Identification of Key Modules and Candidate Genes for Powdery Mildew Resistance of Wheat-Agropyron cristatum Translocation Line WAT-2020-17-6 by WGCNA
Abstract
1. Introduction
2. Results
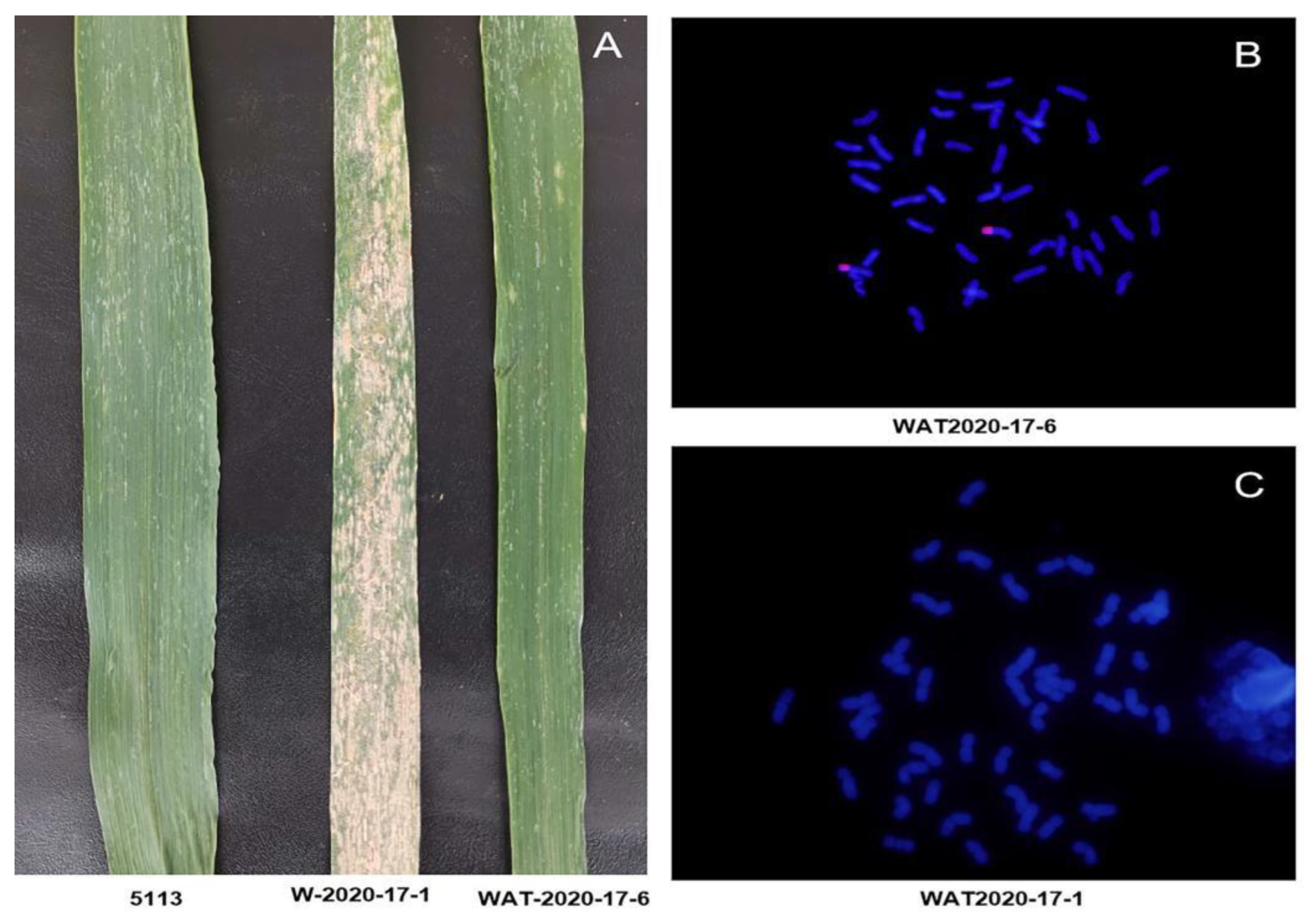
2.1. Identification of Disease Resistance and Cytology
2.2. Data Collection and Preprocessing
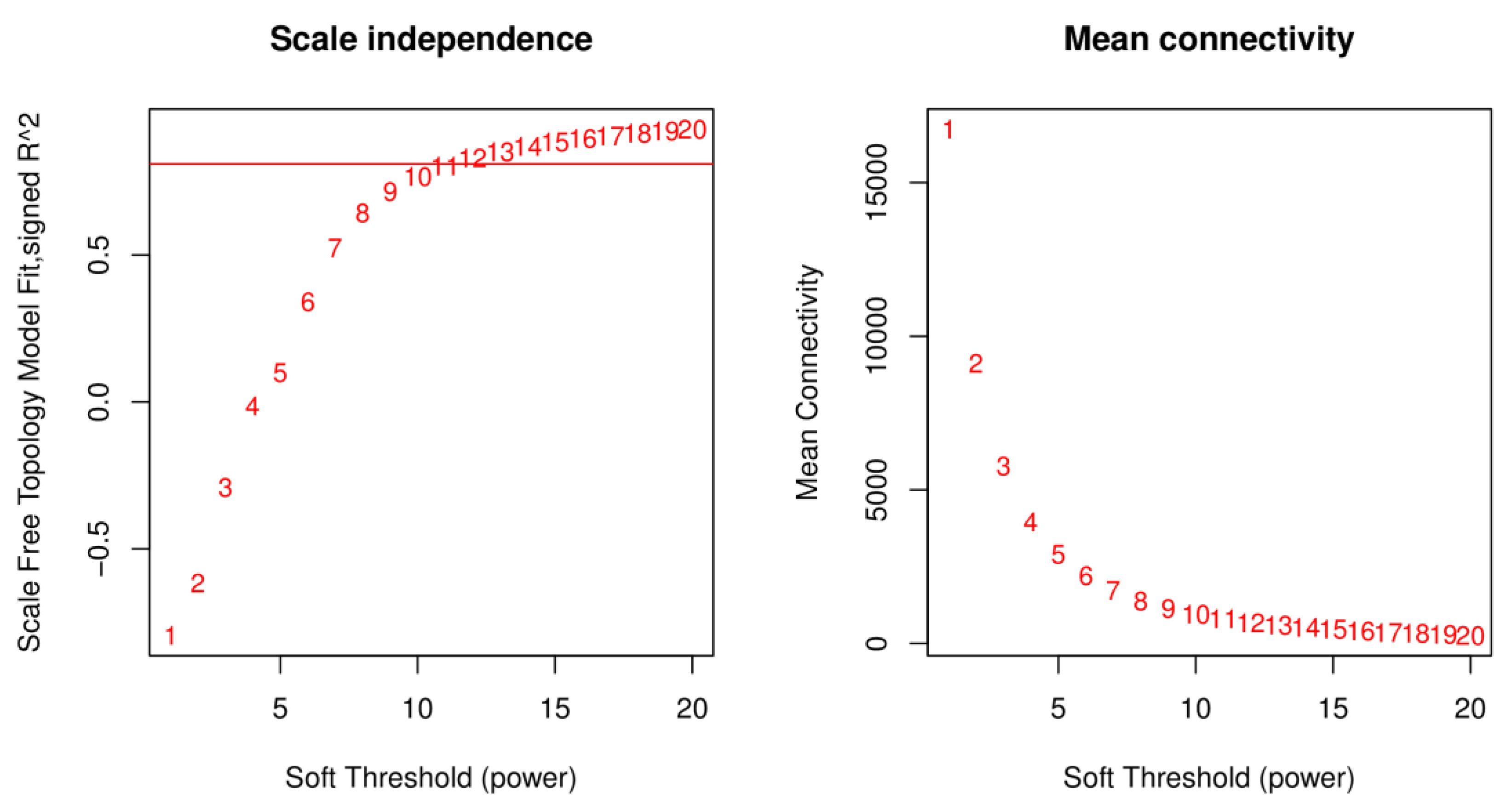
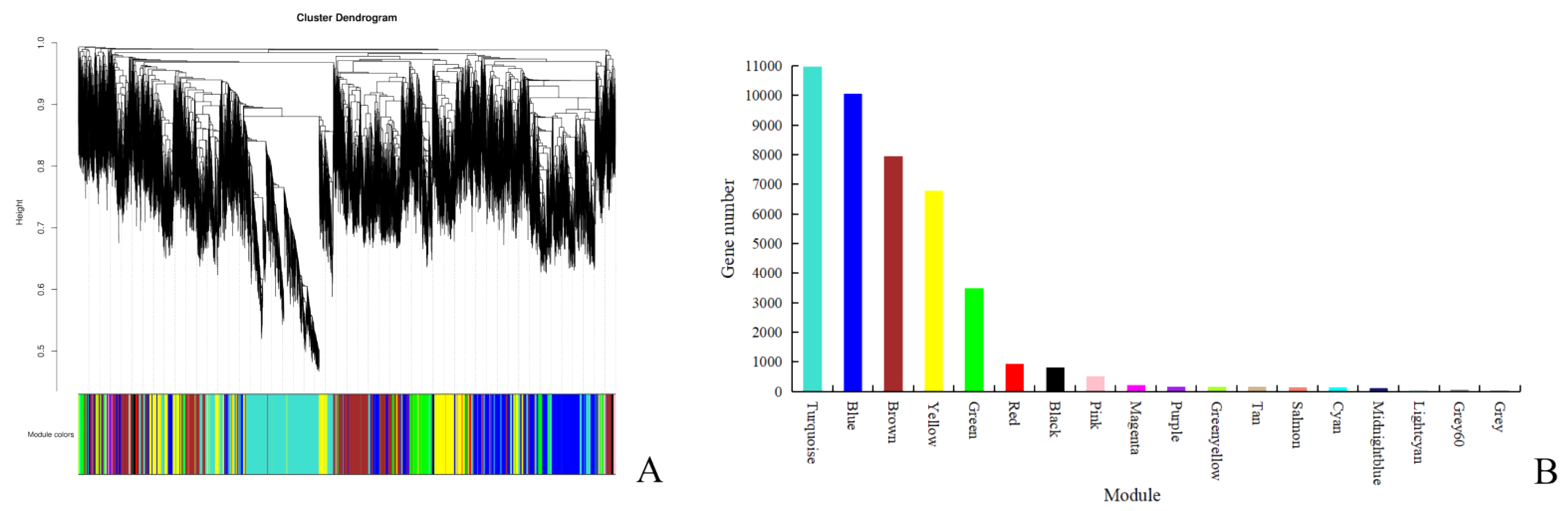
2.3. Weighted Gene Co-Expression Network Construction
2.4. Correlation Analysis to Obtain the Interested Modules
2.5. Enrichment Analysis of Genes in Interested Modules
2.6. Identification of Hub Genes
2.7. Validation of Hub Genes by RT-PCR
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chromosome Preparation
4.3. Infection Experiment, RNA Extraction, and Sequencing
4.4. Data Collection and Preprocessing
4.5. Weighted Gene Co-Expression Network Construction
4.6. Screening and Functional Analysis of Hub Genes
4.7. Validation of Hub Genes by RT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobell, D.B.; Gourdji, S.M. The influence of climate change on global crop productivity. Plant Physiol. 2012, 160, 1686–1697. [Google Scholar] [CrossRef]
- Bekele, S.; Melinda, S.; Hans-Joachim, B.; Etienne, D.; Mathew, R.; Geoffrey, M. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar]
- Mobley, A.R.; Slavin, J.L.; Hornick, B.A. The future of recommendations on grain foods in dietary guidance. J. Nutr. 2013, 143, 1527S–1532S. [Google Scholar] [CrossRef]
- Han, Y. Causes of wheat powdery mildew and comprehensive control measures. New Technol. New Prod. China 2018, 18, 133–134. [Google Scholar]
- Feng, W.; Li, X.; Liu, W.; Wang, X.; Wang, C.; Gou, T. Effects of powdery mildew infection on grain quality traits and yield of winter wheat. J. Triticeae Crops 2014, 34, 1706–1712. [Google Scholar]
- Goyal, A.; Manoharachary, C. Future Challenges in Crop Protection against Fungal Pathogens; Springer: Berlin/Heidelberg, Germany, 2014; p. 364. [Google Scholar]
- Selter, L.; Shatalina, M.; Singla, J.; Keller, B. Identifification and implementation of resistance: Genomics-assisted use of genetic resources for breeding against powdery mildew and stagonospora nodorum blotch in wheat. In Genomics of Plant Genetic Resources; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Ma, P.; Xu, H.; Xu, Y.; Li, L.; Qie, Y.; Luo, Q.; Zhang, X.; Li, X.; Zhou, Y.; An, D. Molecular mapping of a new powdery mildew resistance gene Pm2b in Chinese breeding line KM2939. Theor. Appl. Genet. 