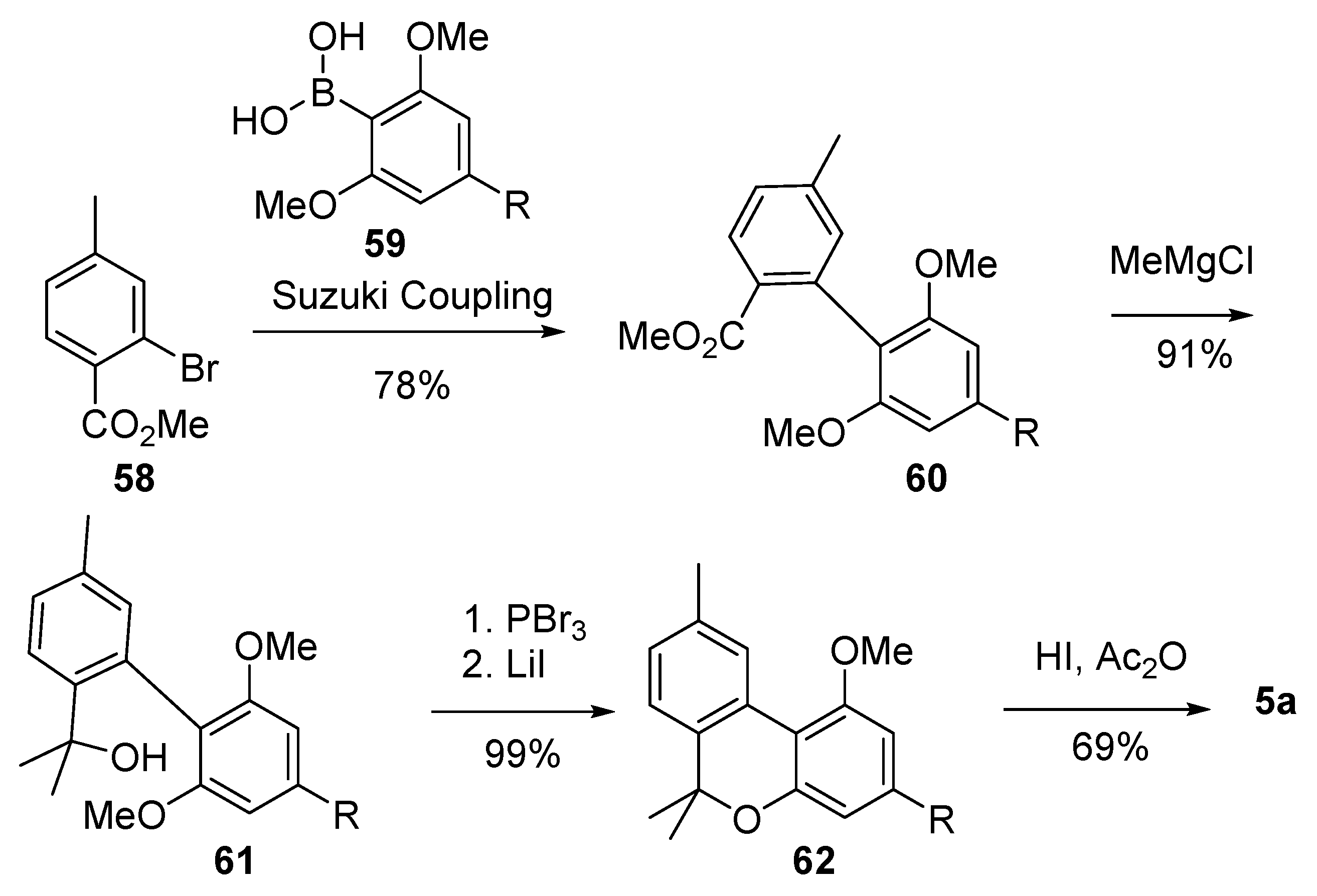
As part of their studies on the synthesis of 6-alkyl-6H-benzo[c]chromenes, Ruchirawat et al. developed a one-pot cyclization/selective ether cleavage reaction which was then applied to the total synthesis of CBN (5a) [62] (Scheme 13). During the event, the product of Suzuki coupling (60) between bromide 58 and boronic acid 59 was gem-dimethylated to 61 before the formation of the B ring, obtained by treatment of the latter with PBr3, followed by the addition of LiI, which led to the corresponding benzo[c]-chromene derivative 62. Final demethylation afforded CBN (5a) in good yields.
