Effects of Plasma-Activated Water on Leaf and Fruit Biochemical Composition and Scion Growth in Apple
Abstract
1. Introduction
2. Results
2.1. Experiment #1
2.1.1. Fruitset from Free Pollination and Yield
2.1.2. The Contents of Primary Nutrients in Apple Leaves
2.1.3. Contents of Primary Nutrients in Apple Fruits
2.1.4. Biochemical Composition of Fruits
2.2. Experiment #2
2.2.1. Graft Survival Rate
2.2.2. Growth of the Grafted Trees in Nursery
3. Materials and Methods
3.1. PAW Preparation
3.2. Location and Conditions of Experiments
3.2.1. Experiment #1
3.2.2. Experiment #2
3.3. Experimental Design and Analysis Methods
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2017, 15, 1700174. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Glinushkin, A.P.; Kalinitchenko, V.P.; Artem’Ev, K.V.; Burmistrov, D.E.; Kozlov, V.A.; Kolik, L.V. Properties and Use of Water Activated by Plasma of Piezoelectric Direct Discharge. Front. Phys. 2021, 8, 616385. [Google Scholar] [CrossRef]
- Belov, S.V.; Danileiko, Y.K.; Egorov, A.B.; Lukanin, V.I.; Semenova, A.A.; Lisitsyn, A.B.; Revutskaya, N.M.; Nasonova, V.V.; Yushina, Y.K.; Tolordava, E.R.; et al. Sterilizer of Knives in the Meat Industry, Working by Activating Aqueous Solutions with Glow Discharge Plasma. Processes 2022, 10, 1536. [Google Scholar] [CrossRef]
- Ashurov, M.K.; Ashurov, E.M.; Astashev, M.E.; Baimler, I.V.; Gudkov, S.V.; Konchekov, E.M.; Lednev, V.N.; Lukina, N.A.; Matveeva, T.A.; Markendudis, A.G.; et al. Development of an Environmentally Friendly Technology for the Treatment of Aqueous Solutions with High-Purity Plasma for the Cultivation of Cotton, Wheat and Strawberries. Chemengineering 2022, 6, 91. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.P.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Sarinont, T.; Katayama, R.; Wada, Y.; Koga, K.; Shiratani, M. Plant Growth Enhancement of Seeds Immersed in Plasma Activated Water. MRS Adv. 2017, 2, 995–1000. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, L.; Hensel, K. Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Process. Polym. 2019, 16, 1800131. [Google Scholar] [CrossRef]
- Koning, L.A.; Veste, M.; Freese, D.; Lebzien, S. Effects of nitrogen and phosphate fertilization on leaf nutrient content, photosynthesis, and growth of the novel bioenergy crop Fallopia sachalinensis cv. ‘Igniscum Candy’. J. Appl. Bot. Food Qual. 2015, 88, 22–28. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.-J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Cortese, E.; Settimi, A.G.; Pettenuzzo, S.; Cappellin, L.; Galenda, A.; Famengo, A.; Dabalà, M.; Antoni, V.; Navazio, L. Plasma-Activated Water Triggers Rapid and Sustained Cytosolic Ca2+ Elevations in Arabidopsis thaliana. Plants 2021, 10, 2516. [Google Scholar] [CrossRef]
- Stoleru, V.; Burlica, R.; Mihalache, G.; Dirlau, D.; Padureanu, S.; Teliban, G.-C.; Astanei, D.; Cojocaru, A.; Beniuga, O.; Patras, A. Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Sci. Rep. 2020, 10, 20920. [Google Scholar] [CrossRef]
- Dou, Y.; Yang, Y.; Mund, N.K.; Wei, Y.; Liu, Y.; Wei, L.; Wang, Y.; Du, P.; Zhou, Y.; Liesche, J.; et al. Comparative Analysis of Herbaceous and Woody Cell Wall Digestibility by Pathogenic Fungi. Molecules 2021, 26, 7220. [Google Scholar] [CrossRef] [PubMed]
