Chemical Composition and In Vitro Evaluation of Antioxidant and Antiproliferative Effects of Volatile Oils Hydrodistilled from Onobrychis carduchorum C.C. Towns., a Kurdish Traditional Plant
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Properties of the Essential Oils
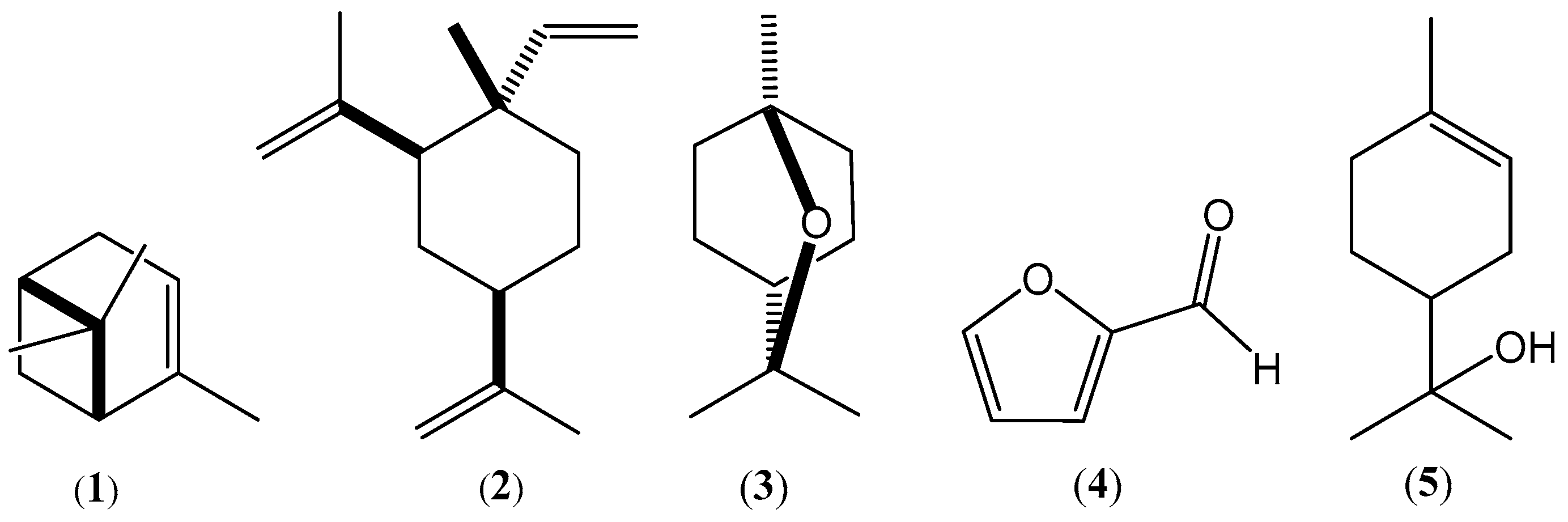
2.2. Chemical Composition of the Essential Oils
2.3. Antiproliferative Activity
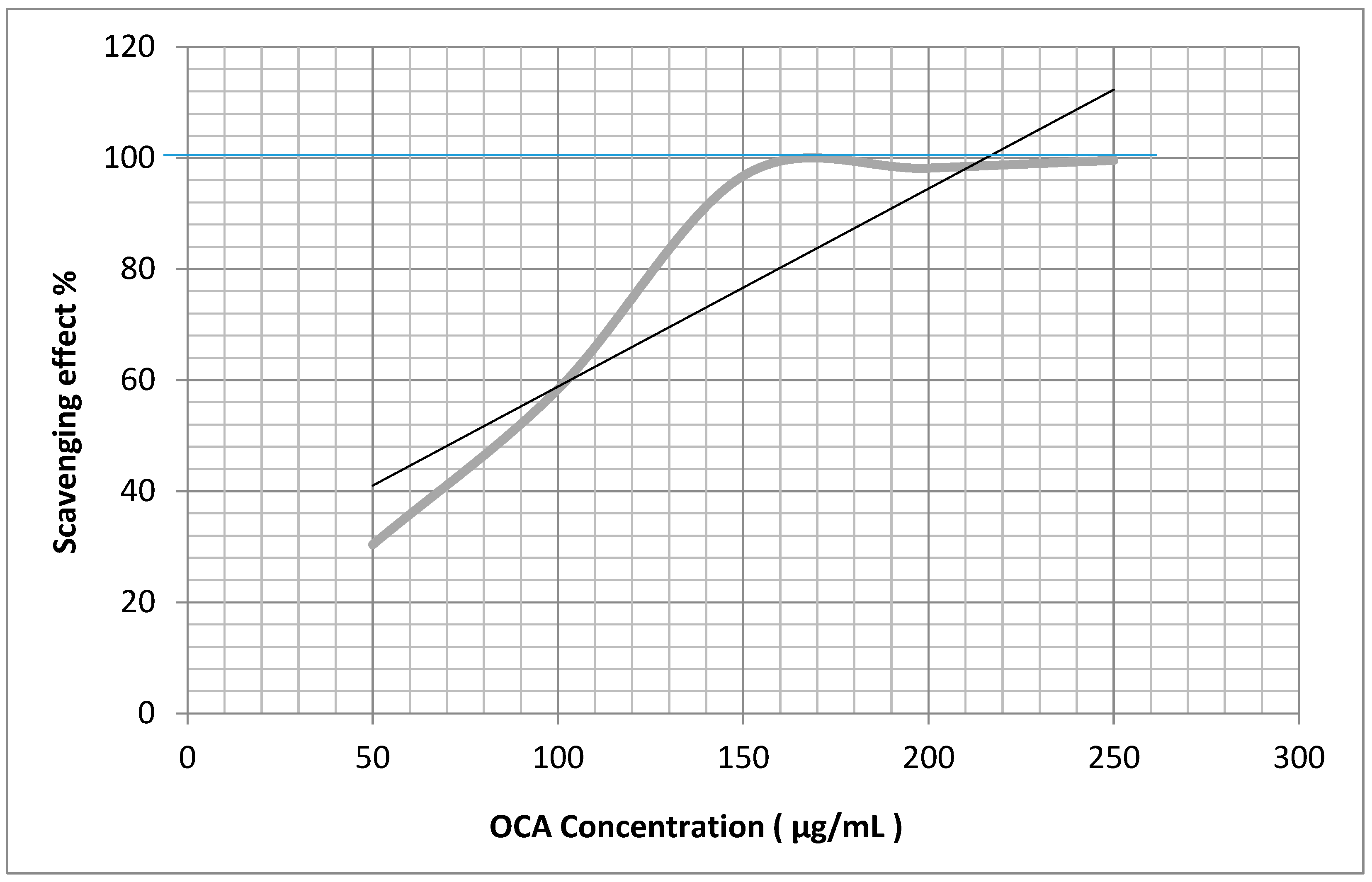
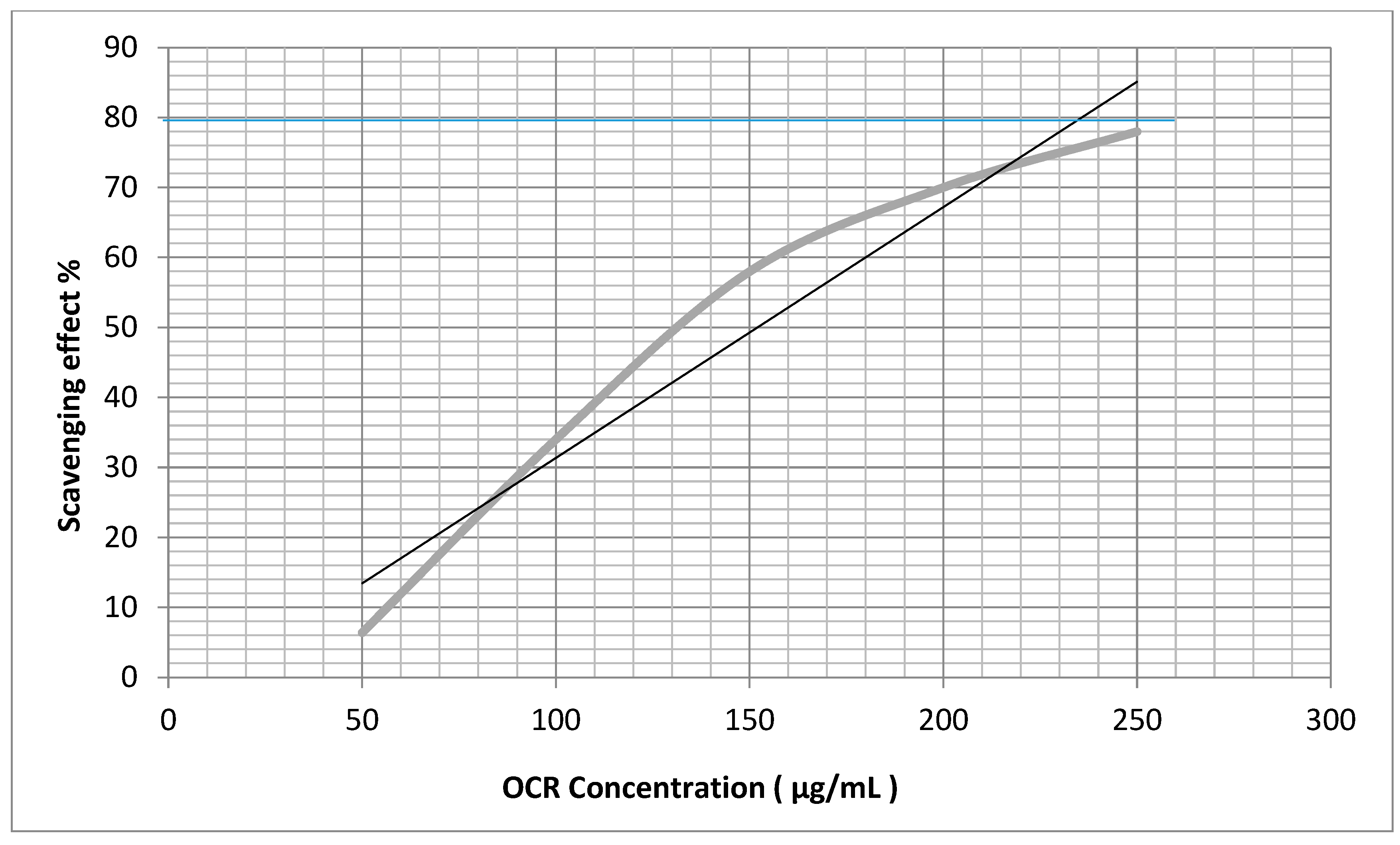
2.4. Antiradical Activities
3. Discussion
4. Materials and Methods
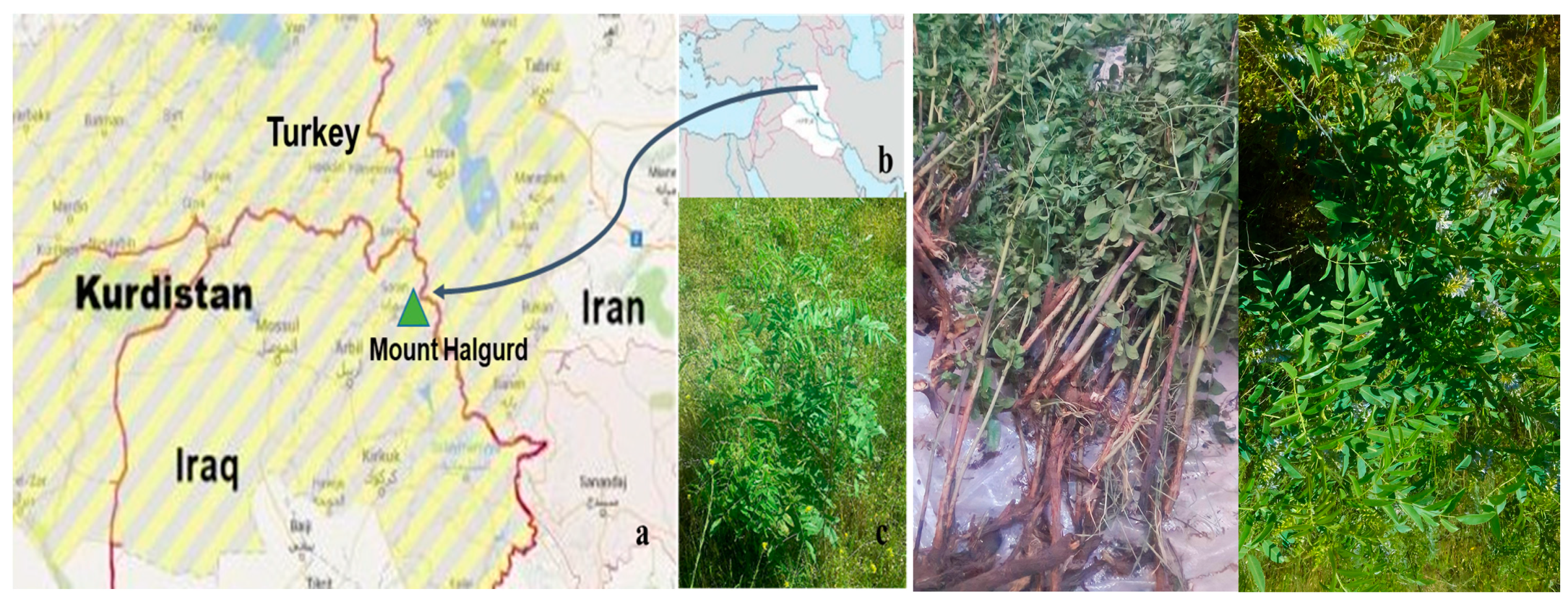
4.1. Plant Material
4.2. Isolation of Volatile Fractions
4.3. GC-MS Analysis
4.4. GC-FID Analysis
4.5. Identification of the Essential Oil Components
4.6. Determination of the Antiproliferative Effects of the Oils (MTS Assay)
4.6.1. Cell Cultures
4.6.2. MTS Assay of the OCA and OCR Oils
4.7. Evaluation of Antiradical and Antioxidant Activities
4.7.1. DPPH Test
4.7.2. H2O2 Test
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pieroni, A.; Sõukand, R.; Amin, H.I.M.; Zahir, H.; Kukk, T. Celebrating Multi-Religious Co-Existence in Central Kurdistan: The Bio-Culturally Diverse Traditional Gathering of Wild Vegetables among Yazidis, Assyrians, and Muslim Kurds. Hum. Ecol. 2018, 46, 217–227. [Google Scholar] [CrossRef]
- Aktoklu, E. Two new varieties and a new record in Onobrychis from Turkey. Turk. J. Bot. 2001, 25, 359–363. [Google Scholar]
