Pre-Harvest Salicylic Acid Application Affects Fruit Quality and Yield under Deficit Irrigation in Aristotelia chilensis (Mol.) Plants
Abstract
:1. Introduction
2. Results
2.1. Environmental Conditions, Applied Water, and Plant Water Status
2.2. ABA Levels in Leaves
2.3. Lipid Peroxidation in Leaves
2.4. Yield and Fruit Quality
2.5. Antioxidant Parameters in Fruits
3. Discussion
4. Materials and Methods
4.1. Experimental Site
4.2. Treatments and Meteorological Measurements
4.3. Plant Water Status
4.4. ABA Determinations in Leaves
4.5. Lipid Peroxidation in Leaves
4.6. Yield and Fruit Quality
4.7. Determination of Antioxidant-Related Parameters in Fruits
4.8. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyer, J. Plant Productivity and Environment. Science 1982, 8, 218–443. [Google Scholar] [CrossRef]
- Pessarakli, M. Plant and Crop Stress, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Zahedi, S.; Sadat, M.; Fahadi, N.; Kadkhodaei, S.; Vaculík, M. Comparative morphological, physiological and molecular analyses of drought-stressed strawberry plants affected by SiO2 and SiO2-NPs foliar spray. Sci. Hortic. 2023, 309, 111686. [Google Scholar] [CrossRef]
- IPCC. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Buendia, E.C., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; IPCC: Geneva, Switzerland, 2019; in press. [Google Scholar]
- Luo, T.; Young, R.; Reig, P. Aqueduct Projected Water Stress Country Rankings. Technical Note; World Resources Institute Report; World Resources Institute: Washington, DC, USA, 2015; Available online: www.wri.org/publication/aqueduct-projected-water-stress-country-rankings (accessed on 15 July 2023).
- Santibáñez, F.; Santibáñez, P.; González, P. Elaboración de una Base Digital del Clima Comunal de Chile: Línea Base (1980–2010) y Proyección Al Año 2050; Ministerio del Medio Ambiente de Chile: Santiago, Chile, 2016. [Google Scholar]
- Garreaud, R.D.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.H.; Veloso-Aguila, D. The Central Chile Mega Drought (2010–2018): A climate dynamics perspective. Int. J. Clim. 2020, 40, 421–439. [Google Scholar] [CrossRef]
- Alvarez-Garreton, C.; Boisier, J.P.; Garreaud, R.; Seibert, J.; Vis, M. Progressive water deficitis during multiyear droughts in basins with long hydrological memory in Chile. Hydrol. Earth Syst. Sci. 2021, 25, 429–446. [Google Scholar] [CrossRef]
- Rosegrant, M.W.; Ringler, C.; Zhu, T. Water for Agriculture: Maintaining Food Security under Growing Scarcity. Annu. Rev. Environ. Resour. 2009, 34, 205–222. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, X.; Gao, X.; Siddique, K.H. Improving/maintaining water-use efficiency and yield of wheat by deficit irrigation: A global meta-analysis. Agric. Water Manag. 2019, 228, 105906. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuño, M.F.; Chaves, M.M. Deficit Irrigation as a Strategy to Save Water: Physiology and Potential Application to Horticulture. J. Integr. Plant Biol. 2007, 49, 1421–1434. [Google Scholar] [CrossRef]
- Lu, J.; Shao, G.; Cui, J.; Wang, X.; Keabetswe, L. Yield, fruit quality and water use efficiency of tomato for processing under regulated deficit irrigation: A meta-analysis. Agric. Water Manag. 2019, 222, 301–312. [Google Scholar] [CrossRef]
- Ertek, A.; Kara, B. Yield and quality of sweet corn under deficit irrigation. Agric. Water Manag. 2013, 129, 138–144. [Google Scholar] [CrossRef]
- Silveira, L.K.; Pavao, G.C.; Santos-Dias, C.T.; Quaggio, J.A.; Matus-Pires, R.C. Deficit irrigation effect on fruit yield, quality, and water use efficiency: A long-term study on Pera-IAC sweet orange. Agric. Water Manag. 2020, 231, 106019. [Google Scholar] [CrossRef]
- Tari, A.F. The effects of different deficit irrigation strategies on yield, quality, and water-use efficiencies of wheat under semi-arid conditions. Agric. Water Manag. 2016, 167, 1–10. [Google Scholar] [CrossRef]
