Quantification and Qualification of Floral Patterns of Coffea arabica L. in Colombia
Abstract
1. Introduction
2. Materials and Methods
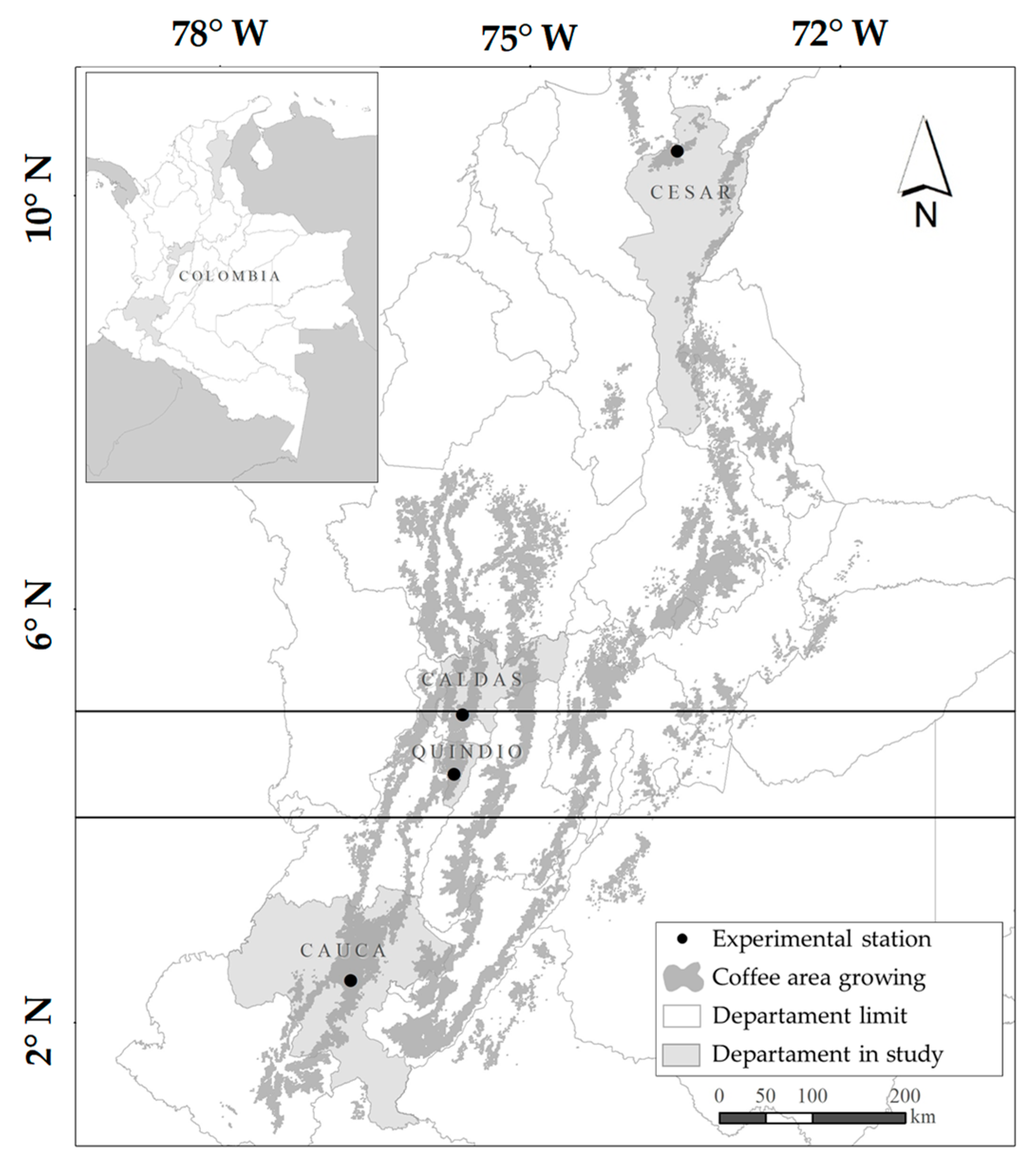
2.1. Study Site and Plant Material
2.2. Data Recording
2.3. Phenological Flowering Descriptors
2.4. Data Analysis
3. Results and Discussion
3.1. Flowering Patterns in Coffee
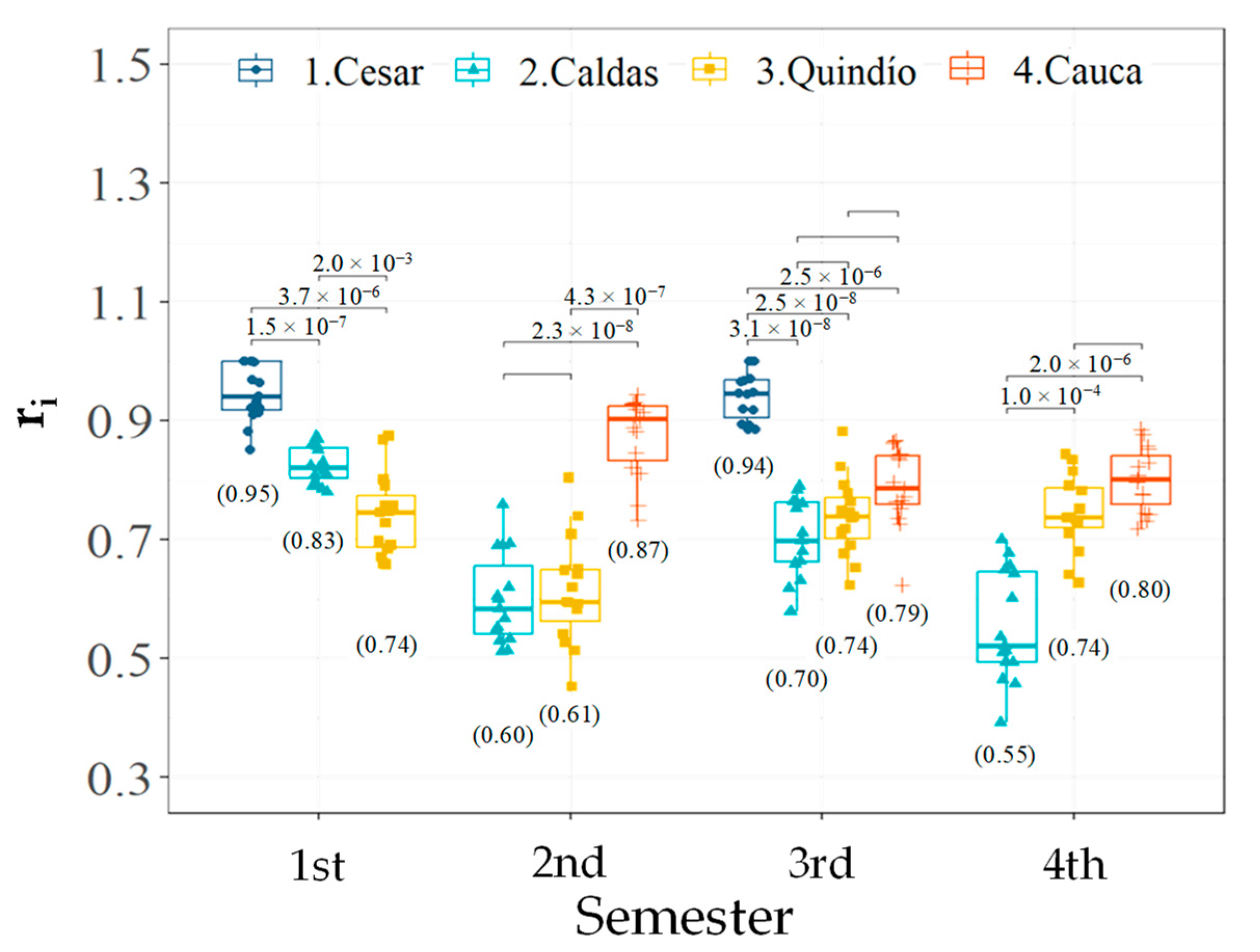
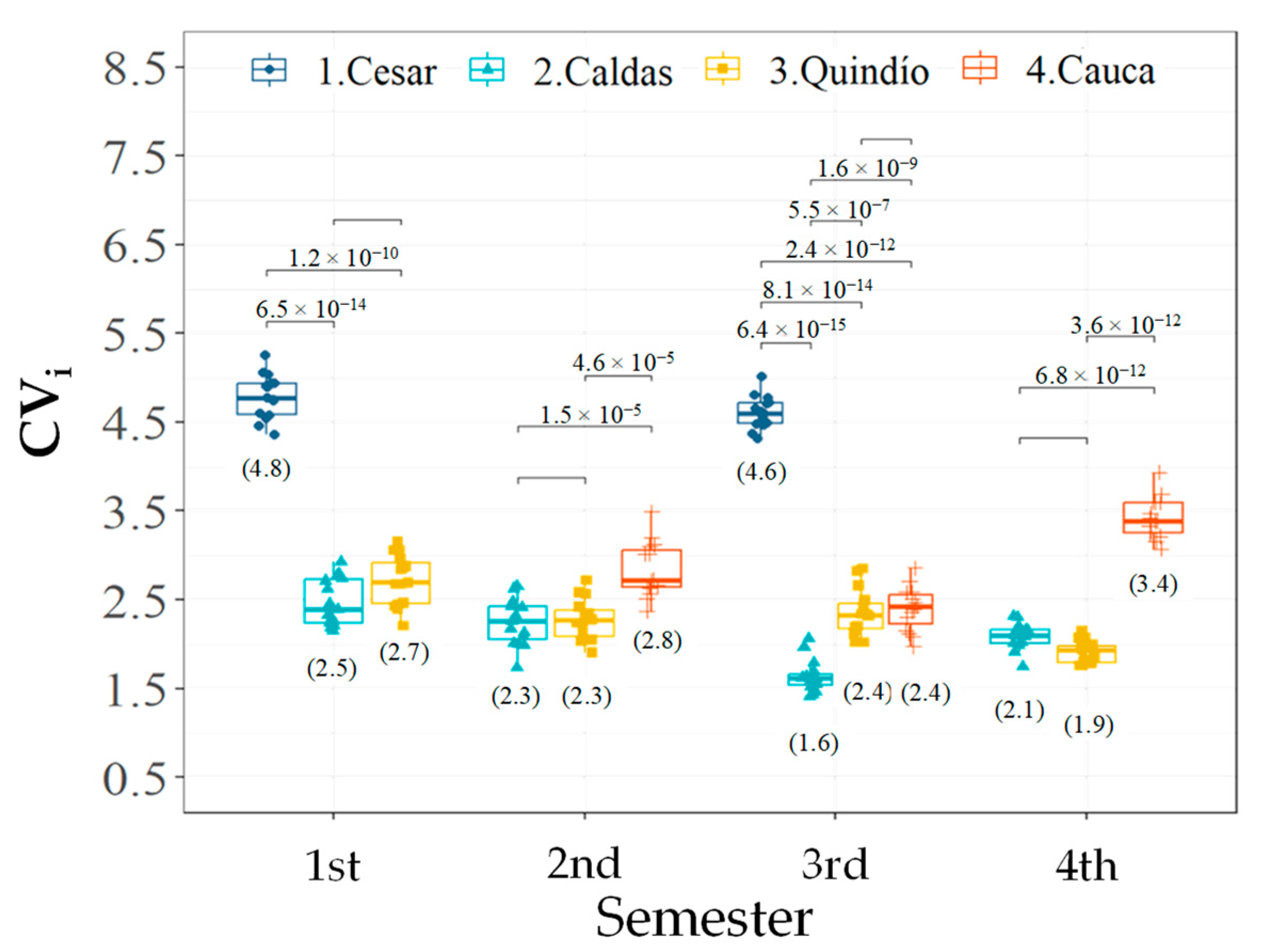
3.2. Effect of Accessions and Geographic Location on Phenological Descriptors of Flowering
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, A.P.; Tosh, J.; Ruch, N.; Fay, M.F. Growing Coffee: Psilanthus (Rubiaceae) Subsumed on the Basis of Molecular and Morphological Data; Implications for the Size, Morphology, Distribution and Evolutionary History of Coffea. Bot. J. Linn. Soc. 2011, 167, 357–377. [Google Scholar] [CrossRef]
- Akaffou, D.S.; Konate, I.; Sie, R.S.; Poncet, V.; Bi, I.A.Z.; Keli, J.; Legnate, H.; de Kochko, A.; Hamon, S.; Hamon, P. Flowering Phenology and Yield-Related Traits in an Interspecific Cross between “Coffea Pseudozanguebariae” Bridson and “C. canephora” Pierre. Aust. J. Crop Sci. 2014, 8, 1272. [Google Scholar]
- ICO Trade Statistics Tables. Available online: http://www.ico.org/trade_statistics.asp?section=Statistics (accessed on 24 August 2021).