- Townsend, C.C.; Guest, E. Flora of Iraq. In Leguminales; Ministry of Agriculture and Agrarian Reform: Baghdad, Iraq, 1974; Volume 3, pp. 471–493. [Google Scholar]
- Rechinger, K.H. Onobrychis (Hedysareae-Papilionaceae II). In Flora Iranica; Rechinger, K.H., Ed.; Akademische Druck: Graz & Wien, Austria, 1984; Volume 157, pp. 387–464. [Google Scholar]
- Davis, P.; Miller, R.; Kit, T. Flora of Turkey; Davis, P., Kit, T., Eds.; Edinburgh University Press: Edinburgh, UK, 1988; Volume 10, pp. 129–131. [Google Scholar]
- Amin, H.I.M.; Ibrahim, M.F.; Hussain, F.H.S.; Sardar, A.S.; Vidari, G. Phytochemistry and ethnopharmacology of some medicinal plants used in the Kurdistan region of Iraq. Nat. Prod. Commun. 2016, 11, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Mükemre, M.; Behçet, L.; Çakılcıoğlu, U. Ethnobotanical study on medicinal plants in villages of Çatak (Van-Turkey). J. Ethnopharm. 2015, 166, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Toluei, Z. Iranian Onobrychis carduchorum (Fabaceae) populations: Morphology, ecology and phylogeography. Plant Ecol. Evol. 2013, 146, 53–67. [Google Scholar] [CrossRef]
- Mabberley, D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classification and Uses, 4th ed.; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Amirahmadii, A.; Khoshsokhan-Mozaffar, M. Molecular and morphological evidence of Onobrychis avanakensis, a new species from Iran. Phytotaxa 2021, 487, 75–81. [Google Scholar] [CrossRef]
