Abstract
Fusarium spp. is a well-studied pathogen with the potential to infect cereals and reduce the yield to maximum if left unchecked. For decades, different control treatments have been tested against different Fusarium spp. and for reducing the mycotoxins they produce and are well documented. Some treatments also involved integrated pest management (IPM) strategies against Fusarium spp. control and mycotoxin degradation produced by them. In this review article, we compiled different control strategies against different Fusarium spp. In addition, special focus is given to the non-thermal plasma (NTP) technique used against Fusarium spp. inactivation. In a separate group, we compiled the literature about the use of NTP in the decontamination of mycotoxins produced by Fusarium spp., and highlighted the possible mechanisms of mycotoxin degradation by NTP. In this review, we concluded that although NTP is an effective treatment, it is a nice area and needs further research. The possibility of a prospective novel IPM strategy against Fusarium spp. is also proposed.
1. Introduction
One of the most important plant-pathogenic groups is Fusarium spp., causing different diseases in agricultural crops. Several devastating diseases such as seedling blight, root rot, and Fusarium crown rot are caused by Fusarium spp. [1]. Different species of Fusarium genera, such as F. graminearum, F. culmorum, F. poae, and F. avenaceum are economically important in Europe [1]. Disease development and mycotoxin production can be influenced by more than one Fusarium spp., which are often found to interact with each other [1,2,3].
Summerell [4] accessed the American Phytopathology Society website only to find that 83 out of 108 plant species, from the list of diseases on agricultural and horticultural crops, have more than one Fusarium disease impacting their production. One of the most important diseases is the Fusarium head blight (FHB) caused predominantly by Fusarium graminearum. Fusarium head blight is a global concern due to its severe impact on grain quality and yield [5], which still needs considerable attention for its devastating effects. Buerstmayr et al. [5] stated in their review that an estimated loss of approximately USD 2.5 billion was attributable to FHB on wheat and barley for the period 1993–2001.
This genus of pathogenic fungi is known not only in agriculture but also in the food industry. There are toxic compounds produced by certain fungi called mycotoxins. The mycotoxins produced by Fusarium spp. that are most studied majorly belong to Trichothecenes (Deoxynivalenol (DON), T-2 and HT-2), Zearalenone (ZEN), and Fumonisins [6,7]. In Europe, Fumagalli [8] stated in their review that the World Mycotoxin Survey, published in 2020, showed that mycotoxin risk was found to be high to severe compared to previous years, with deoxynivalenol (DON) found to be the biggest threat. Fusarium spp. produces deoxynivalenol mycotoxin (predominantly by F. graminearum and F. culmorum), which infects several cereals and can cause yield losses of up to 50% [9]. It has been found that DON mycotoxin can cause vomiting, digestive disorders, oxidative damage, and reproductive toxicities in animals and humans when ingested [7]. Deoxynivalenol also has a toxicological impact on poultry and animal health [10]. Co-contamination of these mycotoxins is also on the rise, while work on identifying new compounds is still ongoing [7]. Due to these reasons, the World Health Organization (WHO) and Food and Agriculture Organization (FAO) have declared a high-priority on mycotoxins [8]. Realizing both that Fusarium spp. is a threat to the agriculture sector and that the toxic effects of mycotoxins are produced by these fungi, there are different ongoing control measures, and some researched in the past against both inactivations of Fusarium spp. and mycotoxin decontamination. However, concerning chemical control, unfortunately, Fusarium spp. is becoming resistant to some chemical fungicides due to their indiscriminate and long-term use [3]. Therefore, it is essential to search for novel, non-chemical control strategies against Fusarium spp. inactivation and mycotoxin decontamination.
One such physical control method, known as the non-thermal plasma/cold plasma (used interchangeably) technique, has started showing promising results in both Fusarium spp. inactivation and mycotoxin decontamination. Physical plasma is the fourth state of matter. It is a partially or fully ionized quasi-neutral substance that is made up of electrons, ions, neutral particles, molecules in the ground or excited state, radical species, and quanta of electromagnetic radiation (UV photons and visible light) [11,12]. These particles exhibit collective behavior. Since this review does not include thermal plasma, only non-thermal plasma (NTP) and its applications are discussed hereafter.
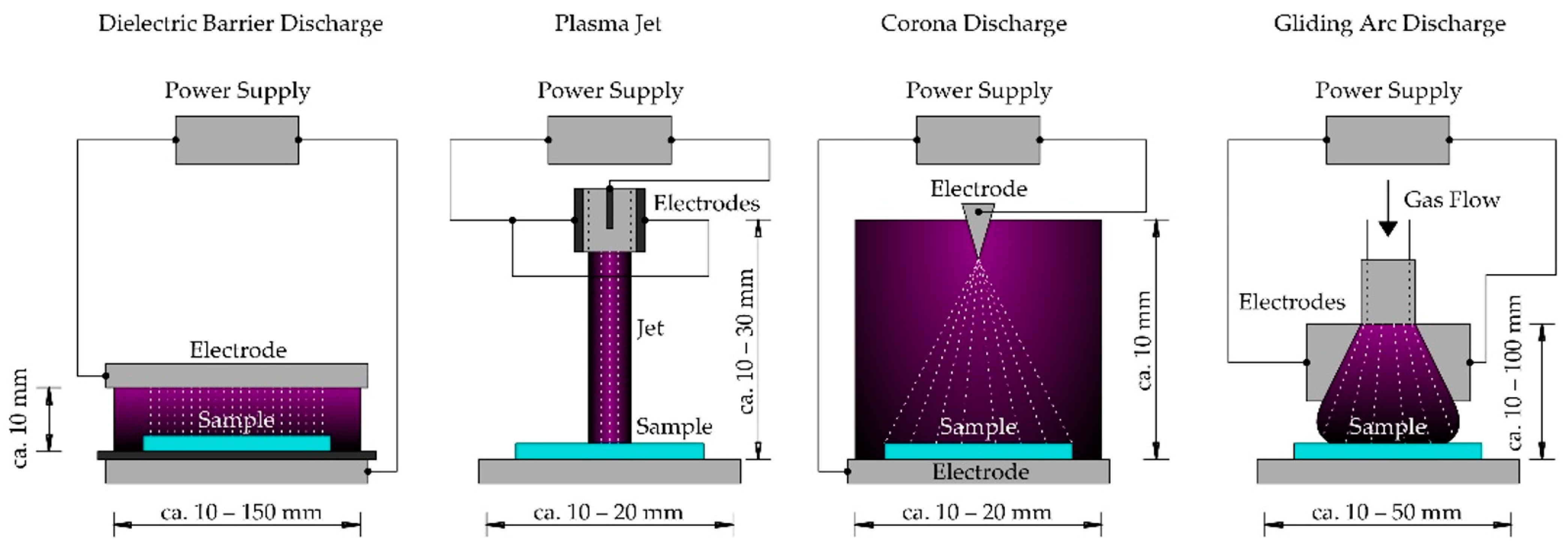
A partially ionized gas with electron temperatures higher than ion temperatures will produce NTP [11]. Non-thermal plasma is gaining attention in various fields due to its ability to generate radicals and reactive species [13]. There are different types of NTP-generating devices, some of which are presented in Figure 1. The descriptions of the NTP devices are taken and explained from Domonkos et al. [12].
Figure 1.
