Simultaneous Action of Silymarin and Dopamine Enhances Defense Mechanisms Related to Antioxidants, Polyamine Metabolic Enzymes, and Tolerance to Cadmium Stress in Phaseolus vulgaris
Abstract
1. Introduction
2. Materials and Methods
2.1. Growing Conditions of the Plant Material
2.2. Evaluation of Growth and Yield Traits
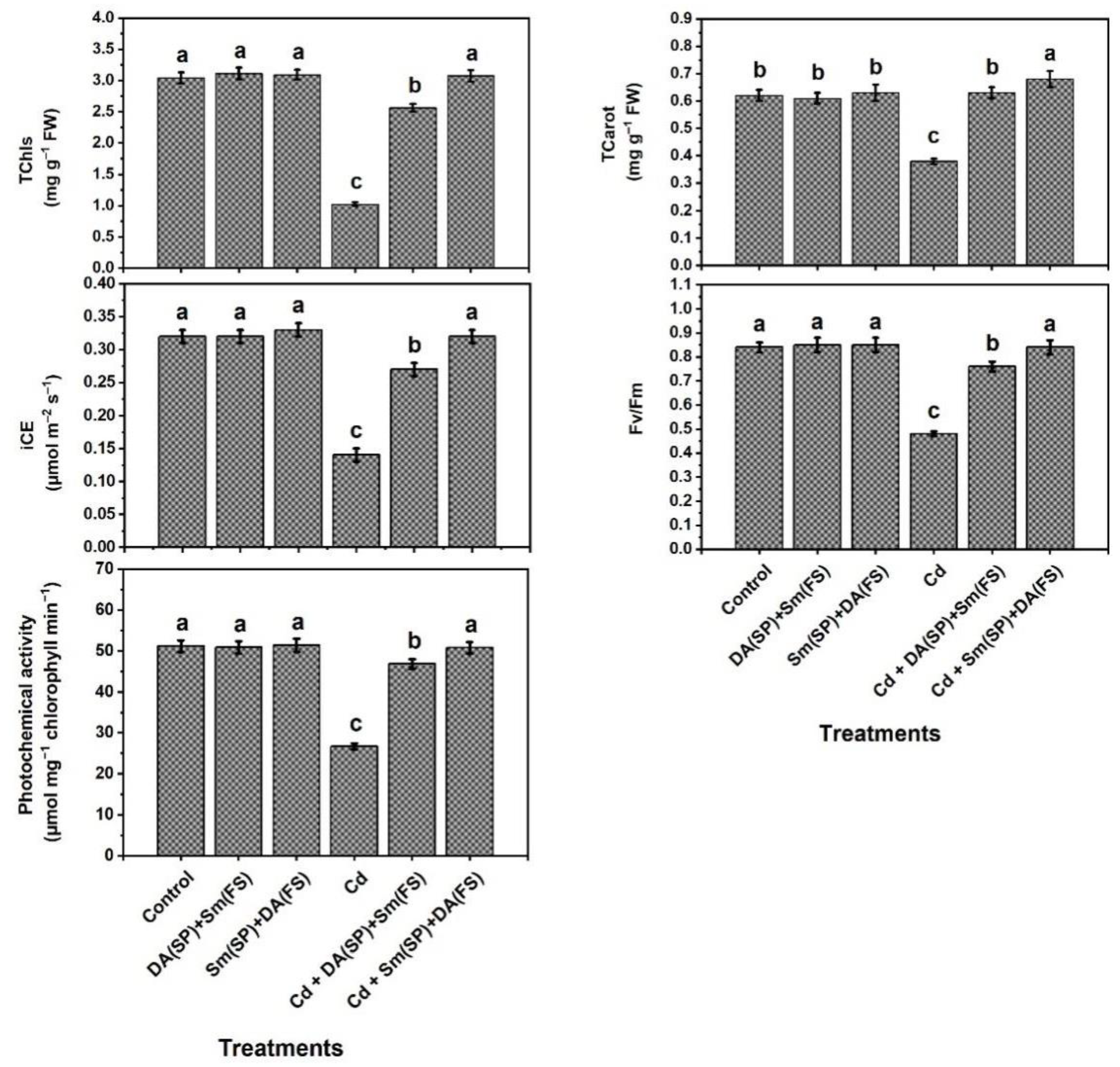
2.3. Evaluation of Photosynthetic Efficiency
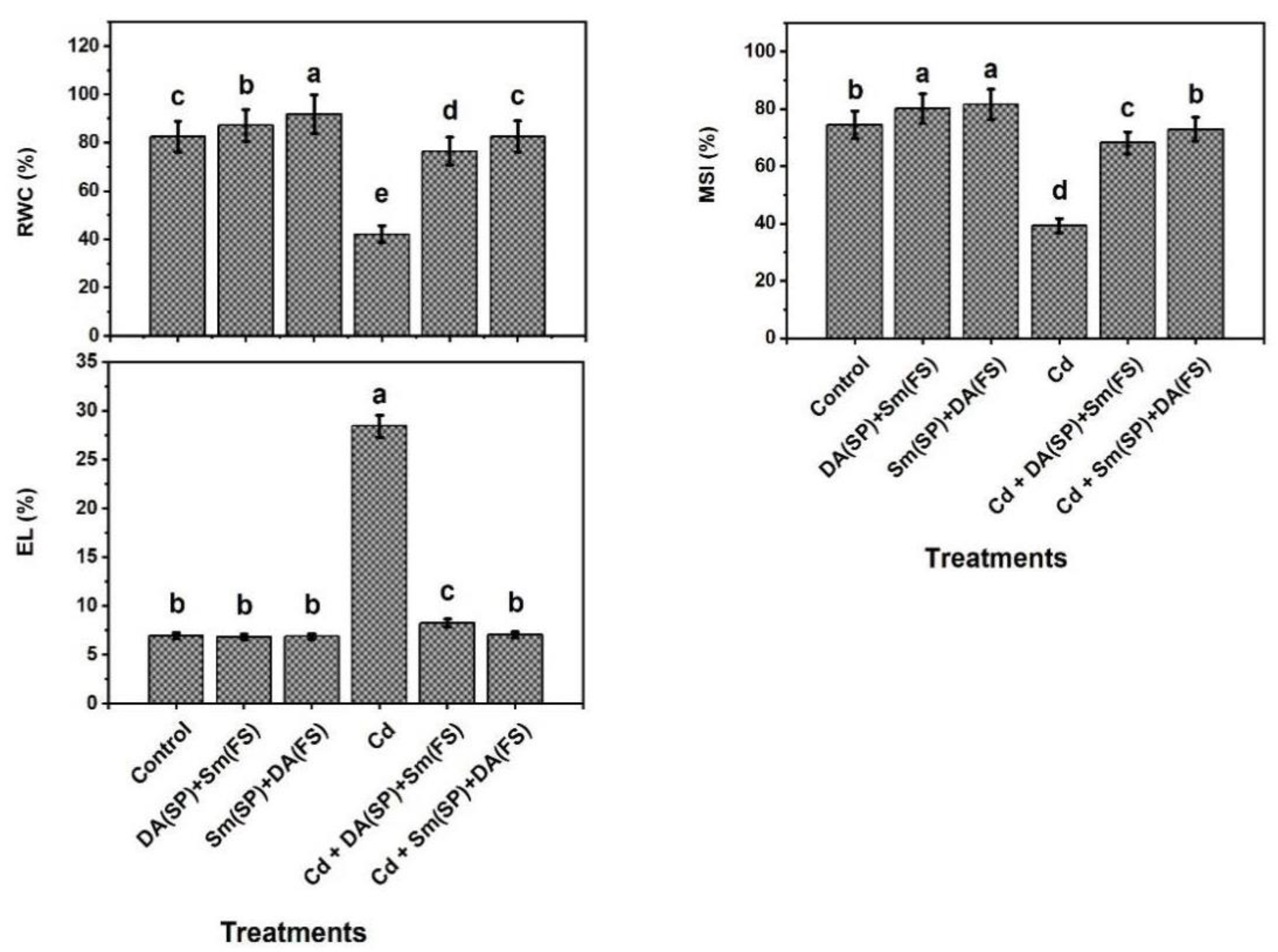
2.4. Determination of Leafy Relative Content of Water (RWC) and Contents of Osmo-Regulatory Compounds
2.5. Determination of Biomarkers of Oxidative Stress and Their Harmful Consequences for Cell Membranes
2.6. Evaluation of Cadmium (Cd), Silymarin (Sm), and Dopamine (DA)
2.7. Determination of Non-Enzymatic Antioxidant Activities
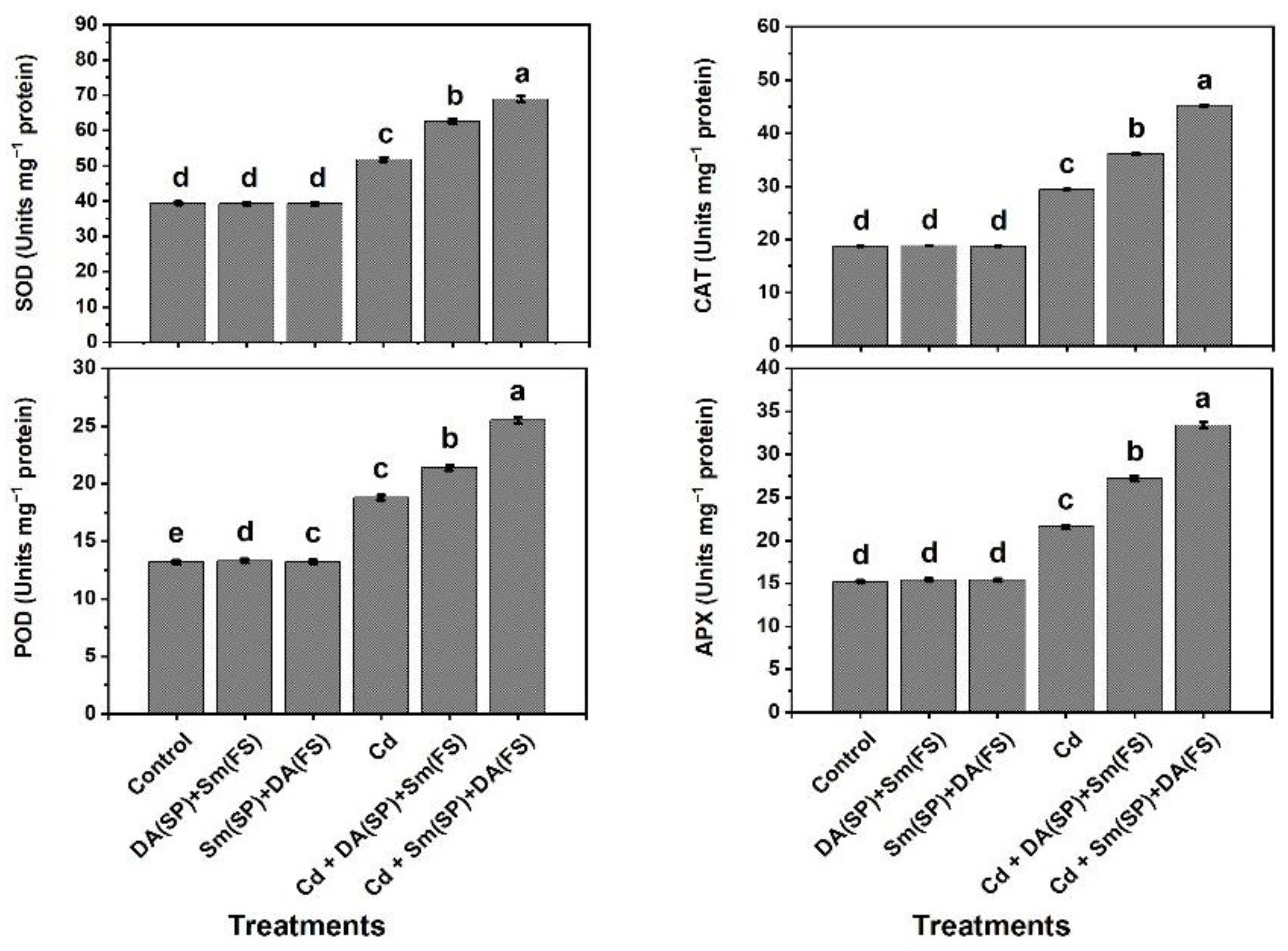
2.8. Evaluation of Antioxidant Enzymes’ Activity
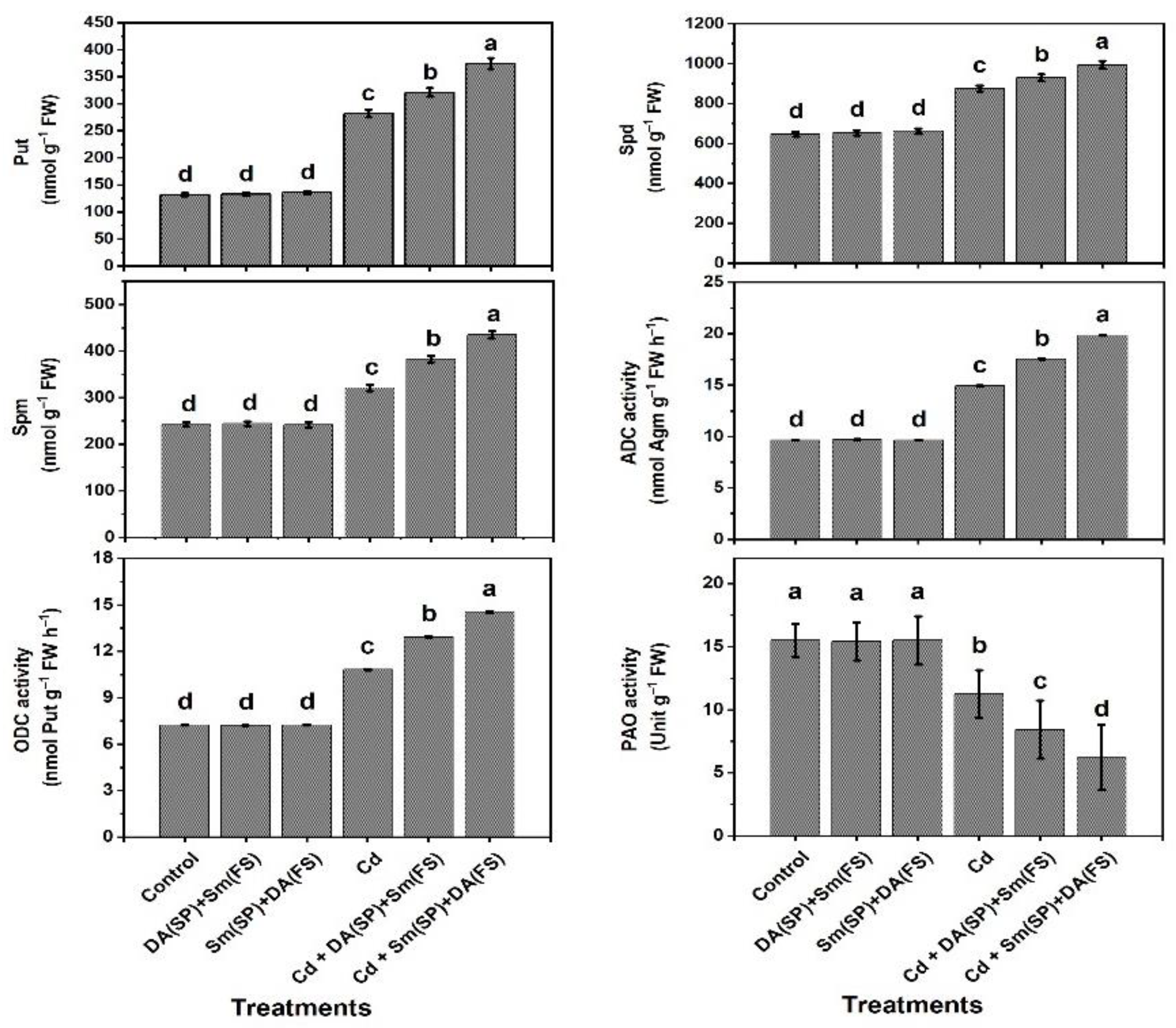
2.9. Evaluation of Polyamine Contents
2.10. Assaying for Polyamine Biosynthetic Enzymes and Polyamine Oxidase (PAO) Activities
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelfattah, M.A.; Rady, M.M.; Belal, H.E.E.; Belal, E.E.; Al-Qthanin, R.; Al-Yasi, H.M.; Ali, E.F. Revitalizing Fertility of Nutrient-Deficient Virgin Sandy Soil Using Leguminous Biocompost Boosts Phaseolus vulgaris Performance. Plants 2021, 10, 1637. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Semida, W.M.; Hemida, K.A.; Abdelhamid, M.T. The effect of compost on growth and yield of Phaseolus vulgaris plants grown under saline soil. Int. J. Recycl. Org. Waste Agric. 2016, 5, 311–321. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Rady, M.M.; Abd El-Mageed, T.A.; Howladar, S.M.; Abdelhamid, M.A. Alleviation of cadmium toxicity in common bean (Phaseolus vulgaris L.) plants by the exogenous application of salicylic acid. J. Hortic. Sci. Biotechnol. 2015, 90, 83–91. [Google Scholar]
- Piršelová, B.; Ondrušková, E. Effect of Cadmium Chloride and Cadmium Nitrate on Growth and Mineral Nutrient Content in the Root of Fava Bean (Vicia faba L.). Plants 2021, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Smeets, K.; Cuypers, A.; Lambrechts, A.; Semane, B.; Hoet, P.; Van Laere, A.; Vangronsveld, J. Induction of oxidative stress and antioxidative mechanisms in Phaseolus vulgaris after Cd application. Plant Physiol. Biochem. 2005, 43, 437–444. [Google Scholar] [CrossRef]
- Hu, J.Z.; Pei, D.L.; Liang, F.; Shi, G.X. Growth responses of Sagittaria sagittifolia L. plants to water contamination with cadmium. Russ. J. Plant Physiol. 2009, 56, 686–694. [Google Scholar] [CrossRef]
- Dobrikova, A.; Apostolova, E.; Adamakis, I.-D.S.; Hanc, A.; Sperdouli, I.; Moustakas, M. Combined Impact of Excess Zinc and Cadmium on Elemental Uptake, Leaf Anatomy and Pigments, Antioxidant Capacity, and Function of Photosynthetic Apparatus in Clary Sage (Salvia sclarea L.). Plants 2022, 11, 2407. [Google Scholar] [CrossRef]
