Growing of the Cretan Therapeutic Herb Origanum Dictamnus in The Urban Fabric: The Effect of Substrate and Cultivation Site on Plant Growth and Potential Toxic Element Accumulation
Abstract
1. Introduction
2. Results
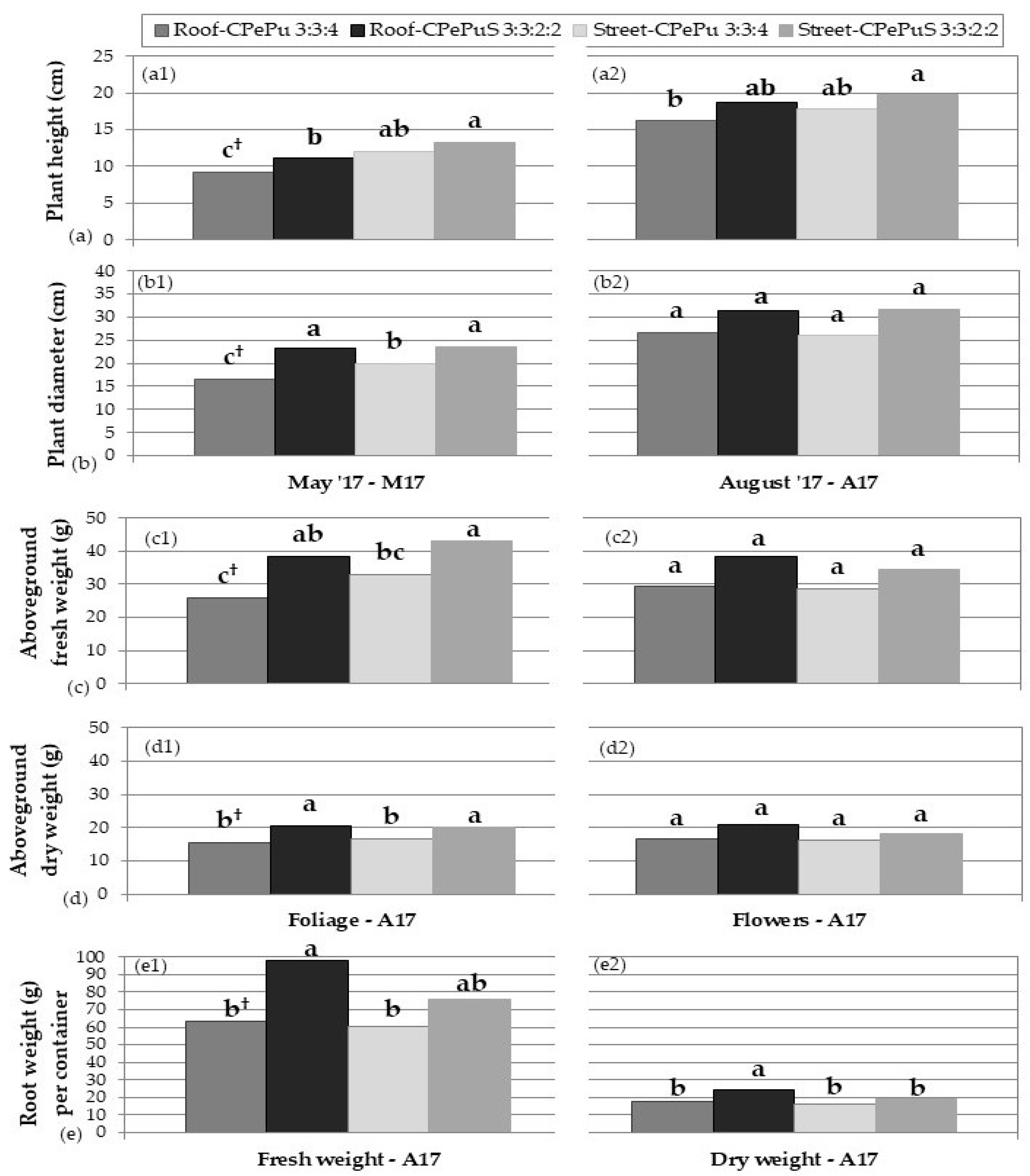
2.1. Plant Growth
2.2. Nutrient Content
2.3. Heavy Metal Accumulation
3. Discussion
3.1. Plant Growth
3.2. Nutrient Content
3.3. Heavy Metal Accumulation
4. Materials and Methods
4.1. Experimental Set-Up (Plant Material, Cultivation System, and Site)
4.2. Substrate
4.3. Irrigation
4.4. Meteorological Data
4.5. Plant Growth Evaluation
4.6. Heavy Metal and Nutrient Determination
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huxley, A.; Taylor, W. Flowers of Greece and the Aegean; Chatto & Windus Ltd.: London, UK, 1977; p. 125. [Google Scholar]
- Blamey, M.; Grey-Wilson, C. Mediterranean Wild Flowers; Harper Collins: London, UK, 1993; p. 399. [Google Scholar]
- Mitropoulou, G.; Fitsiou, E.; Stavropoulou, E.; Papavassilopoulou, E.; Vamvakias, M.; Pappa, A.; Oreopoulou, A.; Kourkoutas, Y. Composition, antimicrobial, antioxidant, and antiproliferative activity of Origanum dictamnus (dittany) essential oil. Microb. Ecol. Health Dis. 2015, 26, 26543. [Google Scholar] [CrossRef] [PubMed]
- Liolios, C.C.; Graikou, K.; Skaltsa, E.; Chinou, I. Dittany of Crete: A botanical and ethnopharmacological review. J. Ethnopharmacol. 2010, 131, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Pirintsos, S.A.; Bariotakis, M.; Kampa, M.; Sourvinos, G.; Lionis, C.; Castanas, E. The Therapeutic Potential of the Essential Oil of Thymbra capitata (L.) Cav., Origanum dictamnus L. and Salvia fruticosa Mill. And a Case of Plant-Based Pharmaceutical Development. Front. Pharmacol. 2020, 11, 522213. [Google Scholar] [CrossRef] [PubMed]