Types of different non-thermal plasma generating devices (adapted from Domonkos et al. [12]).
All the NTP devices in Figure 1 use working gas such as air, oxygen, nitrogen, inert gases (Argon, Helium), and their mixtures. The first is the Dielectric Discharge Barrier which comprises two electrodes, one of which is covered with dielectric material (such as quartz, glass, ceramics, enamel, silicon, rubber, teflon, mica, or plastic) and separated by an insulating dielectric barrier. Voltage is supplied to these electrodes to generate plasma. The sample is kept between the two electrodes.
The second is Plasma Jet. It consists of two concentric cylindrical electrodes. The inner electrode is connected to the power supply, which causes the ionization of the working gas. It produces a high-velocity stream of highly reactive chemical species with weak emitted light and shoots down in the form of a jet onto the sample.
In corona discharge, as the name suggests, the ionized gas creates a crown around the active electrode. Two or more electrodes are attached to the high voltage, and the coronizing electrode is generally in the form of a needle or thin wire.
Gliding arc discharge produces cold plasma, but only under specific conditions. The electrodes are placed in a fast gas flow, which, when supplied with high voltage, discharges the voltages with increased volume and length in the flow direction. The plasma can be applied directly or indirectly on to the target object.
NTP has already shown promising results in the fields of medicine [14,15,16,17,18], in the textile industry [19,20,21], in forestry [22,23], in the food industry [24,25,26], and in the food preservation industry [27,28,29,30]. With so much knowledge about NTP and its established diverse applications, the focus has also shifted towards the agriculture sector (known as ‘plasma agriculture’ [31]) in recent years, as there are calls for new and clean technologies to replace chemical pesticides that are growing around the world. Several research articles have demonstrated that NTP can potentially improve seed germination and crop yield [32,33,34,35,36]. Although it is evident that NTP undoubtedly works well in the enhancement of different crop traits, we shift our focus onto the main topic of this review which is the effective use of NTP in plant protection, especially against Fusarium spp.
Fusarium spp. is one of the infamous plant pathogens that ranks in the list of the top 10 fungal pathogens in molecular plant pathology (Dean et al. [37]) and their derived mycotoxins. Therefore, in this article, (a) the application of NTP against Fusarium spp. inactivation was explored and reviewed, and (b) the decontamination/degradation of mycotoxins produced by Fusarium spp. was also reviewed. The possible underlying mechanisms for both were highlighted and discussed. Given that NTP is a fairly new technique in the agriculture sector, we also propose a combination of NTP and biological control as a possible IPM strategy that may offer better protection against Fusarium spp. and other pathogens, only after conducting extensive research to test their synergy.
2. Results
2.1. Effect of NTP/Cold Plasma on Inactivation of Fusarium spp. (Group 1)
Below in Table 1, we list the articles displaying the success and failures of different plasma techniques against the inactivation of Fusarium spp. spores and mycelium.

Table 1.
Summary of the research on “Non-thermal plasma”/“cold plasma” against inactivation of Fusarium spp. spores or mycelium.
Homa et al. [38] explained the importance of sweet basil as a culinary herb. In addition, they discussed two different diseases, namely basil downy mildew (BDM) caused by Peronospora belbahrii and Fusarium wilt of basil (FOB) caused by Fusarium oxysporum f. sp. basilici, which causes significant damage to this crop. In search of new options to control the FOB disease, they tested cold plasma treatment against FOB mycelium on sweet basil plants and studied seed treatment. In the laboratory test, FOB plugs were treated for a period of 5, 10, and 15 min, while non-treated FOB plugs were used as the control. They found no significant difference in mean mycelial growth when cold plasma jet treatment on mycelium was performed. In the plants experiment, the six-leaf staged plants were inoculated with FOB 1 day before or after the cold plasma jet treatment. They found that prior treatment with cold plasma jet resulted in less stunting of plants. For the seed treatment, the seeds were inoculated with talc/chlamydospore mixture, followed by cold plasma dielectric barrier discharge treatment for 1, 5, 10, or 15 min. They found that as the time increased, the colonies on the seeds decreased, with the maximum reduction seen at 10 and 15 min post-treatment. Subsequent papers also concerned Fusarium oxysporum, where similar results were obtained by Panngom et al. [39] when they tested the effect of non-thermal plasma against F. oxysporum f.sp. lycopersici in a susceptible tomato variety. They not only found that the NTP germination of spores decreased over time after exposure to argon (Ar) plasma for 10 min, but also increased the transcription of pathogenesis related genes, concluding that NTP can also be used to up-regulate resistance mechanisms. This was also confirmed in another study conducted by Go et al. [40], where they found complete inhibition of mycelial growth, spore germination, and up-regulation of membrane-related gene (SHO1). The disinfection of seeds is not limited to cereals or crops but is also used in forestry, as demonstrated by Swiecimska et al. [22] when they treated scots pine seeds against F. oxysporum with non-thermal plasma for different periods viz. 1 s, 3 s, 5 s, 10 s, 15 s, 20 s, 30 s, and 60 s. They found that 3 s of treatment was the optimal time to disinfect the seeds.
Different plasma techniques were tested on inactivating F. culmorum or F. nivale. Zahoranová et al. [41] tested the effect of cold atmospheric pressure plasma on different fungal species, namely Fusarium nivale, F. culmorum, Trichothecium roseum, Aspergillus flavus, A. clavatus, which were used to contaminate wheat seeds artificially. They found that the efficacy of cold atmospheric pressure plasma was greatest on F. nivale and the least on A. clavatus. Zahoranová et al. [42] also investigated the effect of cold atmospheric pressure plasma on native microbiota and three pathogens (Aspergillus flavus, Alternaria alternata and F. culmorum) on the maize surface, and also on germination and growth parameters. They found that after 60 s of treatment, the native microbiota was completely devitalized and F. culmorum was reduced, while A. flavus and A. alternata were reduced after 300 s treatment. The study by Hoppanová et al. [43] also supports the argument that low-temperature plasma inhibited the growth of F. culmorum on the seed surface and, when combined with fungicide, complete inhibition of the fungus was achieved.
Bousba et al. [44] tested a mixture of different plasma working gas (He + air, He + O2, and He + N2O) for the decontamination of fungus in polluted water. They found that different plasma gas mixtures prevented the growth of Fusarium pseudograminearum at different time intervals, and that the combination of He + N2O took the least time (5 min). In cereals, Wang et al. [45] effectively inactivated four major Fusarium graminearum strains (2 to 6 log10 reduction) under in vitro and in vivo conditions using cold atmospheric plasma. Under in vivo conditions, too, cold atmospheric plasma reduced the pathogenicity of F. graminearum.