- Yakovlev, G.P.; Sytin, A.K.; Roskov, Y.R. Legumes of Northern Eurasia: A Checklist; Royal Botanic Gardens, Kew: Richmond, UK, 1996. [Google Scholar]
- Lu, Y.; Sun, Y.; Foo, Y.L.; McNabb, W.C.; Molan, A.L. Phenolic glycosides of forage legume Onobrychis viciifolia. Phytochemistry 2000, 55, 67–75. [Google Scholar] [CrossRef]
- Veitch, N.C.; Regos, I.; Kite, G.C.; Treutter, D. Acylated flavonol glycosides from the forage legume, Onobrychis viciifolia (sainfoin). Phytochemistry 2011, 72, 423–429. [Google Scholar] [CrossRef]
- Ozbek, H.; Acikara, O.B.; Oz, B.E.; Ozbilgin, S.; Kirmizi, N.I.; Ozrenk, B.C.; Tekin, M.; Saltan, G. Antidiabetic activity evaluation of Onobrychis species on alloxan-induced diabetic mice. Braz. J. Pharm. Sci. 2019, 55, e18157. [Google Scholar] [CrossRef]
- Karakoca, K.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Teksen, M. Phenolic compounds, biological and antioxidant activities of Onobrychis armena Boiss. & Huet flower and root extracts. Chiang Mai J. Sci. 2015, 42, 376–392. [Google Scholar]
- Karamian, R.; Asadbegy, M. Antioxidant activity, total phenolic and flavonoid contents of three Onobrychis species from Iran. Pharm. Sci. 2016, 22, 112–119. [Google Scholar] [CrossRef]
- Usta, C.; Yildirim, A.B.; Turker, A.U. Antibacterial and antitumour activities of some plants grown in Turkey. Biotechnol. Biotechnol. Equip. 