Exploring Carob (Ceratonia siliqua L.): A Comprehensive Assessment of Its Characteristics, Ethnomedicinal Uses, Phytochemical Aspects, and Pharmacological Activities
Abstract
:1. Introduction
2. Materials and Methods
3. Systematic and Botanical Classification of the Carob Tree
4. Morphological Description of C. siliqua L.
4.1. Tree
4.2. Fruit and Seed
5. Origin and Geographical Distribution
6. Ecology
7. Production Areas
8. Applications of Carob
8.1. Traditional Uses
8.2. Industrial Applications
9. Phytochemistry of C. siliqua L.
- -
- Total dietary fiber in carob pulp ranges from 30 to 40% [90].
- -
- This fiber is primarily made up of lignin (50–65%), cellulose (15–25%), and hemicellulose (15–25%), with smaller amounts of pectin (0.5–2%), tannins (3–7%), and moisture (4–8%) [91].
- -
- Carob fiber is primarily insoluble and is not easily fermented by gut bacteria [92].
- -
- The soluble fiber content, which can be fermented in the colon, is significantly lower in carob and this portion contains simple carbohydrates [82].
10. Pharmacology of C. siliqua L.
10.1. Antioxidant Activity
10.2. Antibacterial and Antidiarrheal Activity
10.3. Anti-Inflammatory and Antiulcer Activity
10.4. Antihyperglycemic Activity
10.5. Anti-Obesity Activity
10.6. Antiproliferative Activity
10.7. Antidepressant Activity
10.8. Antihypertensive Effect
10.9. Anti-Nociceptive Activity
10.10. Hepatoprotective Activity
11. Toxicology of C. siliqua L.
12. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hadi, M.Y.; Hameed, I.H.; Ibraheam, I.A. Ceratonia siliqua: Characterization, pharmaceutical products and analysis of bioactive compounds: A review. Res. J. Pharm. Technol. 2017, 10, 3585–3589. [Google Scholar] [CrossRef]
- Battle, I.; Tous, J. Carob Tree: Ceratonia siliqua L.—Promoting the Conservation and Use of Underutilized and Neglected Crops. 17; Institute of Plant Genetics and Crops Plant Research, Gatersleben/International Plant Genetic Ressources Institute: Rome, Italy, 1997. [Google Scholar]
- Hillcoat, D.; Lewis, G.; Verdcourt, B. A new species of Ceratonia (Leguminosae-Caesalpinioideae) from Arabia and the Somali Republic. Kew Bull. 1980, 35, 261–271. [Google Scholar] [CrossRef]
- Brassesco, M.E.; Brandão, T.R.S.; Silva, C.L.M.; Pintado, M. Carob bean (Ceratonia siliqua L.): A new perspective for functional food. Trends Food Sci. Technol. 2021, 114, 310–322. [Google Scholar] [CrossRef]
- Basharat, Z.; Afzaal, M.; Saeed, F.; Islam, F.; Hussain, M.; Ikram, A.; Pervaiz, M.U.; Awuchi, C.G. Nutritional and functional profile of carob bean (Ceratonia siliqua): A comprehensive review. Int. J. Food. Prop. 2023, 26, 389–413. [Google Scholar] [CrossRef]
- Loullis, A.; Pinakoulaki, E. Carob as cocoa substitute: A review on composition, health benefits and food applications. Eur. Food Res. Technol. 2018, 244, 959–977. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Jamous, R.M.; Salameh, N.M. Complementary and alternative medicine (CAM) use among hypertensive patients in Palestine. Complement Ther. Clin. Pr. 2013, 19, 256–263. [Google Scholar] [CrossRef]
- Baytop, T. Therapy with Medicinal Plants in Turkey (Past and Present); No: 3255; Istanbul University: Istanbul, Turkey, 1984; p. 359. [Google Scholar]
- Rtibi, K.; Jabri, M.A.; Selmi, S.; Souli, A.; Sebai, H.; El-Benna, J.; Amri, M.; Marzouki, L. Carob pods (Ceratonia siliqua L.) inhibit human neutrophils myeloperoxidase and in vitro ROS-scavenging activity. RSC Adv. 2015, 5, 84207–84215. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Jabri, M.-A.; Mamadou, G.; Limas-Nzouzi, N.; Sebai, H.; El-Benna, J.; Marzouki, L.; Eto, B.; Amri, M. Effects of aqueous extracts from Ceratonia siliqua L. pods on small intestinal motility in rats and jejunal permeability in mice. RSC Adv. 2016, 6, 44345–44353. [Google Scholar] [CrossRef]
- Sbay, H. Le Caroubier au Maroc: Un Arbre D'avenir; Centre de Recherche Forestière du Haut Commissariat aux Eaux et Forêts et à la Lutte contre la Désertification: Rabat, Morocco, 2008. [Google Scholar]
- Gioxari, A.; Amerikanou, C.; Nestoridi, I.; Gourgari, E.; Pratsinis, H.; Kalogeropoulos, N.; Andrikopoulos, N.K.; Kaliora, A.C. Carob: A sustainable opportunity for metabolic health. Foods 2022, 11, 2154. [Google Scholar] [CrossRef] [PubMed]
- Baydoun, S.; Kanj, D.; Raafat, K.; Aboul Ela, M.; Chalak, L.; Arnold-Apostolides, N. Ethnobotanical and economic importance of wild plant species of Jabal Moussa Bioreserve, Lebanon. J. Ecosys. Ecograph. 2017, 7, 245. [Google Scholar] [CrossRef]
- Rankou, H.; M’Sou, S.; Chadburn, H.; Rivers, M.; Ouhammou, A.; Martin, G. Ceratonia siliqua. The IUCN Red List of Threatened Species 2017: E. T202951A112823254; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2019. [Google Scholar] [CrossRef]
- Menale, B.; de Castro, O.; Cascone, C.; Muoio, R.J.J.o.e. Ethnobotanical investigation on medicinal plants in the Vesuvio National Park (Campania, southern Italy). J. Ethnopharmacol. 2016, 192, 320–349. [Google Scholar] [CrossRef]
- Tardío, J.; Pardo-de-Santayana, M.; Morales, R. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Iipumbu, L. Compositional Analysis of Locally Cultivated Carob (Ceratonia siliqua) Cultivars and Development of Nutritional Food Products for a Range of Market Sectors; Stellenbosch University: Stellenbosch, South Africa, 2008; Available online: https://api.semanticscholar.org/CorpusID:129160277 (accessed on 20 January 2023).
- Schopmeyer, C.S. Seeds of Woody Plants in the United States, Agricultural Handbook; US Department of Agriculture, CABI: Boston, MA, USA, 1974; Available online: https://www.cabdirect.org/cabdirect/abstract/19750622387 (accessed on 20 January 2023).