2015, 128, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Hurni, S.; Brunner, S.; Stirnweis, D.; Herren, G.; Peditto, D.; McIntosh, R.A.; Keller, B. The powdery mildew resistance gene Pm8 derived from rye is suppressed by its wheat ortholog Pm3. Plant J. 2014, 79, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Zeller, F.J.; Hsam, S.L. Chromosomal location of a gene suppressing powdery mildew resistance genes Pm8 and Pm17 in common wheat (Triticum aestivum L. em. Thell.). Theor. Appl. Genet. 1996, 93, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Huagang, H.; Shanying, Z.; Renhui, Z.; Zhengning, J.; Yaoyong, J.; Jian, J.; Dan, Q.; Hongjie, L.; Tongde, B. Pm21, Encoding a typical CC-NBS-LRR protein, confers broad-spectrum resistance to wheat powdery mildew disease. Mol. Plant 2018, 11, 879–882. [Google Scholar]
- Zeller, F.J.; Kong, L.; Hartl, L.; Mohler, V.; Hsam, S. Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell.) 7. gene Pm29 in line pova. Euphytica 2002, 123, 187–194. [Google Scholar] [CrossRef]
- Miranda, L.M.; Murphy, J.P.; Marshall, D.; Leath, S. Pm34: A new powdery mildew resistance gene transferred from Aegilops tauschii Coss. to common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2006, 113, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.M.; Murphy, J.P.; Marshall, D.; Cowger, C.; Leath, S. Chromosomal location of Pm35, a novel Aegilops tauschii derived powdery mildew resistance gene introgressed into common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 114, 1451–1456. [Google Scholar] [CrossRef]
- Luo, P.G.; Luo, H.Y.; Chang, Z.J.; Zhang, H.Y.; Zhang, M.; Ren, Z.L. Characterization and chromosomal location of Pm40 in common wheat: A new gene for resistance to powdery mildew derived from Elytrigia intermedium. Theor. Appl. Genet. 2009, 118, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Chang, Z.; Yang, Z.; Yuan, Z.; Zhan, H.; Zhang, X.; Liu, J. Inheritance and mapping of powdery mildew resistance gene Pm43 introgressed from Thinopyrum intermedium into wheat. Theor. Appl. Genet. 2009, 118, 1173–1180. [Google Scholar] [CrossRef]
- Haixian, Z.; Guangrong, L.; Xiaojun, Z.; Xin, L.; Huijuan, G.; Wenping, G.; Juqing, J.; Linyi, Q.; Yongkang, R.; Zujun, Y.; et al. Chromosomal location and comparative genomics analysis of powdery mildew resistance gene Pm51 in a putative wheat-Thinopyrum ponticum introgression line. PLoS ONE 2017, 9, e113455. [Google Scholar]
- Zhang, R.; Sun, B.; Chen, J.; Cao, A.; Xing, L.; Feng, Y.; Lan, C.; Chen, P. Pm55, a developmental-stage and tissue-specific powdery mildew resistance gene introgressed from Dasypyrum villosum into common wheat. Theor. Appl. Genet. 2016, 129, 1975–1984. [Google Scholar] [CrossRef]
