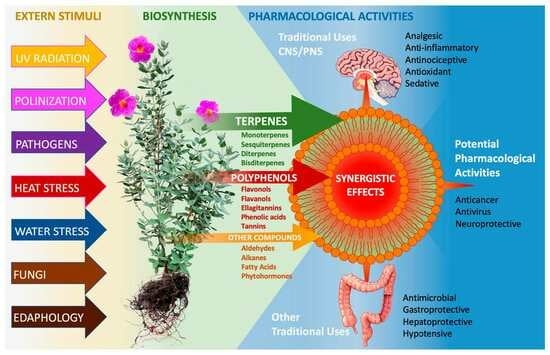
Cistus albidus L.—Review of a Traditional Mediterranean Medicinal Plant with Pharmacological Potential
Abstract
:1. Introduction
2. Distribution
3. Systematics
4. Botanical Characteristics
Vegetative Development
5. Phytochemical Constituents
5.1. Terpenes
5.1.1. Mono- and Sesquiterpenes from the Essential Oils
| No | Compound | Structure/Class | Presence in | Analytical Reference | Pharmacology |
|---|---|---|---|---|---|
| 1 | cis-α-Bergamotene | polycyclic monoterpene hydrocarbon | 🌱✿ | [48,49,53] | n/a |
| 2 | trans-α-Bergamotene | 🌱 | [47,48] | ||
| 3 | Borneol | polycyclic monoterpene alcohol | 🌱 | [52] | blood brain barrier (BBB) permeability improvement, intercellular tight junction (TJ) loosening [54] |
| 4 | Camphor | oxygenated polycyclic monoterpene | 🌱 | [50] | analgesic, antinociceptive [55]; antimicrobial, antiviral [56]; anticancer [57]; antitussive [58]; skin penetration enhancer [59]; |
| 5 | Carene | polycyclic monoterpene hydrocarbon | 🌱✿ | [52] | antiviral [60]; enhances bone mineralization [61]; anti-inflammatory [62]; |
| 6 | Carvacrol | monocyclic monoterpene alcohol | 🌱✿ | [49] | antibacterial (64); antifungal [63]; antioxidant [64]; anticancer [65]; anti-inflammatory, analgesic [66]; antiobesity [67]; hepatoprotective [68]; spasmolytic [69]; vasorelaxant [70]; |
| 7 | β-Cyclocitral | oxygenated monocyclic monoterpene | 🌱 | [53] | n/a |
| 8 | p-Cymene | monocyclic monoterpene hydrocarbon | 🌱 | [49,50] | anti-inflamatory, antinociceptive, antioxidant [71]; antidiabetic [72]; |
| 9 | p-Cymenene | monocyclic monoterpene hydrocarbon | 🌱✿ | [50] | n/a |
| 10 | Isobornyl formate | oxygenated polycyclic monoterpene | 🌱 | [50] | n/a |
| 11 | (D-)Limonene | monocyclic monoterpene hydrocarbon | 🌱✿ | [47,48,49,50] | anticancer, anticholesterol [73]; antidepressant [74]; |
| 12 | Linalool | acyclic monoterpene alcohol | 🌱✿ | [51,52] | antibacterial, antifungal [75]; anxiolytic [76]; anticancer, antioxidant [77]; analgesic [78]; anti-inflammatory [79]; |
| 13 | cis-linalool oxide | oxygenated heteromonocyclic monoterpene | [51] | n/a | |
| 14 | Myrcene | acyclic monoterpene hydrocarbon | 🌱 | [49,51] | analgesic, antinociceptive [80]; |
| 15 | Neryl acetate | acyclic monoterpene hydrocarbon | 🌱✿ | [53] | n/a |
| 16 | (E)-Ocimene | acyclic monoterpene hydrocarbon | 🌱✿ | [49] | anticancer [81]; anticonvulsant [82]; |
| 17 | (Z)-β-Ocimene | [49] | anticancer [81]; antibacterial [83]; | ||
| 18 | α-Phellandrene | monocyclic monoterpene hydrocarbon | 🌱 | [49] | antifungal [84]; antidepressant [85]; anti-inflammatory, antihyperalgesic [85,86], analgesic, antinociceptive [86]; anticancer [87]; |
| 19 | β-Phellandrene | ✿ | [49] | n/a | |