- Ibrahim, W.; Zhu, Y.M.; Chen, Y.; Qiu, C.W.; Zhu, S.; Wu, F. Genotypic differences in leaf secondary metabolism, plant hormones and yield under alone and combined stress of drought and salinity in cotton genotypes. Physiol. Plant. 2019, 165, 343–355. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Rady, M.O.A.; Semida, W.M.; El-mageed, T.A.A.; Hemida, K.A.; Rady, M.M. Up-regulation of antioxidative defense systems by glycine betaine foliar application in onion plants confer tolerance to salinity stress. Sci. Hortic. 2018, 240, 614–622. [Google Scholar] [CrossRef]
- Al-Elwany, O.A.; Mohamed, G.F.; Abdehrahman, H.A.; Rady, M.M.; Abdel Latef, A.H. Exogenous glutathione-mediated tolerance to deficit irrigation stress in salt-affected Capsicum frutescence (L.) plants is connected with higher antioxidant content and proper ion homeostasis. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1957–1979. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Semida, W.M.; Rady, M.M.; Mohamed, G.F.; Hemida, K.A.B.A.A.; Hassan, M.M.; Shami, A. Sequential Application of Antioxidants Rectifies Ion Imbalance and Strengthens Antioxidant Systems in Salt-Stressed Cucumber. Plants 2020, 9, 1783. [Google Scholar] [CrossRef]
- Semida, W.M.; Hemida, K.A.; Rady, M.M. Sequenced ascorbate-proline-glutathione seed treatment elevates cadmium tolerance in cucumber transplants. Ecotoxicol. Environ. Saf. 2018, 154, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.; Alharby, H.F.; Bamagoos, A.A.; Abdelhamid, M.T.; Rady, M.M. Sequenced application of glutathione as an antioxidant with organic biostimulant improves physiological and metabolic adaptation to salinity in wheat. Plant Physiol. Biochem. 2021, 158, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Lan, G.; Sun, Y.; Wang, G.; Sun, Y. Dopamine Alleviates Chilling Stress in Watermelon Seedlings via Modulation of Proline Content, Antioxidant Enzyme Activity, and Polyamine Metabolism. J. Plant Growth Regul. 2021, 40, 277–292. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, T.; Liu, W.; Liu, Y.; Zhao, Y.; Liu, Y.; Li, W.; Ding, K.; Ma, F.; Li, C. Functions of dopamine in plants: A review. Plant Signal. Behav. 2020, 15, 1827782. [Google Scholar] [CrossRef]
- Alharby, H.F.; Al-zahrani, H.S.; Hakeem, K.R.; Alsamadany, H.; Desoky, M. Silymarin-Enriched Biostimulant Foliar Application Minimizes the Toxicity of Cadmium in Maize by Suppressing Oxidative Stress and Elevating Antioxidant Gene Expression. Biomolecules 2021, 11, 465. [Google Scholar] [CrossRef]
- Salman, E.K.; Badr, E.S.; Ghoniem, K.E.; Aboulila, A.A.; Emeran, A.A. Role of silymarin induced rice immunity against blast pathogen Magnaporthe oryzae through regulation of resistance genes expression. Physiol. Mol. Plant Pathol. 2021, 115, 101678. [Google Scholar] [CrossRef]
- Semida, W.M.; Rady, M.M. Pre-soaking in 24-epibrassinolide or salicylic acid improves seed germination, seedling growth, and anti-oxidant capacity in Phaseolus vulgaris L. grown under NaCl stress. J. Hortic. Sci. Biotechnol. 2014, 89, 338–344. [Google Scholar] [CrossRef]