2014, 28, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Clericuzio, M.; Hussain, F.H.S.; Amin, H.I.M.; Bona, E.; Gamalero, E.; Novello, G.; Lappano, R.; Talia, M.; Maggiolini, M.; Bazzicalupo, M.; et al. Cytotoxic, anti-bacterial, and wound-healing activity of prenylated phenols from the Kurdish traditional medicinal plant Onobrychis carduchorum (Fabaceae). Planta Med. Intern. Open 2020, 07, e106–e113. [Google Scholar]
- Karatas, S.; Aktumsek, A.; Uysal, S. Investigation of antioxidant properties, essential oil, and fatty acid composition of Onobrychis armena Boiss. & Huet. Istanbul. J. Pharm. 2022, 52, 164–172. [Google Scholar] [CrossRef]
- Mozaniel, S.O.; Oliviu, V.; Daniela, R.; Eloisa, H. Editorial: Bioactive compounds present in essential oils: Advances and pharmacological applications. Front.Pharmacol. 2023, 14, 1130097. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Tit, D.M.; Bungau, S.G. Antioxidant Activity of essential oils. Antioxidants. 2023, 12, 383. [Google Scholar] [CrossRef]
- Adorjan, B.; Gerhard Buchbauer, G. Biological properties of essential oils: An updated review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Eid, A.M.; Jaradat, N.; Shraim, N. Assessment of anticancer, antimicrobial, antidiabetic, anti-obesity and antioxidant activity of Ocimum basilicum seeds essential oil from Palestine. BMC Complement Med. Ther. 2023, 23, 221. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Robustelli della Cuna, F.; Villa, C.; Corti, M.; Amin, H.I.M.; Faris, P.; Grisoli, P.; Brusotti, G. A chemometric assessment and profiling of the essential oils from Hibiscus sabdariffa L. from Kurdistan, Iraq. Nat. Prod. Res. 2022, 36, 2409–2412. