Cryopreservation of Duckweed Genetic Diversity as Model for Long-Term Preservation of Aquatic Flowering Plants
Abstract
1. Introduction
2. Results and Discussion
2.1. Developing the Method
2.1.1. Attenuation of Toxicity of PVS3: Impact of Temperature and Illumination
2.1.2. Addition of Antioxidants and Other Compounds That Can Support Viability
2.1.3. Breakthrough Regrowth via Elevated Temperature
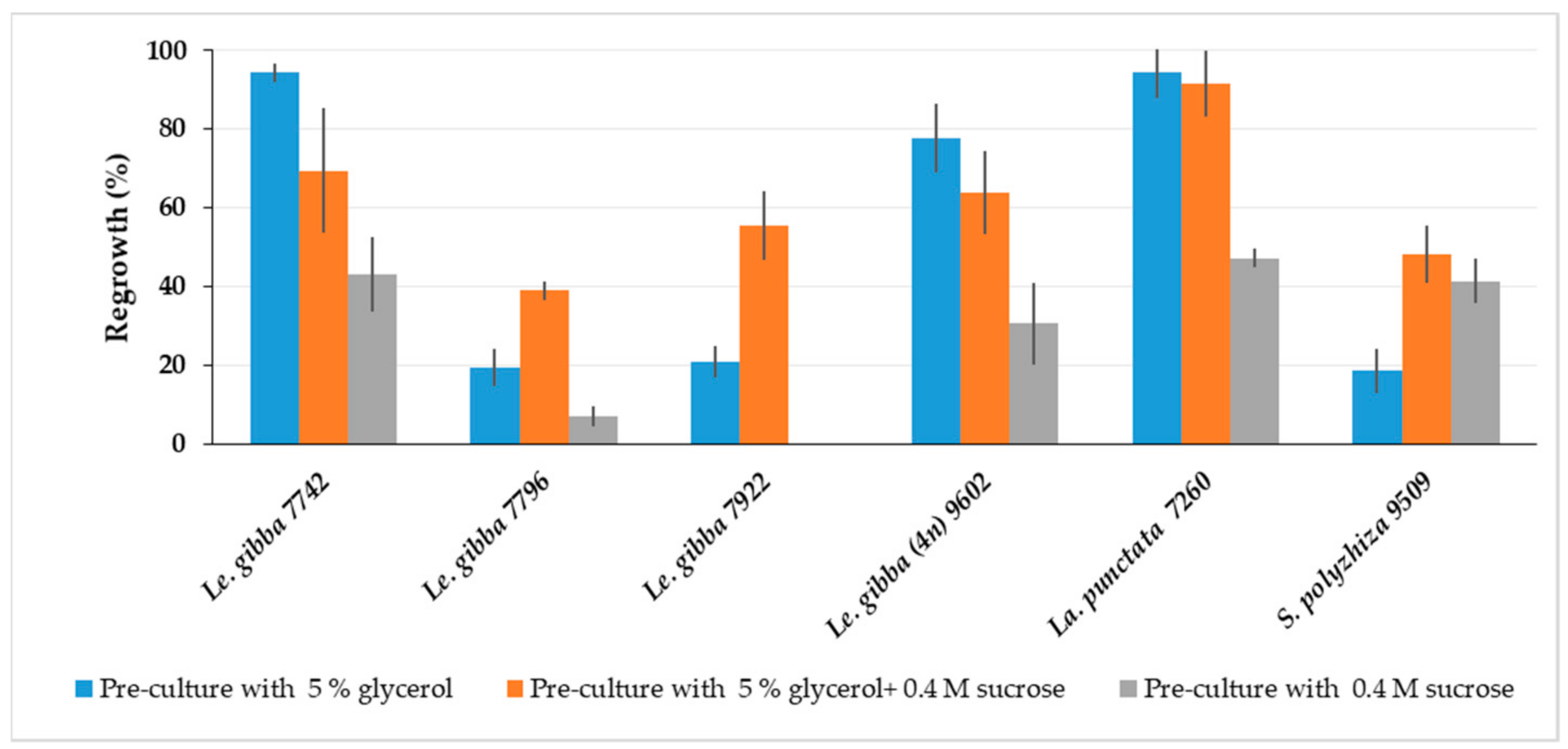
2.1.4. Effect of Adaptation to Cold and High Osmotic Condition at Pre-Culture Stage on Regrowth
2.1.5. Illumination Regime Optimization for Early Regrowth
2.1.6. Vacuum Infiltration Turned Out to Be Dispensable
2.1.7. Substitution of Blotting by Spin-Drying of Duckweed Fronds before Submersing in PVS3
2.1.8. Optimization of Solution Composition for Pre-Cultivation
2.2. Regrowth of Spirodela, Landoltia, Lemna, and Wolffia Accessions after Cryopreservation
2.3. Flow Cytometry Demonstrates the Genome Size Stability after Cryopreservation
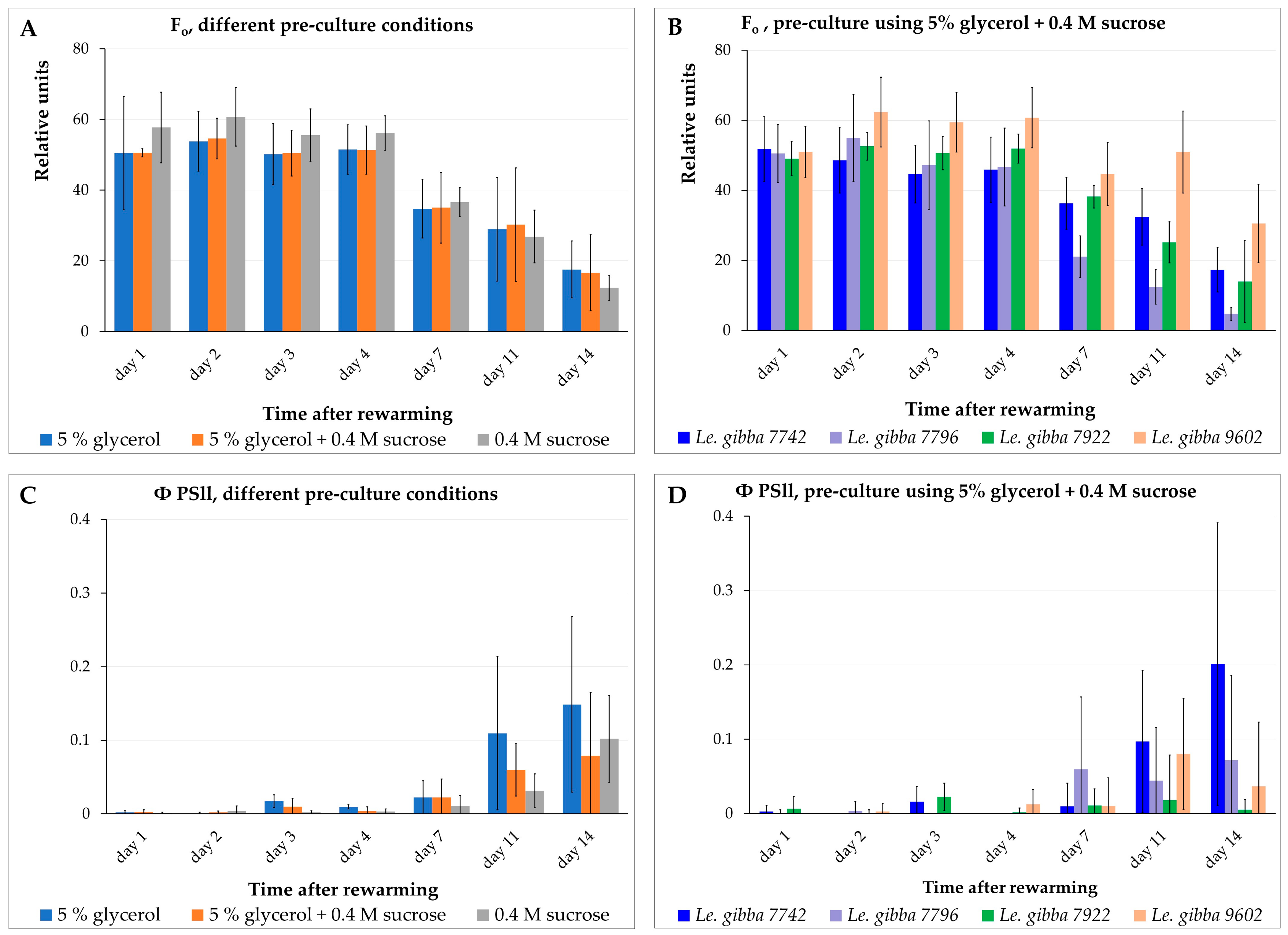
2.4. Monitoring the Photosynthetic Activity of Rewarmed Fronds
2.5. Identification of Duckweed CBFs and Their Involvement in Cryopreservation
2.5.1. Identification and Characterization of CBF/DREB1 Genes in Duckweeds
2.5.2. Dynamics of CBF mRNA Abundance during Cryopreservation
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Nuclear Genome Size Measurement
3.3. Chlorophyll Fluorescence Measurement
3.4. Identification and Analysis of CBF/DREB1 Gene Family Members of Duckweeds
3.5. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time RT–PCR
3.6. Measurement of Spectral Characteristic of the Light Source in the Phytochamber
3.7. Statistical Analysis
3.7.1. Optimization Experiments
3.7.2. Calculation of Average Regrowth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Preliminary Protocol for Cryopreservation Used for First Experiments with Le. gibba 7742
Appendix A.1. Donor Culture Maintenance
Appendix A.2. Pre-Culture
Appendix A.3. PVS3 Preparation
Appendix A.4. Dehydration and Cooling Procedure
- Prepare foil strips (≈7 mm width, ≈70 mm length). Insert the foil strips (with tweezers) to cryo-tubes (2.0 mL) to obtain a “U-form” shape formed by the strip inside the cryo-tubes. Press the side parts of the “U-shaped” foil strip to the sides of the cryo-tube with tweezers (see graphic instruction on Figure S1). Transfer the pre-formed “U-shaped” foil insert to the container for autoclavation.
- Sterilize the prepared “U-shaped” foil insert by autoclavation.
- In the laminar cabinet, insert the autoclaved “U-shaped” foil insert into the sterile cryo-tube using sterile tweezers.