Similar results were obtained by Chang et al. [46] where they tested a different plasma treatment, namely corona discharge air plasma (CDAP), which consists of nitric oxide and nitric dioxide, on the decontaminating microbes on onion. They found that the isolation frequency of Fusarium spp. was less as compared to Alternaria spp. and Botrytis spp. Additionally, different treatment concentrations showed different efficiencies. In the case of Alternaria spp., 2~2.6 ppm of O3 slightly stimulated the mycelial growth, whereas 20~24 ppm of O3 gradually inhibited the fungus. Botrytis spp., on the other hand, showed different time-dependent results. Slight inhibition was seen in Botrytis spp. with a treatment of less than 4 h, while the 8 h of treatment promoted growth, irrespective of O3 concentration. The conidial germination of both the fungi viz. Alternaria and Botrytis spp. was strongly inhibited at 13.7~14.4 ppm of O3. Sandanuwan et al. [47] tested the cold plasma technique against different fungal pathogens of the Cavendish banana fruit. They successfully decreased the percentage disease index (PDI) of Colletotrichum musae, Fusarium semitectum, and Colletotrichum gloeosporioides using cold plasma compared to control and also fungicide treatments. Meanwhile, in pine seeds, Šerá et al. [23] demonstrated that pine seeds contaminated with Fusarium circinatum were completely disinfected using non-thermal plasma technique after 5, 10, 60, 180, and 300 s and the inoculated seeds remained microbe-free for 12 days after 60 s plasma treatment, indicating that seeds can be kept viable with this treatment.
2.2. Effect of Non-Thermal Plasma/Cold Plasma on Degradation/Decontamination of DON Mycotoxin (Group 2)
Below, in Table 2, we list the articles displaying the success of different plasma techniques used to decontaminate Fusarium spp. mycotoxin and mycotoxins from other plant pathogens.

Table 2.
List of the conducted research on “non-thermal plasma”/“cold plasma” in degradation/decontamination of Deoxynivalenol (DON) mycotoxin produced by Fusarium spp.
Ten Bosch et al. [48] tested cold atmospheric pressure plasma on mycotoxin degradation by different microorganisms. The study selected DON, zearalenone, enniatins, fumonisin B1, and T2 toxin produced by Fusarium spp., sterigmatocystin produced by Aspergillus spp., and AAL toxin produced by Alternaria alternata. While they found that 60 s exposure to CAPP resulted in complete degradation of pure mycotoxin, sterigmatocystin offered the highest resistance. They concluded that CAPP is an efficient technique to degrade mycotoxin and the degradation rates may vary due to the mycotoxin structure. The argument is also supported by Abbasian et al. [49], as they succeeded in degrading DON mycotoxin from Fusarium spp. using Argon plasma jet, concluding that certain conditions, such as time and concentration of mycotoxin in food, are required for the plasma treatment to work efficiently.
Wang et al. [45] also investigated the effects of cold atmospheric plasma against DON mycotoxin and also found that the treatment inhibited DON biosynthesis in vitro. Guo et al. [50] tested the effect of cold plasma against DON and ochratoxin A (OTA) mycotoxins. They found that 8 min treatment with cold plasma significantly reduced both DON and OTA mycotoxin. Further they found that mycotoxin reduction was directly proportional to increase in cold plasma treatment time. Qiu et al. [51] investigated the effect of plasma-activated water (PAW) on wheat contaminated with DON mycotoxin. They found a significant decrease in the DON mycotoxin; the number of bacterial, fungal counts, and surviving F. graminearum in wheat was significantly reduced. Other mycotoxins such as T-2 and HT-2 from Fusarium spp. were also tested with the atmospheric cold plasma technique [52]. They artificially spiked the wheat grains with the mycotoxins and were treated with the cold atmospheric plasma for 10 min. The treatment reduced the pure T-2 by 63.63%, while HT-2 concentrations reduced by 51.5%, the spiked T-2 concentration was reduced by 79.8%, and the HT-2 by 70.4%.
3. Discussion
3.1. Effect of NTP/Cold Plasma on Inactivation of Fusarium spp. (Group 1)
The unique quality of NTP is the working gas used either individually or in combination with other gases, according to the study and apparatus. The studies reviewed, in the results, whether the plasma conditions were the same or different, and whether they had the same outcomes of inactivating Fusarium spp. and other fungi in their respective study.
It is evident that NTP has potential plant protection properties and is effective against Fusarium spp. and other studied fungi. For instance, Rüntzel et al. [53] reported that cold plasma treatment of 10–30 min effectively inactivated the fungi (Aspergillus spp. and Penicillium spp.) from the surface of black beans (Phaseolus vulgaris L.). Similar results were confirmed by using plasma-activated water on the inactivation of Penicillium italicum in kumquat [54]. Ahmad et al. [55] tested two plasma treatments viz plasma-activated water and plasma activated H2O2 solution on the spores and mycelium of Colletotrichum gloeosporioides which causes anthracnose disease in pepper (Capsicum annuum L.) seeds. They found that both treatments effectively inhibited the spore and mycelium of C. gloeosporioides. This result is in line with the study conducted [47]. Not only fungi but aerobic bacteria, yeasts, and molds were also inactivated using a corona discharge plasma jet, where 5 min of treatment saw 1.0 log reduction [56]. They also stated that their susceptibility to plasma treatment varied due to different structure and chemical composition of microbes.
It is also crucial to understand the different underlying mechanisms of fungal inactivation caused by NTP, irrespective of the treatment conditions and apparatus used in the respective studies. Several possible theories, such as from Go et al. [40], observed that fungal spores treated with the plasma showed severe structural changes and were crushed and shrunk. They stated that this change was due to the reaction with the active species formed during the plasma process (see [39,40,57]), and further explained in detail that membrane lipids of the microorganism are affected by the reactive species in plasma and that, additionally, the oxidation of amino acids and nucleic acids is detrimental. Supporting this argument, Wang et al. [45] stated that the mode of inactivation was due to the destruction of the cell membrane, accumulation of intracellular ROS, and depolarization of the mitochondrial membrane. Other studies also mentioned cytoplasmic leakage as one of the possible mechanisms of inactivating plant pathogens [58,59]. We hypothesize that Fusarium spp. spores may interact with the reactive species from the plasma gas, and disruption in the spores may lead to cytoplasmic leakage, which needs to be investigated for confirmation in the future.
Homa et al. [38] argue that pathogen deactivation depends on the number of factors, such as the host to be treated, the pathogen(s), the type of cold plasma system, and the degree of exposure of the cold plasma on the host organism. With respect to the cold plasma system, in our review, the most common gas supply was seen to be air or atmospheric plasma.
Scholtz et al. [31] mentioned that it is difficult to compare the results of Shaw et al. [60] and Khun et al. [61] as they use two different plasma treatment conditions (such as gas supply, voltage, current, power, frequency, etc.) on the same reference microbe. It is important to address that studies using similar or the same plasma treatment conditions, for instance, regarding this review, the studies conducted by Swiecimska et al. [22], Zahoranová et al. [41], Zahoranová et al. [42], and Hoppanová et al. [43] can easily be reproduced, compared, and concluded.
Additional biological factors mentioned by Adhikari et al. [62], such as the genus and species of the plant, the microenvironment of the plant–pathogen system, the species and strain of the pathogen, the structure of the cellular envelopes, and the microbial growth phase, could also possibly affect the pathogen deactivation and need to be carefully considered in the future studies to confirm if they have any influence on the outcome.