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Visakh, N.U.; Sasidharan, A.; Pathrose, B.; Olatunji, O.J.; Al-Ansari, A.; Alfarhan, A.; Ramesh, V. Chemical composition, antioxidant, anti-bacterial, and anti-cancer activities of essential oils extracted from Citrus limetta Risso peel waste remains after commercial use. Molecules 2022, 27, 8329. [Google Scholar] [CrossRef] [PubMed]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer properties of essential oils and other natural products. Evid. Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Abdullah, M.M.S.; Mousa, A.A.; Alkhathlan, H.Z. Chemical composition of vegetative parts and flowers essential oils of wild Anvillea garcinii grown in Saudi Arabia. Rec. Nat. Prod. 2016, 10, 251–256. [Google Scholar]
- Khan, M.; Al-Saleem, M.S.M.; Alkhathlan, H.Z. A detailed study on chemical characterization of essential oil components of two Plectranthus species grown in Saudi Arabia. J. Saudi Chem. Soc. 2016, 20, 711–721. [Google Scholar] [CrossRef]
- Khan, M.; Al-Mansour, M.A.; Mousa, A.A.; Alkhathlan, H.Z. Compositional characteristics of the essential oil of Myrtus communis grown in the central part of Saudi Arabia. J. Essent. Oil Res. 2014, 26, 13–18. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST 08, Mass Spectral Library (NIST/EPA/NIH); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2008.