- Tous, J.; Romero, A.; Batlle, I. The Carob tree: Botany, horticulture, and genetic resources. Hortic. Rev. 2013, 41, 385–456. [Google Scholar] [CrossRef]
- Quezel, P.; Santa, S. Nouvelle Flore de l'Algérie et des Régions Désertiques Méridionales, Tome II; Editions du Centre National de la Recherche Scientifique: Paris, France, 1963. [Google Scholar]
- Ramón-Laca, L.; Mabberley, D. The ecological status of the carob-tree (Ceratonia siliqua, Leguminosae) in the Mediterranean. Bot. J. Linn. 2004, 144, 431–436. [Google Scholar] [CrossRef]
- Ferguson, I.K. The pollen morphology of Ceratonia (Leguminosae: Caesalpinioideae). Kew. Bull. 1980, 35, 273–277. [Google Scholar] [CrossRef]
- Benmahioul, B.; Harche, M.K.; Daguin, F. Le caroubier, une espèce méditerranéenne à usages multiples. Forêt Méditerranéenne 2011, 32, 51–58. [Google Scholar]
- Asma, B.; Meriem, H.; Rafika, L. Ethnobotanical knowledge and socio-economic importance of Ceratonia siliqua L (Fabaceae) in the North of Setif (North-East of Algeria). Acta Ecol. Sin. 2022, 43, 712–720. [Google Scholar] [CrossRef]
- Kaderi, M.; Hamouda, G.B.; Zaeir, H.; Hanana, M.; Hamrouni, L. Notes ethnobotanique et phytopharmacologique sur Ceratonia siliqua (L.). Phytothérapie 2015, 13, 144–147. [Google Scholar] [CrossRef]
- Loock, E. The carob or locust tree (Ceratonia siliqua L). J. S. Afr. Assoc. 1940, 4, 78–80. [Google Scholar] [CrossRef]
- Fadl, M.; El-Deen, S.; El-Mahdy, M. Physiological and chemical factors controlling adventitious root initiation in carob Ceratonia siliqua L. stem cuttings. Egypt J. Hortic. 1979, 6, 55–68. [Google Scholar]
- Diamantoglou, S.; Meletiou-Christou, M. Annual changes in carbohydrates in bark and leaves of Ceratonia siliqua L. Port. Acta Biol. A 1980, 16, 197–205. [Google Scholar]
- Von Haselberg, C.D. Vegetative Growth and Flower and Fruit Development in Carob Trees (Ceratonia siliqua L.) with Special Emphasis on Environmental Conditions at Marginal Production Sites in South Portugal. Ph.D. Thesis, University of Berlin, Berlin, Germany, 2000. [Google Scholar] [CrossRef]
- Diamantoglou, S.; Mitrakos, K. Leaf Longevity in Mediterranean Evergreen Sclerophylls. In Components of Productivity of Mediterranean-Climate Regions Basic and Applied Aspects; Tasks for Vegetation Science; Margaris, N.S., Mooney, H.A., Eds.; Springer: Dordrecht, The Netherlands, 1981; Volume 4, pp. 17–19. [Google Scholar] [CrossRef]
- Crescimanno, F.; Dr Michele, A.; Di Lovenzo, R.; Occorso, G.; Raimoudo, A. Aspetti morfologici e carpologici di cultivar di carrubo (Ceratonia siliqua L.). In Proceedings of the II International Carob Symposium, Valencia, Spain; Fito, P., Mulet, A., Eds.; Generalitat Valenciana: Valencia, Spain, 1988; pp. 169–181. [Google Scholar]
- Retana, J.; Ramoneda, J.; Garcia del Pino, F.; Bosch, J. Flowering phenology of carob, Ceratonia siliqua L. (Cesalpinaceae). J. Hortic. Sci. 1994, 69, 97–103. [Google Scholar] [CrossRef]
- Şahin, G.; Taşlıgil, N. Agricultural geography analysis of carob tree (Ceratonia siliqua L.) from Turkey. Turk. JAF Sci. Tech. 2016, 4, 1192–1200. [Google Scholar] [CrossRef]
- Vavilov, N.I. The origin, variation, immunity and breeding of cultivated plants. Soil Sci. 1951, 72, 482. [Google Scholar] [CrossRef]
- De Candolle, A. Origine des Plantes Cultivées, 3rd ed.; Ancienne Librairie Germer Baillière et Cie, Felix Alcan: Paris, France, 1886. [Google Scholar]
- Zohary, M. Geobotanical Foundations of the Middle East; Fischer: Stuttgart, Germany, 1973. [Google Scholar]
- Polhill, R.M. Advances Legume Syst; The University of Chicago Press: Chicago, IL, USA, 1981; pp. 371–374. [Google Scholar]
- Mitrakos, K. The botany of Ceratonia. In Proceedings of the II International Carob Symposium, Valencia, Spain; Fito, P., Mulet, A., Eds.; Generalitat Valenciana: Valencia, Spain, 1988; pp. 209–218. [Google Scholar]
- Berrougui, H. Le caroubier (Ceratonia siliqua L.), une richesse nationale aux vertus médicinales. Maghreb Can. Express 2007, 5, 38. [Google Scholar]
- Gharnit, N.; El Mtili, N.; Ennabili, A.; Sayah, F. Caractérisation foliaire du caroubier (Ceratonia siliqua L.) originaire de la province de Chefchaouen (Nord-ouest du Maroc). J. Bot. Soc. Bot. Fr. 2005, 31, 75–84. [Google Scholar] [CrossRef]
- Ait Chitt, M.; Belmir, M.; Lazrak, A. Production des plants sélectionnés et greffés du caroubier. Bull. Mens. D’information Et De Liaison Du PNTTA MAPM/DERD 2007, 153, 1–4. [Google Scholar]
- Gharnit, N.; El Mtili, N.; Ennabili, A.; Sayah, F. Importance socio-économique du caroubier (Ceratonia siliqua L.) dans la province de Chefchaouen (Nord-ouest du Maroc). J. Bot. Soc. Bot. Fr. 2006, 33, 43–48. [Google Scholar] [CrossRef]
- Boubkar, F.; Rachid, F.; Fatima, A.; Oudou, I.A.; Saida, T.; Said, W. Bioclimatic impact on the carob seeds morphological diversity in Morocco. Biosci Biotech Res. Asia 2021, 18, 207. [Google Scholar] [CrossRef]
- Rima, K. Morpho-ecological and phytochemical characterization of carob tree (Ceratonia siliqua L.) in Algeria. Ph.D. Thesis, University Ziane Achour, Djelfa, Algeria, 2021. [Google Scholar]
- Correia, P.; Gama, F.; Pestana, M.; Martins-Loução, M. Tolerance of young (Ceratonia siliqua L.) carob rootstock to NaCl. Agric. Water Manag. 2010, 97, 910–916. [Google Scholar] [CrossRef]
- FAOSTAT. Crop, Livestock and Food Statistics. FAO of the UN. Available online: https://www.fao.org/faostat/fr/ (accessed on 5 February 2023).