| 20 | α-Pinene | polycyclic monoterpene hydrocarbon | 🌱 | [47,49,51] | antifungal, anti-inflammatory, antioxidant [88]; anticancer [89]; anti-Leishmania [90]; gastroprotective [91,92]; antibacterial [93]; antiviral [94]; neuroprotective [95]; |
| 21 | β-Pinene | 🌱✿ | [47] | anticancer [96]; antimicrobial [97]; gastroprotective [92]; neuroprotective [98]; | |
| 22 | Piperitone | oxygenated monocyclic monoterpene | 𐩕 | [49] | n/a |
| 23 | Sabinene | polycyclic monoterpene hydrocarbon | 🌱 | [49] | n/a |
| 24 | cis-Sabinene hydrate | oxygenated polycyclic monoterpene | [49] | ||
| 25 | Safranal | oxygenated monocyclic monoterpene | 🌱 | [53] | antioxidant [99]; antimicrobial [100]; anticonvulsant [101]; antidepressant, anxiolytic [102]; gastroprotective [103]; |
| 26 | α-Terpinene | monocyclic monoterpene hydrocarbon | 🌱 | [49] | antioxidant [104]; antimicrobial [105]; |
| 27 | Δ-Terpinene | 🌱✿ | [52] | n/a | |
| 28 | γ-Terpinene | ✿ | [49] | antimicrobial [105]; | |
| 29 | α-Terpineol | monocyclic monoterpene alcohol | ✿𐩕 | [49,50,52,53,106] | antioxidant, anticancer, antinociceptive, anticonvulsant, sedative, antibronchitis, antihypertensive, vasorelaxant, cardioprotective [107]; |
| 30 | 4-Terpineol | 🌱 | [49,53] | antimicrobial [108]; gastroprotective [109]; | |
| 31 | Thymol | monocyclic monoterpene hydrocarbon | 🌱✿ | [49,50] | anti-inflammatory, antioxidant, antimicrobial, immunostimulatory, anticancer [110]; cardioprotective [111]; antihypertensive [70]; antihyperglycemic [112]; antinociceptive [113]; gastroprotective [114]; anxiolytic [115]; |
| 32 | Abscisic acid | oxygenated monocyclic sesquiterpene | 🌱 | [116,117] | antidiabetic [118]; antinociceptive [119]; |
| 33 | α-Amorphene | polycyclic sesquiterpene hydrocarbon | 🌱 | [50,53] | n/a |
| 34 | Aromadendrene | polycyclic sesquiterpene hydrocarbon | 🌱✿ | [47,48,53] | antimicrobial [120]; |
| 35 | allo-Aromadendrene | polycyclic sesquiterpene hydrocarbon | 🌱✿ | [6,43,46,48,49,50,51,52,53] | |
| 36 | allo-Aromadendrene epoxide | oxygenated polycyclic sesquiterpene | 🌱 | [53] | |
| 37 | Bisabola-2,10-diene(1-9)oxide | oxygenated polycyclic sesquiterpene | 🌱 | [53] | n/a |
| 38 | β-Bisabolene | monocyclic sesquiterpene hydrocarbon | [48] | anticancer [121] | |
| 39 | epi-α-Bisabolol | monocyclic sesquiterpene alcohol | 🌱✿ | [46,48,49,53] | anti-inflammatory [122]; antimicrobial [123]; anticancer [124]; |
| 40 | α-Bisabolol | 🌱 | [43,53] | n/a | |
| 41 | β-Bisabolol | 🌱✿ | [48] | n/a | |
| 42 | β-Bourbonene | polycyclic sesquiterpene hydrocarbon | 🌱✿ | [6,43,46,47,49,50,51,52,53] | n/a |
| 43 | 1,5-di-epi-Bourbonene (α or β) | 🌱 | [53] | ||
| 44 | Bulnesol | polycyclic sesquiterpene alcohol | 🌱✿ | [49] | n/a |
| 45 | Cadalene | polycyclic sesquiterpene hydrocarbon | 🌱 | [53] | n/a |
| 46 | Cadina-1,4-diene | polycyclic sesquiterpene hydrocarbon | 🌱✿ | [48,53] | n/a |
| 47 | α-Cadinene | polycyclic sesquiterpene hydrocarbon | 🌱 | [50,53] | n/a |