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual [Internet]; 2013 May 1 [updated 2016 July 1]; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Geran, R.I.; Greenberg, N.H.; Macdonald, M.M.; Schumacher, A.M. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep. 1972, 3, 59–61. [Google Scholar]
- Li, X.; Wang, G.; Zhao, J.; Ding, H.; Cunningham, C.; Flynn, D.C.; Reed, E.; Li, Q.Q. Antiproliferative effect of β-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase. CMLS Cell. Mol. Life Sci. 2005, 62, 894–904. [Google Scholar] [CrossRef]
- Dai, Z.J.; Tang, W.; Lu, W.F.; Gao, J.; Kang, H.F.; Ma, X.B.; Min, W.L.; Wang, X.J.; Wu, W.Y. Antiproliferative and apoptotic effects of β-elemene on human hepatoma HepG2 cells. Cancer Cell Int. 2013, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Yao, C.; Zhu, J.; Xie, Y.; Ye, X.Y.; Bai, R.; Xie, T. Anti-tumor drug discovery based on natural product β-elemene: Anti-tumor mechanisms and structural modification. Molecules 2021, 26, 1499. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Wells, P.G.; McCallum, G.P.; Chen, C.S.; Henderson, J.T.; Lee, C.J.; Perstin, J.; Preston, T.J.; Wiley, M.J.; and Wong, A.W. Oxidative stress in developmental origins of disease: Teratogenesis, neurodevelopmental deficits, and cancer. Toxicol. Sci. 2009, 108, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Amin, H.I.M.; Amin, A.A.; Tosi, S.; Mellerio, G.G.; Hussain, F.H.S.; Picco, A.M.; Vidari, G. Chemical composition and antifungal activity of essential oils from flowers, leaves, rhizomes, and bulbs of the wild Iraqi Kurdish plant Iris persica. Nat. Product Commun. 2017, 12, 441–444. [Google Scholar] [CrossRef]
- Amin, H.I.M.; Hussain, F.H.S.; Najmaldin, S.K.; Thu, Z.M.; Ibrahim, M.F.; Gilardoni, G.; Vidari, G. Phytochemistry and bological activities of Iris species growing in Iraqi Kurdistan and phenolic constituents of the traditional plant Iris postii. Molecules 2021, 26, 264. [Google Scholar] [CrossRef]
- Vani, T.; Rajani, M.; Sarkar, S.; Shishoo, C.J. Antioxidant properties of the ayurvedic formulation Triphala and its constituents. Int. J. Pharmac. 1997, 35, 313–317. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.H.; Mohamad, A.B. Hydrogen peroxide scavenging activity of novel coumarins synthesized using different approaches. PLoS ONE 2015, 10, e0132175. [Google Scholar] [CrossRef]
| No. of Identified Compound | Compound Name a | RT b | LRIexp c | LRIlit d | % MS Match e | % in the OCA Oil f ± SD | % in the OCR Oil g ± SD |
|---|---|---|---|---|---|---|---|
| 1 | n-Octane h | 2.12 | 801 | 800 | 94 | 2.26 ± 0.01 | 0.32 ± 0.02 |
| 2 | Hexanal h | 3.88 | 803 | 801 | 93 | 0.13 ± 0.02 | 2.22 ± 0.11 |
| 3 | Furfural h | 3.98 | 825 | 828 | 93 | 7.91 ± 0.06 | 10.44 ± 0.08 |
| 4 | 2-Methylbutanoic acid h | 4.11 | 831 | 832 | 87 | 0.77 ± 0.03 | 0.34 ± 0.03 |
| 5 | Ethyl isovalerate h | 4.31 | 845 | 849 | 89 | Tr | 0.55 ± 0.02 |
| 6 | (2E)-Hexenol h | 5.17 | 855 | 854 | 90 | Tr | 0.05 ± 0.01 |