- Tseliou, M.; Pirintsos, S.A.; Lionis, C.; Castanas, E.; Sourvinos, G. Antiviral effect of an essential oil combination derived from three aromatic plants (Coridothymus capitatus (L.) Rchb. f., Origanum dictamnus L. and Salvia fruticosa Mill.) against viruses causing infections of the upper respiratory tract. J. Herb. Med. 2019, 17–18, 100288. [Google Scholar] [CrossRef]
- Walter, K.S.; Gillett, H.J. 1997 IUCN Red List of Threatened Plants; IUCN (The World Conservation Union): Gland, Switzerland; Cambridge, UK, 1998. [Google Scholar]
- Krigas, N.; Lazari, D.; Maloupa, E.; Stikoudi, M. Introducing Dittany of Crete (Origanum dictamnus L.) to gastronomy: A new culinary concept for a traditionally used medicinal plant. Int. J. Gastron. Food Sci. 2015, 2, 112–118. [Google Scholar] [CrossRef]
- Papafotiou, M.; Pergialioti, N.; Papanastassatos, E.A.; Tassoula, L.; Massas, I.; Kargas, G. Effect of substrate type and depth and the irrigation frequency on growth of semiwoody mediterranean species in green roofs. Acta Hortic. 2013, 990, 481–486. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M.; Evangelopoulos, K. Establishment of the edible and medicinal endemic species Origanum dictamnus on an extensive urban Mediterranean green roof as affected by substrate type and depth. Acta Hortic. 2017, 1189, 461–464. [Google Scholar] [CrossRef]
- Tassoula, L.; Papafotiou, M.; Liakopoulos, G.; Kargas, G. Water use efficiency, growth and anatomic-physiological param-eters of Mediterranean xerophytes as affected by substrate and irrigation on a green roof. Notul. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12283. [Google Scholar] [CrossRef]
- UN (United Nations). World Urbanization Prospects: The 2018 Revision; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2018; pp. 1–126.