- Škarpa, P.; Klofáč, D.; Krčma, F.; Šimečková, J.; Kozáková, Z. Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea mays L.). Water 2020, 12, 3545. [Google Scholar] [CrossRef]
- Popova, A. Comparison of Vitamin C Content of Commercially Available Fresh Fruits. Asian Food Sci. J. 2019, 13, 1–6. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Guan, Q.; Sun, X.; Liu, S.; Hao, K.; Hu, W. The Effects of Cold Plasma-Activated Water Treatment on the Microbial Growth and Antioxidant Properties of Fresh-Cut Pears. Food Bioprocess Technol. 2019, 12, 1842–1851. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Jiang, A.; Sun, X.; Guan, Q.; Hu, W. Effects of plasma-activated water on microbial growth and storage quality of fresh-cut apple. Innov. Food Sci. Emerg. Technol. 2019, 59, 102256. [Google Scholar] [CrossRef]
- Tarabová, B.; Tampieri, F.; Maran, E.; Marotta, E.; Ostrihoňová, A.; Krewing, M.; Machala, Z. Chemical and Antimicrobial Effects of Air Non-Thermal Plasma Processing of Fresh Apple Juice with Focus on Safety Aspects. Foods 2021, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Herron, S.A.; Rubin, M.J.; Ciotir, C.; Crews, T.E.; Van Tassel, D.L.; Miller, A.J. Comparative Analysis of Early Life Stage Traits in Annual and Perennial Phaseolus Crops and Their Wild Relatives. Front. Plant Sci. 2020, 11, 34. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. 3—Physiological Regulation of Vegetative Growth. In Growth Control in Woody Plants; Physiological Ecology; Kozlowski, T.T., Pallardy, S.G., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 73–165. [Google Scholar] [CrossRef]
- Lobo, A.K.M.; Catarino, I.C.A.; Silva, E.A.; Centeno, D.C.; Domingues, D.S. Physiological and Molecular Responses of Woody Plants Exposed to Future Atmospheric CO2 Levels under Abiotic Stresses. Plants 2022, 11, 1880. [Google Scholar] [CrossRef]
- Kilonzo-Nthenge, A.; Liu, S.; Yannam, S.; Patras, A. Atmospheric Cold Plasma Inactivation of Salmonella and Escherichia coli on the Surface of Golden Delicious Apples. Front. Nutr. 2018, 5, 120. [Google Scholar] [CrossRef] [PubMed]
- Park, D.P.; Davis, K.; Gilani, S.; Alonzo, C.-A.; Dobrynin, D.; Friedman, G.; Fridman, A.; Rabinovich, A.; Fridman, G. Reactive nitrogen species produced in water by non-equilibrium plasma increase plant growth rate and nutritional yield. Curr. Appl. Phys. 2013, 13, S19–S29. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.B.; Moreira, A.; Guimarães, C.M. Foliar Fertilization of Crop Plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Grafting_and_Propagating_Fruit_Trees.pdf. Available online: https://static1.squarespace.com/static/5e013c51bf487105fe2e858f/t/617ddca967400a025c697fd0/1635638447282/grafting_and_propagating_fruit_trees.pdf (accessed on 27 September 2022).
- Gimber, M. Whip and Tongue Graft. People’s Trust for Endangered Species. Available online: https://ptes.org/campaigns/traditional-orchard-project/orchard-practical-guides/grafting/whip-and-tongue-graft/ (accessed on 27 September 2022).
- Forestry—Grafting for Forestry Research—Teagasc|Agriculture and Food Development Authority. Available online: https://www.teagasc.ie/news--events/daily/forestry/grafting-for-forestry-research.php (accessed on 27 September 2022).
- Bench-Grafting_Whip-Tongue.pdf. Available online: https://ptes.org/wp-content/uploads/2017/05/Bench-grafting_Whip-tongue.pdf (accessed on 28 September 2022).