- Saha, I.; Ghosh, A.; Dolui, D.; Fujita, M.; Hasanuzzaman, M.; Adak, M.K. Differential Impact of Nitric Oxide and Abscisic Acid on the Cellular and Physiological Functioning of sub1A QTL Bearing Rice Genotype under Salt Stress. Plants 2022, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Desoky, E.M.; Saad, A.M.; El-Saadony, M.T.; Merwad, A.M.; Rady, M.M. Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salt stress in Triticum aestivum (L.) plants. Biocatal. Agric. Biotechnol. 2020, 30, 101878. [Google Scholar] [CrossRef]
- Ghosh, A.; Saha, I.; Fujita, M.; Debnath, S.C.; Hazra, A.K.; Adak, M.K.; Hasanuzzaman, M. Photoactivated TiO2 Nanocomposite Delays the Postharvest Ripening Phenomenon through Ethylene Metabolism and Related Physiological Changes in Capsicum Fruit. Plants 2022, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Desoky, E.M.; Merwad, A.M.A.; ElSayed, A.I.; Rady, M.M. Stimulating antioxidant defenses, antioxidant gene expression, and salt tolerance in Pisum sativum seedling by pretreatment using licorice root extract (LRE) as an organic biostimulant. Plant Physiol. Biochem. 2019, 142, 292–302. [Google Scholar] [CrossRef]
- Rady, M.M.; Talaat, N.; Abdelhamid, M.T.; Shawky, B.T.; Desoky, E.M. Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J. Hortic. Sci. Biotechnol. 2019, 94, 777–789. [Google Scholar] [CrossRef]
- Elateeq, A.A.; Sun, Y.; Nxumalo, W.; Gabr, A.M.M. Biotechnological production of silymarin in Silybum marianum L.: A review. Biocatal. Agric. Biotechnol. 2020, 29, 101775. [Google Scholar] [CrossRef]
- Wadhwa, K.; Pahwa, R.; Kumar, M.; Kumar, S.; Sharma, P.C.; Singh, G.; Verma, R.; Mittal, V.; Singh, I.; Kaushik, D.; et al. Mechanistic Insights into the Pharmacological Significance of Silymarin. Molecules 2022, 27, 5327. [Google Scholar] [CrossRef]
- Tyagi, A.; Bhatia, N.; Condon, M.S.; Bosland, M.C.; Agarwal, C.; Agarwal, R. Antiproliferative and apoptotic effects of silibinin in rat prostate cancer cells. Prostate 2002, 53, 211–217. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, T.; Jain, S. Silymarin- a review on the pharmacodynamics and bioavailability enhancement approaches. J. Pharm. Sci. Technol. 2010, 2, 348–355. [Google Scholar]
- Kshirsagar, A.; Ingawale, D.; Ashok, P.; Vyawahare, N. Silymarin: A comprehensive review. Pharmacogn. Rev. 2009, 3, 126–134. [Google Scholar]
- Zalat, Z.A.; Kohaf, N.A.; Alm El-Din, M.; Elewa, H.A.; Abdel-Latif, M.; El-din, M.A.A.; Elewa, H.A.; Mohamed, M. Silymarin: A promising cardioprotective agent. Azhar Int. J. Pharm. Med. Sci. 2021, 1, 15–23. [Google Scholar] [CrossRef]
- Liang, B.; Gao, T.; Zhao, Q.; Ma, C.; Chen, Q.; Wei, Z.; Li, C.; Li, C.; Ma, F. Effects of exogenous dopamine on the uptake, transport, and resorption of apple ionome under moderate drought. Front. Plant Sci. 2018, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Kulma, A.; Szopa, J. Catecholamines are active compounds in plants. Plant Sci. 2007, 172, 433–440. [Google Scholar] [CrossRef]
- Abdelkader, A.F.; El-khawas, S.; El-Sherif, N.A.S.E.; Hassanein, R.A.; Emam, M.A.; Hassan, R.E. Expression of aquaporin gene (Os PIP1-3) in salt-stressed rice (Oryza sativa L.) plants pre-treated with the neurotransmitter (dopamine). Plant Omics 2012, 5, 532–541. [Google Scholar]