- Bulgari, R.; Petrini, A.; Cocetta, G.; Nicoletto, C.; Ertani, A.; Sambo, P.; Ferrante, A.; Nicola, S. The impact of COVID-19 on horticulture: Critical issues and opportunities derived from an unexpected occurrence. Horticulturae 2021, 7, 124. [Google Scholar] [CrossRef]
- Orsini, F.; Gasperi, D.; Marchetti, L.; Piovene, C.; Draghetti, S.; Ramazzotti, S.; Bazzocchi, G.; Gianquinto, G. Exploring the production capacity of rooftop gardens (RTGs) in urban agriculture: The potential impact on food and nutrition security, biodiversity and other ecosystem services in the city of Bologna. Food Secur. 2014, 6, 781–792. [Google Scholar] [CrossRef]
- Khan, M.M.; Akram, M.T.; Janke, R.; Qadri, R.W.K.; Al-Sadi, A.M.; Farooque, A.A. Urban horticulture for food secure cities through and beyond COVID-19. Sustainability 2020, 12, 9592. [Google Scholar] [CrossRef]
- Evans, D.L.; Falagán, N.; Hardman, C.A.; Kourmpetli, S.; Liu, L.; Mead, B.R.; Davies, J.A.C. Ecosystem service delivery by urban agriculture and green infrastructure—A systematic review. Ecosyst. Serv. 2022, 54, 101405. [Google Scholar] [CrossRef]
- Whittinghill, L.J.; Rowe, D.B. The role of green roof technology in urban agriculture. Renew. Agric. Food Syst. 2012, 27, 314–322. [Google Scholar] [CrossRef]
- Berardi, U.; Ghaffarian Hoseini, A.H.; Ghaffarian Hoseini, A. State-of-the-art analysis of the environmental benefits of green roofs. Appl. Energy 2014, 115, 411–428. [Google Scholar] [CrossRef]
- Shafique, M.; Kim, R.; Rafiq, M. Green roof benefits, opportunities and challenges—A review. Renew. Sustain. Energy Rev. 2018, 90, 757–773. [Google Scholar] [CrossRef]
- Bus, A.; Szelągowska, A. Green Water from Green Roofs—The Ecological and Economic Effects. Sustainability 2021, 13, 2403. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M.; Massas, I.; Chorianopoulou, N. Using the Halophyte Crithmum maritimum in Green Roofs for Sustainable Urban Horticulture: Effect of Substrate and Nutrient Content Analysis including Potentially Toxic Elements. Sustainability 2022, 14, 4713. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N.; Tassoula, L.; Stylias, E.G.; Kalantzis, A.; Dariotis, E. Acclimatization of Mediterranean Native Sages (Salvia spp.) and Interspecific Hybrids in an Urban Green Roof under Regular and Reduced Irrigation. Sustainability 2022, 14, 4978. [Google Scholar] [CrossRef]
- Francini, A.; Romano, D.; Toscano, S.; Ferrante, A. The contribution of Ornamental Plants to Urban Ecosystem Services. Earth 2022, 3, 1258–1274. [Google Scholar] [CrossRef]
- Walters, S.A.; Gajewski, C.; Sadeghpour, A.; Groninger, J.W. Mitigation of Climate Change for Urban Agriculture: Water Management of Culinary Herbs Grown in an Extensive Green Roof Environment. Climate 2022, 10, 180. [Google Scholar] [CrossRef]
- Diamond, M.; Hodge, E. Urban contaminant dynamics: From source to effect. Environ. Sci. Technol. 2007, 41, 3796–3805. [Google Scholar] [CrossRef] [PubMed]
- Blagojenié, N.; Damjanovié-Vratnica, B.; Vukašinovié-Pešié, V.; Durovié, D. Heavy metals content in leaves and extracts of wild-growing Salvia οfficinalis from Montenegro. Pol. J. Environ. Stud. 2009, 18, 167–173. [Google Scholar]
- Suruchi, K.; Jilani, A. Assessment of heavy metal concentration in washed and unwashed vegetables exposed to different degrees of pollution in Agra, India. Electron. J. Environ. Agric. Food Chem. 2011, 10, 2700–2710. [Google Scholar]
- Khandare, A.L.; Validandi, V.; Jamalpur, R.P.; Dheeravath, S.; Kurella, S.; Chauhan, A.; Boiroju, N.K.; Thingnganing, L. Potential Health Risks Associated with the Heavy Metal Content in Commonly Consumed Food from Prakasam District of Andhra Pradesh, India. Biol Trace Elem. Res. 2022, 200, 3453–3461. [Google Scholar] [CrossRef]