3.2. Effect of NTP/Cold Plasma on Degradation/Decontamination of DON Mycotoxin (Group 2)
From the results interpreted, NTP is found to successfully degrade mycotoxins, especially DON produced by Fusarium spp., and mycotoxins from other phytopathogens from different studies. Zhang et al. [63] subjected DON mycotoxin solution to optimized conditions of double dielectric barrier discharge, only to find degradation of 98.94% within 25 min of plasma treatment. Ott et al. [64] also confirmed the degradation of more than 99% of 100 μg DON mycotoxin in aqueous suspensions after 21 min of direct high voltage atmospheric cold plasma treatment, using air to generate reactive oxygen and reactive nitrogen species. They also reported a much lower degradation (33%) of 100 μg DON mycotoxin in powdered form. Janić Hajnal et al. [65] tested atmospheric cold plasma treatment against decontamination of Alternaria toxins (alternariol (AOH), alternariol monomethyl ether (AME), and tentoxin (TEN)) content in wheat flour. They artificially spiked the wheat flour with these toxins and subjected it to cold atmospheric plasma treatment. After 180 s and with treatment performed at 6 mm from the plasma source, the best results were obtained with reductions of 60.6%, 73.8%, and 54.5% for AOH, AME, and TEN, respectively. With such growing evidence, it can be safely said that NTP could have the edge over thermal treatment. To support this argument, Varilla et al. [66] argued that thermal treatment, such as cooking and pasteurization, cannot be a reliable solution for mycotoxin decontamination as some of the mycotoxins are resistant to thermal treatment.
Very few studies have been dedicated to understand the possible mechanisms of how non-thermal plasma degrade mycotoxins. One possible simple mechanism is that the degradation is caused by some energetic particles. For example, ten Bosch et al. [48] found only 60 s is enough for almost complete degradation of many studied mycotoxins. The temperature of the gas and substrate during NTP treatment is usually lower than 60 °C. At such temperatures, even sensitive proteins do not degrade, nevertheless, 60 s was quite sufficient for mycotoxin degradation. They further argued that the mycotoxin type and the matrix greatly influenced the inactivation efficacy of the plasma treatment. In relation to this argument, it is important to understand that different mycotoxins have different chemical structures and may undergo multistep degradation [52,67]. One of the possible reasons also stated by ten Bosch et al. [48] is the reactive species generated in the plasma. Under continuous voltage and due to chemical reactions in the plasma, reactive species such as O, O3, OH, NOX, when they interact with the pure compounds of mycotoxins, lead to the fragmentation of molecular bonds, which further leads to the production of volatile compounds (Iqdiam et al. [52]) that are known to be less toxic [67]. This argument is further supported by Qiu et al. [51] using PAW, where they found that the DON degradation rate was directly proportional to an increase in exposure time, as there was an increase in the concentration of reactive species such as long-lived particles (H2O2, O3, H+, NO2−, NO3−) and short-lived particles (OH, O2−, NO, and ONOOH). Gavahian and Cullen [68] also proposed that several properties of plasma, such as the concentration of oxygen, hydroxyl radicals, the presence of photons, and ultraviolet radiation, could affect mycotoxin degradation using plasma treatment.
Another possibility is from the study of Wang et al. [45]. They found that mycotoxin degradation using cold atmospheric plasma is achieved through reduced acetyl-CoA production, toxisome formation, and key trichothecene biosynthetic gene (TRI) expression, and in vivo by inactivation of fungal spores, thereby reducing DON production.
The mechanism of mycotoxin degradation using NTP in vivo, as proposed by Gavahian and Cullen [68], can be attributed to the principle of “killing two birds with one stone”. They proposed that the reactive species produced by plasma alters the cell membrane and cell walls to release cytoplasm leading to cell inactivation, which does not allow the fungi to produce mycotoxin. Additionally, the plasma reacts with the fungal cell on multiple sites, resulting in loss of functions and eventually apoptosis [69], which Lee et al. [70] support in their study while investigating the effect of plasma on the spores of Cordyceps bassiana.
There have been relatively few articles on the selected topic which are useful. NTP is a very progressive perspective direction in agriculture and plant protection when it comes to finding chemical-free alternatives. Nevertheless, NTP has several limitations. For instance, Šimončicová et al. [71] mentioned that high investment cost, maintenance, and servicing cost are limiting the use of cold atmospheric plasma. Further, up-scaling NTP to the industrial level for decontamination is still far from realization [68].
Another challenge with NTP is the treatment of the uneven surface. Gavahian and Cullen [68] argued that NTP being a surface treatment may not be effective against irregularly shaped or bulky food material. There is a high chance that fungal spores or mycelium may not get treated in irregularly-shaped seeds when treated with NTP, leaving them in the infective state.
There have been some reports that state that NTP has a potential negative impact on food lipids [68]. The studies of Varilla et al. [66] and Jadhav and Annapure [72] confirmed the above argument in their respective studies, as they found that NTP induces lipid oxidation in the meat tissues and fish, thereby turning them inedible. Apart from lipids, oligosaccharides found in juices are also degraded by ozonolysis, triggered by cold plasma treatment [73].
Many research shows no or minimal impact on physical and chemical attributes of some food products. Possible negative effects could be found in other food products entering the human food chain. Such food products need further attention, and research to optimize NTP conditions may be needed. This means there need to be specific guidelines for using NTP treatment on specific food products to enhance their quality. In this way, a compilation of food products with similar or the same treatment can be performed, which can help to upscale the results to an industrial level in the future.
In agriculture, especially in the case of microbial inactivation and/or mycotoxin degradation, perhaps, in the future, a combined strategy of NTP and biological control could be tested as a part of the Integrated Pest Management strategy. We recommend investigating the effects of NTP against different biological control agents/biocontrol agents under laboratory and greenhouse conditions. This will help us to identify if there is a synergistic or antagonistic relationship between the two treatments. Different gases can also be tested to understand the direct effect of gases on these biocontrol agents. These results can be further tested under greenhouse conditions to understand their interaction and efficacies before testing them in the field trials.
4. Materials and Methods
The methodology of the work is based on the analysis and subsequent synthesis of literary sources. We searched the literature available on the Web of Science Core collection database. For convenience, we divided the methodology into two groups viz. Effect of NTP/Cold plasma on inactivation of Fusarium spp. (Group 1) and Effect of NTP/cold plasma on degradation/decontamination of DON mycotoxin (Group 2).