- Swiedrych, A.; Lorenc-Kukula, K.; Skirycz, A.; Szopa, J. The catecholamine biosynthesis route in potato is affected by stress. Plant Physiol. Biochem. 2004, 42, 593–600. [Google Scholar] [CrossRef]
- Li, C.; Sun, X.K.; Chang, C.; Jia, D.F.; Wei, Z.W.; Li, C.Y. Dopamine alleviates salt-induced stress in Malus hupehensis. Physiol. Plant. 2015, 153, 584–602. [Google Scholar] [CrossRef]
- Rady, M.M.; Rehman, H. Supplementing organic biostimulants into growing media enhances growth and nutrient uptake of tomato transplants. Sci. Hortic. 2016, 203, 192–198. [Google Scholar] [CrossRef]
- Konrad, M.L.F.; Silva, J.A.B.; Furlani, P.R.; Machado, E.C. Trocas gasosas e fluorescência da clorofila em seis cultivares de cafeeiro sob estresse de alumínio. Bragantia 2005, 64, 30–37. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll-a and chlorophyll-b, as well as total carotinoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Osman, A.S.; Rady, M.M. Exogenously-applied sulphur and ascorbic acid positively altered their endogenous concentrations, and increased growth and yield in Cucurbita pepo L. plants grown on a newly-reclaimed saline soil. J. Biotechnol. Sci. 2014, 2, 1–9. [Google Scholar]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 207, 205–207. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Emerich, D.W.; Sanchezdiaz, M. Water-Stress Induced Changes in Concentrations of Proline and Total Soluble Sugars in Nodulated Alfalfa (Medicago sativa) Plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Wheeler, R.M.; Stutte, G.W.; Levine, L.H. How far can sodium substitute for potassium in red beet? J. Plant Nutr. 1999, 22, 1745–1761. [Google Scholar] [CrossRef]
- Bessieres, M.A.; Gibon, Y.; Lefeuvre, J.C.; Larher, F. A single-step purification for glycine betaine determination in plant extracts by isocratic HPLC. J. Agric. Food Chem. 1999, 47, 3718–3722. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Khan, A.L.; Shahzad, R.; Asaf, S.; Kang, S.M.; Lee, I.J. Endophytic Paecilomyces formosus LHL10 augments Glycine max L. adaptation to Ni-contamination through affecting endogenous phytohormones and oxidative Stress. Front. Plant Sci. 2017, 8, 870. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Kubiś, J. Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J. Plant Physiol. 2008, 165, 397–406. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation isolated chloroplasts: Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soil, Plants and Water. Soil Sci. 1961, 93, 68. [Google Scholar] [CrossRef]
- Arampatzis, D.A.; Karkanis, A.C.; Tsiropoulos, N.G. Silymarin content and antioxidant activity of seeds of wild Silybum marianum populations growing in Greece. Ann. Appl. Biol. 2019, 174, 61–73. [Google Scholar] [CrossRef]