- Jorquera-Fontena, E.; Tighe-Neira, R.; Bota, J.; Inostroza-Blancheteau, C.; Pastenes, C. Response of sink manipulation in ‘Lapins’ sweet cherry (Prunus avium L.) branches to late-deficit irrigation. Sci. Hortic. 2022, 304, 111323. [Google Scholar] [CrossRef]
- Almutairi, K.; Bryla, D.; Strik, B. Potential of Deficit Irrigation, Irrigation Cutoffs, and Crop Thinning to Maintain Yield and Fruit Quality with Less Water in Northern Highbush Blueberry. HortScience 2017, 52, 625–633. [Google Scholar] [CrossRef]
- Ribera-Fonseca, A.; Jorquera-Fontena, E.; Castro, M.; Acevedo, P.; Parra, J.C.; Reyes-Díaz, M. Exploring VIS/NIR reflectance indices for the estimation of water status in highbush blueberry plants grown under full and deficit irrigation. Sci. Hortic. 2019, 256, 108557. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic Acid Synthesis and Response. Arab. Book 2013, 11, e0166. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 2000, 3, 217–223. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, L.; Shi, Y.; Su, D.; Lu, W.; Cheng, Y.; Li, Z. Stress-responsive tomato gene SlGRAS4 function in drought stress and abscisic acid signaling. Plant Sci. 2020, 304, 110804. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.J. The plant hormones: Their nature, occurrence, and functions. In Plant Hormones; Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 1–15. [Google Scholar]
- Anfang, M.; Shani, E. Transport mechanisms of plant hormones. Curr. Opin. Plant Biol. 2021, 63, 102055. [Google Scholar] [CrossRef] [PubMed]
- Nazar, R.; Umar, S.; Khan, N.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Khan, I.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Yousefvand, P.; Sohrabi, Y.; Heidari, G.; Weisany, W.; Mastinu, A. Salicylic Acid Stimulates Defense Systems in Allium hirtifolium Grown under Water Deficit Stress. Molecules 2022, 27, 3083. [Google Scholar] [CrossRef] [PubMed]
- Wakchaure, G.C.; Minhas, P.S.; Kumar, S.; Khapte, P.S.; Dalvi, S.G.; Rane, J.; Sammi-Reddy, K. Pod quality, yields responses and water productivity of okra (Abelmoschus esculentus L.) as affected by plant growth regulators and deficit irrigation. Agric. Water Manag. 2023, 282, 108267. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, C.; Ren, R.; Jiang, J. Salicylic acid had the potential to enhance tolerance in horticultural crops against abiotic stress. Front. Plant Sci. 2023, 14, 1141918. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A. Flora Silvestre de Chile, Zona Araucana, 5th ed.; Fundación Claudio Gay: Santiago, Chile, 2005. [Google Scholar]
- Yáñez, M.A.; González, B.; Espinoza, S.E.; Vogel, H.; Doll, U. Phenotypic variation of fruit and ecophysiological traits among maqui (Aristotelia chilensis [Molina] Stuntz) provenances established in a common garden. Sci. Rep. 2022, 12, 185. [Google Scholar] [CrossRef]
- Céspedes, C.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Lila, M.A.; Raskin, I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef]
- Pinto, A.; Fuentealba-Sandoval, V.; Dolores-López, M.; Peña-Rojas, K.; Fischer, S. Accumulation of delphinidin derivates and other bioactive compound in wild maqui under different environmental conditions and fruit ripening stages. Ind. Crop. Prod. 2022, 184, 115064. [Google Scholar] [CrossRef]
- Chandía, C.; Urra, C. Análisis Económico de Producción y Comercialización del Maqui Para Apoyar el Micro Emprendimiento Femenino en el Marco del Manejo Sostenible de Los Recursos Naturales en el Sector Rural de la Comuna de El Carmen. Tesis Contador público y auditor, Universidad del Bío-Bío, Chillán, Chile, 2017. Available online: http://repobib.ubiobio.cl/jspui/handle/123456789/2545 (accessed on 15 July 2023).
- ODEPA. Boletín de Frutas y Hortalizas Procesadas; Oficina de Estudios y Políticas Agrarias: Santiago de Chile, Chile, 2019; Available online: https://www.odepa.gob.cl (accessed on 15 July 2023).