- In the laminar cabinet, transfer a portion of the pre-cultivated duckweed fronds to sterile filter paper using sterile tweezers for brief blotting (for 1–2 min).
- Transfer a portion of the blotted duckweed fronds (25–50 fronds) to the cryo-tube, supplemented with the “U-shaped” foil insert by inserting the fronds between “U-shape” parts of the foil insert close to the bottom of the cryo-tube.
- Clamp the edges of the foil strip at the top of the cryo-tube with sterile tweezers to form the foil pack with the fronds inside the cryo-tube (see graphic instruction in Figure S2).
- Add a portion of the 1 mL of PVS3 to the cryo-tube containing duckweed fronds packed in a foil insert.
- Load the opened test tube into the vacuum chamber (exicator after aseptic treatment, placed in the laminar cabinet).
- Apply a vacuum (as deep as possible using a common laboratory vacuum pump).
- Incubate the test tube under vacuum for 15 min at room temperature. Release the vacuum.
- Transfer the cryo-tube from the exicator, and cover the cryo-tube with the sterile cap.
- Incubate for 1 h and 45 min at room temperature.
- Transfer the cryo-tube with the duckweed to liquid nitrogen. Store the sample in liquid nitrogen for at least several days.
Appendix A.5. Rewarming, Washing, and Unloading
- Prepare a glass test tube (sterile, 50 mL, with foil cap) filled with 10 mL of a sterile solution of 1.2 M sucrose (washing solution).
- Prepare the sterile 9 g/L glucose solution, buffered by 1 g/L MES, pH 7.1–7.2 (by KOH).
- Remove the cryo-tube from the liquid nitrogen, and defrost by heating in a water bath set at 40 °C, keeping the tube submersed in the bath until the ice in the tube almost completely melts (visual observation).
- Immediately after defrosting (almost complete ice melting), briefly treat the cryo-tube with the antiseptic solution, open the cryo-tube in the laminar cabinet, and slightly open the foil pack in the cryo-tube with sterile tweezers by separating the edges of the foil strip.
- Immediately transfer the foil pack with duckweed from the cryo-tube to the glass test tube, fill with the 1.2 M sucrose solution (room temperature), and close the test tube using the cap.
- Incubate for 1 h at room temperature. During this time, most of the duckweed fronds should have separated from the foil insert and should start floating on the surface of the washing solution.
- In the laminar cabinet, remove the solution of sucrose from the test tube (leaving the duckweed fronds in the test tube) and add 10 mL of the 9 g/L glucose solution, buffered by 1 g/L MES (pH 7.1–7.2, room temperature) to the test tube.