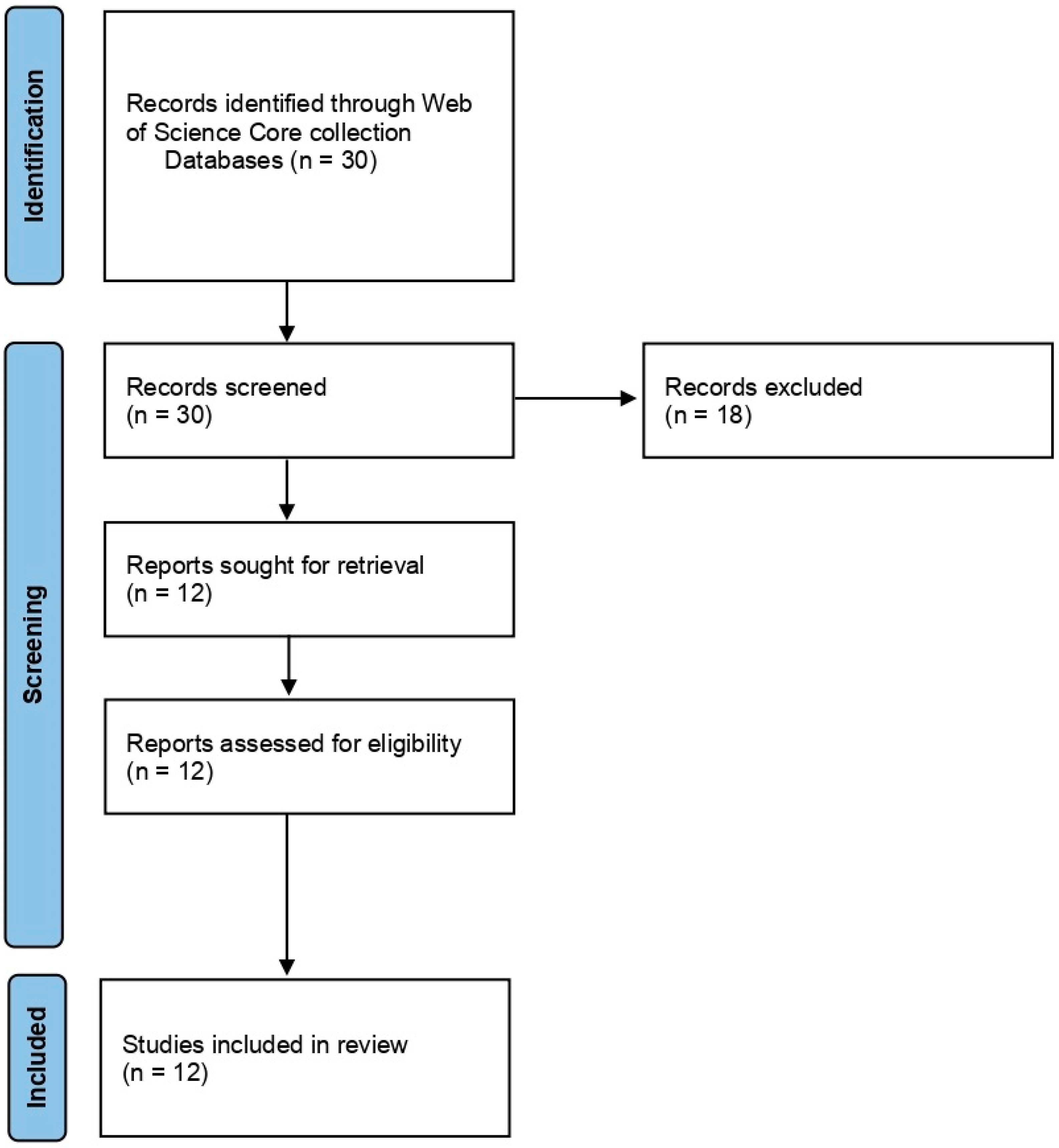
For Group 1, we searched using the terms ‘cold plasma’ and ‘Fusarium’. The search displayed a total of 30 articles, and the results gave both inactivation of spores and mycelium of Fusarium spp. and other fungal species. We filtered the search by excluding the review articles, as we wanted to use them for introduction and discussion, and by excluding the other fungi except for Fusarium spp. We also excluded the results that displayed mycotoxin. Eventually, we selected 12 articles and used them for the systematic review. The articles were selected and reviewed after following PRISMA statement guidelines [74] (Figure 2).
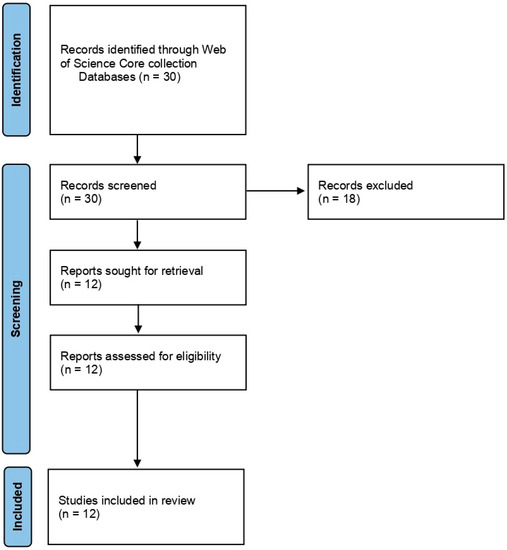
Figure 2.
PRISMA flowchart summarizing the information gathering sequence and selection for the systematic review of Group 1: Effect of NTP/Cold plasma on inactivation of Fusarium spp.
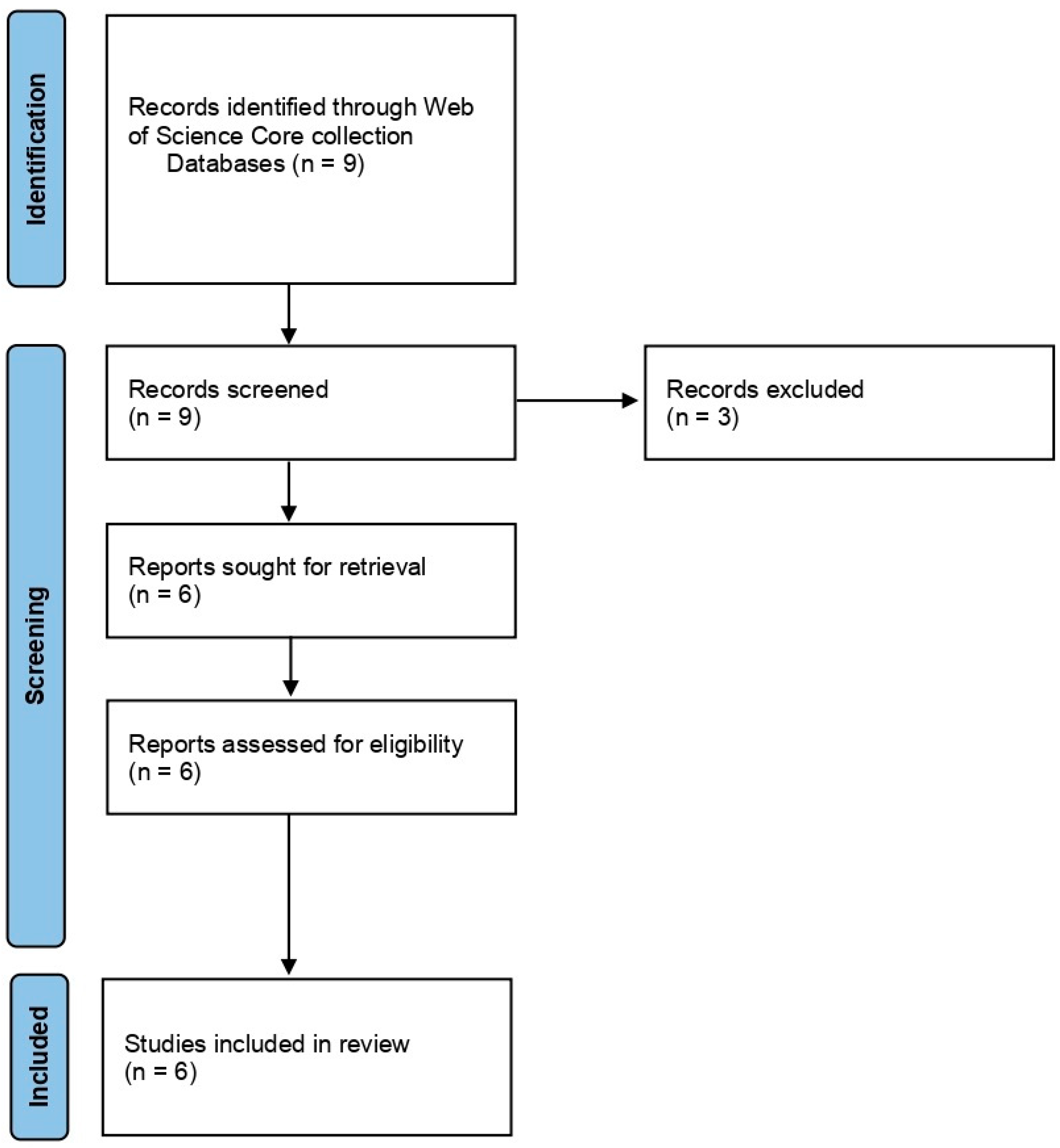
In the case of Group 2, Effect of NTP/cold plasma on degradation/decontamination of DON mycotoxin, we searched the Web of Science core collection database by using the terms ‘cold plasma’ AND ‘Fusarium’ AND ‘deoxynivalenol’. The search returned with nine articles of which we excluded the three review articles. The remaining six articles were considered and used for the systematic review. The articles were selected and reviewed following PRISMA statement guidelines (Page et al., 2021) [74] (Figure 3).