- Vogel, H.; González, B.; Catenacci, G.; Doll, U. Domestication and sustainable production of wild crafted plants with special reference to the Chilean Maqui berry (Aristotelia chilensis). In Proceedings of the 6th International Symposium Breeding Research in Medicinal and Aromatic Plants (BREEDMAP 6), Quedlinburg, Germany, 19–23 June 2016; Abstracts of Oral Presentations and Posters. Julius Kühn-Institut: Quedlinburg, Germany, 2016; pp. S. 50–S. 52. [Google Scholar]
- Fuentealba-Sandoval, V.; Fischer, S.; Pinto, A.A.; Bastías, R.M.; Peña-Rojas, K. Maqui (Aristotelia chilensis (Mol.) Stuntz), towards sustainable canopy management: A review. Ind. Crop. Prod. 2021, 170, 113735. [Google Scholar] [CrossRef]
- González-Villagra, J.; Reyes-Díaz, M.M.; Tighe-Neira, R.; Inostroza-Blancheteau, C.; Escobar, A.L.; Bravo, L.A. Salicylic acid improves antioxidant defense system and photosynthetic performance in Aristotelia chilensis plants subjected to moderate drought stress. Plants 2022, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- González-Villagra, J.; Rodrigues-Salvador, A.; Nunes-Nesi, A.; Cohen, J.D.; Reyes-Díaz, M.M. Age-related mechanism and its relationship with secondary metabolism and abscisic acid in Aristotelia chilensis plants subjected to drought stress. Plant Physiol. Biochem. 2018, 124, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Galmés, J.; Pou, A.; Alsina, M.M.; Tomás, M.; Medrano, H.; Flexas, J. Aquaporin expression in response to different water stress intensities and recovery in Richter-110 (Vitis sp.): Relationship with ecophysiological status. Planta 2007, 226, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Fatma, M.; Gautam, H.; Sehar, Z.; Raseed, F.; Khan, M.; Sofo, A.; Khan, N. Salicylic acid increases photosynthesis of drought grown mustard plants efectively with sufcient n via regulation of ethylene, abscisic acid, and nitrogen use efciency. J. Plant Growth Regul. 2022, 41, 1966–1977. [Google Scholar] [CrossRef]