- Transfer the test tube to the climate chamber, set at +25 ± 1 °C, in a light-protecting box for further unloading from the rest of the components of the PVS3. Incubate for 1 day in darkness.
- In the laminar cabinet, remove the solution of glucose from the test tube (leaving the duckweed fronds in the test tube), and add a fresh portion of 10 mL of the 9 g/L glucose solution (pH 7.1–7.2, room temperature).
- Transfer the test tube to the climate chamber, set at +25 ± 1 °C, in a light-protecting box for further unloading and revitalizing. Incubate for 3 days in darkness.
Appendix A.6. Early Regrowth
- In the laminar cabinet, remove the solution of glucose from the test tube with duckweed and add 10 mL of the liquid SH nutrition medium supplemented with 5 g/L sucrose and 5 g/L glucose (pH 7.1–7.2, room temperature).
- Transfer the test tubes to the climate chamber, set at 12/12 h diurnal light/dark cycle, PPFD approximately 50–60 μmol·m−2·s−1 with the spectrum provided in Figure S13, and 26 °C/25 °C temperature for the light/dark periods. Incubate for further observations.
Appendix B. Optimized Protocol for Cryopreservation of Lemnaceae Representatives
Appendix B.1. Donor Culture Maintenance
Appendix B.2. Pre-Culture
Appendix B.3. PVS3 Preparation
Appendix B.4. Dehydration and Cooling Procedure
- Prepare the foil strips (≈7 mm width, ≈70 mm length). Make 15–20 perforations in the central part of the foil strip using a needle or the sharp and thin end if small tweezers (to accelerate and simplify this operation, we made a special many-needled tool for this purpose from a comb for animals). Insert the foil strips into the plastic centrifugal test tube (2.0 mL) to obtain the “U-shape” form of the strip inside the test tube. Insert a magnetic stirrer bar (7 mm) or another stick with the same diameter and U-shaped end into the test tube to press the foil strip onto the internal surface of the centrifugal test tube (see graphic instruction on Figure S6). Transfer the pre-formed foil insert to the container for autoclavation.
- Sterilize the prepared foil inserts by autoclavation.
- Sterilize swabs by autoclavation.
- In the laminar cabinet, transfer 1 mL of sterile PVS3 solution to the sterile cryo-tubes (2 mL). Close, and precool in ice.
- In the laminar cabinet, insert the autoclaved swabs into the sterile plastic centrifugal test tubes (2 mL) using sterile tweezers. Push the swabs down to the bottom of the centrifugal test tubes.
- In the laminar cabinet, transfer the portions of duckweed fronds from donor cultures (after the pre-cultivation stage) to the sterile centrifugal test tubes, supplemented with cotton wool, using autoclaved foil inserts (as single-use sampler) and sterile tweezers (see graphic instruction on Figure 2).
- Clamp the edges of the foil strips at the top of the centrifugal test tubes with sterile tweezers to form the foil packs with the fronds inside the centrifugal test tubes.
- Close the test tubes, and spin-dry the duckweed fronds by centrifugation for 3 min at 800× g at room temperature.
- In the laminar cabinet, transfer the foil packs with spin-dried duckweed fronds to the precooled-in-ice cryo-tubes filled with 1 mL of PVS3.
- Immediately close the cryo-tubes, and transfer it on ice in darkness.
- Incubate for 4 h on ice in darkness.
- Transfer the cryo-tubes with the duckweed to liquid nitrogen. Perform the operation under weak daylight (or dusk) or weak artificial light. Store the sample in liquid nitrogen for at least several days.
Appendix B.5. Rewarming, Washing, and Unloading
- Prepare a glass test tube (sterile, 50 mL, with foil cap) filled with 10 mL of a sterile 1.2 M sucrose solution (washing solution). Precool and keep on ice.
- Prepare the sterile 9 g/L glucose solution, buffered by 1 g/L MES, pH 7.1–7.2 (by KOH). Precool at ≈10 °C.
- Remove the cryo-tube from liquid nitrogen, and immediately defrost by heating in a water bath set at 40 °C, keeping the tube submersed in the bath until some signs of viscous fluidity of PVS3 in the cryo-tube appears (visual observation). Perform the operations under weak daylight (or dusk) or weak artificial light.
- Immediately after defrosting, briefly treat the cryo-tube with the antiseptic solution, open the cryo-tube in the laminar cabinet, and slightly open the foil pack in the cryo-tube with sterile tweezers by separating the edges of the foil strip. Perform the operations under weak daylight (or dusk) or weak artificial light.
- Immediately transfer the foil pack with duckweed from the cryo-tube to the precooled-in-ice glass test tube, fill with the 1.2 M sucrose solution, and close the test tube using the cap. Perform the operations under weak daylight (or dusk) or weak artificial light.
- Immediately transfer the glass test tube with rewarmed duckweed on ice in darkness.
- Incubate on ice in darkness for 1 h. During this time, most of the duckweed fronds should have separated from the surface of the foil pack and should start floating on the surface of the washing solution.
- In the laminar cabinet, remove the solution of sucrose from the test tube (leaving the duckweed fronds in the test tube), and add 10 mL of the 9 g/L glucose solution, buffered by 1g/L MES (pH 7.1–7.2, precooled at ≈10 °C) to the glass test tube with rewarmed duckweed. Perform the operations under weak daylight (or dusk) or weak artificial light.
- Transfer the test tube to the climate chamber, set at +25 ± 1 °C, in a light-protecting box for further unloading from the rest of the components of the PVS3. Incubate for 1 day in darkness.
- In the laminar cabinet, remove the solution of glucose from the test tube (leaving the duckweed fronds in the test tube), and add a fresh portion of 10 mL of the 9 g/L glucose solution, buffered by 1 g/L MES (pH 7.1–7.2, room temperature). Perform the operations under weak daylight (or dusk) or weak artificial light.
- Transfer the test tube to the climate chamber, set at +25 ± 1 °C, in a light-protecting box for further unloading and revitalizing. Incubate for 3 days in darkness.
Appendix B.6. Early Regrowth
- In the laminar cabinet, remove the solution of glucose from the test tube with duckweed and add 10 mL of the liquid SH nutrition medium supplemented with 5 g/L sucrose and 5 g/L glucose (pH 7.1–7.2). For Wolffia australiana 8730, the liquid SH medium, supplemented with 5 g/L sucrose, 5 g/L glucose, 0.5 g/L casein hydrolysate, and 0.5 g/L yeast extract (pH 7.1–7.2), should be added. Perform the operations under weak daylight (or dusk) or weak artificial light.