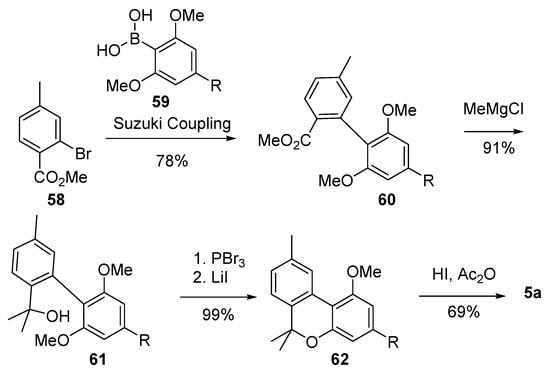
Scheme 13.
Ruchirawat’s synthesis of CBN (5a). R = n-pentyl.
More recently, our research group, inspired by the reactivity studies of natural phytocannabinoids with molecular iodine [48,63], developed the first one-pot total synthesis of CBN (5a) from commercially available starting material exploiting an iodine-mediated deconstructive annulation [49] (Scheme 14). In the event, olivetol (8) and citral (63) reacted in basic condition to afford the corresponding homoisoprenylchromene CBC (4a), which, treated with iodine after removal of the amine catalyst using ionic acidic resin, underwent electroreversion and then hetero Diels–Alder cyclization through a prenylchromene-benzochromane rearrangement, affording a stereoisomeric mixture of THC derivatives 65. The final iodine-catalyzed aromatization was a driving force strong enough to move the overall equilibrium toward the final product CBN (5a), afforded in an outstanding 82% yield.
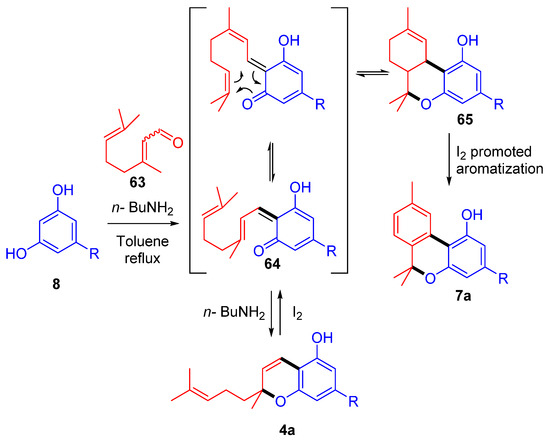
Scheme 14.
Iodine-promoted one-pot total synthesis of CBN (5a) using prenylchromene-benzochromane rearrangement. R = n-pentyl.
4. Biological Profile
The ECS is an extremely important biological system and the main target of the phytocannabinoids: it modulates a wide range of cognitive and physiological processes that are necessary for regulating the body’s homeostatic state [64]. Although the mechanism by which the ECS regulates metabolism is not completely understood, it is thought that its action is largely through cannabinoid ligands activating cyclic AMP/receptor activation-related pathways [65].
The majority of the biological studies conducted on the cannabinome have focused on the “big four” phytocannabinoids; nevertheless, different studies have been conducted to elucidate CBN (5a) pharmacological potential, which have revealed a peculiar and interesting, although sometimes contradictory, biological profile.
4.1. Biochemical Assays
4.1.1. Cannabinoid Receptors (CBs)
CBs are the primary and most studied targets of phytocannabinoids, as well as eponymic receptors. They belong to the G protein-coupled receptor superfamily, and currently two subtypes of cannabinoid receptors are known, namely CB1 and CB2. Generally speaking, the direct activation of CB1 can be characterized by narcotic (euphoric) effects, as well as analgesic, orexic, and anxiety-modulating activities, with the narcotic ones absent in allosteric activators. The activation of CB2 has instead anti-inflammatory and immunity-modulating properties [64]. Despite the fact that CBN (5a) is an agonist of both types of CBs, it exhibits different properties depending on the receptor type. The affinity of CBN (5a) for the CB1 receptors are 10 times lower when compared to that of D9-THC (1a) [66,67,68]; moreover, CBN (5a) is less effective than D9-THC (1a) at inhibiting the CB1 receptor-mediated activity of adenylyl cyclase [66]. The activity on CB2, on the other hand, has been reported to have a changeable profile from study to study; while previously CBN (5a) was considered an agonist with the same potency as D9-THC (1a) [69], it has recently been reported to have a potency between 2–4 times lower [66]. It is possible that the discrepancies are caused by different concentrations of CBN (5a) used in each experiment, as well as by the different conformational states of the receptors in the different tissues involved in the test.
As for CBN (5a) activity on the hypothetical CB3 receptor—the GPR55—there is very little information published, and the few available data show that it has an almost negligible effect in vitro [70].