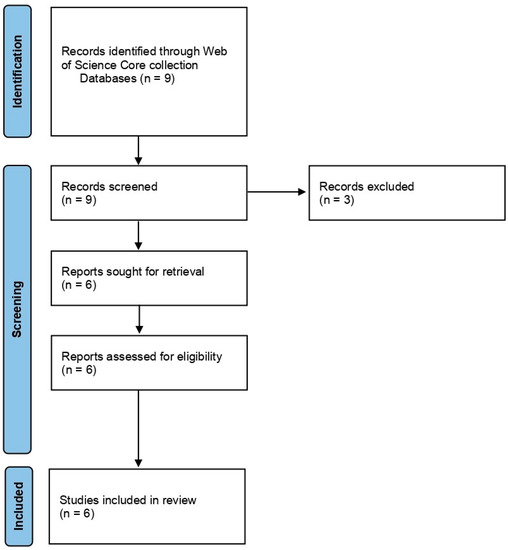
Figure 3.
PRISMA flowchart summarizing the sequence of information gathering and selection for the systematic review of Group 2: Effect of NTP/cold plasma on degradation/decontamination of DON mycotoxin.
5. Conclusions
NTP is undoubtedly one of the most innovative physical control agents of plant pathogens and mycotoxin decontamination. This article attempts to provide a focused view on the use of NTP against the inactivation of Fusarium spp. and other studied plant pathogens, and the degradation of mycotoxins produced by Fusarium spp. and other fungal species. The presented overview shows that rather than the apparatus per se, the plasma treatment conditions such as gas used, voltage, power, and treatment time are the most important conditions that can be used for comparative analysis to study its efficiency. Although there are several possible explanations of the mechanisms of fungal inactivation and mycotoxin degradation, it is still a niche area that needs thorough research and understanding. Additionally, it will be interesting to test NTP and biological control under the Integrated Pest Management framework to find if there is a possibility to achieve synergy that could replace the traditional practices of chemical treatment, and ensure sustainable agriculture in the future.
Author Contributions
Conceptualization, P.D. and B.Š.; methodology, P.D.; resources, P.D. and B.Š.; writing—original draft preparation, P.D.; writing—review and editing, B.Š.; supervision, B.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Comenius University Bratislava project “Synergy Effect of Biological Control and Non-Thermal Plasma Treatment against Fusarium Head Blight in Winter Wheat”.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight from a Microbiome Perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Pathogenomics and Management of Fusarium Diseases in Plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Summerell, B.A. Resolving Fusarium: Current Status of the Genus. Annu. Rev. Phytopathol. 2019, 57, 323–339. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Steiner, B.; Buerstmayr, H. Breeding for Fusarium head blight resistance in wheat—Progress and challenges. Plant Breed. 2020, 139, 429–454. [Google Scholar] [CrossRef]
- D’Mello, J.P.F.; Placinta, C.M.; Macdonald, A.M.C. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Fumagalli, F.; Ottoboni, M.; Pinotti, L.; Cheli, F. Integrated Mycotoxin Management System in the Feed Supply Chain: Innovative Approaches. Toxins 2021, 13, 572. [Google Scholar] [CrossRef]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Gautier, C.; Pinson-Gadais, L.; Richard-Forget, F. Fusarium Mycotoxins Enniatins: An Updated Review of Their Occurrence, the Producing Fusarium Species, and the Abiotic Determinants of Their Accumulation in Crop Harvests. J. Agric. Food Chem. 2020, 68, 4788–4798. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Verschuren, J.; De Clerck, K.; Kiekens, P.; Leys, C. Non-thermal plasma treatment of textiles. Surf. Coat. Technol. 2008, 202, 3427–3449. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine. Agric. Food Ind. Appl. Sci. 2021, 11, 4809. [Google Scholar]
- Du, C.; Yan, J. Surface Sterilization by Atmospheric Pressure Non-thermal Plasma. In Plasma Remediation Technology for Environmental Protection; Du, C., Yan, J., Eds.; Springer: Singapore, 2017; pp. 61–73. [Google Scholar]
- Laskowska, M.; Boguslawska-Was, E.; Kowal, P.; Holub, M.; Dabrowski, W. Efficiency of using non-thermal plasma in microbiology and medicine. Postepy Mikrobiologii 2016, 55, 172–181. [Google Scholar]
- Tanaka, H.; Hori, M. Medical applications of non-thermal atmospheric pressure plasma. J. Clin. Biochem. Nutri. 2017, 60, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Kyzek, S.; Uhrin, F.; Holubová, L.; Tomeková, J.; Ďurovcová, I.; Špačková, J.; Ševčovičová, A.; Gálová, E. Potential Use of Non-thermal Plasma in Oncotherapy. Chemicke Listy 2019, 113, 500–504. [Google Scholar]
- Kajiyama, H.; Utsumi, F.; Nakamura, K.; Tanaka, H.; Toyokuni, S.; Hori, M.; Kikkawa, F. Future perspective of strategic non-thermal plasma therapy for cancer treatment. J. Clin. Biochem. Nutr. 2017, 60, 33–38. [Google Scholar] [CrossRef]
- Gonzalez-Mendoza, B.; Lopez-Callejas, R.; Rodriguez-Mendez, B.G.; Eguiluz, R.P.; Mercado-Cabrera, A.; Valencia-Alvarado, R.; Betancourt-Angeles, M.; Reyes-Frias, M.D.; Reboyo-Barrios, D.; Chavez-Aguilar, E. Healing of wounds in lower extremities employing a non-thermal plasma. Clin. Plasma Med. 2019, 16, 100094. [Google Scholar] [CrossRef]
- Saleem, M.; Naz, M.Y.; Shoukat, B.; Shukrullah, S.; Hussain, Z. Functionality and applications of non-thermal plasma activated textiles: A review. In Proceedings of the 4th International Conference on Materials Science and NanoTechnology (MSNANO), Faisalabad, Pakistan, 3–5 March 2020. [Google Scholar]
- Pandiyaraj, K.N.; Vasu, D.; Ramkumar, M.C.; Deshmukh, R.R.; Ghobeira, R. Improved degradation of textile effluents via the synergetic effects of Cu-CeO2 catalysis and non-thermal atmospheric pressure plasma treatment. Sep. Purif. Technol. 2021, 258, 118037. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Palma, V. Enhanced azo dye removal in aqueous solution by H2O2 assisted non-thermal plasma technology. Environ. Technol. Innov. 2020, 19, 100969. [Google Scholar] [CrossRef]
- Świecimska, M.; Tulik, M.; Šerá, B.; Golińska, P.; Tomeková, J.; Medvecká, V.; Bujdáková, H.; Oszako, T.; Zahoranová, A.; Šerý, M. Non-Thermal Plasma Can Be Used in Disinfection of Scots Pine (Pinus sylvestris L.). Seeds Infected Fusarium Oxysporum. For. 2020, 11, 837. [Google Scholar]
- Šerá, B.; Zahoranová, A.; Bujdáková, H.; Šerý, M. Disinfection from pine seeds contaminated with Fusarium circinatum Nirenberg & O’Donnell using non-thermal plasma treatment. Roman. Rep. Phy. 2019, 71, 701. [Google Scholar]