- Arcila, J.; Buhr, L.; Bleiholder, H.; Hack, H.; Meier, U.; Wicke, H. Application of the Extended BBCH Scale for the Description of the Growth Stages of Coffee (Coffea spp.). Ann. Appl. Biol. 2002, 141, 19–27. [Google Scholar] [CrossRef]
- FNC Estadísticas Cafeteras. Available online: https://federaciondecafeteros.org/wp/estadisticas-cafeteras/ (accessed on 26 April 2023).
- Florez, C.P.; Arias, J.C. Guía para la caracterización de las variedades de café: Claves para su identificación. In Avances Tecnicos Cenicafé; Cenicafé: Manizales, Colombia, 2017; p. 12. [Google Scholar]
- Abernethy, K.; Bush, E.R.; Forget, P.-M.; Mendoza, I.; Morellato, L.P.C. Current Issues in Tropical Phenology: A Synthesis. Biotropica 2018, 50, 477–482. [Google Scholar] [CrossRef]
- Sakai, S.; Kitajima, K. Tropical Phenology: Recent Advances and Perspectives. Ecol. Res. 2019, 34, 50–54. [Google Scholar] [CrossRef]
- DaMatta, F.M. Coffee Tree Growth and Environmental Acclimation. In Achieving Sustainable Cultivation of Coffee; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; pp. 21–48. ISBN 978-1-351-11436-3. [Google Scholar]
- Camayo, G.C.; Chaves, B.; Arcila, J.; Jaramillo, A. Desarrollo floral del cafeto y su relación con las condiciones climáticas de Chinchiná, Caldas. (Coffee tree floral development and its relation with Chinchiná Caldas climatic conditions). Cenicafé 2003, 54, 35–49. [Google Scholar]
- DaMatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. Coffee: Environment and Crop Physiology. In Ecophysiology of Tropical Tree Crops; Nova Science Publishers: New York, NY, USA, 2010; pp. 181–216. ISBN 978-1-60876-392-4. [Google Scholar]
- Arcila, J.; Farfan, F.F.; Moreno, A.M.; Salazar, L.F.; Hincapie, E. Sistemas de Producción de Café en Colombia; Cenicafé: Chinchiná, Colombia, 2007; ISBN 978-958-98193-0-2. [Google Scholar]
- Majerowicz, N.; Söndahl, M.R. Induction and Differentiation of Reproductive Buds in Coffea arabica L. Braz. J. Plant Physiol. 2005, 17, 247–254. [Google Scholar] [CrossRef]
- López, M.E.; Santos, I.S.; de Oliveira, R.R.; Lima, A.A.; Cardon, C.H.; Chalfun-Junior, A. An Overview of the Endogenous and Environmental Factors Related to the Coffea arabica Flowering Process. Beverage Plant Res. 2021, 1, 13. [Google Scholar] [CrossRef]
- Rendón, J.R. Administración de sistemas de producción de café a libre exposición solar. In Manejo Agrónomico de los Sitemas de Producción de Café; 80 años de ciencia para la caficultura colombiana; Cenicafé: Manizales, Colombia, 2020; pp. 34–71. ISBN 978-958-8490-44-1. [Google Scholar]
- Peña, A.J.; Ramírez, V.H.; Jaramillo, A.; Rendón, J.R.; Arcila, J. Effects of Daylength and Soil Humidity on the Flowering of Coffee Coffea arabica L. in Colombia. Rev. Fac. Nac. Agron. Medellín 2011, 64, 5745–5754. [Google Scholar]
- Camayo, G.C.; Arcila, J. Estudio anatómico y morfológico de la diferenciación y desarrollo de las flores del cafeto Coffea arabica L. variedad Colombia. Rev. Cenicafé 1996, 47, 121–139. [Google Scholar]
- Unigarro, C.A.; Ibarra, L.N.; Valencia, C.; Mora, M.; Florez, C.P. Effect of Photoperiod and Thermal Amplitude on Floral Differentiation and Break Dormancy of Floral Buds of C. arabica. In Proceedings of the ASIC COLOMBIA 2014, Quíndio, Colombia, 8–13 September 2014; pp. 190–191. [Google Scholar]
- Rivera, G.; Borchert, R. Induction of Flowering in Tropical Trees by a 30-Min Reduction in Photoperiod: Evidence from Field Observations and Herbarium Specimens. Tree Physiol. 2001, 21, 201–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Öpik, H.; Rolfe, S.A. The Physiology of Flowering Plants, 4th ed.; Willis, A.J., Ed.; Cambridge University Press: Cambridge, UK, 2005; ISBN 978-0-521-66485-1. [Google Scholar]