- Collado-López, S.; Betanzos-Robledo, L.; Téllez-Rojo, M.M.; Lamadrid-Figueroa, H.; Reyes, M.; Ríos, C.; Cantoral, A. Heavy Metals in Unprocessed or Minimally Processed Foods Consumed by Humans Worldwide: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 8651. [Google Scholar] [CrossRef] [PubMed]
- Suruchi, K.; Khanna, P. Assessment of heavy metal contamination in different vegetables grown in and around urban areas. Res. J. Environ. Toxicol. 2011, 5, 162–179. [Google Scholar]
- Gori, A.; Ferrini, F.; Fini, A. Reprint of: Growing healthy food under heavy metal pollution load: Overview and major challenges of tree based edible landscapes. Urban. For. Urban. Green. 2019, 45, 126292. [Google Scholar] [CrossRef]
- Srinivas, N.; Rao, S.R.; Kumar, K.S. Trace metal accumulation in vegetables grown in industrial and semi-urban areas: A case study. Appl. Ecol. Environ. Res. 2009, 7, 131–139. Available online: http://www.ecology.uni-corvinus.hu (accessed on 23 November 2022). [CrossRef]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef]
- Mawari, G.; Kumar, N.; Sarkar, S.; Daga, M.K.; Singh, M.M.; Joshi, T.K.; Khan, N.A. Heavy Metal Accumulation in Fruits and Vegetables and Human Health Risk Assessment: Findings from Maharashtra, India. Environ. Health Insights 2022, 16, 11786302221119151. [Google Scholar] [CrossRef]
- Peris, M.; Micó, C.; Recalatá, L.; Sánchez, R.; Sánchez, J. Heavy metal contents in horticultural crops of a representative area of the European Mediterranean region. Sci. Total Environ. 2007, 378, 42–48. [Google Scholar] [CrossRef]
- Hussain, I.; Ullah, R.; Khurram, M.; Ullah, N.; Baseer, A.; Khan, F.A.; Khan, N.; Khattak, M.R.; Zahoor, M.; Khan, J. Heavy metals and inorganic constituents in medicinal plants of selected districts of Khyber Pakhtoonkhwa, Pakistan. Afr. J. Biotechnbology 2011, 10, 8517–8522. [Google Scholar] [CrossRef]
- Abou-Arab, A.A.K.; Abou Donia, M.A. Heavy metals in egyptian spices and medicinal plants and the effect of processing on their levels. J. Agric. Food Chem. 2000, 48, 2300–2304. [Google Scholar] [CrossRef] [PubMed]
- Antisari, L.V.; Orsini, F.; Marchetti, L.; Vianello, G.; Gianquinto, G. Heavy metal accumulation in vegetables grown in urban gardens. Agron. Sustain. Dev. 2015, 35, 1139–1147. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.R.; Kim, W.I.; Owens, G.; Kim, K.H. Influence of road proximity on the concentrations of heavy metals in Korean urban agricultural soils and crops. Arch. Environ. Contam. Toxicol. 2017, 72, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Papafotiou, M.; Koutri, A.; Massas, I. Heavy metal concentration in sage plants cultivated on an urban green roof or roadside location as affected by substrate type and fertilization. Acta Hortic. 2017, 1189, 439–442. [Google Scholar] [CrossRef]
- Papafotiou, M.; Koutri, A.; Massas, I. Urban green roof versus roadside cultivation: Effect of pollution, substrate type and fertilization on heavy metal concentration in oregano plants. Acta Hortic. 2017, 1189, 443–446. [Google Scholar] [CrossRef]
- Säumel, I.; Kotsyuk, I.; Hölscher, M.; Lenkereit, C.; Weber, F.; Kowarik, I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environ. Pollut. 2012, 165, 124–132. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M.; Massas, I.; Chorianopoulou, N.; Živanović, I. Effect of substrate type and cultivation site in the urban fabric on growth and safety to consume the edible medicinal species Origanum dictamnus. Acta Hortic. 2021, 1327, 487–494. [Google Scholar] [CrossRef]
- FAO; WHO. Food Standards Programme Codex Committee on Contaminants in Foods, Fifth Session; WHO: Geneva, Switzerland, 2011; pp. 64–89.