| 48 | cis-γ-Cadinene | 🌱✿𐩕 | [49] | ||
| 49 | trans-γ-Cadinene | 🌱 | [46] | ||
| 50 | γ-Cadinene | 🌱 | [51,52,53] | ||
| 51 | δ-Cadinene | 🌱✿𐩕 | [43,46,48,49,50,52,53] | ||
| 52 | α-Cadinol | polycycylic sesquiterpene alcohol | 🌱✿ | [43,48,49,53] | antifungal [125]; |
| 53 | T-Cadinol | [46,49,53,126] | anticancer [127] | ||
| 54 | α-Calacorene | polycyclic sesquiterpene hydrocarbon | 🌱 | [50,53] | n/a |
| 55 | β-Calacorene | [53] | |||
| 56 | Calamenene | polycyclic sesquiterpene hydrocarbon | 🌱 | [50,53] | anticancer [127]; |
| 57 | Caryophylladienol I | polycyclic sesquiterpene alcohol | 🌱 | [53] | n/a |
| 58 | Caryophylladienol II | [53] | |||
| 59 | β-Caryophyllene | polycyclic sesquiterpene hydrocarbon | 🌱✿𐩕 | [6,43,46,47,48,49,50,51,52,53] | antioxidant, antimicrobial, antitumor, anticancer [128]; anti-inflammatory, neuroprotective [129]; anxiolytic, antidepressant [130]; anticonvulsant [131]; analgesic [132]; |
| 60 | β-Caryophyllene epoxide | oxygenated polycyclic sesquiterpene | 🌱 | [43,46,48,49,53] | anticancer, analgesic [132]; |
| 61 | Caryophyllenol II | polycyclic sesquiterpene alcohol | 🌱 | [53] | n/a |
| 62 | 8,14-Cedranoxide | oxygenated sesquiterpene | 🌱 | [46] | n/a |
| 63 | α-Cedrene | polycyclic sesquiterpene hydrocarbon | 🌱 | [47] | n/a |
| 64 | α-Copaene | polycyclic sesquiterpene hydrocarbon | 🌱 | [6,46,47,49,50,52,53] | antioxidant, anticancer [133]; neuroprotective [134]; |
| 65 | β-Copaene | 🌱✿𐩕 | [46,50,53] | ||
| 66 | α-Corocalene | polycyclic sesquiterpene hydrocarbon | 🌱 | [53] | n/a |
| 67 | α-Cubebene | polycyclic sesquiterpene hydrocarbon | 🌱✿ | [6,49,53] | antioxidant, neuroprotective [135]; antimicrobial [136]; anti-inflammatory [137]; |
| 68 | β-Cubebene | 🌱 | [6,46,49] | ||
| 69 | Cubebol | polycyclic sesquiterpene alcohol | 🌱 | [53] | n/a |
| 70 | 4-epi-Cubebol | [53] | |||
| 71 | 1,10-di-epiCubenol | polycyclic sesquiterpene alchohol | 🌱✿ | [46,49,53] | n/a |
| 72 | Cubenol | 🌱 | [53] | ||
| 73 | 1-epi-Cubenol | 🌱✿ | [46,49,53] | ||
| 74 | ar-Curcumen-15-al | oxygenated monocyclic sesquiterpene | 🌱 | [46,53] | n/a |
| 75 | ar-Curcumene | monocyclic sesquiterpene hydrocarbon | 🌱✿ | [6,43,46,47,48,49,50,51,52,53,126] | n/a |
| 76 | β-Curcumene | ✿ | [48] | ||
| 77 | γ-Curcumene | 🌱✿ | [48,49] | ||
| 78 | Curcuphenol | monocyclic sesquiterpene alcohol | 🌱✿ | [48,53] | anticancer [138]; |
| 79 | Cyclosativene | polycyclic sesquiterpene hydrocarbon | 🌱✿ | [49] | n/a |
| 80 | Dehydrosesquicineole | oxygenated polycyclic sesquiterpene | 🌱 | [53] | n/a |
| 81 | Bicyclo-Elemene | polycyclic sesquiterpene hydrocarbon | 🌱 | [50] | n/a |
| 82 | β-Elemene | monocyclic sesquiterpene hydrocarbon | 🌱 | [48,53] | anticancer, anti-inflammatory [139]; |
| 83 | γ-Elemene | 🌱 | [48] | ||
| 84 | δ-Elemene | 🌱✿ | [48,49,50] | ||