- Transfer the test tubes to the climate chamber, set at +29 ± 1 °C, for re-growth. Incubate in accordance with the pulsed illumination regime (see Figure 1) with the light spectrum provided in Figure S13 and at a temperature of +29 ± 1 °C for 6 days.
- Transfer the test tubes to the climate chamber, set at a 12/12 h diurnal light/dark cycle, PPFD approximately 50–60 μmol·m−2·s−1 with the spectrum provided in Figure S13, and 26 °C/25 °C temperature for the light/dark periods for further re-growth.
References
- Tippery, N.P.; Les, D.H. Tiny Plants with Enormous Potential: Phylogeny and Evolution of Duckweeds. In The Duckweed Genomes; Cao, X.H., Fourounjian, P., Wang, W., Eds.; Springer: Cham, Switzerland, 2020; pp. 19–38. ISBN 978-3-030-11044-4. [Google Scholar]
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.K.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S.; et al. Return of the Lemnaceae: Duckweed as a model plant system in the genomics and postgenomics era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef]
- Zhou, Y.; Stepanenko, A.; Kishchenko, O.; Xu, J.; Borisjuk, N. Duckweeds for Phytoremediation of Polluted Water. Plants 2023, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.; Kishchenko, O.; Zhou, Y.; Vasylenko, M.; Giritch, A.; Sun, J.; Borisjuk, N.; Kuchuk, M. Robust Agrobacterium-Mediated Transient Expression in Two Duckweed Species (Lemnaceae) Directed by Non-replicating, Replicating, and Cell-to-Cell Spreading Vectors. Front. Bioeng. Biotechnol. 2021, 9, 761073. [Google Scholar] [CrossRef] [PubMed]
- Braglia, L.; Breviario, D.; Gianì, S.; Gavazzi, F.; De Gregori, J.; Morello, L. New Insights into Interspecific Hybridization in Lemna L. Sect. Lemna (Lemnaceae Martinov). Plants 2021, 10, 2767. [Google Scholar] [CrossRef] [PubMed]
- Braglia, L.; Lauria, M.; Appenroth, K.J.; Bog, M.; Breviario, D.; Grasso, A.; Gavazzi, F.; Morello, L. Duckweed Species Genotyping and Interspecific Hybrid Discovery by Tubulin-Based Polymorphism Fingerprinting. Front. Plant Sci. 2021, 12, 625670. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Appenroth, K.J.; Ma, Y.; Shoham, T.; Sree, K.S. Registration of Duckweed Clones/Strains—Future Approach. Duckweed Forum 2020, 8, 35–37. [Google Scholar]
- Stomp, A.M. The duckweeds: A valuable plant for biomanufacturing. In Biotechnology Annual Review; Elsevier: Amsterdam, The Netherlands, 2005; Volume 11, pp. 69–99. [Google Scholar]
- Yang, G.-L.; Feng, D.; Liu, Y.-T.; Lv, S.-M.; Zheng, M.-M.; Tan, A.-J. Research Progress of a Potential Bioreactor: Duckweed. Biomolecules 2021, 11, 93. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.; Li, G.; Khan, S.; Kumar, S.; Yao, L.; Duan, P.; Hou, H. Transformation Development in Duckweeds. In The Duckweed Genomes; Cao, X.H., Fourounjian, P., Wang, W., Eds.; Springer: Cham, Switzerland, 2020; pp. 143–155. ISBN 978-3-030-11044-4. [Google Scholar]
- Yu, L.; Wang, Y.; Xu, S.; Tang, X.; Zhao, J.; Yu, C.; He, G.; Xu, H.; Wang, S.; Tang, Y.; et al. Efficient genetic transformation and CRISPR/Cas9-mediated genome editing in Lemna aequinoctialis. Plant Biotechnol. J. 2019, 17, 2143–2152. [Google Scholar] [CrossRef]
- Shahak, Y.; Posner, H.B.; Avron, M. Evidence for a Block between Plastoquinone and Cytochrome f in a Photosynthetic Mutant of Lemna with Abnormal Flowering Behavior. Plant Physiol. 1976, 57, 577–579. [Google Scholar] [CrossRef][Green Version]
- Tam, Y.Y.; Slovin, J.P.; Cohen, J.D. Selection and Characterization of [alpha]-Methyltryptophan-Resistant Lines of Lemna gibba Showing a Rapid Rate of Indole-3-Acetic Acid Turnover. Plant Physiol. 1995, 107, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Panis, B.; Nagel, M.; Van den Houwe, I. Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef]
- Reed, B.M. (Ed.) Plant Cryopreservation: A Practical Guide; Springer: New York, NY, USA, 2008; ISBN 978-0-387-72275-7. [Google Scholar]
- Volk, G.M.; Walters, C. Plant vitrification solution 2 lowers water content and alters freezing behavior in shoot tips during cryoprotection. Cryobiology 2006, 52, 48–61. [Google Scholar] [CrossRef]
- Kreckel, H.D.; Samuels, F.M.D.; Bonnart, R.; Volk, G.M.; Stich, D.G.; Levinger, N.E. Tracking Permeation of Dimethyl Sulfoxide (DMSO) in Mentha × piperita Shoot Tips Using Coherent Raman Microscopy. Plants 2023, 12, 2247. [Google Scholar] [CrossRef]
- Tunçer, S.; Gurbanov, R.; Sheraj, I.; Solel, E.; Esenturk, O.; Banerjee, S. Low dose dimethyl sulfoxide driven gross molecular changes have the potential to interfere with various cellular processes. Sci. Rep. 2018, 8, 14828. [Google Scholar] [CrossRef] [PubMed]
- Zamecnik, J.; Faltus, M.; Bilavcik, A. Vitrification Solutions for Plant Cryopreservation: Modification and Properties. Plants 2021, 10, 2623. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Borisjuk, N.; Peterson, A.A.; Lv, J.; Qu, G.; Luo, Q.; Shi, L.; Chen, G.; Kishchenko, O.; Zhou, Y.; Shi, J. Structural and Biochemical Properties of Duckweed Surface Cuticle. Front. Chem. 2018, 6, 317. [Google Scholar] [CrossRef]
- Sauter, P.R. Kryokonservierung von Lemnaceae. Ph.D. Thesis, ETH Zürich, Zürich, Switzerland, 1993. [Google Scholar] [CrossRef]
- Parsons, J.; Wingate, V. Methods and Compositions for the Cryopreservation of Duckweed 2011. Patent No. WO2011005502A3, 29 December 2011. [Google Scholar]
- Ito, S.; Tanaka, D.; Oyama, T. Development of Cryopreservation Protocol for a Variety of Duckweed Meristems by Vitrification-cryo-Plate Method. In Proceedings of the 6th International Conference on Duckweed Research and Application ICDRA 2022, Gatersleben, Germany, 29 May–1 June 2022; p. 34. [Google Scholar]