- Papafotiou, M.; Tassoula, L.; Liakopoulos, G.; Kargas, G. Effect of substrate type and irrigation frequency on growth of Mediterranean xerophytes on green roofs. Acta Hortic. 2016, 1108, 309–316. [Google Scholar] [CrossRef]
- Alarcón, J.J.; Morales, M.A.; Ferrández, T.; Sánchez-Blanco, M.J. Effects of water and salt stresses on growth, water relations and gas exchange in Rosmarinus officinalis. J. Hortic. Sci. Biotechnol. 2006, 81, 845–853. [Google Scholar] [CrossRef]
- Savi, T.; Dal Borgo, A.; Love, V.L.; Andri, S.; Tretiach, M.; Nardini, A. Drought versus heat: What’s the major constraint on Mediterranean green roof plants? Sci. Total Environ. 2016, 566, 753–760. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D.; Tribulato, A. Interactive Effects of Drought and Saline Aerosol Stress on Morphological and Physiological Characteristics of Two Ornamental Shrub Species. Horticulturae 2021, 7, 517. [Google Scholar] [CrossRef]
- Tassoula, L.; Papafotiou, M.; Liakopoulos, G. Cultivation of the herb Origanum dictamnus in an urban extensive green roof under different substrate types and irrigation frequencies. Acta Hortic. 2021, 1327, 495–500. [Google Scholar] [CrossRef]
- Ondoño, S.; Martínez-Sánchez, J.J.; Moreno, J.L. Evaluating the growth of several Mediterranean endemic species in artificial substrates: Are these species suitable for their future use in green roofs? Ecol. Eng. 2015, 81, 405–417. [Google Scholar] [CrossRef]
- Azeñas, V.; Janner, I.; Medrano, H.; Gulías, J. Evaluating the establishment performance of six native perennial Mediterranean species for use in extensive green roofs under water-limiting conditions. Urban. For. Urban. Green. 2019, 41, 158–169. [Google Scholar] [CrossRef]
- Ercilla-Montserrat, M.; Muñoz, P.; Montero, J.I.; Gabarrell, X.; Rieradevall, J. A study on air quality and heavy metals content of urban food produced in a Mediterranean city (Barcelona). J. Clean. Prod. 2018, 195, 385–395. [Google Scholar] [CrossRef]
- Yusuf, K.A.; Oluwole, S.O. Heavy metal (Cu, Zn, Pb) contamination of vegetables in urban city: A case study in Lagos. Res. J. Environ. Sci. 2009, 3, 292–298. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.R.; Lim, G.H.; Kim, J.W.; Kim, K.H. Influence of airborne dust on the metal concentrations in crop plants cultivated in a rooftop garden in Seoul. Soil Sci. Plant Nutr. 2015, 61, 88–97. [Google Scholar] [CrossRef]
- Uzu, G.; Sobanska, S.; Sarret, G.; Muñoz, M.; Dumat, C. Foliar lead uptake by lettuce exposed to atmospheric fallouts. Environ. Sci. Technol. 2010, 44, 1036–1042. [Google Scholar] [CrossRef]
- Kinnersley, R.P.; Scott, L.K. Aerial contamination of fruit through wet deposition and particulate dry deposition. J. Environ. Radioact. 2001, 52, 191–213. [Google Scholar] [CrossRef]
- Tomašević, M.; Vukmirović, Z.; Rajšić, S.; Tasić, M.; Stevanović, B. Characterization of trace metal particles deposited on some deciduous tree leaves in an urban area. Chemosphere 2005, 61, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Ferrero, E.; Alessandrini, S.; Balanzino, A. Impact of the electric vehicles on the air pollution from a highway. Appl. Energ. 2016, 169, 450–459. [Google Scholar] [CrossRef]