| 85 | Elemol | monocyclic sesquiterpene alcohol | 🌱 | [43,48,49] | n/a |
| 86 | β-Eudesma 4(15), 7 dien-1β-ol | polycyclic sesquiterpene alcohol | 🌱 | [53] | n/a |
| 87 | α-Eudesmol | 🌱 | [46] | neuroprotective [140]; | |
| 88 | β-Eudesmol | 🌱✿ | [46,48] | anti-allergic, anti-inflammatory [141]; anticancer [142]; | |
| 89 | γ-Eudesmol | 🌱✿ | [48,49] | n/a | |
| 90 | 10-epi-γ-Eudesmol | [48,49] | n/a | ||
| 91 | Kunseaol | monocyclic sesquiterpene alcohol | 🌱 | [53] | n/a |
| 92 | Bicyclo-Germacrene | polycyclic sesquiterpene hydrocarbon | 🌱 | [53] | n/a |
| 93 | Germacrene B | monocyclic sesquiterpene hydrocarbon | 🌱 | [47,48] | |
| 94 | Germacrene D | 🌱✿𐩕 | [6,43,47,48,49,51,52,53] | anticancer [143]; anti-inflammatory, analgesic [144]; antioxidant [145]; | |
| 95 | Iso-Germacrene D | 🌱 | [53] | ||
| 96 | β-Germacrenol | monocyclic sesquiterpene alcohol | 🌱 | [53] | n/a |
| 97 | Globulol | polycyclic sesquiterpene alcohol | 🌱✿ | [46,48] | n/a |
| 98 | α-Guaia-6,10(14)-diene-4β-ol | polycyclic sesquiterpene alcohol | 🌱 | [53] | n/a |
| 99 | Guaiene | polycyclic sesquiterpene hydrocarbon | 🌱 | [46] | n/a |
| 100 | Guaiol | polycyclic sesquiterpene alcohol | 🌱✿ | [48,49] | n/a |
| 101 | α-Gurjunene | polycyclic sesquiterpene hydrocarbon | 🌱✿ | [47,49,51,52] | n/a |
| 102 | β-Gurjunene | [49] | |||
| 103 | β-Himachalene | polycyclic sesquiterpene hydrocarbon | ✿𐩕 | [49] | n/a |
| 104 | α-Humulene | monocyclic sesquiterpene hydrocarbon | 🌱✿ | [6,43,46,47,48,49,51,52,53] | antitumor, anti- inflammatory, antimicrobial [146]; |
| 105 | Iso-Calamendiol | polycyclic sesquiterpene alcohol | 🌱 | [53] | n/a |
| 106 | Iso-Italicene | polycyclic sesquiterpene hydrocarbon | 🌱 | [46] | n/a |
| 107 | Juniper camphor | polycyclic sesquiterpene alcohol | 🌱✿ | [49] | n/a |
| 108 | Ledol | polycyclic sesquiterpene alcohol | 🌱 | [53,106] | n/a |
| 109 | α-Longipinene | polycyclic sesquiterpene hydrocarbon | 🌱 | [46] | n/a |
| 110 | cis-Muurola-4(14),5-diene | polycyclic sesquiterpene hydrocarbon | 🌱✿ | [49,53] | n/a |
| 111 | α-Muurolene | [6,48,49,50,52] | |||
| 112 | γ-Muurolene | [46,49,53] | |||
| 113 | 14-hydroxi-α-Muurolene | polycyclic sesquiterpene alcohol | 🌱 | [46] | n/a |
| 114 | α-Muurolol | 🌱✿ | [49] | ||
| 115 | epi-α-Muurolol | [48] | |||
| 116 | T-Muurolol | [43,46,49,49,53] | |||
| 117 | E-Nerolidol | acyclic sesquiterpene alcohol | 🌱✿ | [48] | antihyperlipidemic, anti-inflammatory, anti-uterine fibroids [147]; anticancer [148]; |
| 118 | (E)-Nuciferol | moncyclic sesquiterpene alcohol | 🌱 | [53] | n/a |
| 119 | β-Oplopenone | oxygenated polycyclic sesquiterpene | 🌱 | [53] | n/a |
| 120 | Salvial-4(14)-en-1-one | oxygenated polycyclic sesquiterpene | 🌱 | [53] | n/a |
| 121 | α-Santalene | polycyclic sesquiterpene hydrocarbon | 🌱 | [50] | n/a |
| 122 | cis-α-Santalol | polycyclic sesquiterpene alcohol | 🌱 | [46] | antihyperglycemic, antioxidant [149]; |