- La, V.; Lee, B.; Islam, M.; Park, S.; Jung, H.; Bae, D.; Kim, T. Characterization of salicylic acid-mediated modulation of the drought stress responses: Reactive oxygen species, proline, and redox state in Brassica napus. Environ. Exp. Bot. 2019, 157, 1–10. [Google Scholar] [CrossRef]
- Fredes, C.; Montenegro, G.; Zoffoli, J.; Robert, P. Polyphenol content and antioxidant activity of maqui (Aristotelia chilensis Stuntz) during fruit development and maturation in central Chile. Chileanjar 2012, 72, 582–589. [Google Scholar] [CrossRef]
- Giménez, M.; Valverde, J.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef] [PubMed]
- García-Pastor, M.; Zapata, P.; Castillo, S.; Martínez-Romero, D.; Guillén, F.; Valero, D.; Serrano, M. The Effects of Salicylic Acid and Its Derivatives on Increasing Pomegranate Fruit Quality and Bioactive Compounds at Harvest and During Storage. Front. Plant Sci. 2020, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- González, B.; Vogel, H.; Razmilic, I.; Wolfram, E. Polyphenol, anthocyanin and antioxidant content in different parts of maqui fruits (Aristotelia chilensis) during ripening and conservation treatments after harvest. Ind. Crop. Prod. 2015, 76, 158–165. [Google Scholar] [CrossRef]
- Khattab, S.; Yap, Y.-K.; El Sherif, F. Salicylic acid foliar spray enhanced Silybum marianum growth and yield, as well as its chemical constituents and Chalcone Synthase gene activity. Horticulturae 2022, 8, 556. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, B.; Zhang, S.; Hao, R.; Xia, Y.; Ma, P.; Dong, J. The SmNPR4-SmTGA5 module regulates SA-mediated phenolic acid biosynthesis in Salvia miltiorrhiza hairy roots. Hortic. Res. 2023, 10, uhad066. [Google Scholar] [CrossRef]