- Borchert, R.; Calle, Z.; Strahler, A.H.; Baertschi, A.; Magill, R.E.; Broadhead, J.S.; Kamau, J.; Njoroge, J.; Muthuri, C. Insolation and Photoperiodic Control of Tree Development near the Equator. New Phytol. 2015, 205, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Yeang, H. Synchronous Flowering of the Rubber Tree (Hevea brasiliensis) Induced by High Solar Radiation Intensity. New Phytol. 2007, 175, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Calle, Z.; Schlumpberger, B.O.; Piedrahita, L.; Leftin, A.; Hammer, S.A.; Tye, A.; Borchert, R. Seasonal Variation in Daily Insolation Induces Synchronous Bud Break and Flowering in the Tropics. Trees 2010, 24, 865–877. [Google Scholar] [CrossRef]
- Carr, M.K.V. The Water Relations and Irrigation Requirements of Coffee. Ex. Agric. 2001, 37, 1–36. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Grantz, D.A.; Meinzer, F.C. Effects of Water Deficit on Flower Opening in Coffee (Coffea arabica L.). Tree Physiol 1992, 10, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Unigarro, C.A.; Flórez, C.P.; Oliveros, C.E.; Cañón, M. Efecto de cuatro inhibidores de etileno en la maduración del fruto de café (Coffea arabica L.) durante precosecha. Rev. Colomb. Cienc. Hortícolas 2018, 12, 500–507. [Google Scholar] [CrossRef]
- Jaramillo, A. El Clima de la Caficultura en Colombia; FNC—Cenicafé: Manizales, Colombia, 2018; ISBN 978-958-8490-21-2. [Google Scholar]
- Adjaloo, M.K.; Oduro, W.; Banful, B.K. Floral Phenology of Upper Amazon Cocoa Trees: Implications for Reproduction and Productivity of Cocoa. ISRN Agron. 2012, 2012, 461674. [Google Scholar] [CrossRef]
- Newstrom, L.E.; Frankie, G.W.; Baker, H.G. A New Classification for Plant Phenology Based on Flowering Patterns in Lowland Tropical Rain Forest Trees at La Selva, Costa Rica. Biotropica 1994, 26, 141. [Google Scholar] [CrossRef]
- Koenig, W.D.; Kelly, D.; Sork, V.L.; Duncan, R.P.; Elkinton, J.S.; Peltonen, M.S.; Westfall, R.D. Dissecting Components of Population-Level Variation in Seed Production and the Evolution of Masting Behavior. Oikos 2003, 102, 581–591. [Google Scholar] [CrossRef]
- Rafferty, N.E.; Diez, J.M.; Bertelsen, C.D. Changing Climate Drives Divergent and Nonlinear Shifts in Flowering Phenology across Elevations. Curr. Biol. 2020, 30, 432–441.e3. [Google Scholar] [CrossRef] [PubMed]
- Michalski, S.G.; Durka, W. Synchronous Pulsed Flowering: Analysis of the Flowering Phenology in Juncus (Juncaceae). Ann. Bot. 2007, 100, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Munguía-Rosas, M.A.; Sosa, V.J. Phenology of Pilosocereus leucocephalus (Cactaceae, Tribe Cereeae): A Columnar Cactus with Asynchronous Pulsed Flowering. Plant Ecol. 2010, 211, 191–201. [Google Scholar] [CrossRef]
- Sakai, S. Phenological Diversity in Tropical Forests. Popul. Ecol. 2001, 43, 77–86. [Google Scholar] [CrossRef]
- Salazar, B.M.; Gunda, D.M.; Lagrimas, A.J.M.; Santos, P.J.A.; Rosario, E.E.D. Profiling and Analysis of Reproductive Phenology of Four Coffee (Coffea spp.) Species in the Philippines Using the BBCH Scale. Philipp. J. Crop Sci. 2019, 44, 10–19. [Google Scholar]
- FNC (Federación Nacional de Cafeteros de Colombia). Atlas Cafetero de Colombia; Imprenta Nacional de Colombia: Bogota, Colombia, 2017; ISBN 978-958-8323-89-3. [Google Scholar]
- Rendón, J.R.; Arcila, J.; Montoya, E.C. Estimación de la producción de café con base en los registros de floración. Rev. Cenicafé 2008, 59, 238–259. [Google Scholar]
- Sáenz, J.R.R.; Restrepo, E.C.M. Cómo registrar las floraciones en los cafetales. In Avances Tecnicos Cenicafé; Cenicafé: Manizales, Colombia, 2015; p. 4. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002; ISBN 978-0-511-07812-5. [Google Scholar]
- Kassambara, A. ANOVA in R: The Ultimate Guide. Available online: https://www.datanovia.com/en/lessons/anova-in-r/ (accessed on 10 April 2023).