- Zhao, J.; Xi, X.; Na, Q.; Wang, S.; Kadry, S.N.; Kumar, P.M. The technological innovation of hybrid and plug-in electric vehicles for environment carbon pollution control. Environ. Imp. Assess. Rev. 2021, 86, 106506. [Google Scholar] [CrossRef]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Craker, L.E.; Xing, B.; Nielsen, N.E.; Wilcox, A. Aromatic plant production on metal contaminated soils. Sci. Total Environ. 2008, 395, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Jeliazkova, E.A.; Kovacheva, N.; Dzhurmanski, A. Metal uptake by medicinal plant species grown in soils contaminated by a smelter. Environ. Exp. Bot. 2008, 64, 207–216. [Google Scholar] [CrossRef]
- Papafotiou, M.; Pergialioti, N.; Tassoula, L.; Massas, I.; Kargas, G. Growth of native aromatic xerophytes in an extensive Mediterranean green roof as affected by substrate type and depth and irrigation frequency. HortScience 2013, 48, 1327–1333. [Google Scholar] [CrossRef]
- Tassoula, L.; Papafotiou, M.; Liakopoulos, G.; Kargas, G. Growth of the native xerophyte Convolvulus cneorum L. on an extensive Mediterranean green roof under different substrate types and irrigation regimens. HortScience 2015, 50, 1118–1124. [Google Scholar] [CrossRef]
- Page, A.L. Methods of Soil Analysis, Part 2, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]


| Significance § (Two-Way ANOVA) | Plant Height (cm) M17/ A17 | Plant Diameter (cm) M17/ A17 | Fresh Weight (g) Foliage/ Flowers A17 | Dry Weight (g) Foliage/ Flowers A17 | Root Weight (g) Per Container Fresh Weight/ Dry Weight A17 |
|---|---|---|---|---|---|
| Fcultivation site | **/ * | */ ** | NS/ NS | NS/ NS | NS/ * |
| Fsubstrate type | **/ NS | **/ NS | **/ NS | **/ NS | */ ** |
| Finteraction | NS/ NS | NS/ NS | NS/ NS | NS/ NS | NS/ NS |
| Fone-way ANOVA | **/ * | **/ NS | **/ NS | **/ NS | */ ** |
| A. LEAVES | |||||
| Cultivation Site | Substrate Type (v/v) | N | P | K | Na |
| Roof | 3C:3Pe:4Pu | 1.8 a † | 0.45 a | 3.7 a | 0.03 c |
| 3C:3Pe:2Pu:2S | 1.7 a | 0.32 b | 2.5 b | 0.03 c | |
| Street | 3C:3Pe:4Pu | 1.8 a | 0.36 b | 3.3 a | 0.12 a |
| 3C:3Pe:2Pu:2S | 2.0 a | 0.36 b | 2.5 b | 0.09 b | |
| Significance § | |||||
| Fcultivation site | NS | - | NS | ** | |
| Fsubstrate type | NS | - | ** | NS | |
| Finteraction | NS | ** | NS | NS | |
| Fone-way ANOVA | NS | ** | ** | ** | |
| B. FLOWERS | |||||
| Cultivation Site | Substrate Type (v/v) | N | P | K | Na |
| Roof | 3C:3Pe:4Pu | 1.2 a † | 0.34 a | 3.3 a | 0.02 b |
| 3C:3Pe:2Pu:2S | 1.3 a | 0.27 a | 2.5 b | 0.02 b | |
| Street | 3C:3Pe:4Pu | 1.4 a | 0.33 a | 2.9 ab | 0.11 a |
| 3C:3Pe:2Pu:2S | 1.5 a | 0.32 a | 2.4 b | 0.04 b | |
| Significance § | |||||
| Fcultivation site | NS | NS | NS | - | |
| Fsubstrate type | NS | NS | ** | - | |
| Finteraction | NS | NS | NS | ** | |
| Fone-way ANOVA | NS | NS | ** | ** | |
| C. ROOTS | |||||
| Cultivation Site | Substrate Type (v/v) | N | P | K | Na |