- Merzouki, A.; Ed-Derfoufi, F.; El Aallali, A.; Molero-Mesa, J. Wild medicinal plants used by local Bouhmed population (Morocco). Fitoterapia 1997, 68, 444–460. [Google Scholar]
- Amico, F.; Sorce, E. Medicinal plants and phytotherapy in Mussomeli area (Caltanissetta, Sicily, Italy). Fitoterapia 1997, 68, 143–159. [Google Scholar]
- Lachkar, N.; Al-Sobarry, M.; El Hajaji, H.; Lamkinsi, T.; Lachkar, M.; Cherrah, Y.; Alaoui, K. Anti-inflammatory and antioxidant effect of Ceratonia siliqua L. methanol barks extract. J. Chem. Pharm. Res. 2016, 8, 202–210. [Google Scholar]
- Rtibi, K.; Selmi, S.; Grami, D.; Amri, M.; Eto, B.; El-Benna, J.; Sebai, H.; Marzouki, L. Chemical constituents and pharmacological actions of carob pods and leaves (Ceratonia siliqua L.) on the gastrointestinal tract: A review. Biomed. Pharmacother. 2017, 93, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Mrabti, H.N.; Jaradat, N.; Kachmar, M.R.; Ed-Dra, A.; Ouahbi, A.; Cherrah, Y.; Faouzi, M.E.A. Integrative herbal treatments of diabetes in Beni Mellal region of Morocco. J. Integr. Med. 2019, 17, 93–99. [Google Scholar] [CrossRef]
- Ouhaddou, H.; Boubaker, H.; Msanda, F.; El Mousadik, A. An ethnobotanical study of medicinal plants of the Agadir Ida Ou Tanane province (southwest Morocco). J. App. Biosci. 2014, 84, 7707–7722. [Google Scholar] [CrossRef]
- Ennabili, A.; Gharnit, N.; El Hamdouni, E.M. Inventory and Social Interest of Medicinal, Aromatic and Honey-Plants from Mokrisset Region (NW of Morocco); Studia Botanica Ediciones; Universidad Salamanca: Salamanca, Spain, 2000; Volume 19. [Google Scholar]
- El-Hilaly, J.; Hmammouchi, M.; Lyoussi, B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco). J. Ethnopharmacol. 2003, 86, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Orch, H.; Zidane, L.; Douira, A. Ethnobotanical study of the plants used in the treatment of the digestive diseases by the riverine population of the forest of Izarène. Int. J. Recent. Sci. Res. 2017, 8, 15213–15220. [Google Scholar]
- AbouZid, S.F.; Mohamed, A.A. Survey on medicinal plants and spices used in Beni-Sueif, Upper Egypt. J. Ethnobiol. Ethnomedicine 2011, 7, 18. [Google Scholar] [CrossRef]
- Afifi-Yazar, F.U.; Kasabri, V.; Abu-Dahab, R. Medicinal plants from Jordan in the treatment of diabetes: Traditional uses vs. in vitro and in vivo evaluations–part 2. Planta Med. 2011, 77, 1210–1220. [Google Scholar] [CrossRef]
- Oran, S.; Al-Eisawi, D. Ethnobotanical survey of the medicinal plants in the central mountains (North-South) in Jordan. J. Bio. Env. Sci. 2015, 6, 381–400. [Google Scholar]
- Baydoun, S.; Chalak, L.; Dalleh, H.; Arnold, N. Ethnopharmacological survey of medicinal plants used in traditional medicine by the communities of Mount Hermon, Lebanon. J. Ethnopharmacol. 2015, 173, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Baumel, A.; Mirleau, P.; Viruel, J.; Bou Dagher Kharrat, M.; La Malfa, S.; Ouahmane, L.; Diadema, K.; Moakhar, M.; Sanguin, H.; Médail, F. Assessment of plant species diversity associated with the carob tree (Ceratonia siliqua, Fabaceae) at the Mediterranean scale. Plant Ecol. Evol. 2018, 151, 185–193. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Al-Shafie', J.H.; Elgharabah, W.A.; Kherfan, F.A.; Qarariah, K.H.; Khdair, I.S.; Soos, I.M.; Musleh, A.A.; Isa, B.A. Traditional knowledge of wild edible plants used in Palestine (Northern West Bank): A comparative study. J. Ethnobiol. Ethnomed. 2008, 4, 13. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ethnopharmacobotanical study on the medicinal plants used by herbalists in Sulaymaniyah Province, Kurdistan, Iraq. J. Ethnobiol. Ethnomed. 2016, 12, 8. [Google Scholar] [CrossRef]
- Fakir, H.; Korkmaz, M.; Güller, B. Medicinal plant diversity of western Mediterrenean region in Turkey. J. Appl. Biol. Sci. 2009, 3, 33–43. [Google Scholar]
- Özkum, D.; Akı, Ö.; Toklu, H.Z. Herbal medicine use among diabetes mellitus patients in Northern Cyprus. Age 2013, 18, 1652–1664. [Google Scholar] [CrossRef]
- Öztürk, M.; Seҫmen, O.; Gucel, S.; Sakcali, S. An overview of economic and medicinal importance of carob plants (Ceratonia siliqua L.) in the Mediterranean basin. Acta Hortic. 2012, 964, 197–203. [Google Scholar] [CrossRef]
- Saad, B.; Said, O. Greco-Arab and Islamic Herbal Medicine: Traditional System, Ethics, Safety, Efficacy, and Regulatory Issues; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Fatima, S.; Shahid, M.; Mand, D.; Parveen, S.; Naaz, I. Potential effects of unani herbs in the management of obesity and its related comorbidities–a systematic review. World J. Pharm. Res. 2017, 6, 1475–1485. [Google Scholar] [CrossRef]
- Tansaz, M.; Memarzadehzavareh, H.; Qaraaty, M.; Eftekhar, T.; Tabarrai, M.; Kamalinejad, M. Menorrhagia management in Iranian traditional medicine. J. Evid. Based Complement Altern. Med. 2016, 21, 71–76. [Google Scholar] [CrossRef]
- Lentini, F.; Venza, F. Wild food plants of popular use in Sicily. J. Ethnobiol. Ethnomed. 2007, 3, 15. [Google Scholar] [CrossRef]
- Montesano, V.; Negro, D.; Sarli, G.; De Lisi, A.; Laghetti, G.; Hammer, K. Notes about the uses of plants by one of the last healers in the Basilicata Region (South Italy). J. Ethnobiol. Ethnomed. 2012, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Mekhoukhe, A.; Kicher, H.; Ladjouzi, A.; Medouni-Haroune, L.; Brahmi, F.; Medouni-Adrar, S.; Madani, K. Antioxidant activity of carob seeds and chemical composition of their bean gum by–products. J. Complement. Integr. Med. 2019, 16, 158. [Google Scholar] [CrossRef] [PubMed]
- Krokou, A.; Stylianou, M.; Agapiou, A. Assessing the volatile profile of carob tree (Ceratonia siliqua L.). Env. Sci. Pollut. Res. 2019, 26, 35365–35374. [Google Scholar] [CrossRef] [PubMed]
- Vladimir, L.; Yuliya, S.; Irina, Z.; Tatʹyana, K.; Yuriy, R.; Ana, K. Economic effect of innovative flour-based functional foods production. Foods Raw. Mater. 2018, 6, 474–482. [Google Scholar] [CrossRef]
- Sbay, H.; Abourouh, M. Apport des Espèces à Usages Multiples Pour le Développement Durable: Cas du Pin Pignon et du Caroubier; Centre de Recherche Forestière Haut-Commissariat aux Eaux et Forêts et à la Lutte Contre la Désertification: Rabat, Morocco, 2006; pp. 1–9. [Google Scholar]
- Zhu, B.-J.; Zayed, M.Z.; Zhu, H.-X.; Zhao, J.; Li, S.-P. Functional polysaccharides of carob fruit: A review. Chin. Med. 2019, 14, 40. [Google Scholar] [CrossRef]
- Bengoechea, C.; Romero, A.; Villanueva, A.; Moreno, G.; Alaiz, M.; Millán, F.; Guerrero, A.; Puppo, M. Composition and structure of carob (Ceratonia siliqua L.) germ proteins. Food Chem. 2008, 107, 675–683. [Google Scholar] [CrossRef]
- Custódio, L.; Escapa, A.L.; Fernandes, E.; Fajardo, A.; Aligué, R.; Alberício, F.; Neng, N.; Nogueira, J.M.F.; Romano, A. Phytochemical profile, antioxidant and cytotoxic activities of the carob tree (Ceratonia siliqua L.) germ flour extracts. Plant Foods Hum. Nut. 2011, 66, 78–84. [Google Scholar] [CrossRef]