- Nadarajan, J.; Pritchard, H.W. Biophysical Characteristics of Successful Oilseed Embryo Cryoprotection and Cryopreservation Using Vacuum Infiltration Vitrification: An Innovation in Plant Cell Preservation. PLoS ONE 2014, 9, e96169. [Google Scholar] [CrossRef] [PubMed]
- Volk, G. Application of Functional Genomics and Proteomics to Plant Cryopreservation. Curr. Genom. 2010, 11, 24–29. [Google Scholar] [CrossRef]
- Bruňáková, K.; Čellárová, E. Conservation Strategies in the Genus Hypericum via Cryogenic Treatment. Front. Plant Sci. 2016, 7, 558. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Kulus, D.; de Souza, A.V.; Kaviani, B.; Vicente, E.F. Cryopreservation of Agronomic Plant Germplasm Using Vitrification-Based Methods: An Overview of Selected Case Studies. Int. J. Mol. Sci. 2021, 22, 6157. [Google Scholar] [CrossRef] [PubMed]
- Barrero-Gil, J.; Salinas, J. Gene Regulatory Networks Mediating Cold Acclimation: The CBF Pathway. In Survival Strategies in Extreme Cold and Desiccation; Iwaya-Inoue, M., Sakurai, M., Uemura, M., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1081, pp. 3–22. ISBN 978-9-81131-243-4. [Google Scholar]
- Vyse, K.; Schaarschmidt, S.; Erban, A.; Kopka, J.; Zuther, E. Specific CBF transcription factors and cold-responsive genes fine-tune the early triggering response after acquisition of cold priming and memory. Physiol. Plant. 2022, 174, e13740. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Shi, Y.; Peng, Y.; Jia, Y.; Yan, Y.; Dong, X.; Li, H.; Dong, J.; Li, J.; Gong, Z.; et al. Cold-Induced CBF–PIF3 Interaction Enhances Freezing Tolerance by Stabilizing the phyB Thermosensor in Arabidopsis. Mol. Plant 2020, 13, 894–906. [Google Scholar] [CrossRef]
- Hu, Z.; Ban, Q.; Hao, J.; Zhu, X.; Cheng, Y.; Mao, J.; Lin, M.; Xia, E.; Li, Y. Genome-Wide Characterization of the C-repeat Binding Factor (CBF) Gene Family Involved in the Response to Abiotic Stresses in Tea Plant (Camellia sinensis). Front. Plant Sci. 2020, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ding, Y.; Shi, Y.; Zhang, X.; Gong, Z.; Yang, S. The cbfs triple mutants reveal the essential functions of CBF s in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016, 212, 345–353. [Google Scholar] [CrossRef]
- Baker, C.R.; Stewart, J.J.; Amstutz, C.L.; Ching, L.G.; Johnson, J.D.; Niyogi, K.K.; Adams, W.W.; Demmig-Adams, B. Genotype-dependent contribution of CBF transcription factors to long-term acclimation to high light and cool temperature. Plant Cell Environ. 2022, 45, 392–411. [Google Scholar] [CrossRef] [PubMed]
- Lemon, G.D.; Posluszny, U. Comparative Shoot Development and Evolution in the Lemnaceae. Int. J. Plant Sci. 2000, 161, 733–748. [Google Scholar] [CrossRef]
- Ren, L.; Wang, M.-R.; Wang, Q.-C. ROS-induced oxidative stress in plant cryopreservation: Occurrence and alleviation. Planta 2021, 254, 124. [Google Scholar] [CrossRef]
- Volk, G.M.; Harris, J.L.; Rotindo, K.E. Survival of mint shoot tips after exposure to cryoprotectant solution components. Cryobiology 2006, 52, 305–308. [Google Scholar] [CrossRef]
- Senula, A.; Nagel, M. Cryopreservation of Plant Shoot Tips of Potato, Mint, Garlic, and Shallot Using Plant Vitrification Solution 3. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W.F., Oldenhof, H., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2180, pp. 647–661. ISBN 978-1-07-160782-4. [Google Scholar]
- Lukić, M.; Funnekotter, B.; Considine, M.J.; Bunn, E.; Mancera, R.L. Evaluation of Vacuum Infiltration Vitrification and Cryo-mesh Cryopreservation Techniques with Arabidopsis thaliana Shoot Tips. Cryo Lett. 2022, 43, 328–333. [Google Scholar] [CrossRef]
- Normah, M.N.; Sulong, N.; Reed, B.M. Cryopreservation of shoot tips of recalcitrant and tropical species: Advances and strategies. Cryobiology 2019, 87, 425–445. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.A.; Kalisz-Nowak, B.; Korohoda, W.; Wieckowski, S.; Kilarski, W.; Kozlowska, M. Contractile elements of Lemna trisulca L. glycerinated cell models during chloroplast translocations. Biochem. Cell Biol. 2000, 78, 503–510. [Google Scholar] [CrossRef]
- Yamada, T.; Marubashi, W.; Niwa, M. Detection of Four Lethality Types in Interspecific Crosses among Nicotiana Species through the Use of Three Rescue Methods for Lethality. Breed. Sci. 1999, 49, 203–210. [Google Scholar] [CrossRef]
- Folgado, R.; Panis, B.; Sergeant, K.; Renaut, J.; Swennen, R.; Hausman, J.-F. Unravelling the effect of sucrose and cold pretreatment on cryopreservation of potato through sugar analysis and proteomics. Cryobiology 2015, 71, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Queval, G.; Issakidis-Bourguet, E.; Hoeberichts, F.A.; Vandorpe, M.; Gakière, B.; Vanacker, H.; Miginiac-Maslow, M.; Van Breusegem, F.; Noctor, G. Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cel: Photoperiod and H2O2 signalling. Plant J. 2007, 52, 640–657. [Google Scholar] [CrossRef]
- Challabathula, D.; Puthur, J.T.; Bartels, D. Surviving metabolic arrest: Photosynthesis during desiccation and rehydration in resurrection plants: Surviving Metabolic Arrest. Ann. N. Y. Acad. Sci. 2016, 1365, 89–99. [Google Scholar] [CrossRef]
- Rogers, H.; Munné-Bosch, S. Production and Scavenging of Reactive Oxygen Species and Redox Signaling during Leaf and Flower Senescence: Similar but Different. Plant Physiol. 2016, 171, 1560–1568. [Google Scholar] [CrossRef]
- Mechela, A.; Schwenkert, S.; Soll, J. A brief history of thylakoid biogenesis. Open Biol. 2019, 9, 180237. [Google Scholar] [CrossRef]