- Sharma, A.; Zheng, B. Molecular Responses during Plant Grafting and Its Regulation by Auxins, Cytokinins, and Gibberellins. Biomolecules 2019, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, C.W.; Meyerowitz, E.M. Plant grafting. Curr. Biol. 2015, 25, R183–R188. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A Technique to Modify Ion Accumulation in Horticultural Crops. Front. Plant Sci. 2016, 7, 01457. [Google Scholar] [CrossRef]
- Melnyk, C.W. Plant grafting: Insights into tissue regeneration. Regeneration 2016, 4, 3–14. [Google Scholar] [CrossRef]
- Harada, T. Grafting and RNA transport via phloem tissue in horticultural plants. Sci. Hortic. 2010, 125, 545–550. [Google Scholar] [CrossRef]
- Habibi, F.; Liu, T.; Folta, K.; Sarkhosh, A. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hortic. Res. 2022, 9, uhac032. [Google Scholar] [CrossRef]
- Belov, S.V.; Gudkov, S.V.; Danyleiko, Y.K.; Egorov, A.B.; Lukanin, V.I.; Sidorov, V.A.; Tsvetkov, V.B. A Device for Biological Activation of Aqueous Solutions Using Glow Discharge Plasma in Water Vapor. Biomed. Eng. 2021, 55, 97–102. [Google Scholar] [CrossRef]
- Belov, S.V.; Danyleiko, Y.K.; Glinushkin, A.P.; Kalinitchenko, V.P.; Egorov, A.V.; Sidorov, V.A.; Konchekov, E.M.; Gudkov, S.V.; Dorokhov, A.S.; Lobachevsky, Y.P.; et al. An Activated Potassium Phosphate Fertilizer Solution for Stimulating the Growth of Agricultural Plants. Front. Phys. 2021, 8, 618320. [Google Scholar] [CrossRef]
- Izmailov, A.; Khort, D.; Filippov, R.; Pishchalnikov, R.Y.; Simakin, A.V.; Shogenov, Y. Improvement of Winter Graft Techniques Using Cold Plasma and Plasma-Treated Solution on Cherry Cultures. Appl. Sci. 2022, 12, 4953. [Google Scholar] [CrossRef]
- Sergeichev, K.F.; Lukina, N.A.; Arutyunyan, N.R. Atmospheric-Pressure Microwave Plasma Torch for CVD Technology of Diamond Synthesis. Plasma Phys. Rep. 2019, 45, 551–560. [Google Scholar] [CrossRef]