- Hao, M.; Liu, M.; Luo, J.; Fan, C.; Yi, Y.; Zhang, L.; Yuan, Z.; Ning, S.; Zheng, Y.; Liu, D. Introgression of powdery mildew resistance gene Pm56 on rye chromosome arm 6RS into wheat. Front. Plant Sci. 2018, 9, 1040. [Google Scholar] [CrossRef]
- Wiersma, A.T.; Pulman, J.A.; Brown, L.K.; Cowger, C.; Olson, E.L. Identification of Pm58 from Aegilops tauschii. Theor. Appl. Genet. 2017, 130, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Fan, Y.; Kong, L.; Wang, Z.; Wu, J.; Xing, L.; Cao, A.; Feng, Y. Pm62, an adult-plant powdery mildew resistance gene introgressed from Dasypyrum villosum chromosome arm 2VL into wheat. Theor. Appl. Genet. 2018, 131, 2613–2620. [Google Scholar] [CrossRef]
- Li, H.; Dong, Z.; Ma, C.; Xia, Q.; Tian, X.; Sehgal, S.; Koo, D.; Friebe, B.; Ma, P.; Liu, W. A spontaneous wheat-Aegilops longissima translocation carrying Pm66 confers resistance to powdery mildew. Theor. Appl. Genet. 2020, 133, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Ruiqi, Z.; Chuanxi, X.; Huanqing, M.; Ruonan, Y.; Xiangru, M.; Lingna, K.; Liping, X.; Jizhong, W.; Yigao, F.; Aizhong, C. Pm67, a new powdery mildew resistance gene transferred from Dasypyrum villosum chromosome 1V to common wheat (Triticum aestivum L.). Crop J. 2021, 9, 882–888. [Google Scholar]
- Li, L.; Yang, X.; Li, X.; Dong, Y. Study and utilization of wild relatives of wheat in China. Rev. China Agric. Sci. Technol. 2000, 2, 73–76. [Google Scholar]
- Li, H.; Jiang, B.; Wang, J.; Lu, Y.; Zhang, J.; Pan, C.; Yang, X.; Li, X.; Liu, W.; Li, L. Mapping of novel powdery mildew resistance gene(s) from Agropyron cristatum chromosome 2P. Theor. Appl. Genet. 2017, 130, 109–121. [Google Scholar] [CrossRef]
- Wu, J.; Yang, X.; Wang, H.; Li, H.; Li, L.; Li, X.; Liu, W. The introgression of chromosome 6P specifying for increased numbers of florets and kernels from Agropyron cristatum into wheat. Theor. Appl. Genet. 2006, 114, 13–20. [Google Scholar] [CrossRef]
- Pan, C.; Li, Q.; Lu, Y.; Zhang, J.; Yang, X.; Li, X.; Li, L.; Liu, W. Chromosomal localization of genes conferring desirable agronomic traits from Agropyron cristatum chromosome 1P. PLoS ONE 2017, 12, e0175265. [Google Scholar] [CrossRef]
- Luan, Y.; Wang, X.; Liu, W.; Li, C.; Zhang, J.; Gao, A.; Wang, Y.; Yang, X.; Li, L. Production and identification of wheat-Agropyron cristatum 6P translocation lines. Planta 2010, 232, 501–510. [Google Scholar] [CrossRef]