- Fredes, C.; Montenegro, G.; Zoffoli, J.P.; Santander, F.; Robert, P. Comparison of the phenolic content, total anthocyanin content and antioxidant capacity of polyphenol-rich fruits grown in Chile. Cienc. Investig. Agrar. 2014, 41, 49–61. [Google Scholar]
- Retamal-Salgado, J.; Adaos, G.; Cedeño-García, G.; Ospino-Olivella, S.; Vergara-Retamales, R.; Dolores-López, M.; Olivares, R.; Hirzel, J.; Olivares-Soto, H.; Betancur, M. Preharvest Applications of Oxalic Acid and Salicylic Acid Increase Fruit Firmness and Polyphenolic Content in Blueberry (Vaccinium corymbosum L.). Horticulturae 2023, 9, 639. [Google Scholar] [CrossRef]
- Mellisho, C.D.; Egea, I.; Galindo, A.; Rodríguez, P.; Rodríguez, J.; Conejero, W.; Romojaro, F.; Torrecillas, A. Pomegranate (Punica granatum L.) fruit response to different deficit irrigation conditions. Agric. Water Manag. 2012, 114, 30–36. [Google Scholar] [CrossRef]
- Pérez-Pastor, A.; Domingo, R.; Torrecillas, A.; Ruiz-Sánchez, M.C. Response of apricot trees to deficit irrigation strategies. Irrig. Sci. 2009, 27, 231–242. [Google Scholar] [CrossRef]
- Samperio, A.; Prieto, M.H.; Blanco-Cipollone, F.; Vivas, A.; Monino, M.J. Effects of postharvest deficit irrigation in ‘Red Beaut’ Japanese plum: Tree water status, vegetative growth, fruit yield, quality and economic return. Agric. Water Manag. 2015, 150, 92–102. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Tanany, M.M. Efficacy of foliar applications of salicylic acid, zinc, and potassium on reducing fruit drop, yield improvement, and quality of balady mandarins. Egypt. J. Hort. 2016, 43, 371–388. [Google Scholar]
- Kumara, N.; Singh, H.; Kaur, N.; Kaur, K. CPPU and salicylic acid application improved fruit retention, yield, and fruit quality of mango cv. Dusehri. J. Hortic. Sci. Biotechnol. 2023, 98, 608–621. [Google Scholar] [CrossRef]
- Yuan, R.; Mao, L.; Min, T.; Zhao, Y.; Duan, Y.; Wang, H.; Lin, Q. Salicylic acid treatment inhibits ethylene synthesis and starch-sugar conversion to maintain apple fruit quality during shelf life. Sci. Hortic. 2023, 308, 111586. [Google Scholar] [CrossRef]