- Sergeichev, K.F.; Lukina, N.A.; Apasheva, L.M.; Ovcharenko, E.N.; Lobanov, A.V. Water Activated by a Microwave Plasma Argon Jet as a Factor Stimulating the Germination of Plant Seeds. Russ. J. Phys. Chem. B 2022, 16, 84–89. [Google Scholar] [CrossRef]
- Mineev, V.G.; Sychev, V.G.; Amelyanchik, O.A.; Bolsheva, T.N.; Gomonova, N.F.; Durynina, E.P.; Egorov, V.S.; Egorova, E.V.; Edemskaya, N.L.; Karpova, E.A.; et al. Educational Aid on Agricultural Chemistry, 2nd ed.; Publishing House of Lomonosov Moscow State University: Moskow, Russia, 2001; 688p. (In Russian) [Google Scholar]
- Dennis, F.G. Factors Affecting Yield in Apple, with Emphasis on ‘Delicious’. In Horticultural Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1979; pp. 395–422. [Google Scholar] [CrossRef]
- Kuzin, A.I.; Kashirskaya, N.Y.; Kochkina, A.M.; Kushner, A.V. Correction of Potassium Fertigation Rate of Apple Tree (Malus domestica Borkh.) in Central Russia during the Growing Season. Plants 2020, 9, 1366. [Google Scholar] [CrossRef]
- Guo, D.; Liu, H.; Zhou, L.; Xie, J.; He, C. Plasma-activated water production and its application in agriculture. J. Sci. Food Agric. 2021, 101, 4891–4899. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Calonego, J.C.; Foloni, J.S.S. Potassium Leaching from Millet Straw as Affected by Rainfall and Potassium Rates. Commun. Soil Sci. Plant Anal. 2005, 36, 1063–1074. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Viskelis, J.; Viskelis, P.; Liaudanskas, M.; Janulis, V. Changes in the Biochemical Composition and Physicochemical Properties of Apples Stored in Controlled Atmosphere Conditions. Appl. Sci. 2021, 11, 6215. [Google Scholar] [CrossRef]
- Rehman, A.; Deyuan, Z.; Hussain, I.; Iqbal, M.S.; Yang, Y.; Jingdong, L. Prediction of Major Agricultural Fruits Production in Pakistan by Using an Econometric Analysis and Machine Learning Technique. Int. J. Fruit Sci. 2018, 18, 445–461. [Google Scholar] [CrossRef]