- Mandal, R.; Singh, A.; Singh, A.P. Recent developments in cold plasma decontamination technology in the food industry. Trends. Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Marar, T.; Patil, S. Non-thermal plasma: An advanced technology for food industry. Food Sci. Technol. Internat. 2020, 26, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Kulawik, P.; Tiwari, B.K. Recent advancements in the application of non-thermal plasma technology for the seafood industry. Crit. Review. Food Sci. Nutri. 2019, 59, 3199–3210. [Google Scholar] [CrossRef] [PubMed]
- Birania, S.; Attkan, A.K.; Kumar, S.; Kumar, N.; Singh, V.K. Cold plasma in food processing and preservation: A review. J. Food Process Eng. 2022, 45, 14110. [Google Scholar] [CrossRef]
- Orellana, L.E.; Plaza, M.D.; Perez, F.; Cedeno, Y.; Perales, O. Non-thermal Methods for Food Preservation. In Food Microbiology and Food Safety; Juneja, V., Dwivedi, H., Sofos, J., Eds.; Springer: New York, NY, USA, 2017; pp. 299–326. [Google Scholar]
- Asl, P.J.; Rajulapati, V.; Gavahian, M.; Kapusta, I.; Putnik, P.; Khaneghah, A.M.; Marszalek, K. Non-thermal plasma technique for preservation of fresh foods: A review. Food Cont. 2022, 134, 108560. [Google Scholar] [CrossRef]
- Chacha, J.S.; Zhang, L.Y.; Ofoedu, C.E.; Suleiman, R.A.; Dotto, J.M.; Roobab, U.; Agunbiade, A.O.; Duguma, H.T.; Mkojera, B.T.; Hossaini, S.M.; et al. Revisiting Non-Thermal Food Processing and Preservation Methods-Action Mechanisms, Pros and Cons: A Technological Update (2016–2021). Foods 2021, 10, 1430. [Google Scholar] [CrossRef]
- Scholtz, V.; Jirešová, J.; Šerá, B.; Julák, J. A Review of Microbial Decontamination of Cereals by Non-Thermal Plasma. Foods 2021, 10, 2927. [Google Scholar] [CrossRef]
- Zhang, B.; Li, R.H.; Yan, J.C. Study on activation and improvement of crop seeds by the application of plasma treating seeds equipment. Arch. Biochem. Biophys. 2018, 655, 37–42. [Google Scholar] [CrossRef]
- Perez-Piza, M.C.; Prevosto, L.; Grijalba, P.E.; Zilli, C.G.; Cejas, E.; Mancinelli, B.; Balestrasse, K.B. Improvement of growth and yield of soybean plants through the application of non-thermal plasmas to seeds with different health status. Heliyon 2019, 5, 01495. [Google Scholar] [CrossRef]
- Perez-Piza, M.C.; Cejas, E.; Zilli, C.; Prevosto, L.; Mancinelli, B.; Santa-Cruz, D.; Yannarelli, G.; Balestrasse, K. Enhancement of soybean nodulation by seed treatment with non-thermal plasmas. Sci. Rep. 2020, 10, 4917. [Google Scholar] [CrossRef] [PubMed]
- Yemeli, G.B.N.; Janda, M.; Machala, Z. Non-thermal Plasma as a Priming Tool to Improve the Yield of Pea in Outdoor Conditions. Plasma Chem. Plasma Process. 2022, 42, 1143–1168. [Google Scholar] [CrossRef]
- Perez-Piza, M.C.; Clausen, L.; Cejas, E.; Ferreyra, M.; Chamorro-Garces, J.C.; Fina, B.; Zilli, C.; Vallecorsa, P.; Prevosto, L.; Balestrasse, K. Non-thermal plasma application improves germination, establishment and productivity of Gatton panic grass (Megathyrsus maximus) without compromising forage quality. Crop Pasture Sci. 2022, 73, 1188–1199. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pierto, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant. Path. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Homa, K.; Barney, W.P.; Davis, W.P.; Guerrero, D.; Berger, M.J.; Lopez, J.L.; Wyenandt, C.A.; Simon, J.E. Cold Plasma Treatment Strategies for the Control of Fusarium oxysporum f. sp. basilici in Sweet Basil. HortScience 2021, 56, 42–51. [Google Scholar] [CrossRef]
- Panngom, K.; Lee, S.H.; Park, D.H.; Sim, G.B.; Kim, Y.H.; Uhm, H.S.; Park, G.; Choi, E.H. Non-thermal plasma treatment diminishes Fungal Viability and Up-Regulates Resistance Genes in a Plant Host. PLoS ONE 2014, 9, e99300. [Google Scholar] [CrossRef]
- Go, S.M.; Park, M.R.; Kim, H.S.; Choi, W.S.; Jeong, R.D. Antifungal effect of non-thermal atmospheric plasma and its application for control of postharvest Fusarium oxysporum decay of paprika. Food Cont. 2019, 98, 245–252. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of cold atmospheric pressure plasma on maize seeds: Enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Hoppanová, L.; Medvecká, V.; Dylíková, J.; Hudecová, D.; Kaliňáková, B.; Kryštofová, S.; Zahoranová, A. Low-temperature plasma applications in chemical fungicide treatment reduction. Acta Chimica Slovaca 2020, 13, 26–33. [Google Scholar] [CrossRef]
- Bousba, H.E.; Saoudi, M.; Sahli, S.; Namous, W.S.E.; Benterrouche, L. Effect of Different Plasma Working Gas Mixtures on the Decontamination of Fungus Polluted Water. In Proceeding of the 6th International Symposium on Dielectric Materials and Applications (ISyDMA), Calais, France, 15–17 December 2021. [Google Scholar]
- Wang, Y.Q.; Li, B.; Shang, H.H.; Ma, R.N.; Zhu, Y.P.; Yang, X.D.; Ju, S.Y.; Zhao, W.B.; Sun, H.; Zhuang, J.; et al. Effective inhibition of fungal growth, deoxynivalenol biosynthesis and pathogenicity in cereal pathogen Fusarium spp. by cold atmospheric plasma. Chem. Eng. J. 2022, 437, 135307. [Google Scholar]
- Chang, E.H.; Bae, Y.S.; Shin, I.S.; Choi, H.J.; Lee, J.H.; Choi, J.W. Microbial Decontamination of Onion by Corona Discharge Air Plasma during Cold Storage. J. Food Qual. 2018, 2018, 3481806. [Google Scholar] [CrossRef]
- Sandanuwan, T.; Attygalle, D.; Amarasinghe, S.; Weragoda, S.C.; Ranaweera, B.; Rathnayake, K.; Alankara, W. Shelf Life Extension of Cavendish Banana Fruit Using Cold Plasma Treatment. In Proceedings of the 6th International Multidisciplinary Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 28–30 July 2020. [Google Scholar]