- Sarricolea, P.; Herrera-Ossandon, M.; Meseguer-Ruiz, O. Climatic regionalisation of continental Chile. J. Maps 2017, 13, 66–73. [Google Scholar] [CrossRef]
- CIREN. Estudio Agrológico Región de Los Ríos; CIREN: Havana, Cuba, 2017. [Google Scholar]
- Vogel, H.; Peñailillo, P.; Catenacci, G.; González, B. Maqui (Aristotelia chilensis): Morpho-phenological characterization to design high-yielding cultivation techniques. J. Appl. Res. Med. Aromat. Plants 2014, 1, 123–133. [Google Scholar] [CrossRef]
- Begg, J.E.; Turner, N.C. Water Potential Gradients in Field Tobacco. Plant Physiol. 1970, 46, 343–346. [Google Scholar] [CrossRef]
- Tillmann, M.; Tang, Q.; Cohen, J.D. Protocol: Analytical methods for visualizing the indolic precursor network leading to auxin biosynthesis. Plant Methods 2021, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, P.I.; Havlicek, L.; Vagner, M.; Malbeck, J.; Kaminek, M. Purification and determination of plant hormones auxin and abscisic acid using solid phase extraction and two-dimensional high performance liquid chromatography. J. Chromatogr. A 2005, 1075, 159–166. [Google Scholar] [CrossRef]
- Cohen, J.D.; Tang, Q.; Hegeman, A.D. Chapter Nine-Using targeted metabolomics to elucidate the indole auxin network in plants. Meth. Enzymol. 2022, 676, 239–278. [Google Scholar]
- Du, Z.; Bramalage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Chinnici, F.; Bendini, A.; Gaiani, A.; Riponi, C. Radical Scavenging Activities of Peels and Pulps from cv. Golden Delicious Apples as Related to Their Phenolic Composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Strack, D.; Wray, V. Anthocyanins. In Methods in Plant Biology. Plant Phenolics; Harborne, J.B., Ed.; Academic Press/Harcourt Brace Jovanovich: London, UK, 1989; Volume 1. [Google Scholar]