- Johnson, D. Influence of phosphorus sprays on the storage quality of apples. Acta Hortic. 1980, 92, 327–328. [Google Scholar] [CrossRef]
- Gupta, N.; Jawandha, S.K.; Gill, P.S. Effect of calcium on cold storage and post-storage quality of peach. J. Food Sci. Technol. 2011, 48, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, J.; Zang, N.; Yin, Z.; Wang, A. Effects of calcium application on apple fruit softening during storage revealed by proteomics and phosphoproteomics. Hortic. Plant J. 2022, 8, 408–422. [Google Scholar] [CrossRef]
- Fallahi, E.; Conway, W.S.; Hickey, K.D.; Sams, C.E. The Role of Calcium and Nitrogen in Postharvest Quality and Disease Resistance of Apples. Hortscience 1997, 32, 831–835. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Kolik, L.V.; Danilejko, Y.K.; Belov, S.V.; Artem’Ev, K.V.; Astashev, M.E.; Pavlik, T.I.; Lukanin, V.I.; Kutyrev, A.I.; Smirnov, I.G.; et al. Enhancement of the Plant Grafting Technique with Dielectric Barrier Discharge Cold Atmospheric Plasma and Plasma-Treated Solution. Plants 2022, 11, 1373. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Adhikari, M.; Park, G. The Effects of Plasma on Plant Growth, Development, and Sustainability. Appl. Sci. 2020, 10, 6045. [Google Scholar] [CrossRef]
- Mujahid, Z.; Tounekti, T.; Khemira, H. Cold plasma treatment to release dormancy and improve growth in grape buds: A promising alternative to natural chilling and rest breaking chemicals. Sci. Rep. 2020, 10, 2667. [Google Scholar] [CrossRef] [PubMed]
- Danilejko, Y.K.; Belov, S.V.; Egorov, A.B.; Lukanin, V.I.; Sidorov, V.A.; Apasheva, L.M.; Dushkov, V.Y.; Budnik, M.I.; Belyakov, A.M.; Kulik, K.N.; et al. Increase of Productivity and Neutralization of Pathological Processes in Plants of Grain and Fruit Crops with the Help of Aqueous Solutions Activated by Plasma of High-Frequency Glow Discharge. Plants 2021, 10, 2161. [Google Scholar] [CrossRef]