- Köpnick, C.; Grübe, M.; Stock, J.; Senula, A.; Mock, H.-P.; Nagel, M. Changes of soluble sugars and ATP content during DMSO droplet freezing and PVS3 droplet vitrification of potato shoot tips. Cryobiology 2018, 85, 79–86. [Google Scholar] [CrossRef]
- Madsen, J.D. Biomass Techniques for Monitoring and Assessing Control of Aquatic Vegetation. Lake Reserv. Manag. 1993, 7, 141–154. [Google Scholar] [CrossRef]
- Turner, S.; Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K.; Tan, B. Stereochemical arrangement of hydroxyl groups in sugar and polyalcohol molecules as an important factor in effective cryopreservation. Plant Sci. 2001, 160, 489–497. [Google Scholar] [CrossRef]
- Sarin, L.P.; Sree, K.S.; Bóka, K.; Keresztes, Á.; Fuchs, J.; Tyagi, A.K.; Khurana, J.P.; Appenroth, K.-J. Characterisation of a Spontaneous Mutant of Lemna gibba G3 (Lemnaceae). Plants 2023, 12, 2525. [Google Scholar] [CrossRef]
- Ernst, E.; Abramson, B.; Acosta, K.; Hoang, P.T.N.; Mateo-Elizalde, C.; Schubert, V.; Pasaribu, B.; Hartwick, N.; Colt, K.; Aylward, A.; et al. The Genomes and Epigenomes of Aquatic Plants (Lemnaceae) Promote Triploid Hybridization and Clonal Repro-duction. Plant Biol. 2023, 2023, 551673. [Google Scholar] [CrossRef]
- Sror, H.A.M.; Tischendorf, G.; Sieg, F.; Schmitt, J.M.; Hincha, D.K. Cryoprotectin protects thylakoids during a freeze–thaw cycle by a mechanism involving stable membrane binding. Cryobiology 2003, 47, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Sershen; Berjak, P.; Pammenter, N.W.; Wesley-Smith, J. The effects of various parameters during processing for cryopreservation on the ultrastructure and viability of recalcitrant zygotic embryos of Amaryllis belladonna. Protoplasma 2012, 249, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Heringer, A.S.; Steinmacher, D.A.; Schmidt, É.C.; Bouzon, Z.L.; Guerra, M.P. Survival and ultrastructural features of peach palm (Bactris gasipaes, Kunth) somatic embryos submitted to cryopreservation through vitrification. Protoplasma 2013, 250, 1185–1193. [Google Scholar] [CrossRef]
- Stock, J.; Bräutigam, A.; Melzer, M.; Bienert, G.P.; Bunk, B.; Nagel, M.; Overmann, J.; Keller, E.R.J.; Mock, H.-P. The transcription factor WRKY22 is required during cryo-stress acclimation in Arabidopsis shoot tips. J. Exp. Bot. 2020, 71, 4993–5009. [Google Scholar] [CrossRef] [PubMed]
- Ingle, R.A.; Collett, H.; Cooper, K.; Takahashi, Y.; Farrant, J.M.; Illing, N. Chloroplast biogenesis during rehydration of the resurrection plant Xerophyta humilis: Parallels to the etioplast-chloroplast transition. Plant Cell Environ. 2008, 31, 1813–1824. [Google Scholar] [CrossRef]
- Charuvi, D.; Kiss, V.; Nevo, R.; Shimoni, E.; Adam, Z.; Reich, Z. Gain and Loss of Photosynthetic Membranes during Plastid Differentiation in the Shoot Apex of Arabidopsis. Plant Cell 2012, 24, 1143–1157. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, M.-R.; Li, J.; Cui, Z.; Volk, G.M.; Wang, Q. Cryobiotechnology: A Double-Edged Sword for Obligate Plant Pathogens. Plant Dis. 2019, 103, 1058–1067. [Google Scholar] [CrossRef]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. DNA-Binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-Wide Analysis of the ERF Gene Family in Arabidopsis and Rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.A.; Farré, E.M.; Thomashow, M.F. CIRCADIAN CLOCK-ASSOCIATED 1 and LATE ELONGATED HYPOCOTYL regulate expression of the C-REPEAT BINDING FACTOR (CBF) pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 7241–7246. [Google Scholar] [CrossRef] [PubMed]
- Kidokoro, S.; Konoura, I.; Soma, F.; Suzuki, T.; Miyakawa, T.; Tanokura, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Clock-regulated coactivators selectively control gene expression in response to different temperature stress conditions in Arabidopsis. Proc. Natl. Acad. Sci. USA 2023, 120, e2216183120. [Google Scholar] [CrossRef] [PubMed]
- Maibam, P.; Nawkar, G.M.; Park, J.H.; Sahi, V.P.; Lee, S.Y.; Kang, C.H. The Influence of Light Quality, Circadian Rhythm, and Photoperiod on the CBF-Mediated Freezing Tolerance. Int. J. Mol. Sci. 2013, 14, 11527–11543. [Google Scholar] [CrossRef]
- Lee, E.S.; Park, J.H.; Wi, S.D.; Kang, C.H.; Chi, Y.H.; Chae, H.B.; Paeng, S.K.; Ji, M.G.; Kim, W.-Y.; Kim, M.G.; et al. Redox-dependent structural switch and CBF activation confer freezing tolerance in plants. Nat. Plants 2021, 7, 914–922. [Google Scholar] [CrossRef]
- Doležel, J.; Greilhuber, J.; Suda, J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef]
- Doležel, J.; Bartoš, J.; Voglmayr, H.; Greilhuber, J. Letter to the editor. Cytometry 2003, 51, 127–128. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Tschiersch, H.; Junker, A.; Meyer, R.C.; Altmann, T. Establishment of integrated protocols for automated high throughput kinetic chlorophyll fluorescence analyses. Plant Methods 2017, 13, 54. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.; Lyons, E.; Bomhoff, M.; Lenards, A. Comparative Genomics of Grass Genomes Using CoGe. In Handbook of Plant and Crop Physiology; CRC Press: Boca Raton, FL, USA, 2014; pp. 797–816. ISBN 978-0-429-09788-1. [Google Scholar]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, w202–w208. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Serra, F.; Bork, P. ETE 3: Reconstruction, Analysis, and Visualization of Phylogenomic Data. Mol. Biol. Evol. 2016, 33, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- GenomeNet. Available online: https://www.genome.jp/tools/ete/ (accessed on 1 May 2022).