4.1.2. Transient Receptor Potential Channels (TRPs)
Several types of animal cells contain TRP channels, which are ionic channels located primarily on their plasma membranes. Various chemical and physical stimuli (as an example, heat and cold somatosensation) are transduced by these membrane proteins, and pain signals are also generated by them. They are also capable of causing and sustaining inflammation as a result of their activation [71].
Several types of TRPs are modulated by CBN (5a): it acts as an agonist at TRPV1, TRPV2, TRPV3 and TRPV4 channels, stimulating the Ca2+ influx, as well as activating Ca2+-dependent pathways in the cells [71]. Regarding other TRP variants, CBN (5a) inhibits the icilin-induced activation of TRPM8 (also known as the cold and menthol receptor 1, CMR1) by acting as a potent antagonist [71]. The natural compound has also been demonstrated to stimulate the TRPA1 channel in a potent and effective manner, exhibiting an EC50 value of 0.18 ± 0.02 mmol [71].
4.2. Pre-Clinical and Clinical Assays
It does not appear that CBN (5a) has been extensively investigated preclinically or clinically (no human pharmacokinetic and metabolism data have been reported), although the published results indicate that CBN (5a) has a promising pharmacological profile. Despite the positive results of the studies that have been published so far, the results are often still open to doubt, since a large number of the research did not test the most suitable pathological endpoints and did not achieve the outcomes that were expected.
4.2.1. Analgesic and Anti-Inflammatory Activity
As an analgesic and anti-inflammatory agent, CBN (5a) has been found to be potentially useful in the treatment of pain. Previous researches have reported that CBN (5a) can mitigate the symptoms of myofascial pain disorders, including temporomandibular disorders and fibromyalgia in rats by reducing the mechanical sensitivity induced by intramuscular injections of nerve growth factor in the masseter muscles [72], with an increasing activity when administered in a 1:1 mixture with CBD (2a). CBN (5a) could attenuate the production of interleukins 2, 4, 5, 13 and decrease allergen mucus production in OVA-sensitized and challenged A/J mice, classifying it as a potential treatment for allergic airway diseases [73]. The use of CBN (5a) for the treatment of glaucoma was also suggested since it prevents inflammation which causes elevated intraocular pressure [74]. Moreover, preliminary studies have demonstrated that CBN acts as an antioxidant and decreases cell damage in a cell culture model of Huntington disease [75].
4.2.2. Antibacterial Activity
In the same way that other cannabinoids have been shown to be effective against a number of antibiotic-resistant bacteria, e.g., CBG (3a) and CBC (4a), CBN (5a) also appears to have antibacterial properties; it has been proven to be highly effective against multiple bacteria that are resistant to antibiotics, including methicillin-resistant Staphylococcus aureus (MRSA), making it a potentially effective treatment for staph infections [76].
4.2.3. Orexigenic Activity
As well as stimulating hyperphagia, CBN (5a) increases food consumption and feeding time in rats [77,78]. However, since CBN (5a) is not a narcotic, it may have greater potential as an orexic drug in conditions where the stimulation of appetite is beneficial, such as terminal cancer and HIV infection.
4.2.4. Treatment of Epidermolysis Bullosa
The use of CBN (5a) has recently been shown to be effective in the treatment of epidermolysis bullosa, a group of rare medical conditions characterized by easy blistering of the skin and mucous membranes. Phase 2 studies undertaken by InMed Pharmaceuticals are currently in progress [79] as a follow-up of the previous Phase 1 studies conducted by the same, showing that the CBN (5a) based preparation in development was safe and well-tolerated on induced open epidermal wounds, causing no systemic or serious adverse effects [80].
4.2.5. Sleep Induction
As already shown in Section 2, high concentrations of CBN (5a) are found in aged cannabis products, since it is the main degradation product of D9-THC (1a). As a result of unclear reasons, CBN (5a) has been associated with the induction of sleep, causing the cannabinoid to be marketed under the name “the sleepy cannabinoid in old weed” at dosages generally less than 5 mg/die [81]. Despite this, sleep studies involving CBN (5a) have not reached a clear conclusion as to whether this claim is true.