- Arampatzis, D.A.; Karkanis, A.C.; Tsiropoulos, N.G. Impact of Plant Density and Mepiquat Chloride on Growth, Yield, and Silymarin Content of Silybum marianum Grown under Mediterranean Semi-Arid Conditions. Agronomy 2019, 9, 669. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, T.H.; Hong, S.H.; Kim, H.G. Direct detection of tetrahydrobiopterin (BH4) and dopamine in rat brain using liquid chromatography coupled electrospray tandem mass spectrometry. Biochem. Biophys. Res. Commun. 2012, 419, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Kampfenkel, K.; Vanmontagu, M.; Inze, D. Extraction and Determination of Ascorbate and Dehydroascorbate from Plant Tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Griffth, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2 vinyl pyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Konings, E.J.; Roomans, H.H.; Beljaars, P.R. Liquid chromatographic determination of tocopherols and tocotrienols in margarine, infant foods, and vegetables. J. AOAC Int. 1996, 79, 902–906. [Google Scholar] [CrossRef]
- Ching, L.S.; Mohamed, S. Alpha-tocopherol content in 62 edible tropical plants. J. Agric. Food Chem. 2001, 49, 3101–3105. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Ali, Q.; Ali, S.; Iqbal, N.; Javed, M.T.; Rizwan, M.; Khaliq, R.; Shahid, S.; Perveen, R.; Alamri, S.A.; Alyemeni, M.N.; et al. Alpha-tocopherol fertigation confers growth physio-biochemical and qualitative yield enhancement in feld grown water defcit wheat (Triticum aestivum L.). Sci. Rep. 2019, 9, 12924. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Chandlee, J.M.; Scandalios, J.G. Analysis of variants afecting the catalase developmental program in maize scutellum. Theor. Appl. Genet. 1984, 69, 71–77. [Google Scholar] [CrossRef]
- Asada, K.; Takahashi, M. Production and scavenging of active oxygen in chloroplasts. In Photoinhibition; Kyle, D.J., Osmond, C.B., Arntzen, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 227–287. [Google Scholar]
- Kotzabasis, K.; Christakis-Hampsas, M.D.; Roubelakis-Angelakis, K.A. A narrow-bore HPLC method for the identifcation and quantitation of free, conjugated, and bound polyamines. Anal. Biochem. 1993, 214, 484. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Sun, C.; Liu, Y.; Zhang, W. Relationship between polyamine in roots and salt tolerance of barley seedlings. Acta Bot. Sin. 2003, 45, 295–300. [Google Scholar]
- Song, Y.; Diao, Q.; Qi, H. Putrescine enhances chilling tolerance of tomato (Lycopersicon esculentum Mill.) through modulating antioxidant systems. Acta Physiol. Plant. 2014, 36, 3013–3027. [Google Scholar] [CrossRef]
- Song, Y.; Diao, Q.; Qi, H. Polyamine metabolism and biosynthetic genes expression in tomato (Lycopersicon esculentum Mill.) seedlings during cold acclimation. Plant Growth Regul. 2014, 75, 21–32. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: Singapore, 1984. [Google Scholar]
- Desoky, E.M.; Elrys, A.S.; Mansour, E.; Eid, R.S.M.; Selem, E.; Rady, M.M.; Ali, E.F.; Mersal, G.A.M.; Semida, W.M. Application of biostimulants promotes growth and productivity by fortifying the antioxidant machinery and suppressing oxidative stress in faba bean under various abiotic stresses. Sci. Hortic. 2021, 288, 110340. [Google Scholar] [CrossRef]
- Gheshlaghpour, J.; Asghari, B.; Khademian, R.; Sedaghati, B. Silicon alleviates cadmium stress in basil (Ocimum basilicum L.) through alteration of phytochemical and physiological characteristics. Ind. Crops Prod. 2021, 163, 113338. [Google Scholar] [CrossRef]