- Li, H.; Lv, M.; Song, L.; Zhang, J.; Gao, A.; Li, L.; Liu, W. Production and identification of wheat-Agropyron cristatum 2P translocation lines. PLoS ONE 2016, 11, e145928. [Google Scholar] [CrossRef]
- Ye, X.; Lu, Y.; Liu, W.; Chen, G.; Han, H.; Zhang, J.; Yang, X.; Li, X.; Gao, A.; Li, L. The effects of chromosome 6P on fertile tiller number of wheat as revealed in wheat-Agropyron cristatum chromosome 5A/6P translocation lines. Theor. Appl. Genet. 2015, 128, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Jiang, L.; Han, H.; Gao, A.; Yang, X.; Li, L.; Liu, W. Efficient induction of wheat-Agropyron cristatum 6P translocation lines and GISH detection. PLoS ONE 2013, 8, e69501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Liu, W.; Wu, X.; Yang, X.; Li, X.; Lu, Y.; Li, L. An intercalary translocation from Agropyron cristatum 6P chromosome into common wheat confers enhanced kernel number per spike. Planta 2016, 244, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, J.; Han, H.; Zhang, J.; Ma, H.; Zhang, Z.; Lu, Y.; Liu, W.; Yang, X.; Li, X.; et al. Full-length transcriptome sequences of Agropyron cristatum facilitate the prediction of putative genes for thousand-grain weight in a wheat-A. cristatum translocation line. BMC Genom. 2019, 20, 1025. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lyu, M.; Han, H.; Zhou, S.; Lu, Y.; Liu, W.; Yang, X.; Li, X.; Zhang, J.; Liu, X.; et al. Identification and fine mapping of alien fragments associated with enhanced grain weight from Agropyron cristatum chromosome 7P in common wheat backgrounds. Theor. Appl. Genet. 2021, 134, 3759–3772. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yao, M.; Zhang, J.; Song, L.; Liu, W.; Yang, X.; Li, X.; Li, L. Genetic analysis of a novel broad-spectrum powdery mildew resistance gene from the wheat-Agropyron cristatum introgression line Pubing 74. Planta 2016, 244, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, S.; Liu, W.; Song, L.; Zhang, J.; Han, H.; Yang, X.; Lin, Y.; Li, X.; Li, L. Molecular Cytogenetic Analysis of the introgression between Agropyron cristatum P genome and wheat genome. Int. J. Mol. Sci. 2021, 22, 11208. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Yang, X.; Liu, W.; Li, X.; Han, H.; Zhou, S. Research progress of remote hybridization and new germplasm creation between wheat and Agropyro. In Proceedings of the 10th National Conference on Wheat Genomics and Molecular Breeding, Yantai, China, 11 August 2019. [Google Scholar]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Zhang, B.; Horvath, S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 2005, 4, e17. [Google Scholar] [CrossRef]