CBN (5a) was reported to increase barbiturate-induced sleep time in one study [82], but this result was not replicated in disaccordance with a previous study [83]. Nevertheless, as shown in a murine study [84], CBN (5a), combined with D9-THC (1a), increased sedation synergistically, and this observation was reproduced in a small clinical trial involving five male volunteers who received oral CBN (50 mg), D9-THC (12.5 mg), or two different combinations of them (12.5 mg Δ9-THC + 25 mg CBN or 25 mg D9-THC and 50 mg CBN), separated by a one-week washout period [84]. As well as pain threshold and skin sensitivity, which are not affected by CBN alone, the combination did not alter the effects of D9-THC (1a) on heart, blood pressure, and body temperature [84]. Nonetheless, the combination led to modest increases in some subjective outcomes (drowsiness and dizziness). It is therefore unsubstantiated that CBN (5a) has sleep-inducing properties, from a clinical perspective [85].
5. Conclusions
The studies reviewed demonstrate the significant contribution that CBN (5a) has made to the development of knowledge pertaining, from chemical and biological perspectives, to the field of cannabinoids, even if in a sometimes indirect and particular manner. Since the beginning of the chemistry associated with the study of this class of compounds, CBN (5a) has played a crucial role, actively participating in the clarification of their biological properties, the elucidation of the structure of the major cannabinoids, and the development of innovative and efficient extraction and purification processes.
As a result of the subsequent isolation of the major cannabinoids, particularly CBD (2a) and ∆9-THC (1a), and the discovery of the remarkable biological activity associated with them, CBN (5a) has unfortunately often been overlooked in favor of its analogues which demonstrate a more marked and promising biological profile; however, it has recently regained prominence following the discovery of the ECS and the multitude of targets that comprise it and provide new avenues for the study and treatment of a broad spectrum of diseases. Despite the large number of biological studies conducted on this compound, further research is necessary: extensive data on pharmacokinetics and pharmacodynamics are required, especially on larger mammals, as well as a complete screening on the large number of secondary targets correlated to the ECS.
The peculiar biphenyl structure of CBN (5a) has made it the target compound for the validation of numerous and diversified synthetic techniques, keeping it in high regard from the 1940s to the present day, and a number of important synthetic methodologies have been developed through the study of its chemistry, including a straightforward one-pot protocol for its synthesis from commercially available source materials.
The current simplicity of synthesis, both of CBN (5a) and its analogues, combined with the largely unknown biological properties, make this class of benzo[c]-chromenic structured compounds one of the most powerful platforms for exploration of the biological space associated with the chemotype of cannabinoids.
Author Contributions
Conceptualization, D.C., C.M. and D.M.; methodology, D.M.; software, C.M.; validation, D.C.; formal analysis, H.I.M.A.; investigation, H.I.M.A.; resources, D.C., A.M.; data curation, D.C.; writing—original draft preparation, D.C.; writing—review and editing, C.M., D.M.; visualization, A.M.; supervision, A.M.; project administration, D.C.; funding acquisition, D.C., A.M. All authors have read and agreed to the published version of the manuscript.
Funding
Research on phytocannabinoids at the laboratories of Novara was funded by MIUR Italy (PRIN2017, Project 2017WN73PL, bioactivity-directed exploration of the phytocannabinoid chemical space).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karche, T.; Singh, M.R. The Application of Hemp (Cannabissativa L.) for a Green Economy: A Review. Turk. J. Bot. 2019, 43, 710–723. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Salami, S.A.; Jones, A.M.P. New Insight into Ornamental Applications of Cannabis: Perspectives and Challenges. Plants 2022, 11, 2383. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Bifulco, M. Medical Cannabis: A Plurimillennial History of an Evergreen. J. Cell. Physiol. 2019, 234, 8342–8351. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.R.; Henriques, A.T.; Limberger, R.P. Medical Cannabis Regulation: An Overview of Models around the World with Emphasis on the Brazilian Scenario. J. Cannabis Res. 2022, 4, 33. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sandoval, E.A.; Fincham, J.E.; Kolano, A.L.; Sharpe, B.N.; Alvarado-Vázquez, P.A. Cannabis for Chronic Pain: Challenges and Considerations. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Pepe, M.; Alizadeh, M.; Rakei, A.; Baiton, A.; Phineas Jones, A.M. Recent Advances in Cannabis Biotechnology. Ind. Crops Prod. 2020, 158, 113026. [Google Scholar] [CrossRef]