- El-Haskoury, R.; Kriaa, W.; Lyoussi, B.; Makni, M. Ceratonia siliqua honeys from Morocco: Physicochemical properties, mineral contents, and antioxidant activities. J. Food Drug. Anal. 2018, 26, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Haddarah, A. L’influence des Cultivars Sur Les Propriétés Fonctionnelles de la Caroube Libanaise. Ph.D. Thesis, Université de Lorraine, Paris, France, 2013. [Google Scholar]
- Owen, R.; Haubner, R.; Hull, W.; Erben, G.; Spiegelhalder, B.; Bartsch, H.; Haber, B. Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem. Toxicol. 2003, 41, 1727–1738. [Google Scholar] [CrossRef]
- Fadel, F.; Fattouch, S.; Tahrouch, S.; Lahmar, R.; Benddou, A.; Hatimi, A. The phenolic compounds of Ceratonia siliqua pulps and seeds (Les composes phénoliques des pulpes et des graines de Ceratonia siliqua). J. Mater. Env. Sci. 2011, 2, 285–292. [Google Scholar]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional components of carob fruit: Linking the chemical and biological space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Macia, A.; Romero, M.-P.; Trullols, E.; Morello, J.-R.; Angles, N.; Motilva, M.-J. Rapid determination of phenolic compounds and alkaloids of carob flour by improved liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2009, 57, 7239–7244. [Google Scholar] [CrossRef] [PubMed]
- Corsi, L.; Avallone, R.; Cosenza, F.; Farina, F.; Baraldi, C.; Baraldi, M. Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line. Fitoterapia 2002, 73, 674–684. [Google Scholar] [CrossRef]
- Avallone, R.; Plessi, M.; Baraldi, M.; Monzani, A. Determination of chemical composition of carob (Ceratonia siliqua): Protein, fat, carbohydrates, and tannins. J. Food Compos. Anal. 1997, 10, 166–172. [Google Scholar] [CrossRef]
- Rtibi, K.; Jabri, M.-A.; Selmi, S.; Sebai, H.; Amri, M.; El-Benna, J.; Marzouki, L. Ceratonia siliqua leaves exert a strong ROS-scavenging effect in human neutrophils, inhibit myeloperoxydase in vitro and protect against intestinal fluid and electrolytes secretion in rats. RSC Adv. 2016, 6, 65483–65493. [Google Scholar] [CrossRef]
- El Hajaji, H.; Lachkar, N.; Alaoui, K.; Cherrah, Y.; Farah, A.; Ennabili, A.; El Bali, B.; Lachkar, M. Antioxidant activity, phytochemical screening, and total phenolic content of extracts from three genders of carob tree barks growing in Morocco. Arab. J. Chem. 2011, 4, 321–324. [Google Scholar] [CrossRef]
- Papagiannopoulos, M.; Wollseifen, H.R.; Mellenthin, A.; Haber, B.; Galensa, R. Identification and quantification of polyphenols in Carob Fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MS n. J. Agric. Food Chem. 2004, 52, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Abidar, S.; Boiangiu, R.S.; Dumitru, G.; Todirascu-Ciornea, E.; Amakran, A.; Cioanca, O.; Hritcu, L.; Nhiri, M. The aqueous extract from Ceratonia siliqua leaves protects against 6-hydroxydopamine in zebrafish: Understanding the underlying mechanism. Antioxidants 2020, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Haber, B. Carob fiber benefits and applications. Cereal Foods World 2002, 47, 365. [Google Scholar]
- Marco, A.M.; De Mora, B.R.; Diaz, C.S. Method of Making Natural Carob Fiber. US Patent 5609905A, USA, 11 March 1997. Available online: https://patents.google.com/patent/US5609905A/en (accessed on 14 May 2023).
- Nasar-Abbas, S.M.; e-Huma, Z.; Vu, T.H.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob kibble: A bioactive-rich food ingredient. Compr. Rev. Food Sci. Food Saf. 2016, 15, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Khlifa, M.; Bahloul, A.; Kitane, S. Determination of chemical composition of carob pod (Ceratonia siliqua L.) and its morphological study. J. Mater. Env. Sci. 2013, 4, 348–353. [Google Scholar]
- Petit, M.; Pinilla, J. Production and purification of a sugar syrup from carob pods. LWT–Food Sci. Tech. 1995, 28, 145–152. [Google Scholar] [CrossRef]
- Fidan, H.; Stankov, S.; Petkova, N.; Petkova, Z.; Iliev, A.; Stoyanova, M.; Ivanova, T.; Zhelyazkov, N.; Ibrahim, S.; Stoyanova, A. Evaluation of chemical composition, antioxidant potential and functional properties of carob (Ceratonia siliqua L.) seeds. J. Food Sci. Technol. 2020, 57, 2404–2413. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Torun, H.; Glew, R.H.; Bak, Z.D.; Chuang, L.T.; Presley, J.M.; Andrews, R. Nutrient content of carob pod (Ceratonia siliqua L.) flour prepared commercially and domestically. Plant. Foods Hum. Nutr. 2009, 64, 286. [Google Scholar] [CrossRef] [PubMed]
- Sigge, G.; Iipumbu, L.; Britz, T. Proximate composition of carob cultivars growing in South Africa. S. Afr. J. Plant Soil 2011, 28, 17–22. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Torun, H.; Ayaz, S.; Correia, P.J.; Alaiz, M.; Sanz, C.; Grúz, J.; Strnad, M. Determination of chemical composition of anatolian carob pod (Ceratonia siliqua L.): Sugars, amino and organic acids, minerals and phenolic compounds. J. Food Qual. 2007, 30, 1040–1055. [Google Scholar] [CrossRef]
- Smith, B.M.; Bean, S.R.; Schober, T.J.; Tilley, M.; Herald, T.J.; Aramouni, F. Composition and molecular weight distribution of carob germ protein fractions. J. Agric. Food Chem. 2010, 58, 7794–7800. [Google Scholar] [CrossRef] [PubMed]
- Cebolla, Á.; Moreno, M.d.L.; Coto, L.; Sousa, C. Gluten immunogenic peptides as standard for the evaluation of potential harmful prolamin content in food and human specimen. Nutrients 2018, 10, 1927. [Google Scholar] [CrossRef] [PubMed]
- Mahtout, R.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Gracia, I.; Hernández-Fernández, F.J.; Pérez de los Ríos, A.; Zaidia, F.; Sanchez-Segado, S.; Lozano-Blanco, L.J. Algerian carob tree products: A comprehensive valorization analysis and future prospects. Sustainability 2018, 10, 90. [Google Scholar] [CrossRef]
- Ghanemi, F.Z.; Belarbi, M. Phytochemistry and Pharmacology of Ceratonia siliqua L. leaves. J. Nat. Prod. Res. App. 2021, 1, 69–82. [Google Scholar] [CrossRef]
- Musa Özcan, M.; Arslan, D.; Gökçalik, H. Some compositional properties and mineral contents of carob (Ceratonia siliqua) fruit, flour and syrup. Int. J. Food Sci. Nutr. Eng. 2007, 58, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Dakia, P.A.; Wathelet, B.; Paquot, M. Isolation and chemical evaluation of carob (Ceratonia siliqua L.) seed germ. Food Chem. 2007, 102, 1368–1374. [Google Scholar] [CrossRef]
- El Hajaji, H.; Farah, A.; Ennabili, A.; Bousta, D.; Greche, H.; El Bali, B.; Lachkar, M. Etude comparative de la composition minérale des constituants de trois catégories de Ceratonia siliqua L. (Comparative study of the mineral composition of the constituents of three varieties of Ceratonia siliqua L.). J. Mater. Env. Sci. 2013, 4, 165–170. [Google Scholar]