- Lin, C.T.; Xu, T.; Xing, S.L.; Zhao, L.; Sun, R.Z.; Liu, Y.; Moore, J.P.; Deng, X. Weighted Gene Co-expression Network analysis (WGCNA) reveals the hub role of protein ubiquitination in the acquisition of desiccation tolerance in Boea hygrometrica. Plant Cell Physiol. 2019, 60, 2707–2719. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Han, K.; Zhang, G.; Wang, J.; Xie, K.; Xue, Q. Genome-wide analysis of lncRNA and mRNA expression during differentiation of abdominal preadipocytes in the chicken. G3-Genes Genom. Genet. 2017, 7, 953–966. [Google Scholar] [CrossRef]
- Zheng, J.; He, C.; Qin, Y.; Lin, G.; Park, W.D.; Sun, M.; Li, J.; Lu, X.; Zhang, C.; Yeh, C.; et al. Co-expression analysis aids in the identification of genes in the cuticular wax pathway in maize. Plant J. 2019, 97, 530–542. [Google Scholar] [CrossRef]
- Li, T.; Wang, W.; Chen, O.; Yao, S.; Zeng, K. Recent advances in understanding the role of AP2/ERF transcription factors in regulating postharvest diseases of fruits and vegetables. Food Sci. 2022, 43, 312–319. [Google Scholar]
- Xu, T.; Xia, D.; Wang, J.; Jiang, S.; Song, J. Research progress of F-box protein involved in plant stress. Biotechnol. Bull. 2021, 37, 205–211. [Google Scholar]
- Pi, B.; Ruan, Y.; Huang, Y. Research progress and bioinformatics analysis of RR-TZF family of plant tandem CCCH zinc finger proteins. Mol. Plant Breed. 2019, 17, 2171–2177. [Google Scholar]
- Wei, X.; Lan, H. Advances in the regulation of plant MYB transcription factors in secondary metabolism and stress response. Biotechnol. Bull. 2022, 38, 12. [Google Scholar]
- Ren, Z. Issues and suggestions about technique and policy for crop breeding in Sichuan region. Southwest China J. Agric. Sci. 1995, 119–125. [Google Scholar]
- Hao, C.; Wang, L.; Zhang, X.; You, G.; Dong, Y. Evolution of genetic diversity of cultivated wheat varieties. Sci. China Ser. C 2005, 27–34. [Google Scholar]
- Xu, G.; Wang, Y.; Chen, J.; Guo, C. The study of inducing wheat-Thinopyrum Elongatum 7E chromosome translocations by gametocidal chromosome. Chin. Master’s Full-Text Database 2008, 10, 88–93. [Google Scholar]
- Dong, Y. Genepools of common wheat. Acta Triticale Crops 2000, 20, 78–81. [Google Scholar]
- Ren, T.; Tang, Z.; Fu, S.; Yan, B.; Tan, F.; Ren, Z.; Li, Z. Molecular cytogenetic characterization of novel wheat-rye T1RS.1BL translocation lines with high resistance to diseases and great agronomic traits. Front Plant Sci. 2017, 8, 799. [Google Scholar] [CrossRef]