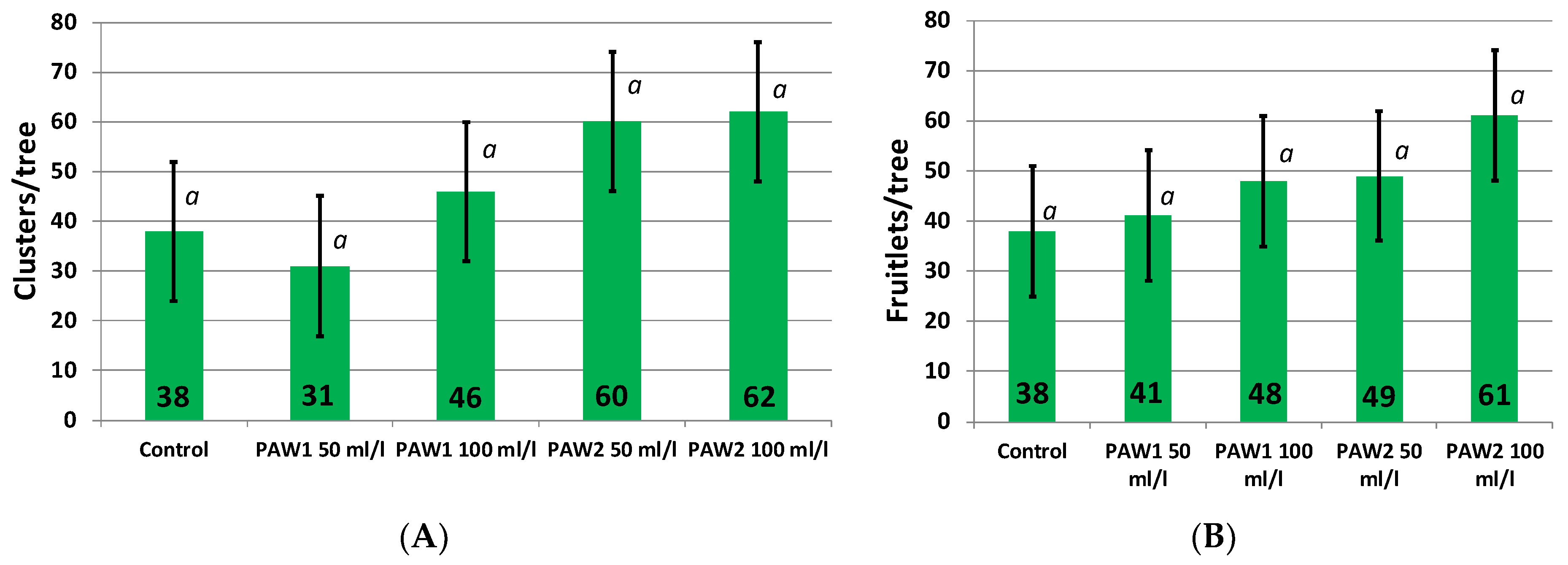
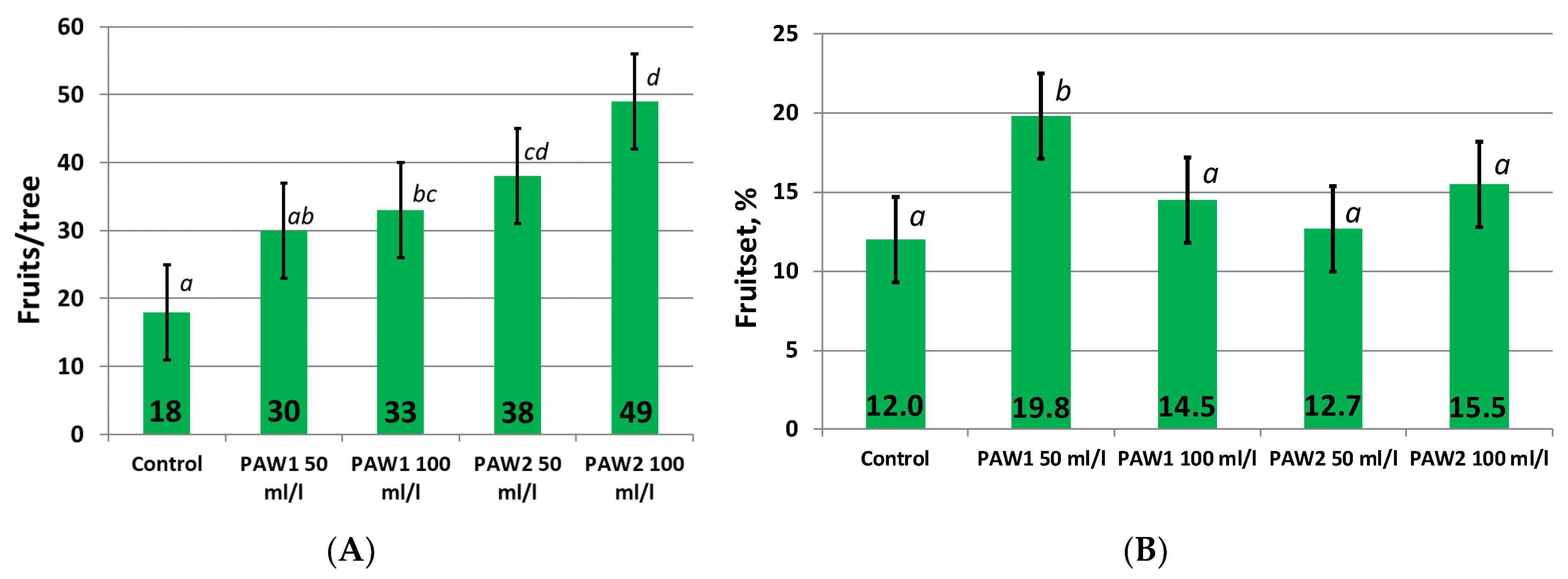
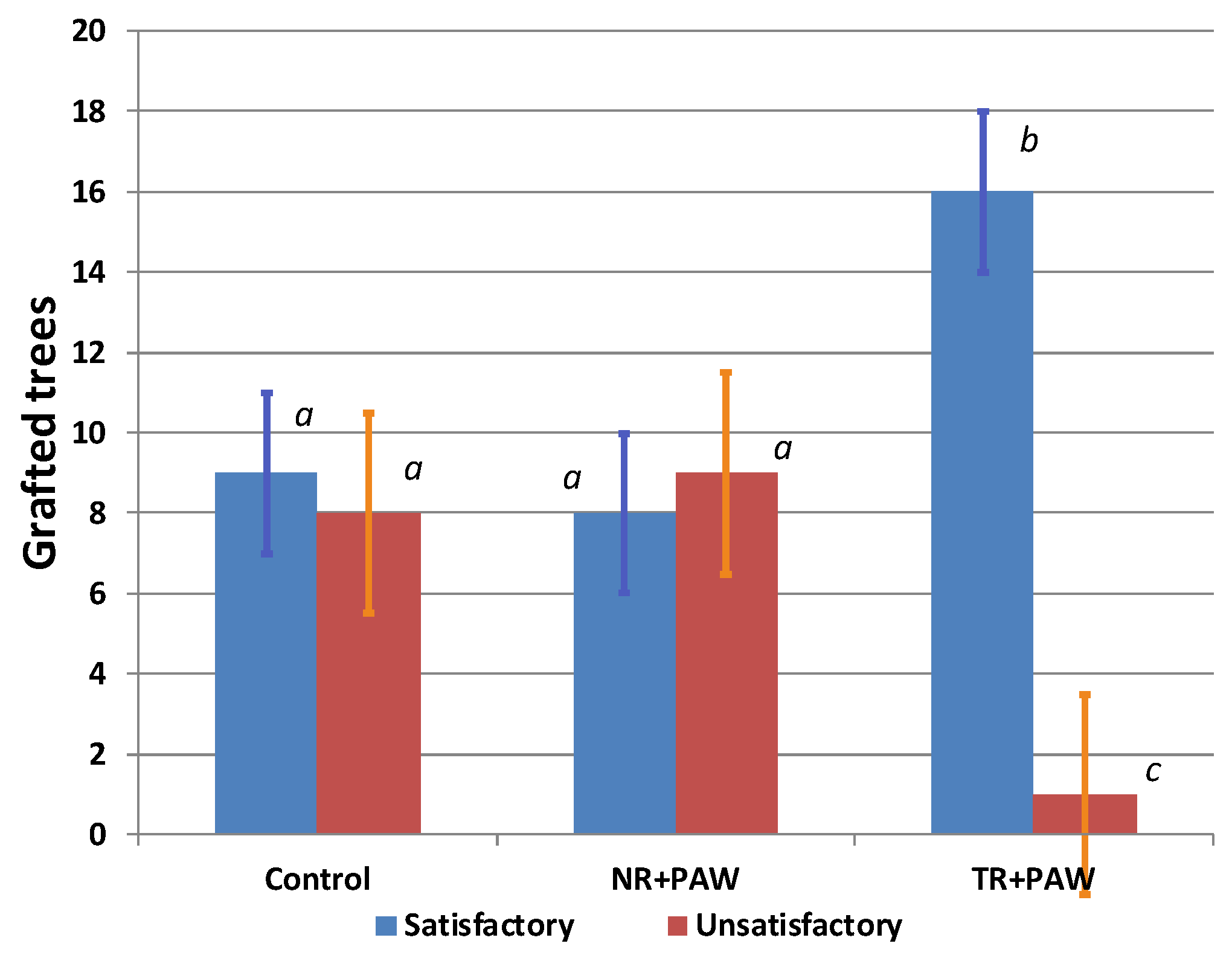
| Treatments | Fruit Average Mass, g | Yield, t/ha |
|---|---|---|
| Control 1 | 163.5 a,* | 7.2 a |
| PAW1 50 mL/L | 142.1 a | 7.6 a |
| PAW1 100 mL/L | 130.6 b | 8.1 a |
| PAW2 50 mL/L | 150.1 a | 10.9 b |
| PAW2 100 mL/L | 146.8 a | 13.9 b |
| LSD05 ** | 25.3 | 2.7 |
| Treatments | N | P | K | Ca |
|---|---|---|---|---|
| Control 1 | 1.49 a,* | 0.27 a | 0.89 a | 1.15 a |
| PAW1 50 mL/L | 2.13 b | 0.26 a | 0.83 a | 1.22 a |
| PAW1 100 mL/L | 1.27 a | 0.23 a | 0.87 a | 1.20 a |
| PAW2 50 mL/L | 2.12 b | 0.29 a | 1.05 b | 1.88 b |
| PAW2 100 mL/L | 1.61 a | 0.39 b | 1.24 b | 1.28 a |
| LSD05 ** | 0.44 | 0.09 | 0.13 | 0.35 |
| Treatments | N | P | K | Ca |
|---|---|---|---|---|
| Control 1 | 0,39 a,* | 0.082 a | 0.66 a | 0.0267 |
| PAW1 50 mL/L | 0.26 a | 0.08 a | 0.62 a | 0.0333 |
| PAW1 100 mL/L | 0.39 a | 0.176 b | 0.91 b | 0.0467 * |
| PAW2 50 mL/L | 0.39 a | 0.373 c | 0.70 a | 0.0667 * |
| PAW2 100 mL/L | 0.37 a | 0.229 d | 0.63 a | 0.0300 |
| LSD05 ** | 0.11 | 0.040 | 0.16 | 0.0096 |
| Treatments | Ascorbic Acid, mg 100 g−1 | Monosaccharides, % d.m. | Disaccharides, % d.m. | Titratable Acidity, % | Dry Mass (d.m.), % |
|---|---|---|---|---|---|
| Control 1 | 6.24 a,* | 6.93 a | 2.40 a | 1.01 a | 14.3 a |
| PAW1 50 mL/L | 7.38 b | 7.81 a | 1.52 b | 1.39 c | 16.2 b |
| PAW1 100 mL/L | 8.83 c | 7.44 a | 1.97 b | 1.16 b | 16.1 b |
| PAW2 50 mL/L | 6.89 a | 7.24 a | 1.48 b | 1.39 c | 15.6 b |
| PAW2 100 mL/L | 6.68 a | 7.24 a | 1.42 b | 0.94 a | 15.5 b |
| LSD05 ** | 1.09 | 0.97 | 0.41 | 0.08 | 0.9 |
| Treatments | Died Scions | Grafted Tree Development | ||
|---|---|---|---|---|
| Weak | Average | Good | ||
| Control | 3 a,* | 6 a | 3 a | 5 b |
| NR+PAW | 2 a | 6 a | 6 b | 3 a |
| TR+PAW | 5 b | 11 b | 1 a | 0 a |
| LSD05 ** | 2 | 3 | 2 | 1 |
| Treatments | Average Annual Shoot Elongation | Cumulative Annual Shoot Elongation |