- Oziyci, H.R.; Tetik, N.; Turhan, I.; Yatmaz, E.; Ucgun, K.; Akgul, H.; Gubbuk, H.; Karhan, M. Mineral composition of pods and seeds of wild and grafted carob (Ceratonia siliqua L.) fruits. Sci. Hortic. 2014, 167, 149–152. [Google Scholar] [CrossRef]
- El Bouzdoudi, B.; Saïdi, R.; Khalid, E.; El Mzibri, M.; Nejjar, A.; El Kbiach, M.; Lamarti, A. Mineral composition of mature carob (Ceratonia siliqua L.) Pod: A Study. Int. J. Food Sci. Nutr. Eng. 2017, 7, 91–103. [Google Scholar] [CrossRef]
- Dallali, S.; Aloui, F.; Selmi, H.; Sebei, H. Comparison of the chemical composition and the antioxidant activity of the leaves of Carob tree (Ceratonia siliqua L.) collected in three sites of Djebel Zaghouan (Tunisia). J. New Sci. Agric. Biotechnol. CIRS 2018, 21, 3429–3438. [Google Scholar]
- De Falco, B.; Grauso, L.; Fiore, A.; Bonanomi, G.; Lanzotti, V. Metabolomics and chemometrics of seven aromatic plants: Carob, eucalyptus, laurel, mint, myrtle, rosemary and strawberry tree. Phytochem. Anal. 2022, 33, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; El-Kersh, D.M.; Ehrlich, A.; Choucry, M.A.; El-Seedi, H.; Frolov, A.; Wessjohann, L.A. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem. 2019, 283, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Samia, O.; Nesrine, M.; Feten, Z.K.; Riadh, K. Tunisian Ceratonia siliqua: Phytochemical Analysis, Antioxidant Activity, Preparation and Characterization of Carob Emulsion System. Eur. J. Nutr. Food Saf. 2022, 14, 41–52. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Abduvakhidov, A.; Caputo, P.; Crupi, P.; Muraglia, M.; Oliviero Rossi, C.; Clodoveo, M.L.; Aiello, F.; Restuccia, D. Kefir enriched with carob (Ceratonia siliqua L.) leaves extract as a new ingredient during a gluten-free bread-making process. Fermentation 2022, 8, 305. [Google Scholar] [CrossRef]
- Alqudah, A.; Qnais, E.Y.; Wedyan, M.A.; Oqal, M.; Alqudah, M.; AbuDalo, R.; Nabil, A.-H. Ceratonia siliqua leaves ethanol extracts exert anti-nociceptive and anti-inflammatory effects. Heliyon 2022, 8, e10400. [Google Scholar] [CrossRef] [PubMed]
- Abidar, S.; Yildiz, O.; Degirmenci, A.; Amakran, A.; El Maadoudi, M.; Nhiri, M. Glucose-mediated protein glycation: Contribution of methanolic extract of Ceratonia siliqua L. in protection and in vitro potential inhibition of acetylcholinesterase. J. Food Biochem. 2019, 43, e13009. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, F.Z.; Belarbi, M.; Fluckiger, A.; Nani, A.; Dumont, A.; De Rosny, C.; Aboura, I.; Khan, A.S.; Murtaza, B.; Benammar, C. Carob leaf polyphenols trigger intrinsic apoptotic pathway and induce cell cycle arrest in colon cancer cells. J. Funct. Foods 2017, 33, 112–121. [Google Scholar] [CrossRef]
- Aissani, N.; Coroneo, V.; Fattouch, S.; Caboni, P. Inhibitory effect of carob (Ceratonia siliqua) leaves methanolic extract on Listeria monocytogenes. J. Agric. Food Chem. 2012, 60, 9954–9958. [Google Scholar] [CrossRef] [PubMed]
- Hsouna, A.B.; Trigui, M.; Jarraya, R.M.; Damak, M.; Jaoua, S. Identification of phenolic compounds by high performance liquid chromatography/mass spectrometry (HPLC/MS) and in vitro evaluation of the antioxidant and antimicrobial activities of Ceratonia siliqua leaves extracts. J. Med. Plant. Res. 2015, 9, 479–485. [Google Scholar] [CrossRef]
- Eldahshan, O.A. Isolation and structure elucidation of phenolic compounds of carob leaves grown in Egypt. Curr. Res. J. Biol. Sci. 2011, 3, 52–55. [Google Scholar]
- Rasheed, D.M.; El-Kersh, D.M.; Farag, M.A. Ceratonia siliqua (carob-locust bean) outgoing and potential trends of phytochemical, economic and medicinal merits. In Wild Fruits: Composition, Nutritional Value and Products, 1st ed.; Mariod, A.A., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 481–498. [Google Scholar] [CrossRef]
- Karmous, I.; Taheur, F.B.; Zuverza-Mena, N.; Jebahi, S.; Vaidya, S.; Tlahig, S.; Mhadhbi, M.; Gorai, M.; Raouafi, A.; Debara, M. Phytosynthesis of Zinc Oxide Nanoparticles Using Ceratonia siliqua L. and Evidence of Antimicrobial Activity. Plants 2022, 11, 3079. [Google Scholar] [CrossRef]
- Soleimanzadeh, A.; Kian, M.; Moradi, S.; Mahmoudi, S. Carob (Ceratonia siliqua L.) fruit hydro-alcoholic extract alleviates reproductive toxicity of lead in male mice: Evidence on sperm parameters, sex hormones, oxidative stress biomarkers and expression of Nrf2 and iNOS. Avicenna J. Phytomed. 2020, 10, 35. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6941692/ (accessed on 30 March 2023).
- Rashed, K. Phytochemical and biological effects of Ceratonia siliqua L: A review. Int. J. Pharm. Sci. Res. Inter. 2021, 9, 1–8. Available online: https://www.ijipsr.com/index.php/IJIPSR/article/view/39/25 (accessed on 30 March 2023).
- Gregoriou, G.; Neophytou, C.M.; Vasincu, A.; Gregoriou, Y.; Hadjipakkou, H.; Pinakoulaki, E.; Christodoulou, M.C.; Ioannou, G.D.; Stavrou, I.J.; Christou, A. Anti-cancer activity and phenolic content of extracts derived from Cypriot carob (Ceratonia siliqua L.) pods using different solvents. Molecules 2021, 26, 5017. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Georgiou, E. Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: Extraction optimization and application in food models. Foods 2020, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Ghorbaninejad, Z.; Eghbali, A.; Ghorbaninejad, M.; Ayyari, M.; Zuchowski, J.; Kowalczyk, M.; Baharvand, H.; Shahverdi, A.; Eftekhari-Yazdi, P.; Esfandiari, F. Carob extract induces spermatogenesis in an infertile mouse model via upregulation of Prm1, Plzf, Bcl-6b, Dazl, Ngn3, Stra8, and Smc1b. J. Ethnopharmacol. 2023, 301, 115–760. [Google Scholar] [CrossRef] [PubMed]
- Richane, A.; Rim, B.M.; Riadh, K.; Khaoula, A.; Nizar, M.; Hanen, B.I. Variability of phenolic compounds and antioxidant activities of ten Ceratonia siliqua L. provenances. Biochem. Syst. Ecol. 2022, 104, 104486. [Google Scholar] [CrossRef]
- Martić, N.; Zahorec, J.; Stilinović, N.; Andrejić-Višnjić, B.; Pavlić, B.; Kladar, N.; Šoronja-Simović, D.; Šereš, Z.; Vujčić, M.; Horvat, O. Hepatoprotective effect of carob pulp flour (Ceratonia siliqua L.) extract obtained by optimized microwave-assisted extraction. Pharmaceutics 2022, 14, 657. [Google Scholar] [CrossRef]
- Darwish, W.S.; Khadr, A.E.S.; Kamel, M.A.E.N.; Abd Eldaim, M.A.; El Sayed, I.E.T.; Abdel-Bary, H.M.; Ullah, S.; Ghareeb, D.A. Phytochemical characterization and evaluation of biological activities of egyptian carob pods (Ceratonia siliqua L.) aqueous extract: In vitro study. Plants 2021, 10, 2626. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, F.E.; Silva, J.C.E.; Cacciola, F.; Asraoui, F.; Tayeq, H.; Ben Amar, Y.M.; Lovillo, M.P.; Chouaibi, N.; Brigui, J. Evaluation of Different Extraction Methods on the Phenolic Profile and the Antioxidant Potential of Ceratonia siliqua L. Pods Extracts. Molecules 2022, 27, 6163. [Google Scholar] [CrossRef] [PubMed]