- Han, D.; Kang, Z. Current status and future strategy in breeding wheat for resistance to stripe rust in China. Plant Prot. 2018, 44, 1–12. [Google Scholar]
- Alam, M.A.; Fei, X.; Changyou, W.; Wanquan, J. Powdery mildew resistance genes in wheat: Identification and genetic analysis. J. Mol. Biol. Res. 2011, 1, 20. [Google Scholar] [CrossRef]
- Han, Q.; Yang, M.; Huang, Z.; Yan, L.; Hu, X. Effects of wheat powdery mildew on the photosynthetic physiological characteristics of wheat seedlings. Genom. Appl. Biol. 2017, 36, 4373–4379. [Google Scholar]
- Shao, W.; Shi, J.; Zhang, P.; Lang, M. Research progress of ERF transcription factors in regulating biological stress responses. Biotechnol. Bull. 2021, 37, 136–143. [Google Scholar]
- Liu, D.F.; Chen, X.J.; Liu, J.Q.; Ye, J.C.; Guo, Z.J. The rice ERF transcription factor OsERF922 negatively regulates resistance to Magnaporthe oryzae and salt tolerance. J. Exp. Bot. 2012, 63, 3899–3911. [Google Scholar] [CrossRef]
- Lionetti, V.; Cervone, F.; Bellincampi, D. Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J. Plant Physiol. 2012, 169, 1623–1630. [Google Scholar] [CrossRef]
- Leng, Y.J.; Yang, Y.L.; Ren, D.Y.; Huang, L.C.; Dai, L.P.; Wang, Y.Q.; Chen, L.; Tu, Z.J.; Gao, Y.H.; Li, X.Y.; et al. A Rice PECTATE LYASE-LIKE gene is required for plant growth and leaf senescence. Plant Physiol. 2017, 174, 1151–1166. [Google Scholar] [CrossRef]
- Raffaele, S.; Rivas, S.; Roby, D. An essential role for salicylic acid in AtMYB30-mediated control of the hypersensitive cell death program in Arabidopsis. FEBS Lett. 2006, 580, 3498–3504. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.E.; Barley, R.; Curtis, M.; Arroyo, J.M.; Dunham, M.; Hudson, A.; Martienssen, R.A. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 2000, 408, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Waites, R.; Selvadurai, H.R.; Oliver, I.R.; Hudson, A. The phantastica gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell 1998, 93, 779–789. [Google Scholar] [CrossRef]
- Timmermans, M.C.; Hudson, A.; Becraft, P.W.; Nelson, T. ROUGH SHEATH2: A Myb protein that represses knox homeobox genes in maize lateral organ primordia. Science 1999, 284, 151–153. [Google Scholar]
- Li, Q.F.; Lu, Y.Q.; Pan, C.L.; Zhang, J.P.; Liu, W.H.; Yang, X.M.; Li, X.Q.; Xi, Y.J.; Li, L.H. Characterization of a novel wheat-Agropyron cristatum 2P disomic addition line with powdery mildew resistance. Crop Sci. 2016, 56, 2390–2400. [Google Scholar] [CrossRef]