| Roof | 3C:3Pe:4Pu | 1.4 b † | 0.28 a | 1.8 a | 0.28 b |
| 3C:3Pe:2Pu:2S | 1.5 b | 0.20 a | 0.8 c | 0.12 c | |
| Street | 3C:3Pe:4Pu | 1.8 a | 0.23 a | 1.2 b | 0.47 a |
| 3C:3Pe:2Pu:2S | 1.6 ab | 0.20 a | 0.7 c | 0.30 b | |
| Significance § | |||||
| Fcultivation site | * | NS | - | ** | |
| Fsubstrate type | NS | NS | - | ** | |
| Finteraction | NS | NS | ** | NS | |
| Fone-way ANOVA | * | NS | ** | ** | |
| Cu * | Pb * | Ni * | Mn * | Zn * | Fe * | N | P-Olsen | Kexch | Naexch | |
|---|---|---|---|---|---|---|---|---|---|---|
| Soil | 0.159 | 0.459 | 0.038 | 1.222 | 0.19 | 0.74 | 0.091 | 11.52 ** | 60 ** | 380 ** |
| Grape marc compost | 0.248 | 0.759 | 0.045 | 0.428 | 0.213 | 4.646 | 2.814 | 0.5 | 1.96 | 0.16 |
| Three-Way ANOVA | Leaves | Flowers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Pb | Ni | Mn | Zn | Fe | Cu | Pb | Ni | Mn | Zn | Fe | |
| Roof | 5.7 b z | 17.9 | 4.3 b | 33.3 | 32.4 | 172.0 | 5.1 b z | 18.6 | 5.0 a | 31.6 | 28.4 | 103.9 |
| Street | 20.8 a | 28.9 | 6.2 a | 37.6 | 88.6 | 436.2 | 11.9 a | 19.3 | 6.0 a | 33.9 | 46.5 | 206.5 |
| 3C:3Pe:4Pu | 12.7 a | 22.5 a | 5.0 a | 29.9 b | 58.7 a | 261.1 b | 8.5 a | 18.2 a | 6.3 a | 27.7 | 36.9 | 166.3 a |
| 3C:3Pe:2Pu:2S | 13.8 a | 24.2 a | 5.5 a | 41.0 a | 62.2 a | 347.1 a | 8.6 a | 19.7 a | 4.7 a | 37.8 | 37.9 | 144.1 a |
| Washed | 11.9 b | 22.5 | 4.7 b | 35.0 a | 54.1 | 225.6 | 8.0 a | 18.1 | 5.4 a | 32.5 | 32.4 | 124.8 |
| Unwashed | 14.5 a | 24.2 | 5.8 a | 35.9 a | 66.9 | 382.6 | 9.1 a | 19.8 | 5.6 a | 33.0 | 42.4 | 185.6 |
| Significance § | ||||||||||||
| Fcultivation site | ** | ** | ** | NS | ||||||||
| Fsubstrate type | NS | NS | NS | ** | NS | * | NS | NS | NS | NS | ||
| Fwashing | * | ** | NS | NS | ||||||||
| Fcult. site × substr. type | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | * | NS |
| Fcult. site × washing | NS | * | NS | * | ** | * | NS | ** | NS | * | ** | * |
| Fsubstr. type × washing | NS | NS | NS | NS | NS | NS | NS | NS | NS | * | NS | NS |
| Fcultivation site × substrate type × washing | NS | NS | NS | NS | NS | NS | NS | NS | NS | ** | NS | NS |
| A. LEAVES | |||||||
| Cultivation Site | Substrate Type (v/v) | Cu W/UW | Pb W/UW | Ni W/UW | Mn W/UW | Zn W/UW | Fe W/UW |
| Roof | 3C:3Pe:4Pu | 5.6 b †/ 5.9 b | 16.9 b z/ 15.8 c z | 4.0 b z/ 3.8 c z | 32.6 bc z/ 25.3 c | 35.4 b/ 33.1 b | 113.3 c/ 137.1 b |
| 3C:3Pe:2Pu:2S | 5.8 b/ 5.6 b | 19.0 b z/ 19.8 b z | 4.0 b z/ 5.4 b z | 36.6 ab z/ 38.8 ab z | 32.0 b/ 29.1 b | 168.9 bc/ 268.8 b | |
| Street | 3C:3Pe:4Pu | 15.4 a/ 23.8 a | 26.5 a z/ 31.0 a z | 5.5 a z/ 6.9 a z | 28.5 c/ 33.4 b z | 69.8 a z/ 96.7 a z | 276.3 ab/ 517.8 a |
| 3C:3Pe:2Pu:2S | 21.0 a/ 22.9 a | 27.7 a z/ 30.3 a z | 5.4 a z/ 7.1 a z | 45.4 a z/ 46.1 a z | 79.2 a z/ 108.7 a z | 343.9 a/ 606.8 a | |
| Significance § | |||||||
| Fcultivation site | **/ ** | **/ ** | **/ ** | -/ ** | **/ ** | **/ ** | |
| Fsubstrate type | NS/ NS | NS / NS | NS/ NS | -/ ** | NS/ NS | NS/ NS | |
| Finteraction | NS/ NS | NS/ NS | NS/ NS | */ NS | NS/ NS | NS/ NS | |