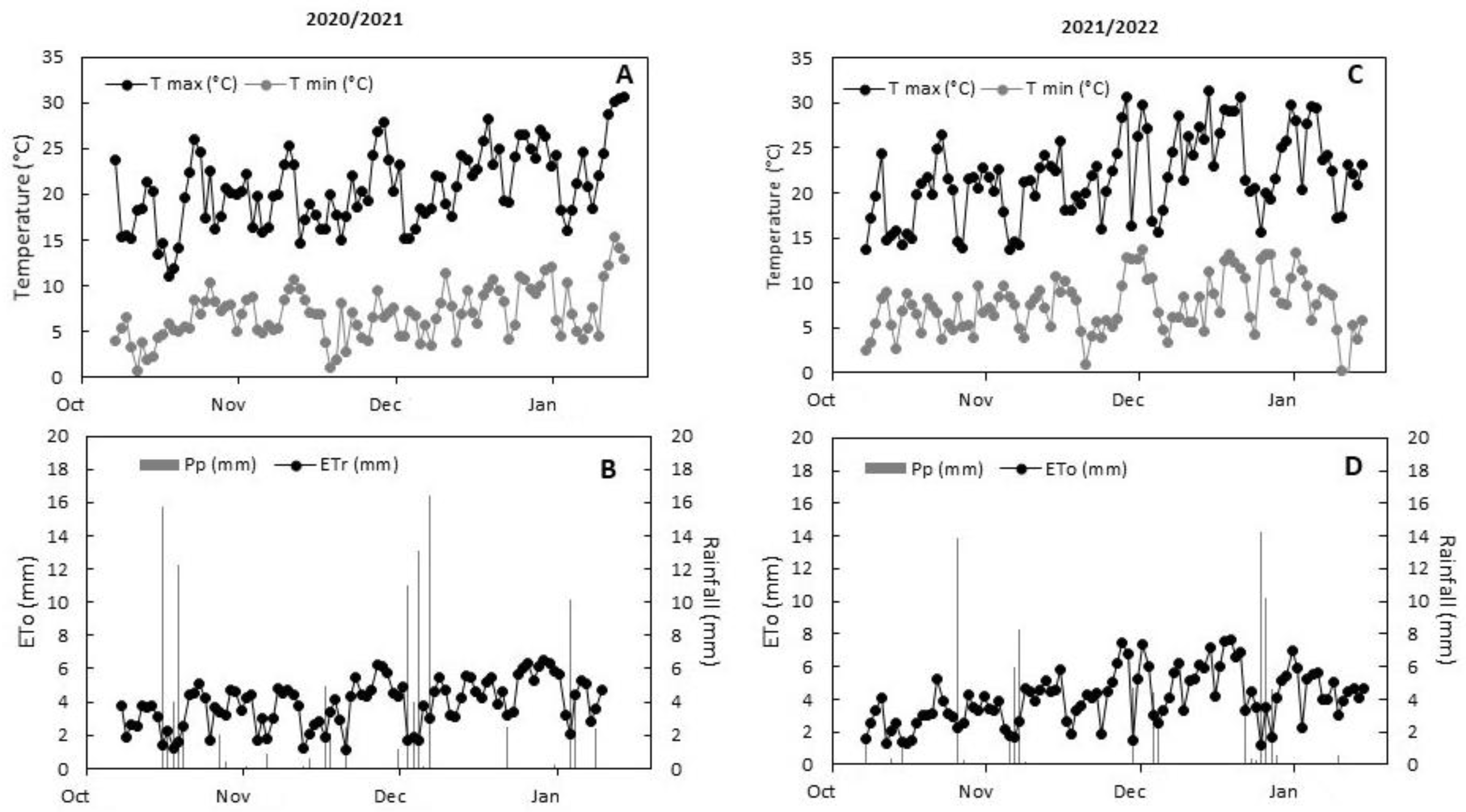
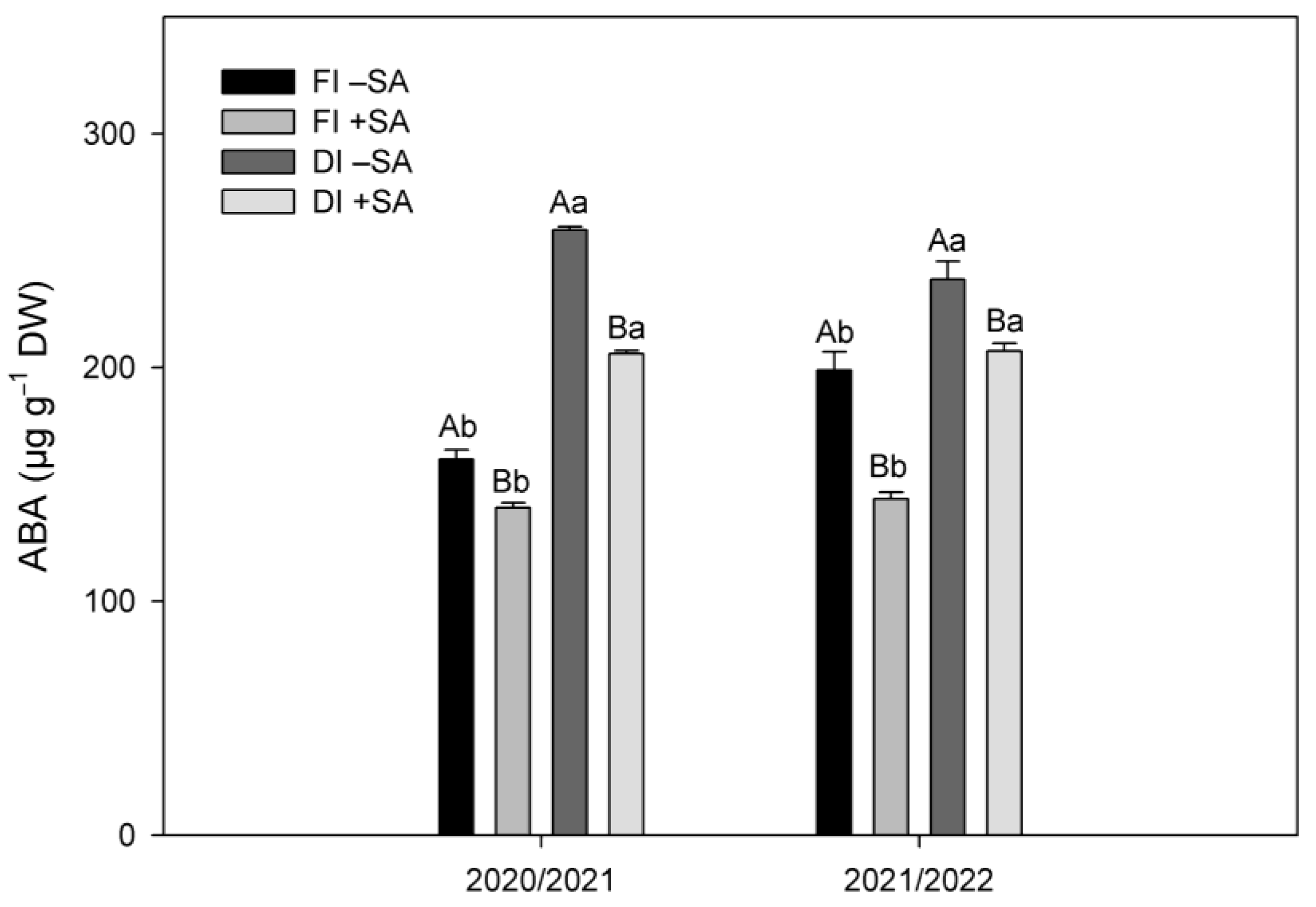

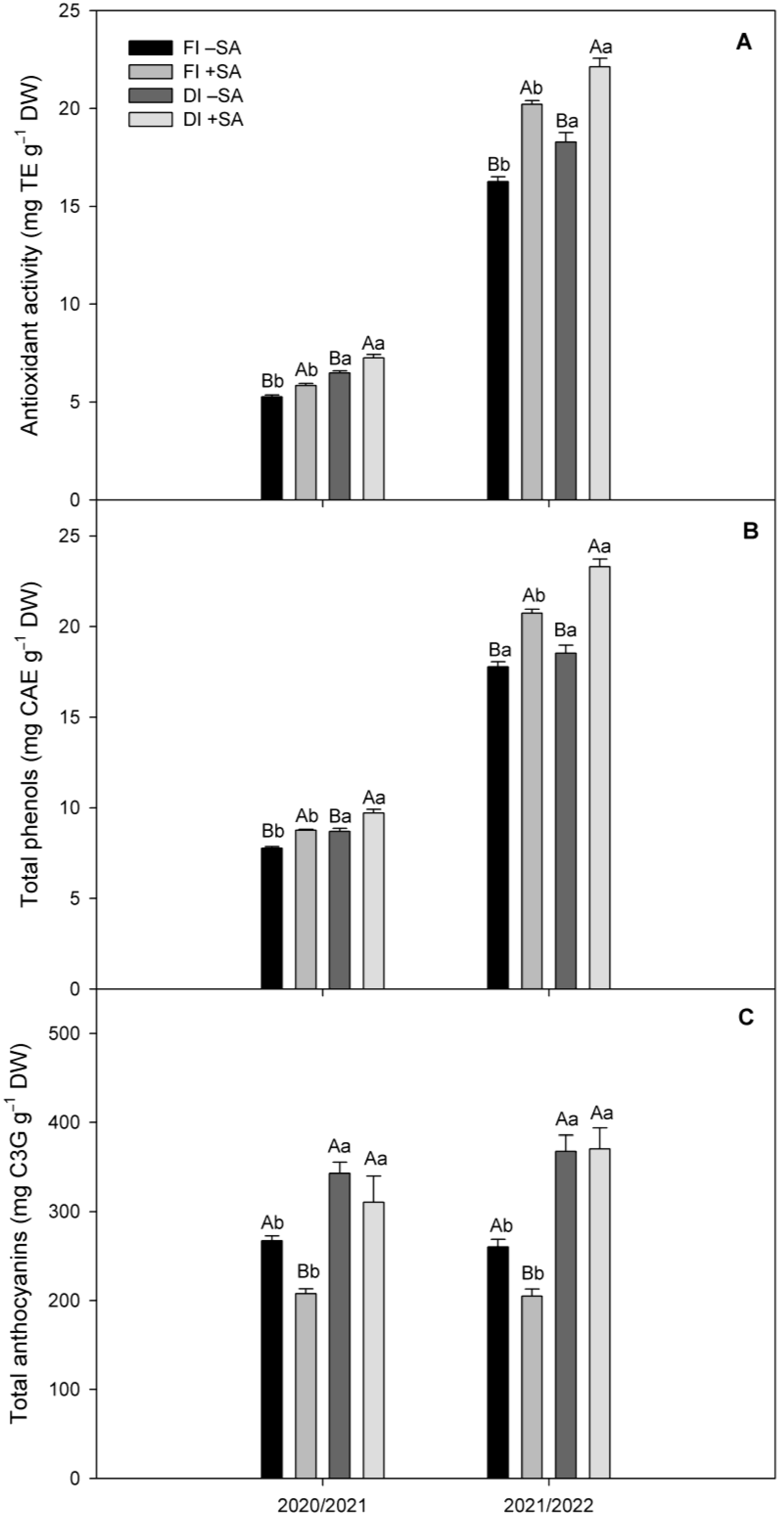
| Cumulative Values | Season 2020/2021 | Season 2020/2021 | ||
|---|---|---|---|---|
| FI | DI | FI | DI | |
| Irrigation (m3 ha−1) | 2717 | 1630 | 2991 | 1795 |
| Rainfall (m3 ha−1) | 115 | 115 | 80 | 80 |
| Total water (m3 ha−1) | 2832 | 1699 | 3071 | 1875 |
| Treatment | Season 2020/2021 | Season 2020/2021 |
|---|---|---|
| FI −SA | −0.67 ± 0.02 Aa | −0.65 ± 0.04 Aa |
| FI +SA | −0.59 ± 0.02 Aa | −0.58 ± 0.03 Aa |
| DI −SA | −1.31 ± 0.07 Ab | −1.33 ± 0.04 Ab |
| DI +SA | −1.11 ± 0.07 Bb | −1.17 ± 0.02 Bb |
| Fruit Fresh Weight (g of 10 Fruits) | Fruit Dry Weight (g of 10 Fruits) | Yield per Plant (kg) | Total Yield (kg ha−1) | |
|---|---|---|---|---|
| Season 2020/2021 | ||||
| FI −SA | 2.21 ± 0.02 Aa | 0.77 ± 0.04 Aa | 2.33 ± 0.2 Aa | 3881.7 ± 0.4 Aa |
| FI +SA | 2.35 ± 0.07Aa | 0.79 ± 0.07 Aa | 2.47 ± 0.3 Aa | 4115.0 ± 0.5 Aa |
| DI −SA | 1.53 ± 0.06 Bb | 0.54 ± 0.05 Bb | 1.75 ± 0.1 Bb | 2915.5 ± 0.3 Bb |
| DI +SA | 2.32 ± 0.13 Aa | 0.83 ± 0.01 Aa | 2.15 ± 0.6 Aa | 3581.9 ± 1.0 Aa |
| Season 2021/2022 | ||||
| FI −SA | 2.89 ± 0.53 Aa | 0.78 ± 0.02 Aa | 2.32 ± 0.2 Aa | 3865.1 ± 0.5 Aa |
| FI +SA | 2.84 ± 0.28 Aa | 0.81 ± 0.01 Aa | 2.51 ± 0.3 Aa | 4181.6 ± 0.4 Aa |
| DI −SA | 1.70 ± 0.42 Bb | 0.59 ± 0.06 Bb | 1.67 ± 0.4 Bb | 2782.4 ± 0.6 Bb |
| DI +SA | 3.02 ± 0.34 Aa | 0.80 ± 0.05 Aa | 2.27 ± 0.6 Aa | 3781.8 ± 0.4 Aa |
| Equatorial Diameter (mm) | Polar Diameter (mm) | Total Soluble Solids (°Brix) | Titratable Acidity (% Citric Acid) | |
|---|---|---|---|---|
| Season 2020/2021 | ||||
| FI −SA | 6.75 ± 0.18 Aa | 6.83 ± 0.18 Aa | 19.20 ± 1.71 Ab | 1.22 ± 0.12 Aa |
| FI +SA | 6.76 ± 0.12 Aa | 7.07 ± 0.09 Aa | 24.57 ± 2.01 Ab | 1.18 ± 0.07 Aa |
| DI −SA | 5.87 ± 0.11 Bb | 6.33 ± 0.15 Aa | 28.23 ± 1.86 Aa | 0.81 ± 0.01 Aa |