|---|---|---|
| Control 2 | 85.6 a,* | 862.7 a |
| NR+PAW | 86.7 a | 880.7 a |
| TR+PAW | 80.5 b | 722.3 c |
| LSD05 ** | 5.5 | 76.7 |
| PAW Type | Exposure Time, min | Electrical Conductivity, mS/cm | pH | Redox, μV | NO3−, mM | H2O2, mM |
|---|---|---|---|---|---|---|
| PAW1 | 360 | 24.9 ± 1.2 | 8.3 ± 0.2 | 598 ± 26 | 22.05 ± 0.98 | 7.12 ± 0.68 |
| PAW2 | 240 | 14.0 ± 1.0 | 4.5 ± 0.2 | 560 ± 18 | 87.00 ± 5.00 | 0.11 ± 0.01 |
| Month | Temperature, °C | Precipitation, mm |
|---|---|---|
| April | 9.5 | 52.4 |
| May | 11.9 | 44.8 |
| June | 20.6 | 47.2 |
| July | 21.8 | 79.4 |
| August | 23.7 | 23.0 |
| September | 11.7 | 121.0 |
| October | 7.9 | 43.0 |
| Mean IV–X | 15.3 | 58.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzin, A.; Solovchenko, A.; Khort, D.; Filippov, R.; Lukanin, V.; Lukina, N.; Astashev, M.; Konchekov, E. Effects of Plasma-Activated Water on Leaf and Fruit Biochemical Composition and Scion Growth in Apple. Plants 2023, 12, 385. https://doi.org/10.3390/plants12020385
Kuzin A, Solovchenko A, Khort D, Filippov R, Lukanin V, Lukina N, Astashev M, Konchekov E. Effects of Plasma-Activated Water on Leaf and Fruit Biochemical Composition and Scion Growth in Apple. Plants. 2023; 12(2):385. https://doi.org/10.3390/plants12020385
Chicago/Turabian StyleKuzin, Andrei, Alexei Solovchenko, Dmitry Khort, Rostislav Filippov, Vladimir Lukanin, Natalya Lukina, Maxim Astashev, and Evgeny Konchekov. 2023. "Effects of Plasma-Activated Water on Leaf and Fruit Biochemical Composition and Scion Growth in Apple" Plants 12, no. 2: 385. https://doi.org/10.3390/plants12020385
APA StyleKuzin, A., Solovchenko, A., Khort, D., Filippov, R., Lukanin, V., Lukina, N., Astashev, M., & Konchekov, E. (2023). Effects of Plasma-Activated Water on Leaf and Fruit Biochemical Composition and Scion Growth in Apple. Plants, 12(2), 385. https://doi.org/10.3390/plants12020385