- Hediji, H.; Kharbech, O.; Massoud, M.; Ben Boukari, N.; Debez, A.; Chaibi, W.; Chaoui, A.; Djebali, W. Salicylic acid mitigates cadmium toxicity in bean (Phaseolus vulgaris L.) seedlings by modulating cellular redox status. Environ. Exp. Bot. 2021, 186, 104432. [Google Scholar] [CrossRef]
- Wei, T.; Sun, Y.; Yashir, N.; Li, X.; Guo, J.; Liu, X.; Jia, H.L.; Ren, X.; Hua, L. Inoculation with Rhizobacteria Enhanced Tolerance of Tomato (Solanum lycopersicum L.) Plants in Response to Cadmium Stress. J. Plant Growth Regul. 2022, 41, 445–460. [Google Scholar] [CrossRef]
- Sardar, R.; Ahmed, S.; Yasin, N.A. Role of exogenously applied putrescine in amelioration of cadmium stress in Coriandrum sativum by modulating antioxidant system. Int. J. Phytoremediation 2022, 24, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Hemida, K.A. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol. Environ. Saf. 2015, 119, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, K.; Du Laing, G.; Van Damme, E.J.M. Sodium selenate treatment using a combination of seed priming and foliar spray alleviates salinity stress in rice. Front. Plant Sci. 2019, 10, 116. [Google Scholar] [CrossRef]
- Lan, G.; Jiao, C.; Wang, G.; Sun, Y.; Sun, Y. Effects of dopamine on growth, carbon metabolism, and nitrogen metabolism in cucumber under nitrate stress. Sci. Hortic. 2020, 260, 108790. [Google Scholar] [CrossRef]
- Zahra, N.; Wahid, A.; Hafeez, M.B.; Lalarukh, I.; Batool, A.; Uzair, M.; El-Sheikh, M.A.; Alansi, S.; Kaushik, P. Effect of Salinity and Plant Growth Promoters on Secondary Metabolism and Growth of Milk Thistle Ecotypes. Life 2022, 12, 1530. [Google Scholar] [CrossRef]
- Keshavarz Afshar, R.; Chaichi, M.R.; Ansari Jovini, M.; Jahanzad, E.; Hashemi, M. Accumulation of silymarin in milk thistle seeds under drought stress. Planta 2015, 242, 539–543. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Zhang, X.; Liu, C.; Liang, W.; Li, C.; Ma, F.; Li, C. Exogenous dopamine application promotes alkali tolerance of apple seedlings. Plants 2019, 8, 580. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Desoky, E.M.; Osman, A.M.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Jodko-Piórecka, K.; Sikora, B.; Kluzek, M.; Przybylski, P.; Litwinienko, G. Antiradical Activity of Dopamine, L-DOPA, Adrenaline, and Noradrenaline in Water/Methanol and in Liposomal Systems. J. Org. Chem. 2022, 87, 1791–1804. [Google Scholar] [CrossRef]
- Guidotti, B.B.; Gomes, B.R.; de cássia Siqueira-Soares, R.D.; Soares, A.R.; Ferrarese-Filho, O. The effects of dopamine on root growth and enzyme activity in soybean seedlings. Plant Signal. Behav. 2013, 8, e25477. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.W.; Li, C.Y.; Ma, C.Q.; Wei, Z.W.; Wang, Q.; Huang, D. Dopamine alleviates nutrient deficiency-induced stress in Malus hupehensis. Plant Physiol. Biochem. 