- Amrani, A.; Bouakline, H.; Elkabous, M.; Brahmi, M.; Karzazi, Y.; El Bachiri, A.; Tahani, A. Ceratonia siliqua L seeds extract: Experimental analysis and simulation study. Mater. Today Proc. 2023, 72, 3705–3711. [Google Scholar] [CrossRef]
- Atta, A.H.; Atta, S.A.; Khattab, M.; Abd El-Aziz, T.H.; Mouneir, S.M.; Ibrahim, M.; Nasr, S.M.; Ramadan, S. Ceratonia siliqua pods (Carob) methanol extract alleviates doxorubicin–induced nephrotoxicity via antioxidant, anti-inflammatory and anti-apoptotic pathways. Env. Sci. Pollut. Res. 2023, 30, 83421–83438. [Google Scholar] [CrossRef] [PubMed]
- Ayache, S.B.; Reis, F.S.; Dias, M.I.; Pereira, C.; Glamočlija, J.; Soković, M.; Saafi, E.B.; Ferreira, I.C.; Barros, L.; Achour, L. Chemical characterization of carob seeds (Ceratonia siliqua L.) and use of different extraction techniques to promote its bioactivity. Food Chem. 2021, 351, 129263. [Google Scholar] [CrossRef]
- Yahiaoui, K.; Bouchenak, O.; Boumaza, S.; Toubal, S.; Blizak, D.; Nouani, A.; Arab, K. Characterization and assessment of the antimicrobial function of total polyphenol extracts from pulps, leaves and seeds of two Ceratonia siliqua L. varieties. Alger. J. Envir. Sci. Tech. 2021, 7, 3429–3438. [Google Scholar]
- Ben Ayache, S.; Behija Saafi, E.; Emhemmed, F.; Flamini, G.; Achour, L.; Muller, C.D. Biological activities of aqueous extracts from carob plant (Ceratonia siliqua L.) by antioxidant, analgesic and proapoptotic properties evaluation. Molecules 2020, 25, 3120. [Google Scholar] [CrossRef] [PubMed]
- Lakkab, I.; El Hajaji, H.; Lachkar, N.; Lefter, R.; Ciobica, A.; El Bali, B.; Lachkar, M. Ceratonia siliqua L. seed peels: Phytochemical profile, antioxidant activity, and effect on mood disorders. J. Funct. Foods 2019, 54, 457–465. [Google Scholar] [CrossRef]
- Ouahioune, L.A.; Wrona, M.; Becerril, R.; Salafranca, J.; Nerín, C.; Djenane, D. Ceratonia siliqua L. kibbles, seeds and leaves as a source of volatile bioactive compounds for antioxidant food biopackaging applications. Food Packag. Shelf Life 2022, 31, 100764. [Google Scholar] [CrossRef]
- El Hajaji, H.; Lachkar, N.; Alaoui, K.; Cherrah, Y.; Farah, A.; Ennabili, A.; El Bali, B.; Lachkar, M. Antioxidant properties and total phenolic content of three varieties of carob tree leaves from Morocco. Rec. Nat. Prod. 2010, 4, 193. [Google Scholar]
- Saci, F.; Louaileche, H.; Gali, L.; Bensouici, C. Changes in anticholinesterase, antioxidant activities and related bioactive compounds of carob pulp (Ceratonia siliqua L.) during ripening stages. J. Food Meas. Charact. 2020, 14, 937–945. Available online: https://link.springer.com/article/10.1007/s11694-019-00344-9 (accessed on 14 April 2023). [CrossRef]
- Rtibi; Selmi, S.; Grami, D.; Saidani, K.; Sebai, H.; Amri, M.; Eto, B.; Marzouki, L. Ceratonia siliqua L. (immature carob bean) inhibits intestinal glucose absorption, improves glucose tolerance and protects against alloxan-induced diabetes in rat. J. Sci. Food Agric. 2017, 97, 2664–2670. [Google Scholar] [CrossRef]
- Carbas, B.; Salinas, M.V.; Serrano, C.; Passarinho, J.A.; Puppo, M.C.; Ricardo, C.; Brites, C. Chemical composition and antioxidant activity of commercial flours from Ceratonia siliqua and Prosopis spp. J. Food Meas. Charact. 2019, 13, 305–311. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Rtibi, K.; Jabri, M.-A.; Selmi, S.; Sebai, H.; Marie, J.-C.; Amri, M.; Marzouki, L.; El-Benna, J. Preventive effect of carob (Ceratonia siliqua L.) in dextran sulfate sodium-induced ulcerative colitis in rat. RSC Adv. 2016, 6, 19992–20000. Available online: https://pubs.rsc.org/en/content/articlelanding/2016/ra/c5ra21388f/unauth (accessed on 3 May 2023). [CrossRef]
- Serairi-Beji, R.; Mekki-Zouiten, L.; Tekaya-Manoubi, L.; Loueslati, M.; Guemira, F.; Mansour, A.B. Could carob pulp be incorporated in oral rehydration solution for the treatment of acute diarrhoea? Med. Trop. 2000, 60, 125–128. Available online: https://www.cabdirect.org/cabdirect/abstract/20003009680 (accessed on 3 May 2023).
- Ikram, A.; Waseem, K.; Wajeeha Zafar, K.u.; Ali, A.; Afzal, M.F.; Aziz, A.; Faiz ul Rasool, I.; Al-Farga, A.; Aqlan, F.; Koraqi, H. Nutritional, biochemical, and clinical applications of carob: A review. Food Sci. Nutr. 2023, 11, 3641–3654. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Trigui, M.; Mansour, R.B.; Jarraya, R.M.; Damak, M.; Jaoua, S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int. J. Food Microbiol. 2011, 148, 66–72. [Google Scholar] [CrossRef]
- Bakhtaoui, F.-Z.; Lakmichi, H.; Chait, A.; Gadhi, C.A. In vivo Gastro-protective effects of five Moroccan medicinal plants against gastric ulcer. Am. J. Phytomedicine Clin. Ther. 2014, 2, 1262–1276. [Google Scholar]
- Chao, E.C.; Henry, R.R. SGLT2 inhibition—A novel strategy for diabetes treatment. Nat. Rev. Drug. Discov. 2010, 9, 551–559. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Grami, D.; Sebai, H.; Marzouki, L. In vitro α-amylase/α-glucosidase inhibitory activities and in vivo improving glucose tolerance and hypoglycemic effect of Ceratonia siliqua leaves aqueous extract. EC Nutr. 2018, 13, 171–179. [Google Scholar]
- Qasem, M.A.; Noordin, M.I.; Arya, A.; Alsalahi, A.; Jayash, S.N. Evaluation of the glycemic effect of Ceratonia siliqua pods (Carob) on a streptozotocin-nicotinamide induced diabetic rat model. PeerJ. 2018, 6, e4788. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Patarra, J.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. In vitro antioxidant and inhibitory activity of water decoctions of carob tree (Ceratonia siliqua L.) on cholinesterases, α-amylase and α-glucosidase. Nat. Prod. Res. 2015, 29, 2155–2159. [Google Scholar] [CrossRef] [PubMed]
- Azab, A. D-Pinitol—Active Natural Product from Carob with Notable Insulin Regulation. Nutrients 2022, 14, 1453. [Google Scholar] [CrossRef] [PubMed]
- Lasa, A.; Silván, J.M.; Martínez Villaluenga, C.; Dueñas, M.; Martín Diana, A.B.; Rico, D.; Fernández-Quintela, A.; Aguirre, L. In vitro anti-obesity and ani-inflammatory effects of Ceratonia siliqua L. extracts. In Proceedings of the 11th World Congress on Polyphenol Applications, Viena, Austria, 20–21 June 2017; Available online: http://hdl.handle.net/10261/172239 (accessed on 30 May 2023).
- Aguirre, L.; Martín Diana, A.B.; Martínez Villaluenga, C.; Milton-Laskibar, I.; Luis, D.A.d.; Lasa, A.; Portillo, M.; Rico, D. In vitro and in vivo anti-obesity effects of carob and wakame combination. In Proceedings of the 25th European Congress on Obesity, Viena, Austria, 23–26 May 2018; Available online: http://hdl.handle.net/10261/171804 (accessed on 30 May 2023).