| 7 | 1-Hexanol h | 5.53 | 862 | 863 | 89 | 0.83 ± 0.02 | 0.24 ± 0.02 |
| 8 | Heptanal h | 6.34 | 903 | 901 | 89 | 2.01 ± 0.01 | 0.83 ± 0.03 |
| 9 | Methional h | 7.03 | 911 | 909 | 90 | 1.45 ± 0.03 | Tr |
| 10 | 2-Acetylfuran h | 7.21 | 915 | 909 | 90 | Tr | 2.14 ± 0.06 |
| 11 | α-Pinene h | 7.93 | 935 | 932 | 88 | 23.11 ± 0.06 | 4.76 ± 0.12 |
| 12 | 3-Methylcyclohexanone h | 7.98 | 946 | 945 | 78 | 0.77 ± 0.01 | Tr |
| 13 | Benzaldehyde h | 8.01 | 959 | 952 | 95 | 0.32 ± 0.02 | 0.54 ± 0.04 |
| 14 | β-Pinene h | 8.18 | 971 | 974 | 90 | 0.29 ± 0.02 | 4.37 ± 0.14 |
| 15 | Isomaltol | 8.26 | 985 | 980 | 94 | 0.07 ± 0.01 | 0.03 ± 0.01 |
| 16 | 1,4-Cineole h | 8.34 | 996 | 991 | 83 | 0.17 ± 0.01 | Tr |
| Unidentified | 9.11 | 1006 | - | - | Tr | 0.43 ± 0.02 | |
| 17 | α-Terpinene h | 9.81 | 1018 | 1014 | 90 | 1.65 ± 0.04 | Tr |
| 18 | Limonene h | 10.23 | 1022 | 1024 | 88 | 4.13 ± 0.06 | 2.89 ± 0.07 |
| 19 | 1,8-Cineole h | 10.59 | 1024 | 1026 | 93 | 12.15 ± 0.05 | 15.79 ± 0.12 |
| Unidentified | 11.22 | 1045 | - | - | 0.54 ± 0.03 | 0.67 ± 0.03 | |
| 20 | m-Cresol h | 11.39 | 1069 | 1072 | 74 | 0.54 ± 0.03 | Tr |
| 21 | cis-Linalool oxide h (furanoic) | 11.74 | 1078 | 1067 | 83 | 1.23 ± 0.04 | 1.33 ± 0.06 |
| 22 | trans-Linalool oxide h (furanoic) | 12.09 | 1081 | 1084 | 67 | 0.32 ± 0.02 | Tr |
| Unidentified | 13.19 | 1089 | - | - | 0.42 ± 0.02 | 0.34 ± 0.03 | |
| 23 | Linalool h | 13.67 | 1100 | 1095 | 80 | 2.70 ± 0.04 | 7.45 ± 0.06 |
| 24 | Nonanal h | 13.74 | 1105 | 1100 | 64 | 0.43 ± 0.02 | Tr |
| 25 | Methyl octanoate h | 13.91 | 1130 | 1123 | 68 | Tr | 2.3 ± 0.07 |
| 26 | 1,4-Dimethoxybenzene h | 14.04 | 1163 | 1161 | 82 | 0.28 ± 0.04 | 0.07 ± 0.01 |
| 27 | Octanoic acid h | 14.22 | 1171 | 1167 | 89 | 1.41 ± 0.01 | 2.45 ± 0.03 |
| 28 | Terpinen-4-ol h | 14.39 | 1177 | 1174 | 90 | 6.32 ± 0.05 | 3.61 ± 0.07 |
| 29 | α-Terpineol h | 15.57 | 1190 | 1186 | 81 | 1.32 ± 0.05 | 7.74 ± 0.07 |
| 30 | Decanal h | 15.89 | 1205 | 1201 | 90 | 0.08 ± 0.02 | 0.05 ± 0.01 |
| 31 | (Z)-Ocimenone | 16.12 | 1231 | 1226 | 90 | 1.32 ± 0.04 | Tr |
| Unidentified | 16.88 | 1243 | - | - | Tr | 0.67 ± 0.02 | |
| 32 | 2-Phenylethyl acetate h | 17.65 | 1255 | 1260 | 79 | 1.30 ± 0.02 | 0.15 ± 0.01 |
| 33 | Cinnamaldehyde h | 17.77 | 1258 | 1267 | 51 | Tr | 0.18 ± 0.02 |
| 34 | Carvacrol h | 17.90 | 1297 | 1298 | 94 | 1.06 ± 0.03 | 6.41 ± 0.05 |
| 35 | Undecanal h | 17.98 | 1299 | 1305 | 84 | 0.23 ± 0.04 | 0.03 ± 0.01 |
| Unidentified | 18.94 | 1365 | - | - | Tr | 1.77 ± 0.09 | |
| 36 | β-Elemene h | 18.98 | 1385 | 1389 | 91 | 17.33 ± 0.07 | 10.14 ± 0.12 |
| 37 | γ-Curcumene | 19.09 | 1482 | 1481 | 95 | 0.03 ± 0.01 | Tr |
| 38 | β-Ionone h | 19.33 | 1495 | 1486 | 82 | 1.35 ± 0.03 | 2.65 ± 0.03 |
| Unidentified | 20.77 | 1520 | - | - | Tr | 0.65 ± 0.04 | |
| 39 | α-Cadinene | 21.73 | 1540 | 1537 | 88 | 0.04 ± 0.01 | 0.11 ± 0.01 |
| 40 | Zierone | 22.45 | 1580 | 1574 | 83 | 0.11 ± 0.03 | 0.17 ± 0.03 |
| Unidentified | 23.11 | 1583 | - | - | 0.12 ± 0.03 | 0.42 ± 0.02 | |
| 41 | Salvial-4(14)-en-1-one | 24.25 | 1591 | 1594 | 81 | 0.03 ± 0.01 | 0.04 ± 0.01 |