2017, 119, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Sreedharan, S.; Shekhawat, U.K.S.; Ganapathi, T.R. Transgenic banana plants overexpressing a native plasma membrane aquaporin MusaPIP1;2 display high tolerance levels to different abiotic stresses. Plant Biotechnol. J. 2013, 11, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.A.; Marey, R.A.; El-Mageed, T.A.A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Rady, M.M.; Taha, R.S.; Semida, W.M.; Alharby, H.F. Modulation of salt stress effects on Vicia faba L. plants grown on a reclaimed-saline soil by salicylic acid application. Rom. Agric. Res. 2017, 34, 175–185. [Google Scholar]
- Semida, W.M.; El-Mageed, T.A.A.; Mohamed, S.E.; El-Sawah, N.A. Combined effect of deficit irrigation and foliar-applied salicylic acid on physiological responses, yield, and water-use efficiency of onion plants in saline calcareous soil. Arch. Agron. Soil Sci. 2017, 63, 1227–1239. [Google Scholar] [CrossRef]
- Iqbal, N.; Nazar, R.; Khan, N.A. Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies, Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Pal, M.; Tajti, J.; Szalai, G.; Peeva, V.; Vegh, B.; Janda, T. Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci. Rep. 2018, 8, 12812–12839. [Google Scholar] [CrossRef]
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How Do Plants Respond to Combined Drought and Salinity Stress?—A Systematic Review. Plants 2022, 11, 2884. [Google Scholar] [CrossRef]
- Rady, M.M.; Boriek, S.H.K.; Abd El-Mageed, T.A.; Seif El-Yazal, M.A.; Ali, E.F.; Hassan, F.A.S.; Abdelkhalik, A. Exogenous Gibberellic Acid or Dilute Bee Honey Boosts Drought Stress Tolerance in Vicia faba by Rebalancing Osmoprotectants, Antioxidants, Nutrients, and Phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, Q.; Shah, F.A.; Liu, W.; Wang, D.; Huang, S.; Fu, S.; Wu, L. Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Skórzyńska-Polit, E.; Dra̧żkiewicz, M.; Krupa, Z. The activity of the antioxidative system in cadmium-treated Arabidopsis thaliana. Biol. Plant. 2004, 47, 71–78. [Google Scholar] [CrossRef]
- Desoky, E.M.; Merwad, A.M.; Semida, W.M.; Ibrahim, S.A.; El-Saadony, M.T.; Rady, M.M. Heavy metals-resistant bacteria (HM-RB): Potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol. Environ. Saf. 2020, 198, 110685. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Desoky, E.M.; El-Maghraby, L.M.M.; Awad, A.E.; Abdo, A.I.; Rady, M.M.; Semida, W.M. Fennel and ammi seed extracts modulate antioxidant defence system and alleviate salinity stress in cowpea (Vigna unguiculata). Sci. Hortic. 2020, 272, 109576. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-mageed, T.A.; Howladar, S.M.; Rady, M.M. Foliar-applied ɑ-tocopherol enhances salt-tolerance in onion plants by improving antioxidant defence system. Aust. J. Crop Sci. 2016, 10, 1030–1039. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Borusiewicz, A.; Lozowicka, B. Fluazinam and its mixtures induce diversified changes of crucial biochemical and antioxidant profile in leafy vegetable. Sci. Hort. 2022, 298, 110988. [Google Scholar] [CrossRef]
- Semida, W.M.; Taha, R.S.; Abdelhamid, M.T.; Rady, M.M. Foliar-applied α-tocopherol enhances salt-tolerance in Vicia faba L. plants grown under saline conditions. S. Afr. J. Bot. 2014, 95, 24–31. [Google Scholar] [CrossRef]