- Han, F.; Gao, Z.; Yu, W.; Birchler, J.A. Minichromosome analysis of chromosome pairing, disjunction, and sister chromatid cohesion in maize. Plant Cell 2007, 19, 3853–3863. [Google Scholar] [CrossRef] [PubMed]
- Sheng, B.; Duan, X. Improvement of “Grade 0~9 method” for recording powdery mildew of wheat adults. Beijing Agric. Sci. 1991, 1, 38–39. [Google Scholar]
- An, D.G.; Zheng, Q.; Zhou, Y.L.; Ma, P.T.; Lv, Z.L.; Li, L.H.; Li, B.; Luo, Q.L.; Xu, H.X.; Xu, Y.F. Molecular cytogenetic characterization of a new wheat-rye 4R chromosome translocation line resistant to powdery mildew. Chromosome Res. 2013, 21, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Parkhomchuk, D.; Borodina, T.; Amstislavskiy, V.; Banaru, M.; Hallen, L.; Krobitsch, S.; Lehrach, H.; Soldatov, A. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 2009, 37, e123. [Google Scholar] [CrossRef] [PubMed]
- Kroll, K.W.; Mokaram, N.E.; Pelletier, A.R.; Frankhouser, D.E.; Westphal, M.S.; Stump, P.A.; Stump, C.L.; Bundschuh, R.; Blachly, J.S.; Yan, P. Quality control for RNA-Seq (QuaCRS): An integrated quality control pipeline. Cancer Inf. 2014, 13, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Balyan, H.; Gahlaut, V. QTL Analysis for drought tolerance in wheat: Present status and future possibilities. Agronomy 2017, 7, 5. [Google Scholar] [CrossRef]
- Li, Y.C.; Meng, F.R.; Zhang, C.Y.; Zhang, N.; Sun, M.S.; Ren, J.P.; Niu, H.B.; Wang, X.; Yin, J. Comparative analysis of water stress-responsive transcriptomes in drought-susceptible and -tolerant wheat (Triticum aestivum L.). J. Plant Biol. 2012, 55, 349–360. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- WJ, W. Intergeneric crosses between Triticum and Agropyron. Sci. Agric. 1940, 21, 198–232. [Google Scholar]
- Lu, C.; Pu, Y.; Liu, Y.; Li, Y.; Qu, J.; Huang, H.; Dai, S. Comparative transcriptomics and weighted gene co-expression correlation network analysis (WGCNA) reveal potential regulation mechanism of carotenoid accumulation in Chrysanthemum x morifolium. Plant Physiol. Biochem. 2019, 142, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, X.; Liang, S.; Tang, S.; Wu, S.; Huang, T.; Mo, Z.; Wang, Q. Identifying miRNA and gene modules of colon cancer associated with pathological stage by weighted gene co-expression network analysis. Oncotargets Ther. 2018, 11, 2815–2830. [Google Scholar] [CrossRef] [PubMed]




| Module | Hub Gene ID | Gene ID in Aarbidopisis | Gene Function |
|---|---|---|---|
| Midnightblue | TraesCS1A02G225200 | AT3G11560.2 | LETM1-like protein |