- Andrade, C. The P Value and Statistical Significance: Misunderstandings, Explanations, Challenges, and Alternatives. Indian J. Psychol. Med. 2019, 41, 210–215. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. The Proposal to Lower P Value Thresholds to 0.005. JAMA 2018, 319, 1429–1430. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World beyond “p < 0.05” . Am. Stat. 2019, 73, 1–19. [Google Scholar] [CrossRef]
- Levshina, N. How to Do Linguistics with R Data Exploration and Statistical Analysis; John Benjamins Publishing Company: Amsterdam, The Netherlands; Philadelphia, PA, USA, 2015; ISBN 978-90-272-6845-7. [Google Scholar]
- Fox, J.; Weisberg, S.; Price, B.; Adler, D.; Bates, D.; Baud-Bovy, G.; Bolker, B.; Ellison, S.; Firth, D.; Friendly, M.; et al. Car: Companion to Applied Regression. Available online: https://cran.r-project.org/web/packages/car/index.html (accessed on 2 May 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Houston, TX, USA, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research. Available online: https://cran.r-project.org/web/packages/psych/index.html (accessed on 15 July 2022).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 2 May 2021).
- R Core Team. R: A Language and Environment for Statistical Computing—Reference Index; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Fournier, L.A.; Herrera, M.E. Una década de observaciones fenológicas en café (Coffea arabica L.) en Ciudad Colón, Costa Rica. Rev. Biol. Trop. 1983, 31, 307–310. [Google Scholar]
- Cannell, M.G.R. Physiology of the Coffee Crop. In Coffee; Clifford, M.N., Willson, K.C., Eds.; Springer: Boston, MA, USA, 1985; pp. 108–134. ISBN 978-1-4615-6659-5. [Google Scholar]
- Drinnan, J.E.; Menzel, C.M. Temperature Affects Vegetative Growth and Flowering of Coffee (Coffea arabica L.). J. Hortic. Sci. 1995, 70, 25–34. [Google Scholar] [CrossRef]
- de Camargo, Â.P. Florescimento e frutificação de café arábica nas diferentes regiões (cafeeiras) do Brasil. Pesqui. Agropecuária Bras. 1985, 20, 831–839. [Google Scholar]
- Mayer, J.L.S.; Carmello-Guerreiro, S.M.; Mazzafera, P. A Functional Role for the Colleters of Coffee Flowers. AoB Plants 2013, 5, plt029. [Google Scholar] [CrossRef]
- Lima, A.A.; Santos, I.S.; Torres, M.E.L.; Cardon, C.H.; Caldeira, C.F.; Lima, R.R.; Davies, W.J.; Dodd, I.C.; Chalfun-Junior, A. Drought and Re-Watering Modify Ethylene Production and Sensitivity, and Are Associated with Coffee Anthesis. Environ. Exp. Bot. 2021, 181, 104289. [Google Scholar] [CrossRef]
- Proenca, C.E.B.; Gibbs, P.E. Reproductive Biology of Eight Sympatric Myrtaceae from Central Brazil. New Phytol. 1994, 126, 343–354. [Google Scholar] [CrossRef]
- Arroyo-Pérez, E.; Jiménez-Sierra, C.L.; Zavala-Hurtado, J.A.; Flores-Rivas, J.D.; Salgado-Ugarte, I.H. Fenología, sincronía floral y éxito reproductivo de Neolloydia conoidea (Cactaceae). Bot. Sci. 2019, 97, 579–587. [Google Scholar] [CrossRef]