- ten Bosch, L.; Pfohl, K.; Avramidis, G.; Wieneke, S.; Viol, W.; Karlovsky, P. Plasma-Based Degradation of Mycotoxins Produced by Fusarium, Aspergillus and Alternaria Species. Toxins 2017, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, E.G.; Bayat, M.; Nosrati, A.C.; Hashemi, S.J.; Ghoranneviss, M. Study of the effect of plasma jet on Fusarium isolates with ability to produce DON toxins. Wor. Fam. Medic. 2017, 15, 204–207. [Google Scholar] [CrossRef]
- Guo, J.; He, Z.P.; Ma, C.; Li, W.T.; Wang, J.Y.; Lin, F.C.; Liu, X.Q.; Li, L. Evaluation of cold plasma for decontamination of molds and mycotoxins in rice grain. Food Chem. 2022, 402, 134159. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, X.L.; Zhang, J.Y.; Ding, Y.T.; Lyu, F. Effects of tempering with plasma activated water on the degradation of deoxynivalenol and quality properties of wheat. Food Res. Int. 2022, 162, 112070. [Google Scholar] [CrossRef]
- Iqdiam, B.M.; Feizollahi, E.; Arif, M.F.; Jeganathan, B.; Vasanthan, T.; Thilakarathna, M.S.; Roopesh, M.S. Reduction of T-2 and HT-2 mycotoxins by atmospheric cold plasma and its impact on quality changes and germination of wheat grains. J. Food. Sci. 2021, 86, 1354–1371. [Google Scholar] [CrossRef]
- Rüntzel, C.L.; da Silva, J.R.; da Silva, B.A.; Moecke, E.S.; Scussel, V.M. Effect of cold plasma on black beans (Phaseolus vulgaris L.), fungi inactivation and micro-structures stability. Emr. J. Food. Agric. 2019, 31, 864–873. [Google Scholar] [CrossRef]
- Guo, J.; Qin, D.; Li, W.; Wu, F.; Li, L.; Liu, X. Inactivation of Penicillium italicum on kumquat via plasma-activated water and its effects on quality attributes. Int. J. Food Microbiol. 2021, 343, 109090. [Google Scholar] [CrossRef]
- Ahmad, A.; Sripong, K.; Uthairatanakij, A.; Photchanachai, S.; Pankasemsuk, T.; Jitareerat, P. Decontamination of seed borne disease in pepper (Capsicum annuum L.) seed and the enhancement of seed quality by the emulated plasma technology. Scient. Hortic. 2022, 291, 110568. [Google Scholar] [CrossRef]
- Park, H.; Puligundla, P.; Mok, C. Cold plasma decontamination of brown rice grains: Impact on biochemical and sensory qualities of their corresponding seedlings and aqueous tea infusions. LWT 2020, 131, 109508. [Google Scholar] [CrossRef]
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal plasma inactivation of food-borne pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef]
- Xiong, Z.; Lu, X.P.; Feng, A.; Pan, Y.; Ostrikov, K. Highly effective fungal inactivation in He+O2 atmospheric-pressure nonequilibrium plasmas. Phys. Plasmas. 2010, 17, 123502. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, D.; Song, Y.; Zhou, R.; Jinhai, N. Inactivation of the Tomato Pathogen Cladosporium fulvum by an Atmospheric-Pressure Cold Plasma Jet. Plasma Process. Polym. 2014, 11, 1028–1036. [Google Scholar] [CrossRef]
- Shaw, A.; Seri, P.; Borghi, C.A.; Shama, G.; Iza, F. A reference protocol for comparing the biocidal properties of gas plasma generating devices. J. Phys. D Appl. Phys. 2015, 48, 484001. [Google Scholar] [CrossRef]
- Khun, J.; Scholtz, V.; Hozák, P.; Fitl, P.; Julák, J. Various DC-driven point-to-plain discharges as non-thermal plasma sources and their bactericidal effects. Plasma Sources Sci. Technol. 2018, 27, 065002. [Google Scholar] [CrossRef]
- Adhikari, B.; Pangomm, K.; Veerana, M.; Mitra, S.; Park, G. Plant Disease Control by Non Thermal Atmospheric-Pressure Plasma. Front. Plant Sci. 2020, 11, 77. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, Z.; Xing, C.; Chen, H.; Zhnag, J.; Yan, M. Degradation of Deoxynivalenol in wheat by double dielectric barrier discharge cold plasma: Identification and pathway of degradation products. J. Sci. Food Agric. 2022, accepted. [Google Scholar] [CrossRef]
- Ott, L.C.; Appleton, H.J.; Shi, H.; Keener, K.; Mellata, M. High voltage atmospheric cold plasma treatment inactivates Aspergillus flavus spores and deoxynivalenol toxin. Food Microbiol. 2021, 95, 103669. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Vukić, M.; Pezo, L.; Orčić, D.; Puač, N.; Škoro, N.; Milidrag, A.; Šoronja Simović, D. Effect of Atmospheric Cold Plasma Treatments on Reduction of Alternaria Toxins Content in Wheat Flour. Toxins 2019, 11, 704. [Google Scholar] [CrossRef]
- Varilla, C.; Marcone, M.; Annor, G.A. Potential of Cold Plasma Technology in Ensuring the Safety of Foods and Agricultural Produce: A Review. Foods 2020, 9, 1435. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A review of recent innovative strategies for controlling mycotoxins in foods. Food Cont. 2023, 144, 109350. [Google Scholar] [CrossRef]
- Gavahian, M.; Cullen, P.J. Cold Plasma as an Emerging Technique for Mycotoxin-Free Food: Efficacy, Mechanisms, and Trends. Food Rev. Internat. 2019, 36, 193–214. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, J.-H.; Sun, D.-W. Blocking and degradation of aflatoxins by cold plasma treatments: Applications and mechanisms. Trends Food Sci. Technol. 2021, 109, 647–661. [Google Scholar] [CrossRef]
- Lee, G.J.; Sim, G.B.; Choi, E.H.; Kwon, Y.-W.; Kim, J.Y.; Jang, S.; Kim, S.H. Optical and structural properties of plasma-treated Cordyceps bassiana spores as studied by circular dichroism, absorption, and fluorescence spectroscopy. J. Appl. Phys. 2015, 117, 023303. [Google Scholar] [CrossRef]
- Šimončicová, J.; Kryštofová, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical applications of plasma treatments: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5117–5129. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U. Consequences of non-thermal cold plasma treatment on meat and dairy lipids—A review. Future Foods 2021, 4, 10095. [Google Scholar] [CrossRef]
- Almeida, F.D.L.; Cavalcante, R.S.; Cullen, P.J.; Frias, J.; Bourke, P.; Fernandes, F.A.N.; Rodrigues, S. Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innov. Food Sci. Emerg. Technol. 2015, 32, 127–135. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).