- Rico, D.; Martín-Diana, A.B.; Martínez-Villaluenga, C.; Aguirre, L.; Silván, J.M.; Dueñas, M.; De Luis, D.; Lasa, A. In vitro approach for evaluation of carob by-products as source bioactive ingredients with potential to attenuate metabolic syndrome (MetS). Heliyon 2019, 5, e01175. [Google Scholar] [CrossRef]
- Aboura, I.; Nani, A.; Belarbi, M.; Murtaza, B.; Fluckiger, A.; Dumont, A.; Benammar, C.; Tounsi, M.S.; Ghiringhelli, F.; Rialland, M. Protective effects of polyphenol-rich infusions from carob (Ceratonia siliqua) leaves and cladodes of Opuntia ficus-indica against inflammation associated with diet-induced obesity and DSS-induced colitis in Swiss mice. Biomed. Pharmacother. 2017, 96, 1022–1035. [Google Scholar] [CrossRef]
- Custódio, L.; Escapa, A.L.; Fernandes, E.; Fajardo, A.; Aligué, R.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. In vitro cytotoxic effects and apoptosis induction by a methanol leaf extract of carob tree (Ceratonia siliqua L.). J. Med. Plant. Res. 2011, 5, 1987–1996. [Google Scholar]
- Agrawal, A.; Mohan, M.; Kasture, S.; Foddis, C.; Frau, M.A.; Loi, M.C.; Maxia, A. Antidepressant activity of Ceratonia siliqua L. fruit extract, a source of polyphenols. Nat. Prod. Res. 2011, 25, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Avallone, R.; Cosenza, F.; Farina, F.; Baraldi, C.; Baraldi, M. Extraction and purification from Ceratonia siliqua of compounds acting on central and peripheral benzodiazepine receptors. Fitoterapia 2002, 73, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Hamid, I.; Janbaz, K.H.; Iqbal, R.; Akhtar, M.F.; Saleem, A.; Ali, S.; Sharif, A.; Akhtar, B.; Javaid, Z.; Sohail, K. Therapeutic potential of Ceratonia siliqua extract for the management of asthma and hypertension. Cell. Mol. Biol. 2021, 67, 6–15. [Google Scholar] [CrossRef]
- de la Fuente-Fernández, M.; González-Hedström, D.; Amor, S.; Tejera-Muñoz, A.; Fernández, N.; Monge, L.; Almodóvar, P.; Andrés-Delgado, L.; Santamaría, L.; Prodanov, M. Supplementation with a carob (Ceratonia siliqua L.) fruit extract attenuates the cardiometabolic alterations associated with metabolic syndrome in mice. Antioxidants 2020, 9, 339. [Google Scholar] [CrossRef]
- Birketvedt, G.S. Northern White Kidney Bean Extract and Ceratonia siliqua extract in Combination with Green Tea Extract in the Treatment of Excess Weight and Obesity. U.S. Patent 15/137,967, 27 October 2016. Available online: https://patents.google.com/patent/US20160310552A1/en (accessed on 2 June 2023).
- Gulay, M.; Yildiz-Gulay, O.; Ata, A.; Balic, A.; Demirtas, A. Toxicological evaluation of carob (Ceratonia siliqua) bean extracts in male New Zealand White rabbits. J. Anim. Vet. Adv. 2012, 11, 1853–1857. Available online: https://www.cabdirect.org/globalhealth/abstract/20123235138 (accessed on 2 June 2023). [CrossRef]
- Abajy, M.Y.; Nayal, R.; Alqubaji, M.; Abdrabbo, Y. Efficacy of Carob Leaves and Pods in Reducing Cyclophosphamide-Induced Toxicity on Bone Marrow and Peripheral Blood Leukocytes. Int. J. Life Sci. Pharma Res. 2022, 12, 8–15. [Google Scholar] [CrossRef]

| Country | Cultivated Area (ha) | Production (Tons) | Yield (t/ha) |
|---|---|---|---|
| Morocco Turkey Lebanon Algeria | 10,389 1078 400 688 | 21,976.85 20,633 4351.1 3219 | 2.11 19.14 0.01 4.67 |
| Tunisia America Ukraine Jordan | 408 80 95 0 | 818.17 311.93 200.24 0 | 2.00 3.89 2.10 0 |
| Total | 1682,467 | 138,051,995 | 32.92 |
| Country | Ethnomedicinal/Food Uses | Part and/or Method of Use | References |
|---|---|---|---|
| Morocco | Diarrhea/toxic for fish | Fruits, barks | [53] |
| Intestinal parasites, digestive system, antidiarrheal | |||
| Digestive, skin, nervous | Leaves, fruits decoction or raw | [54] | |
| Diabetes | Seeds, leaves or fruits infusion, powder, or decoction | [51,52] | |
| Digestive diseases | Leaves, seeds decoction or powder | [51] | |
| Fruits powder | [55] | ||
| Tunisia | Food | Pulp | [25] |
| Gastrointestinal disorders, Diarrhea | Fruits | [50] | |
| Egypt | Diarrhea | Fruits, leaves infusion | [56] |
| Jordan | Diabetes | Leaves decoction cold or hot beverage of the pods | [57] |
| Cough | [58] | ||
| Lebanon | Sweetener | Molasse of ripened fruits | [59] |
| Food | Pods | ||
| Medicine | Not indicated | [60] | |
| Palestine | Food | Raw fruits | [61] |
| Hypertension | Raw or cooked fruits, seeds | [7] | |
| Iraq | Abdominal pain, diarrhea | Fruits | [62] |
| Turkey | Diuretic, purgative | Fresh fruits | [63] |
| Diarrhea | Leaves | [44] | |
| Cyprus | Diabetes | Fruits decoction | [64] |
| Laxative, demulcent | Pods | [65] | |