| Fone-way ANOVA | **/ ** | **/ ** | **/ ** | **/ ** | **/ ** | **/ ** | |
| B. FLOWERS | |||||||
| Cultivation Site | Substrate Type (v/v) | Cu W/UW | Pb W/UW | Ni W/UW | Mn W/UW | Zn W/UW | Fe W/UW |
| Roof | 3C:3Pe:4Pu | 5.1 b †/ 4.9 b | 17.6 a z/ 20.0 a z | 6.5 a z/ 4.6 a z | 31.7 bc z/ 22.9 c | 30.6 c/ 29.6 b | 101.0 a/ 111.2 b |
| 3C:3Pe:2Pu:2S | 5.2 b/ 5.1 b | 20.8 a z/ 19.0 a z | 4.1 a z/ 4.9 a z | 34.0 ab z/ 37.7 a z | 24.8 d/ 28.5 b | 80.3 a/ 123.3 b | |
| Street | 3C:3Pe:4Pu | 10.2 a/ 13.6 a | 17.4 a z/ 20.9 a z | 6.7 a z/ 7.6 a z | 25.7 c/ 30.5 b z | 35.2 b/ 52.2 a | 170.8 a/ 283.4 a |
| 3C:3Pe:2Pu:2S | 11.4 a/ 12.6 a | 16.5 a z/ 22.5 a z | 4.5 a z/ 5.4 a z | 38.5 a z/ 40.8 a z | 39.0 a/ 59.3 a | 148.2 a/ 224.6 a | |
| Significance § | |||||||
| Fcultivation site | **/ ** | NS/ * | NS/ NS | -/ ** | -/ ** | */ ** | |
| Fsubstrate type | NS/ NS | NS/ NS | NS/ NS | -/ ** | -/ NS | NS/ NS | |
| Finteraction | NS/ NS | NS/ NS | NS/ NS | */ NS | **/ NS | NS/ NS | |
| Fone-way ANOVA | **/ ** | NS/ NS | NS/ NS | **/ ** | **/ ** | NS/ ** | |
| Cultivation Site | Substrate Type (v/v) | Cu | Pb | Ni | Mn | Zn | Fe |
|---|---|---|---|---|---|---|---|
| Roof | 3C:3Pe:4Pu | 13.3 a † | 20.8 a z | 10.0 a z | 54.0 b z | 43.1 a | 440.7 b |
| 3C:3Pe:2Pu:2S | 12.4 a | 26.1 a z | 14.3 a z | 88.0 a z | 32.7 a | 1.478.0 a | |
| Street | 3C:3Pe:4Pu | 17.4 a | 23.8 a z | 10.1 a z | 51.8 b z | 65.2 a z | 534.8 b |
| 3C:3Pe:2Pu:2S | 16.9 a | 24.9 a z | 16.2 a z | 81.0 a z | 48.8 a | 1389.2 a | |
| Significance § | |||||||
| Fcultivation site | * | NS | NS | NS | NS | NS | |
| Fsubstrate type | NS | * | NS | ** | NS | ** | |
| Finteraction | NS | NS | NS | NS | NS | NS | |
| Fone-way ANOVA | NS | NS | NS | ** | NS | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, A.N.; Papafotiou, M.; Massas, I.; Chorianopoulou, N. Growing of the Cretan Therapeutic Herb Origanum Dictamnus in The Urban Fabric: The Effect of Substrate and Cultivation Site on Plant Growth and Potential Toxic Element Accumulation. Plants 2023, 12, 336. https://doi.org/10.3390/plants12020336
Martini AN, Papafotiou M, Massas I, Chorianopoulou N. Growing of the Cretan Therapeutic Herb Origanum Dictamnus in The Urban Fabric: The Effect of Substrate and Cultivation Site on Plant Growth and Potential Toxic Element Accumulation. Plants. 2023; 12(2):336. https://doi.org/10.3390/plants12020336
Chicago/Turabian StyleMartini, Aikaterini N., Maria Papafotiou, Ioannis Massas, and Nikoleta Chorianopoulou. 2023. "Growing of the Cretan Therapeutic Herb Origanum Dictamnus in The Urban Fabric: The Effect of Substrate and Cultivation Site on Plant Growth and Potential Toxic Element Accumulation" Plants 12, no. 2: 336. https://doi.org/10.3390/plants12020336
APA StyleMartini, A. N., Papafotiou, M., Massas, I., & Chorianopoulou, N. (2023). Growing of the Cretan Therapeutic Herb Origanum Dictamnus in The Urban Fabric: The Effect of Substrate and Cultivation Site on Plant Growth and Potential Toxic Element Accumulation. Plants, 12(2), 336. https://doi.org/10.3390/plants12020336