| Midnightblue | TraesCS6A02G281300 | AT1G68550.1 | Encodes a member of the ERF (ethylene response factor) sub-family B-6 of ERF/AP2 transcription factor family |
| Blue | novel.8020 | AT3G02030.2 | Transferase |
| Blue | TraesCS6D02G008600 | AT1G50640.1 | Encodes a member of the ERF (ethylene response factor) sub-family B-1 of ERF/AP2 transcription factor family (ATERF-3) |
| Brown | novel.10169 | AT4G03205.2 | Coproporphyrinogen III oxidase |
| Brown | TraesCS1B02G274700 | AT5G63120.2 | P-loop containing nucleoside triphosphate hydrolases superfamily protein |
| Lightcyan | TraesCS5D02G450500 | AT4G13710.1 | Pectin lyase-like superfamily protein |
| Lightcyan | TraesCS7D02G526100 | AT1G62250.1 | Orotidine 5-phosphate decarboxylase |
| Turquoise | TraesCS5D02G265500 | AT4G13960.1 | F-box/RNI-like superfamily protein |
| Turquoise | TraesCS5B02G426500 | AT5G44260.1 | Encodes a Tandem CCCH Zinc Finger protein |
| Tan | TraesCS5A02G332900 | AT4G37180.2 | UIF1 is a nuclear and cytoplasmically localized myb-domain containing a member of the GARP G2-like sub-family of transcription factors. |
| Tan | novel.7975 | AT1G59840.1 | Co-factor assembly of complex C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, M.; Wang, X.; Long, J.; Bai, S.; Cui, Y.; Wang, Z.; Liu, C.; Liu, F.; Wang, Z.; Li, Q. Identification of Key Modules and Candidate Genes for Powdery Mildew Resistance of Wheat-Agropyron cristatum Translocation Line WAT-2020-17-6 by WGCNA. Plants 2023, 12, 335. https://doi.org/10.3390/plants12020335
Yao M, Wang X, Long J, Bai S, Cui Y, Wang Z, Liu C, Liu F, Wang Z, Li Q. Identification of Key Modules and Candidate Genes for Powdery Mildew Resistance of Wheat-Agropyron cristatum Translocation Line WAT-2020-17-6 by WGCNA. Plants. 2023; 12(2):335. https://doi.org/10.3390/plants12020335
Chicago/Turabian StyleYao, Mingming, Xinhua Wang, Jiaohui Long, Shuangyu Bai, Yuanyuan Cui, Zhaoyi Wang, Caixia Liu, Fenglou Liu, Zhangjun Wang, and Qingfeng Li. 2023. "Identification of Key Modules and Candidate Genes for Powdery Mildew Resistance of Wheat-Agropyron cristatum Translocation Line WAT-2020-17-6 by WGCNA" Plants 12, no. 2: 335. https://doi.org/10.3390/plants12020335
APA StyleYao, M., Wang, X., Long, J., Bai, S., Cui, Y., Wang, Z., Liu, C., Liu, F., Wang, Z., & Li, Q. (2023). Identification of Key Modules and Candidate Genes for Powdery Mildew Resistance of Wheat-Agropyron cristatum Translocation Line WAT-2020-17-6 by WGCNA. Plants, 12(2), 335. https://doi.org/10.3390/plants12020335