- Nguyen, G.-N.; Jordan, E.N.; Kayser, O. Synthetic Strategies for Rare Cannabinoids Derived from Cannabis sativa. J. Nat. Prod. 2022, 85, 1555–1568. [Google Scholar] [CrossRef]
- Atakan, Z. Cannabis, a Complex Plant: Different Compounds and Different Effects on Individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. New Approaches and Challenges to Targeting the Endocannabinoid System. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Anokwuru, C.P.; Makolo, F.L.; Sandasi, M.; Tankeu, S.Y.; Elisha, I.L.; Agoni, C.; Combrinck, S.; Viljoen, A. Cannabigerol: A Bibliometric Overview and Review of Research on an Important Phytocannabinoid. Phytochem. Rev. 2022, 21, 1523–1547. [Google Scholar] [CrossRef]
- Pollastro, F.; Caprioglio, D.; Del Prete, D.; Rogati, F.; Minassi, A.; Taglialatela-Scafati, O.; Munoz, E.; Appendino, G. Cannabichromene. Nat. Prod. Commun. 2018, 13, 1934578X1801300. [Google Scholar] [CrossRef]
- Iversen, L. The Pharmacology of Delta-9-Tetrahydrocannabinol (THC); Oxford University Press: Oxford, UK, 2018; Volume 1. [Google Scholar]
- Sholler, D.J.; Schoene, L.; Spindle, T.R. Therapeutic Efficacy of Cannabidiol (CBD): A Review of the Evidence From Clinical Trials and Human Laboratory Studies. Curr. Addict. Rep. 2020, 7, 405–412. [Google Scholar] [CrossRef]
- Maiocchi, A.; Barbieri, J.; Fasano, V.; Passarella, D. Stereoselective Synthetic Strategies to (−)-Cannabidiol. ChemistrySelect 2022, 7, e202202400. [Google Scholar] [CrossRef]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef]
- Bloemendal, V.R.L.J.; van Hest, J.C.M.; Rutjes, F.P.J.T. Synthetic Pathways to Tetrahydrocannabinol (THC): An Overview. Org. Biomol. Chem. 2020, 18, 3203–3215. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Caprioglio, D.; Amin, H.I.M.; Taglialatela-Scafati, O.; Muñoz, E.; Appendino, G. Minor Phytocannabinoids: A Misleading Name but a Promising Opportunity for Biomedical Research. Biomolecules 2022, 12, 1084. [Google Scholar] [CrossRef]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor Cannabinoids: Biosynthesis, Molecular Pharmacology and Potential Therapeutic Uses. Front. Pharmacol. 2021, 12, 777804. [Google Scholar] [CrossRef]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The Biosynthesis of the Cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef]
- Garrett, E.R.; Gouyette, A.J.; Roseboom, H. Stability of Tetrahydrocannabinols II. J. Pharm. Sci. 1978, 67, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bastola, K.; Hazekamp, A.; Verpoorte, R. Synthesis and Spectroscopic Characterization of Cannabinolic Acid. Planta Med. 2007, 73, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Pain, S. A Potted History. Nature 2015, 525, S10–S11. [Google Scholar] [CrossRef] [PubMed]
- Lavrieux, M.; Jacob, J.; Disnar, J.-R.; Bréheret, J.-G.; Le Milbeau, C.; Miras, Y.; Andrieu-Ponel, V. Sedimentary Cannabinol Tracks the History of Hemp Retting. Geology 2013, 41, 751–754. [Google Scholar] [CrossRef]
- Russo, E.B. History of Cannabis and Its Preparations in Saga, Science, and Sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Appendino, G. The Early History of Cannabinoid Research. Rendiconti Lincei. Scienze Fisiche e Naturali 2020, 31, 919–929. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoid Pharmacology: The First 66 Years: Cannabinoid Pharmacology. Br. J. Pharmacol. 2006, 147, S163–S171. [Google Scholar] [CrossRef]
- Wood, T.B.; Spivey, W.T.N.; Easterfield, T.H. XL.—Charas. The Resin of Indian Hemp. J. Chem. Soc. Trans. 1896, 69, 539–546. [Google Scholar] [CrossRef]
- Caprioglio, D.; Mattoteia, D.; Taglialatela-Scafati, O.; Muñoz, E.; Appendino, G. Cannabinoquinones: Synthesis and Biological Profile. Biomolecules 2021, 11, 991. [Google Scholar] [CrossRef]
- Wood, T.B.; Spivey, W.T.N.; Easterfield, T.H. III.—Cannabinol. Part I. J. Chem. Soc. Trans. 1899, 75, 20–36. [Google Scholar] [CrossRef]
- Madan, H.G.; Randall, W.B.; Shaw, S.; Simpson, M.; Newton Spivey, W.T. Obituary notices: Sir Joseph Henry Gilbert, Ph.D., M.A., L.L.D., Sc.D., F.R.S., 1817–1901. J. Chem. Soc. Trans. 1902, 81, 625–636. [Google Scholar] [CrossRef]
- Walton, R. Marihuana, America’s New Drug Problem; Lippincott: New York, NY, USA, 1938. [Google Scholar]
- Brian, R. Davis “Easterfield, Thomas Hill”, Dictionary of New Zealand Biography, First Published in 1996. Te Ar—The Encyclopedia of New Zealand. Available online: https://teara.govt.nz/en/biographies/3e1/easterfield-thomas-hill (accessed on 15 September 2022).