- Katoh, K.; Kuma, K.I.; Toh, H.; Miyata, T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Rombauts, S.; Déhais, P.; Van Montagu, M.; Rouzé, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 45e–445e. [Google Scholar] [CrossRef]
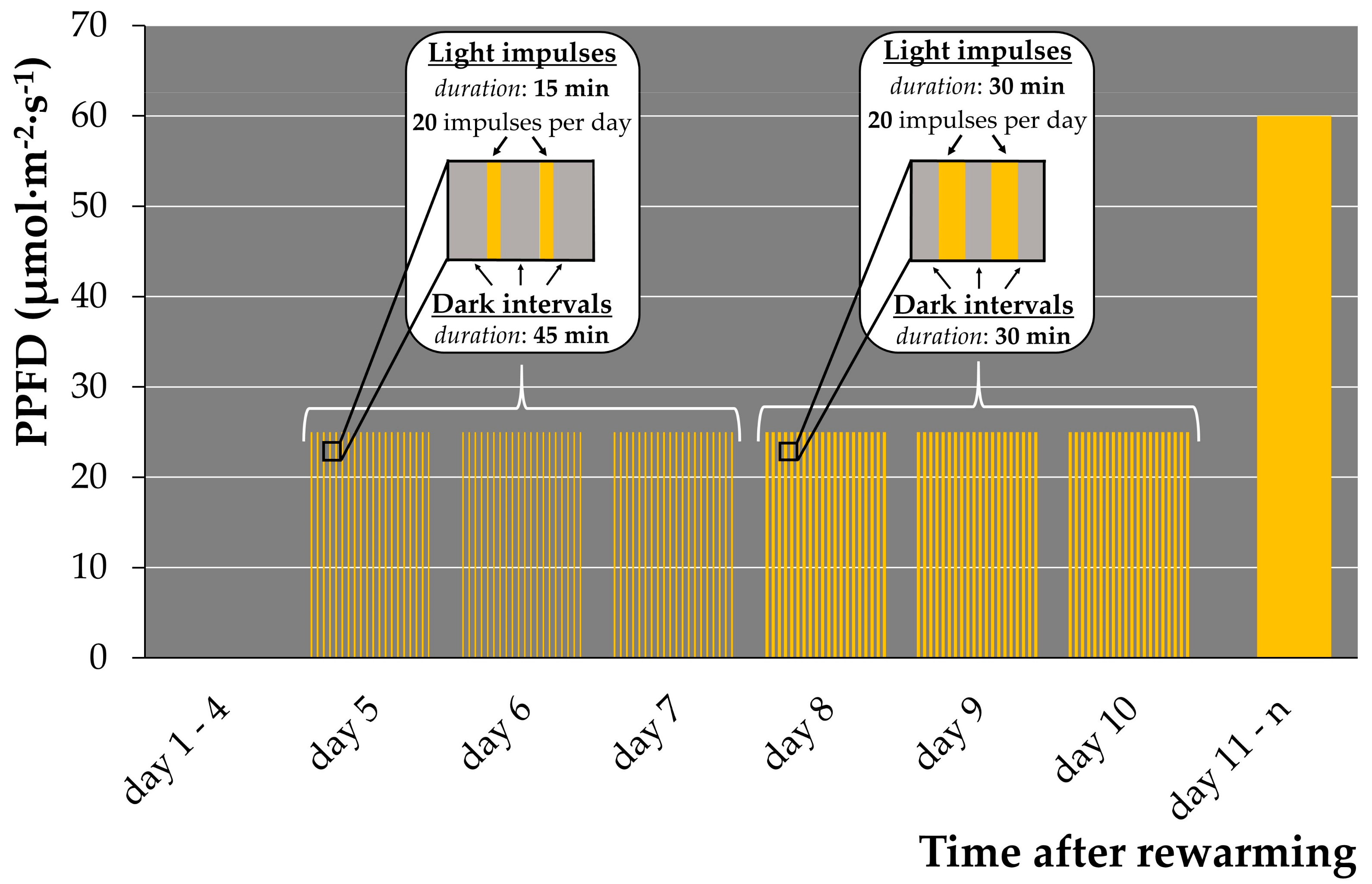
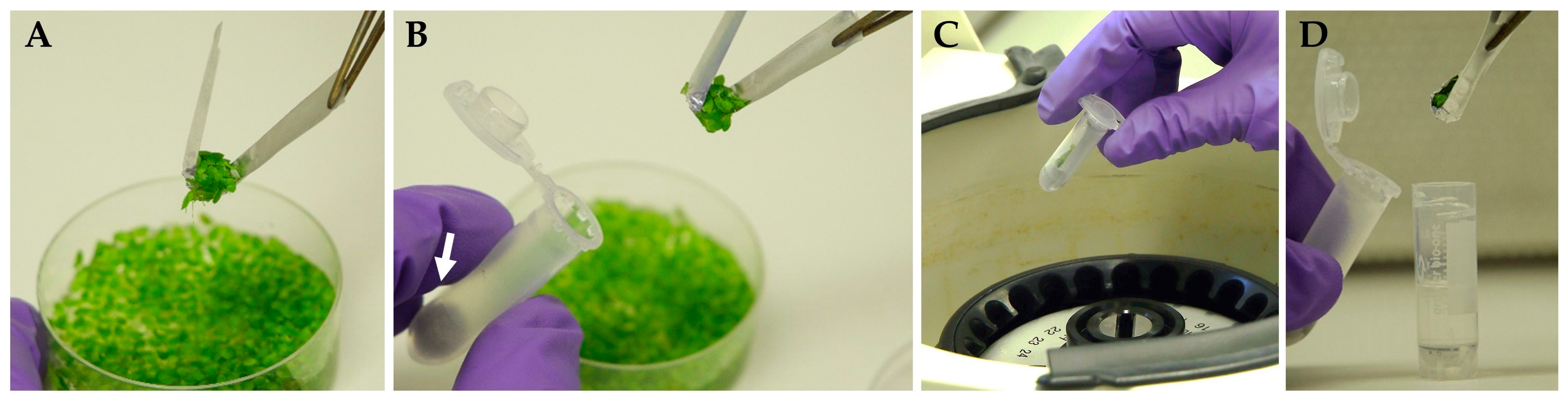


| Illumination Regime | Duration of Light Impulse | Duration of Dark Intervals | Number of Light Impulses per Day | PPFD, μmol·m−2·s−1 | Number of Regrowth Events after Cryopreservation, per CRYO-Tube (25–50 Fronds) |
|---|---|---|---|---|---|
| “15/45” | 15 min | 45 min | 20 | 25–30 | 9–34 |
| “30/30” | 30 min | 30 min | 20 | 25–30 | 3–16 |
| “60/60” | 60 min | 60 min | 8 | 25–30 | 1–5 |
| Attenuated (control) | 2–4 |
| Pre-Treatment Condition | Number of Regrowth Events after Cryopreservation, per Cryo-Tube (25–50 Fronds in Cryo-Tube) |
|---|---|
| V: 5 min | 0 |
| V: 15 min | 0 |
| V: 15 min + AP: 15 min | 0 |
| V: 15 min + AP: 45 min | 0–1 |
| V: 15 min + AP: 1 h 45 min | 9–35 |
| V: 15 min + AP: 2 h 45 min | 7–34 |
| V: 15 min + AP: 3 h 45 min | 9–32 |
| AP: 5 min | 0 |
| AP: 15 min | 0 |
| AP: 30 min | 0 |
| AP: 1 h | 0 |
| AP: 2 h | 1–12 |
| AP: 3 h | 3–29 |
| AP: 4 h | 8–35 |
| Clone ID | Species | Karyotype | Genome Size (Mbp) | ||
|---|---|---|---|---|---|
| Before Cryopreservation | After Cryopreservation | ||||
| 1 | 9602 | Lemna gibba | 4 n * | 1176 | 1120 |
| 2 | 7641 | Lemna gibba × Lemna minor | 1 n × 1 n *** | 558 | 571 |
| 3 | 8434 | Lemna minor × Lemna turionifera | 1 n × 1 n *** | 453 | 451 |
| 4 | 8627 | Lemna minor × Lemna turionifera | 2 n × 1 n ** | 635 | 623 |
| 5 | 9533 | Lemna minor | 3 n *** | 600 | 594 |
| Clone ID | Genus | Species | Continent/Region | Country | State/City | |
|---|---|---|---|---|---|---|
| 1 | 5548 | Spirodela | polyrhiza | Asia | China | Jansu, Huaian |
| 2 | 9509 | Spirodela | polyrhiza | Europe | Germany | Lotschen, Stadtroda 2002 |
| 3 | 7260 | Landoltia | punctata | Australia | Australia | Victoria, Tyrendarra |
| 4 | 5562 | Landoltia | punctata | Asia | Israel | Kfar Hayarok, Sharon Plain |
| 5 | 7742 | Lemna | gibba | Europe | Italy | Sicilia |
| 6 | 7749 | Lemna | gibba | Europe | Belgium | Liege, Terwagne |
| 7 | 7796 | Lemna | gibba | Europe | Italy | Sicilia |
| 8 | 7922 | Lemna | gibba | South America | Argentina | Buenos Aires |