| DI +SA | 6.71 ± 0.07 Aa | 6.98 ± 0.13 Aa | 31.23 ± 0.70 Aa | 1.02 ± 0.14 Aa |
| Season 2021/2022 | ||||
| FI −SA | 7.26 ± 0.20 Aa | 6.85 ± 0.15 Aa | 22.53 ± 0.64 Ab | 1.20 ± 0.10 Aa |
| FI +SA | 7.41 ± 0.13 Aa | 6.88 ± 0.12 Aa | 27.07 ± 1.05 Ab | 1.07 ± 0.05 Aa |
| DI −SA | 5.82 ± 0.15 Bb | 6.44 ± 0.13 Aa | 29.05 ± 0.55 Aa | 1.14 ± 0.01 Aa |
| DI +SA | 7.53 ± 0.13 Aa | 7.04 ± 0.06 Aa | 32.47 ± 2.17 Aa | 1.23 ± 0.03 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Villagra, J.; Bravo, L.A.; Reyes-Díaz, M.; Cohen, J.D.; Ribera-Fonseca, A.; López-Olivari, R.; Jorquera-Fontena, E.; Tighe-Neira, R. Pre-Harvest Salicylic Acid Application Affects Fruit Quality and Yield under Deficit Irrigation in Aristotelia chilensis (Mol.) Plants. Plants 2023, 12, 3279. https://doi.org/10.3390/plants12183279
González-Villagra J, Bravo LA, Reyes-Díaz M, Cohen JD, Ribera-Fonseca A, López-Olivari R, Jorquera-Fontena E, Tighe-Neira R. Pre-Harvest Salicylic Acid Application Affects Fruit Quality and Yield under Deficit Irrigation in Aristotelia chilensis (Mol.) Plants. Plants. 2023; 12(18):3279. https://doi.org/10.3390/plants12183279
Chicago/Turabian StyleGonzález-Villagra, Jorge, León A. Bravo, Marjorie Reyes-Díaz, Jerry D. Cohen, Alejandra Ribera-Fonseca, Rafael López-Olivari, Emilio Jorquera-Fontena, and Ricardo Tighe-Neira. 2023. "Pre-Harvest Salicylic Acid Application Affects Fruit Quality and Yield under Deficit Irrigation in Aristotelia chilensis (Mol.) Plants" Plants 12, no. 18: 3279. https://doi.org/10.3390/plants12183279
APA StyleGonzález-Villagra, J., Bravo, L. A., Reyes-Díaz, M., Cohen, J. D., Ribera-Fonseca, A., López-Olivari, R., Jorquera-Fontena, E., & Tighe-Neira, R. (2023). Pre-Harvest Salicylic Acid Application Affects Fruit Quality and Yield under Deficit Irrigation in Aristotelia chilensis (Mol.) Plants. Plants, 12(18), 3279. https://doi.org/10.3390/plants12183279