- Mills, J.H. Cannabis Britannica: Empire, Trade, and Prohibition, 1800–1928; Oxford University Press: Oxford, UK, 2005; ISBN 978-0-19-927881-7. [Google Scholar]
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of Molecular Chirality. Angew. Chem. Int. Ed. Engl. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Cahn, R.S. 326. Cannabis Indica Resin. Part IV. The Synthesis of Some 2: 2-Dimethyldibenzopyrans, and Confirmation of the Structure of Cannabinol. J. Chem. Soc. Resumed 1933, 1400–1405. [Google Scholar] [CrossRef]
- Mechoulam, R. Todd’s Achievement. Nature 1997, 386, 755. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.; Pease, D.C.; Clark, J.H. Isolation of Cannabinol, Cannabidiol and Quebrachitol from Red Oil of Minnesota Wild Hemp. J. Am. Chem. Soc. 1940, 62, 2194–2196. [Google Scholar] [CrossRef]
- Work, T.S.; Bergel, F.; Todd, A.R. The Active Principles of Cannabis Indica Resin. I. Biochem. J. 1939, 33, 123–127. [Google Scholar] [CrossRef]
- Jacob, A.; Todd, A.R. 119. Cannabis Indica. Part II. Isolation of Cannabidiol from Egyptian Hashish. Observations on the Structure of Cannabinol. J. Chem. Soc. Resumed 1940, 649–653. [Google Scholar] [CrossRef]
- Adams, R.; Baker, B.R.; Wearn, R.B. Structure of Cannabinol. III. Synthesis of Cannabinol, 1-Hydroxy-3-n-Amyl-6,6,9-Trimethyl-6-Dibenzopyran 1. J. Am. Chem. Soc. 1940, 62, 2204–2207. [Google Scholar] [CrossRef]
- Hanessian, S.; Margarita, R.; Hall, A.; Johnstone, S.; Tremblay, M.; Parlanti, L. New and Old Challenges in Total Synthesis. From Concept to Practice. Pure Appl. Chem. 2003, 75, 209–221. [Google Scholar] [CrossRef][Green Version]
- Wianowska, D.; Dawidowicz, A.L.; Kowalczyk, M. Transformations of Tetrahydrocannabinol, Tetrahydrocannabinolic Acid and Cannabinol during Their Extraction from Cannabis sativa L. J. Anal. Chem. 2015, 70, 920–925. [Google Scholar] [CrossRef]
- Hidayati, N.; Saefumillah, A.; Cahyana, A.H. Simple Isolation Method of Cannabinol from Cannabis sativa to Produce Secondary Reference Standard Analysis Material. IOP Conf. Ser. Mater. Sci. Eng. 2020, 902, 012063. [Google Scholar] [CrossRef]
- Meltzer, P.C.; Dalzell, H.C.; Razdan, R.K. An Improved Synthesis of Cannabinol and Cannabiorcol. Synthesis 1981, 1981, 985–987. [Google Scholar] [CrossRef]
- Mechoulam, R.; Yagnitinsky, B.; Gaoni, Y. Hashish. XII. Stereoelectronic Factor in the Chloranil Dehydrogenation of Cannabinoids. Total Synthesis of Dl-Cannabichromene. J. Am. Chem. Soc. 1968, 90, 2418–2420. [Google Scholar] [CrossRef] [PubMed]
- Pollastro, F.; Caprioglio, D.; Marotta, P.; Moriello, A.S.; De Petrocellis, L.; Taglialatela-Scafati, O.; Appendino, G. Iodine-Promoted Aromatization of p-Menthane-Type Phytocannabinoids. J. Nat. Prod. 2018, 81, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Caprioglio, D.; Mattoteia, D.; Minassi, A.; Pollastro, F.; Lopatriello, A.; Muňoz, E.; Taglialatela-Scafati, O.; Appendino, G. One-Pot Total Synthesis of Cannabinol via Iodine-Mediated Deconstructive Annulation. Org. Lett. 2019, 21, 6122–6125. [Google Scholar] [CrossRef] [PubMed]
- Costantino, L.; Barlocco, D. Privileged Structures as Leads in Medicinal Chemistry. Curr. Med. Chem. 2006, 13, 65–85. [Google Scholar] [CrossRef]
- Novák, J.; Salemink, C.A. Cannabis Xxiv. A New Convenient Synthesis of Cannabinol. Tetrahedron Lett. 1982, 23, 253–254. [Google Scholar] [CrossRef]
- Hattori, T.; Suzuki, T.; Miyano, S. A Practical and Efficient Method for the Construction of the Biphenyl Framework; Nucleophilic Aromatic Substitution on 2-Methoxybenzoates with Aryl Grignard Reagents. J. Chem. Soc. Chem. Commun. 1991, 1375–1376. [Google Scholar] [CrossRef]