| 9 | 9602 | Lemna | gibba (4n) | Europe | Italy | Sicilia |
| 10 | 7641 | Lemna | gibba (1n) × minor (1n) | Asia | Israel | Hadera, Kirket Batih |
| 11 | 8623 | Lemna | minor | Europe | Denmark | Ijland Alborg |
| 12 | 9441 | Lemna | minor | Europe | Germany | Marburg St |
| 13 | 5500 | Lemna | minor | Europe | Ireland | Blarney, County Cork |
| 14 | 8434 | Lemna | minor (1n) × turionifera (1n) | North America | Canada | Ontario |
| 15 | 8627 | Lemna | minor (2n) × turionifera (1n) | Europe | Denmark | Sjaelland, Copenhagen, Slangerup |
| 16 | 9533 | Lemna | minor (3n) | Europe | Macedonia | Krusje |
| 17 | 6717 | Lemna | minuta | Central America | Guatemala | Chinaltenango, Chocoyos |
| 18 | 9260 | Lemna | minuta | Europe | Italy | Sicilia, Catania, Bot. Garden |
| 19 | 9434 | Lemna | turionifera | Asia | Russia | Shelekhov, region Baikal lake |
| 20 | 8845 | Lemna | valdiviana | South America | Brazil | Rio de Janeiro, Sao Conrado |
| 21 | 8730 | Wolffia | australiana | Australia | Australia | New South Wales, Singleton, Doughboy Hollow |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterson, A.; Kishchenko, O.; Kuhlmann, M.; Tschiersch, H.; Fuchs, J.; Tikhenko, N.; Schubert, I.; Nagel, M. Cryopreservation of Duckweed Genetic Diversity as Model for Long-Term Preservation of Aquatic Flowering Plants. Plants 2023, 12, 3302. https://doi.org/10.3390/plants12183302
Peterson A, Kishchenko O, Kuhlmann M, Tschiersch H, Fuchs J, Tikhenko N, Schubert I, Nagel M. Cryopreservation of Duckweed Genetic Diversity as Model for Long-Term Preservation of Aquatic Flowering Plants. Plants. 2023; 12(18):3302. https://doi.org/10.3390/plants12183302
Chicago/Turabian StylePeterson, Anton, Olena Kishchenko, Markus Kuhlmann, Henning Tschiersch, Joerg Fuchs, Natalia Tikhenko, Ingo Schubert, and Manuela Nagel. 2023. "Cryopreservation of Duckweed Genetic Diversity as Model for Long-Term Preservation of Aquatic Flowering Plants" Plants 12, no. 18: 3302. https://doi.org/10.3390/plants12183302
APA StylePeterson, A., Kishchenko, O., Kuhlmann, M., Tschiersch, H., Fuchs, J., Tikhenko, N., Schubert, I., & Nagel, M. (2023). Cryopreservation of Duckweed Genetic Diversity as Model for Long-Term Preservation of Aquatic Flowering Plants. Plants, 12(18), 3302. https://doi.org/10.3390/plants12183302