| 42 | Tetradecanoic acid | 25.33 | 1777 | 1767 | 88 | 0.98 ± 0.05 | 0.55 ± 0.02 |
| 43 | Octadecanoic acid h | 25.97 | 2165 | 2169 | 95 | 2.15 ± 0.06 | 2.39 ± 0.07 |
| Chemical Classes of Identified Compounds (Total Number in the OCA + OCR Oils) | % in OCA | % in OCR |
|---|---|---|
| Terpenoids: | ||
| Monoterpene hydrocarbons (4) | 29.18 | 12.02 |
| Oxygenated monoterpenoids (8) | 25.53 | 35.92 |
| Sesquiterpene hydrocarbons (3) | 17.40 | 10.25 |
| Oxygenated sesquiterpenoids (2) | 0.14 | 0.21 |
| Others: | ||
| Hydrocarbons (1) | 2.26 | 0.32 |
| Aldehydes (9) | 12.56 | 14.29 |
| Ketones (4) | 2.19 | 4.82 |
| Alcohols (2) | 0.83 | 0.29 |
| Carboxylic acids (4) | 5.31 | 5.73 |
| Esters (3) | 1.30 | 3.00 |
| Miscellaneous aromatic derivatives (3) | 1.88 | 6.48 |
| Total identified compounds (43) | 98.58 | 93.33 |
| Sample | T-47D a | MDA-MB-453 b | BG-1 c | A549 d | HEK-293 e | HFF-1 f |
|---|---|---|---|---|---|---|
| OCA | 12.1 ± 0.11 * | >50 | 11.2 ± 0.2 * | 10.2 ± 0.3 * | 26.1 ± 0.21 * | >50 |
| OCR | 16.3 ± 0.15 * | 14.5 ± 0.2 * | 23.4 ± 0.6 * | 16.4 ± 0.4 * | 30.8 ± 0.1 * | 32.5 ± 0.3 * |
| Cisplatin | 5.10 ± 0.04 | 3.09 ± 0.06 | 3.69 ± 0.11 | 3.96 ± 0.08 | 7.3 ± 0.07 | 7.09 ± 0.11 |
| Sample | DPPH | H2O2 |
|---|---|---|
| OCA | 79.8 ± 0.5 * | 394.1 ± 0.2 * |
| OCR | 153.3 ± 0.6 * | 311.7 ± 0.5 * |
| Ascorbic acid | 19.84 ± 0.12 | 36.51 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, H.I.M.; Abdoulrahman, K.; Sadraddin, A.S.; Smail, H.A.; Jawhar, Z.H.; Dilawer Issa, K.; Armijos, C.; Vidari, G. Chemical Composition and In Vitro Evaluation of Antioxidant and Antiproliferative Effects of Volatile Oils Hydrodistilled from Onobrychis carduchorum C.C. Towns., a Kurdish Traditional Plant. Plants 2023, 12, 3013. https://doi.org/10.3390/plants12163013
Amin HIM, Abdoulrahman K, Sadraddin AS, Smail HA, Jawhar ZH, Dilawer Issa K, Armijos C, Vidari G. Chemical Composition and In Vitro Evaluation of Antioxidant and Antiproliferative Effects of Volatile Oils Hydrodistilled from Onobrychis carduchorum C.C. Towns., a Kurdish Traditional Plant. Plants. 2023; 12(16):3013. https://doi.org/10.3390/plants12163013
Chicago/Turabian StyleAmin, Hawraz Ibrahim M., Kamaran Abdoulrahman, Azad S. Sadraddin, Heman A. Smail, Zanko Hassan Jawhar, Kovan Dilawer Issa, Chabaco Armijos, and Giovanni Vidari. 2023. "Chemical Composition and In Vitro Evaluation of Antioxidant and Antiproliferative Effects of Volatile Oils Hydrodistilled from Onobrychis carduchorum C.C. Towns., a Kurdish Traditional Plant" Plants 12, no. 16: 3013. https://doi.org/10.3390/plants12163013
APA StyleAmin, H. I. M., Abdoulrahman, K., Sadraddin, A. S., Smail, H. A., Jawhar, Z. H., Dilawer Issa, K., Armijos, C., & Vidari, G. (2023). Chemical Composition and In Vitro Evaluation of Antioxidant and Antiproliferative Effects of Volatile Oils Hydrodistilled from Onobrychis carduchorum C.C. Towns., a Kurdish Traditional Plant. Plants, 12(16), 3013. https://doi.org/10.3390/plants12163013