- Nüllen, M.; Göttlich, R. Synthesis of Cannabinol by a Modified Ullmann-Ziegler Cross-Coupling. Synlett 2013, 24, 1109–1112. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.-J.; Wang, J.-Y.; Su, Y.-M.; Wang, X.-S. Pd-Catalyzed C–H Lactonization for Expedient Synthesis of Biaryl Lactones and Total Synthesis of Cannabinol. Org. Lett. 2013, 15, 2574–2577. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-D.; Li, B.; Wang, D.-Y.; Gao, Y.-R.; Guo, S.-H.; Pan, G.-F.; Wang, Y.-Q. Synthesis of 6 H -Benzo[ c ]Chromenes via Palladium-Catalyzed Intramolecular Dehydrogenative Coupling of Two Aryl C–H Bonds. Org. Lett. 2017, 19, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Kloss, F.; Neuwirth, T.; Haensch, V.G.; Hertweck, C. Metal-Free Synthesis of Pharmaceutically Important Biaryls by Photosplicing. Angew. Chem. Int. Ed. 2018, 57, 14476–14481. [Google Scholar] [CrossRef]
- Teske, J.A.; Deiters, A. A Cyclotrimerization Route to Cannabinoids. Org. Lett. 2008, 10, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Nandaluru, P.R.; Bodwell, G.J. Multicomponent Synthesis of 6 H -Dibenzo[b,d]Pyran-6-Ones and a Total Synthesis of Cannabinol. Org. Lett. 2012, 14, 310–313. [Google Scholar] [CrossRef]
- Minuti, L.; Temperini, A.; Ballerini, E. High-Pressure-Promoted Diels–Alder Approach to Biaryls: Application to the Synthesis of the Cannabinols Family. J. Org. Chem. 2012, 77, 7923–7931. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Dong, J.; Wang, J.; Song, L.; Song, C.; Chang, J. An Intramolecular Pyranone Diels-Alder Cycloaddition Approach to Cannabinol. Adv. Synth. Catal. 2014, 356, 1337–1342. [Google Scholar] [CrossRef]
- Mou, C.; Zhu, T.; Zheng, P.; Yang, S.; Song, B.-A.; Chi, Y.R. Green and Rapid Access to Benzocoumarins via Direct Benzene Construction through Base-Mediated Formal [4+2] Reaction and Air Oxidation. Adv. Synth. Catal. 2016, 358, 707–712. [Google Scholar] [CrossRef]
- Norseeda, K.; Tummatorn, J.; Krajangsri, S.; Thongsornkleeb, C.; Ruchirawat, S. Synthesis of 6-Alkyl-6 H -Benzo[ c ]Chromene Derivatives by Cyclization/Selective Ether Cleavage in One Pot: Total Synthesis of Cannabinol. Asian J. Org. Chem. 2016, 5, 792–800. [Google Scholar] [CrossRef]
- Lopatriello, A.; Caprioglio, D.; Minassi, A.; Schiano Moriello, A.; Formisano, C.; De Petrocellis, L.; Appendino, G.; Taglialatela-Scafati, O. Iodine-Mediated Cyclization of Cannabigerol (CBG) Expands the Cannabinoid Biological and Chemical Space. Bioorg. Med. Chem. 2018, 26, 4532–4536. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Di Marzo, V. An Introduction to the Endocannabinoid System: From the Early to the Latest Concepts. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 1–15. [Google Scholar] [CrossRef]
- Moreno, E.; Cavic, M.; Canela, E.I. Functional Fine-Tuning of Metabolic Pathways by the Endocannabinoid System—Implications for Health and Disease. Int. J. Mol. Sci. 2021, 22, 3661. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.-H.; Vogel, Z.; Barg, J.; Bayewitch, M.; Levy, R.; Hanuš, L.; Breuer, A.; Mechoulam, R. Cannabinol Derivatives: Binding to Cannabinoid Receptors and Inhibition of Adenylylcyclase. J. Med. Chem. 1997, 40, 3228–3233. [Google Scholar] [CrossRef] [PubMed]