- Yang, J.; Wang, P.; Li, S.; Liu, T.; Hu, X. Polyamine Oxidase Triggers H2O2-Mediated Spermidine Improved Oxidative Stress Tolerance of Tomato Seedlings Subjected to Saline-Alkaline Stress. Int. J. Mol. Sci. 2022, 23, 1625. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, Y.; Rady, M.M. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol. Environ. Saf. 2019, 182, 109378. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; Giri, K. Molecular phylogenomic study and the role of exogenous spermidine in the metabolic adjustment of endogenous polyamine in two rice cultivars under salt stress. Gene 2017, 609, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Singh, R.P. Cadmium-induced Changes in Diamine Oxidase Activity and Polyamine Levels in Vigna radiata Wilczek Seedlings. J. Plant Physiol. 2000, 156, 704–710. [Google Scholar] [CrossRef]
- Gao, C.; Sheteiwy, M.S.; Lin, C.; Guan, Y.; Ulhassan, Z.; Hu, J. Spermidine Suppressed the Inhibitory Effects of Polyamines Inhibitors Combination in Maize (Zea mays L.) Seedlings under Chilling Stress. Plants 2021, 10, 2421. [Google Scholar] [CrossRef]






| Treatment | Sm (mg kg−1 DW) | DA (μg kg−1 DW) |
|---|---|---|
| Control | 11.1 ± 0.4 d | 18.7 ± 0.02 d |
| DA (SP) + Sm (FS) | 28.9 ± 0.9 b | 18.8 ± 0.03 d |
| Sm (SP) + DA (FS) | 15.7 ± 0.6 c | 18.7 ± 0.03 d |
| Cadmium (Cd) | 15.8 ± 0.6 c | 29.4 ± 0.05 c |
| Cd + DA (SP) + Sm (FS) | 36.2 ± 1.2 a | 36.1 ± 0.07 b |
| Cd + Sm (SP) + DA (FS) | 28.4 ± 0.8 b | 45.2 ± 0.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulmajeed, A.M.; Alharbi, B.M.; Alharby, H.F.; Abualresh, A.M.; Badawy, G.A.; Semida, W.M.; Rady, M.M. Simultaneous Action of Silymarin and Dopamine Enhances Defense Mechanisms Related to Antioxidants, Polyamine Metabolic Enzymes, and Tolerance to Cadmium Stress in Phaseolus vulgaris. Plants 2022, 11, 3069. https://doi.org/10.3390/plants11223069
Abdulmajeed AM, Alharbi BM, Alharby HF, Abualresh AM, Badawy GA, Semida WM, Rady MM. Simultaneous Action of Silymarin and Dopamine Enhances Defense Mechanisms Related to Antioxidants, Polyamine Metabolic Enzymes, and Tolerance to Cadmium Stress in Phaseolus vulgaris. Plants. 2022; 11(22):3069. https://doi.org/10.3390/plants11223069
Chicago/Turabian StyleAbdulmajeed, Awatif M., Basmah M. Alharbi, Hesham F. Alharby, Amani M. Abualresh, Ghada A. Badawy, Wael M. Semida, and Mostafa M. Rady. 2022. "Simultaneous Action of Silymarin and Dopamine Enhances Defense Mechanisms Related to Antioxidants, Polyamine Metabolic Enzymes, and Tolerance to Cadmium Stress in Phaseolus vulgaris" Plants 11, no. 22: 3069. https://doi.org/10.3390/plants11223069
APA StyleAbdulmajeed, A. M., Alharbi, B. M., Alharby, H. F., Abualresh, A. M., Badawy, G. A., Semida, W. M., & Rady, M. M. (2022). Simultaneous Action of Silymarin and Dopamine Enhances Defense Mechanisms Related to Antioxidants, Polyamine Metabolic Enzymes, and Tolerance to Cadmium Stress in Phaseolus vulgaris. Plants, 11(22), 3069. https://doi.org/10.3390/plants11223069