- Qiu, S.; Xu, X.; Liu, S.; Liu, W.; Liu, J.; Nie, M.; Shi, F.; Zhang, Y.; Weiner, J.; Li, B. Latitudinal Pattern of Flowering Synchrony in an Invasive Wind-Pollinated Plant. Proc. R. Soc. B 2018, 285, 20181072. [Google Scholar] [CrossRef]
- Rojas-Sandoval, J.; Meléndez-Ackerman, E. Reproductive Phenology of the Caribbean Cactus Harrisia portoricensis: Rainfall and Temperature Associations. Botany 2011, 89, 861–871. [Google Scholar] [CrossRef]



| DEP | Sem | TempMean | TempMax | TempMin | Insolation | Daylength | Rainfall | DaysRain |
|---|---|---|---|---|---|---|---|---|
| Cesar | 1st | 20.4 | 27.7 | 14.9 | 71,475 | 2142 | 680 | 58 |
| 2nd | 21.8 | 28.0 | 17.2 | 78,602 | 2285 | 1852 | 100 | |
| 3rd | 22.9 | 27.5 | 17.2 | 71,463 | 2142 | 656 | 46 | |
| 4th | 21.3 | 28.0 | 16.5 | 78,614 | 2285 | 1171 | 77 | |
| Mean | 21.6 | 27.8 | 16.5 | 75,039 | 2214 | 1090 | 70 | |
| Caldas | 1st | 20.9 | 28.1 | 16.7 | 74,647 | 2169 | 1501 | 101 |
| 2nd | 21.2 | 28.6 | 17.0 | 77,194 | 2256 | 1793 | 118 | |
| 3rd | 20.9 | 28.1 | 16.9 | 74,643 | 2169 | 1514 | 104 | |
| 4th | 21.2 | 28.6 | 16.9 | 77,198 | 2256 | 1273 | 107 | |
| Mean | 21.1 | 28.4 | 16.9 | 75,921 | 2213 | 1520 | 108 | |
| Quindío | 1st | 21.7 | 29.1 | 17.2 | 74,947 | 2172 | 1359 | 88 |
| 2nd | 22.2 | 29.8 | 17.6 | 77,008 | 2253 | 952 | 73 | |
| 3rd | 21.7 | 29.5 | 17.4 | 74,944 | 2172 | 1707 | 92 | |
| 4th | 22.0 | 29.7 | 17.5 | 77,011 | 2253 | 1040 | 75 | |
| Mean | 21.9 | 29.5 | 17.4 | 75,978 | 2213 | 1265 | 82 | |
| Cauca | 1st | 19.3 | 24.9 | 15.4 | 75,930 | 2182 | 1420 | 107 |
| 2nd | 19.0 | 26.8 | 13.7 | 76,309 | 2242 | 748 | 65 | |
| 3rd | 18.9 | 26.3 | 15.1 | 75,930 | 2182 | 1529 | 110 | |
| 4th | 19.1 | 27.1 | 13.8 | 76,309 | 2242 | 601 | 71 | |
| Mean | 19.1 | 26.3 | 14.5 | 76,120 | 2212 | 1075 | 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unigarro, C.A.; Imbachi, L.C.; Darghan, A.E.; Flórez-Ramos, C.P. Quantification and Qualification of Floral Patterns of Coffea arabica L. in Colombia. Plants 2023, 12, 3332. https://doi.org/10.3390/plants12183332
Unigarro CA, Imbachi LC, Darghan AE, Flórez-Ramos CP. Quantification and Qualification of Floral Patterns of Coffea arabica L. in Colombia. Plants. 2023; 12(18):3332. https://doi.org/10.3390/plants12183332
Chicago/Turabian StyleUnigarro, Carlos Andres, Luis Carlos Imbachi, Aquiles Enrique Darghan, and Claudia Patricia Flórez-Ramos. 2023. "Quantification and Qualification of Floral Patterns of Coffea arabica L. in Colombia" Plants 12, no. 18: 3332. https://doi.org/10.3390/plants12183332
APA StyleUnigarro, C. A., Imbachi, L. C., Darghan, A. E., & Flórez-Ramos, C. P. (2023). Quantification and Qualification of Floral Patterns of Coffea arabica L. in Colombia. Plants, 12(18), 3332. https://doi.org/10.3390/plants12183332