| Greco-Arab | Diabetes, herpes, lip sores | Leaves decoction | [66] |
| India | Hypocholesterolemic, hypolipidemic, hypoglycemic, demulcent, resolvent | Not indicated | [67] |
| Iran | Menorrhagia | Patient should sit in a pot of a decoction of several plants | [68] |
| Spain | Chocolate or coffee substitute, preservative for olives | Fruits, leaves | [16] |
| Sicily | Food | Raw or boiled fruits | [69] |
| Italy | Emollient | Decoction with Ficus carica L. and Malva sylvestris L. | [70] |
| Class | Compounds | Part of the Plant | References |
|---|---|---|---|
| Phenols | Resorcinol | Leaves, pods, pulp, seeds | [102] |
| Vanillin, fraxidin, 2,4- bis(dimethylbenzyl)-6-butylphenol | Leaves | [102,109] | |
| Alizarin, hydroquinone, lignan bis(trihydroxyphenyl)methanone | Pods | [110,111] | |
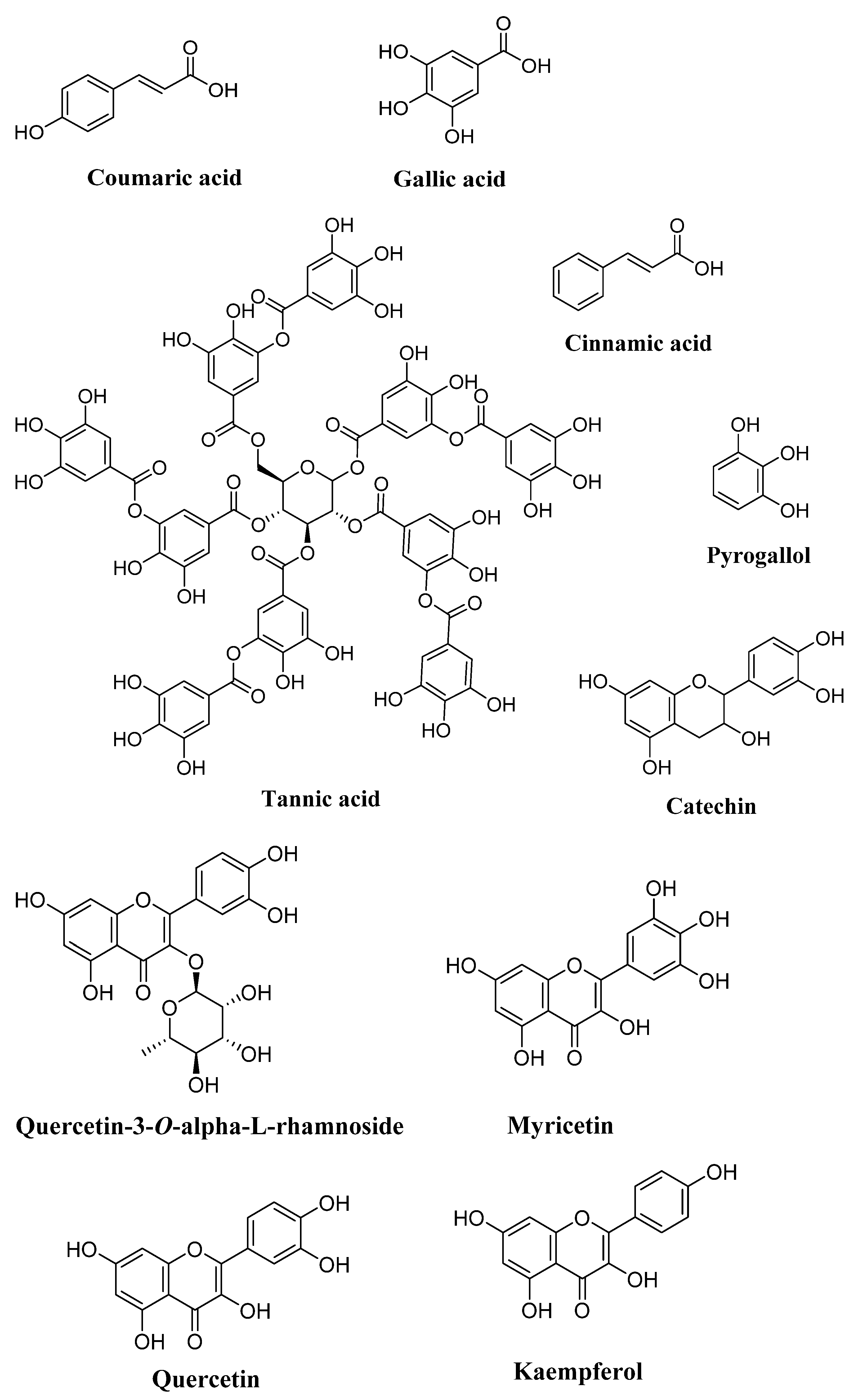
| Phenolic acids | Gallic acid, chlorogenic acid, syringic acid, ferulic acid, coumaric acid, cinnamic acid | Leaves, pods, pulp, seeds | [4,5,89,102,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130] |
| 4-Hydroxybenzoic acid, caffeic acid, vanillic acid, gentisic acid | Leaves, pods, pulp | [5,89,102,112,113,114,119,121,122,125,126,127,128,129] | |
| Tannic acid | Leaves, pods, seeds | [4,122] | |
| Ellagic acid, rosmarinic acid | Pods, pulp, seeds | [111,119,125,126,127] | |
| Sinapic acid | Pulp, seeds | [111,126] | |
| Pyrogallol, methyl gallate, benzoic acid, protocatechuic acid | Pods, pulp | [4,5,110,111,119,120,122,128,129] | |
| Quinic acid | Leaves, pods | [109,120] | |
| Transferulic acid, O-feruloylrutinose, O- feruloylrutinose isomer, p-coumaroyl- galloylhexose, O-p-coumaroylrutinose, siliquapyranone | Pods | [110,120,125] | |
| 4-Hydroxy-coumaric acid | Leaves | [112,113] | |
| 5-Caffeoylquinic acid, myristic acid, ascorbic acid | Pulp | [111,126,131] | |
| Flavonoids | Epicatechin, quercetin, kaempferol, luteolin, catechin, apigenin | Leaves, pods, pulp, seeds | [4,5,89,102,110,111,112,113,115,116,118,119,120,121,122,123,124,125,126,127,128,130,131,132] |
| Epigallocatechin gallate, rutin, myricetin, naringenin | Leaves, pods, pulp | [4,5,110,111,112,113,114,116,117,118,119,120,121,123,124,125,126,127,128] | |
| Iso-rhamnetin | Leaves, pods, seeds | [102,110,111,119,125] | |
| Leucoanthocyanins | Leaves, pulp, seeds | [133] | |
| Genistein | Leaves, pods | [5,119] | |
| Quercitrin, catechin tannins | Leaves, pulp | [112,113,115,127,133] | |
| Anthocyanins | Pods, pulp, seeds | [5,134,135] | |
| Myricitrin, daidzein, flavonol, morin | Leaves | [102,112,113,114,116] | |
| Rhamnosides, chrysoeriol, tricetin dimethyl ether, (iso)schaftoside-4′-O- glucoside, gallocatechin, chrysoeriol-O- deoxyheoxoside, dihydroxyflavanone hexoside, tetrahydroxy flavanone, trihy- droxy flavone (apigenin isomer), kamp- feride, methoxykampferol, dihydroxy flavanone, tricetin dimethyl ether, cirsi- liol, flavone glycosides, hydroxytyrosol | Pods | [5,110,119,120,122] | |
| Crismaritin, catechol, isoquercetrin, flavonols 3′,4′,5,7-OH, 2-hexadecanol scutellarin tetramethyl ether, silybin B, hydroxytyrosol, catechin gallate | Pulp | [4,126,129,131] | |
| Apigenin flavone, chrysin aglycones | Seeds | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahmani, W.; Elaouni, N.; Abousalim, A.; Akissi, Z.L.E.; Legssyer, A.; Ziyyat, A.; Sahpaz, S. Exploring Carob (Ceratonia siliqua L.): A Comprehensive Assessment of Its Characteristics, Ethnomedicinal Uses, Phytochemical Aspects, and Pharmacological Activities. Plants 2023, 12, 3303. https://doi.org/10.3390/plants12183303
Dahmani W, Elaouni N, Abousalim A, Akissi ZLE, Legssyer A, Ziyyat A, Sahpaz S. Exploring Carob (Ceratonia siliqua L.): A Comprehensive Assessment of Its Characteristics, Ethnomedicinal Uses, Phytochemical Aspects, and Pharmacological Activities. Plants. 2023; 12(18):3303. https://doi.org/10.3390/plants12183303
Chicago/Turabian StyleDahmani, Widad, Nabia Elaouni, Abdelhadi Abousalim, Zachée Louis Evariste Akissi, Abdelkhaleq Legssyer, Abderrahim Ziyyat, and Sevser Sahpaz. 2023. "Exploring Carob (Ceratonia siliqua L.): A Comprehensive Assessment of Its Characteristics, Ethnomedicinal Uses, Phytochemical Aspects, and Pharmacological Activities" Plants 12, no. 18: 3303. https://doi.org/10.3390/plants12183303
APA StyleDahmani, W., Elaouni, N., Abousalim, A., Akissi, Z. L. E., Legssyer, A., Ziyyat, A., & Sahpaz, S. (2023). Exploring Carob (Ceratonia siliqua L.): A Comprehensive Assessment of Its Characteristics, Ethnomedicinal Uses, Phytochemical Aspects, and Pharmacological Activities. Plants, 12(18), 3303. https://doi.org/10.3390/plants12183303