- Riedel, G.; Fadda, P.; McKillop-Smith, S.; Pertwee, R.G.; Platt, B.; Robinson, L. Synthetic and Plant-Derived Cannabinoid Receptor Antagonists Show Hypophagic Properties in Fasted and Non-Fasted Mice: Cannabinoid Antagonists as Anorectic Agents. Br. J. Pharmacol. 2009, 156, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Kopstick, R.L.; Limebeer, C.L.; Parker, L.A. Tetrahydrocannabinolic Acid Reduces Nausea-Induced Conditioned Gaping in Rats and Vomiting in Suncus murinus: THCA, Emesis and Nausea. Br. J. Pharmacol. 2013, 170, 641–648. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.-O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The Orphan Receptor GPR55 Is a Novel Cannabinoid Receptor: GPR55, a Novel Cannabinoid Receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of Cannabinoids and Cannabinoid-Enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes: Novel Pharmacology of Minor Plant Cannabinoids. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Wong, H.; Cairns, B.E. Cannabidiol, Cannabinol and Their Combinations Act as Peripheral Analgesics in a Rat Model of Myofascial Pain. Arch. Oral Biol. 2019, 104, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Jan, T.-R.; Farraj, A.K.; Harkema, J.R.; Kaminski, N.E. Attenuation of the Ovalbumin-Induced Allergic Airway Response by Cannabinoid Treatment in A/J Mice ☆. Toxicol. Appl. Pharmacol. 2003, 188, 24–35. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Harland, E.; Murphy, J.C.; Wirth, P.; Waller, C.W. Cannabinoids in Glaucoma: A Primary Screening Procedure. J. Clin. Pharmacol. 1981, 21, 472S–478S. [Google Scholar] [CrossRef]
- Aiken, C.T.; Tobin, A.J.; Schweitzer, E.S. A Cell-Based Screen for Drugs to Treat Huntington’s Disease. Neurobiol. Dis. 2004, 16, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial Cannabinoids from Cannabis sativa: A Structure−Activity Study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Farrimond, J.A.; Whalley, B.J.; Williams, C.M. Cannabinol and Cannabidiol Exert Opposing Effects on Rat Feeding Patterns. Psychopharmacology 2012, 223, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Farrimond, J.A.; Whalley, B.J.; Williams, C.M. Non-Δ9tetrahydrocannabinol Phytocannabinoids Stimulate Feeding in Rats. Behav. Pharmacol. 2012, 23, 113–117. [Google Scholar] [CrossRef]
- InMed Announces Update on Phase 2 Clinical Trial Investigating INM-755 Cannabinol Cream for Epidermolysis Bullosa. Available online: https://investors.inmedpharma.com/news-releases/news-release-details/inmed-announces-update-phase-2-clinical-trial-investigating-inm (accessed on 15 September 2022).
- InMed Announces Results of Second Phase 1 Clinical Trial of INM-755 CBN Cream in Healthy Subjects. Available online: https://investors.inmedpharma.com/2021-01-08-InMed-Announces-Results-of-Second-Phase-1-Clinical-Trial-of-INM-755-CBN-Cream-in-Healthy-Subjects?_ga=2.241590278.568845992.1663078970-2025599024.1663078970 (accessed on 15 September 2022).
- Corroon, J. Cannabinol and Sleep: Separating Fact from Fiction. Cannabis Cannabinoid Res. 2021, 6, 366–371. [Google Scholar] [CrossRef]
- Yoshida, H.; Usami, N.; Ohishi, Y.; Watanabe, K.; Yamamoto, I.; Yoshimura, H. Synthesis and Pharmacological Effects in Mice of Halogenated Cannabinol Derivatives. Chem. Pharm. Bull. 1995, 43, 335–337. [Google Scholar] [CrossRef]
- Chesher, G.B.; Jackson, D.M.; Starmer, G.A. Interaction of Cannabis and General Anaesthetic Agents in Mice. Br. J. Pharmacol. 1974, 50, 593–599. [Google Scholar] [CrossRef]
- Takahashi, R.N.; Karniol, I.G. Pharmacological interaction between cannabinol and δ9-tetrahydrocannabinol. Psychopharmacologia 1975, 41, 277–284. [Google Scholar] [CrossRef]
- Karniol, I.G.; Shirakawa, I.; Takahashi, R.N.; Knobel, E.; Musty, R.E. Effects of ∆9-Tetrahydrocannabinol and Cannabinol in Man. Pharmacology 1975, 13, 502–512. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).