| 123 | Selin-11-en-4-α-ol | polycyclic sesquiterpene alcohol | 🌱 | [106] | anxiolytic, sedative [150]; |
| 124 | Selina-3,7(11)-diene | polycyclic sesquiterpene hydrocarbon | 🌱 | [50] | n/a |
| 125 | α-Selinene | polycyclic sesquiterpene hydrocarbon | 🌱 | [50] | n/a |
| 126 | β-Sesquiphellandrene | monocyclic sesquiterpene hydrocarbon | 🌱✿ | [43,47,49,53] | anticancer [151]; antioxidant [152]; |
| 127 | trans-Sesquisabinene hydrate | polycyclic sesquiterpene alcohol | 🌱 | [48] | n/a |
| 128 | Shyobunone | oxygenated monocyclic sesquiterpene | 🌱 | [6,53] | neuroprotective, acetyl-cholinesterase inhibition [153]; |
| 129 | 6-epi-Shyobunone | [53] | |||
| 130 | iso-Shyobunone | [53] | |||
| 131 | Spathulenol | polycyclic sesquiterpene alcohol | 🌱✿ | [46,48,49,53] | neuroprotective [154]; antibacterial, antioxidant, anti-inflammatory, anticancer [155]; |
| 132 | Spathulenol isomer | 🌱 | [48] | ||
| 133 | ar-Turmerol | monocyclic sesquiterpene alcohol | 🌱✿ | [46,48] | n/a |
| 134 | Valerianol | polycyclic sesquiterpene alcohol | 🌱✿ | [49] | n/a |
| 135 | Viridiflorol | polycyclic sesquiterpene alcohol | 🌱✿ | [48,53] | anti-arthritic, analgesic, antinociceptive [156]; anticancaer [157]; antioxidant, antibacterial, anti-inflammatory [158]; |
| 136 | Xanthorrhizol | monocyclic sesquiterpene alcohol | 🌱 | [46,53] | anticancer [159]; antimicrobial, antibacterial [160]; antihypolipidemic [161]; anti-inflammatory [162]; |
| 137 | α-Ylangene | polycyclic sesquiterpene hydrocarbon | 🌱 | [50] | n/a |
| 138 | β-Ylangene | 🌱 | [53] | ||
| 139 | α-Zingiberene | monocyclic sesquiterpene hydrocarbon | 🌱✿𐩕 | [6,43,46,47,48,49,50,51,52,53] | analgesic, neuroprotective [163]; anticancer [164]; anti-inflammatory [165]; |
| 140 | Zingiberenol | monocyclic sesquiterpene alcohol | 🌱 | [53] | n/a |
| 141 | 15,16-Dinorlabd-8(20)-en-13-one | oxygenated diterpene | 🌱 | [106] | n/a |
| 142 | Geranyl-p-cymene | monocyclic diterpene hydrocarbon | 🌱 | [53] | n/a |
| 143 | Geranyl α-terpinene | monocyclic diterpene hydrocarbon | 🌱 | [53] | n/a |
| 144 | Geranyl linalool | acyclic diterpene alcohol | 🌱 | [53] | n/a |
| 145 | Gibberellin | oxygenated polycyclic diterpene | 🌱 | [29] | n/a |
| 146 | Manool | polycyclic diterpene alcohol | 🌱 | [106] | antioxidant, anti-inflammatory [166]; anticancer [167]; antihypertensive [168]; cardioprotective [169]; |
| 147 | Manoyl oxide | polycyclic oxygenated diterpene | 🌱 | [126] | anticancer [170]; |
| 148 | 13-epi-Manoyl oxide | [47,53,126] | |||
| 149 | Methyl Neoabietate | oxygenated polycyclic diterpene | 🌱 | [47] | n/a |
| 150 | lutein | oxygenated polycyclic tetraterpene | 🌱 | [29] | antioxidant, anti-inflammatory, neuroprotective [171]; anticancer [172]; hepatoprotective [173]; cardiopreotective [174]; |
| 151 | neoxanthin | oxygenated polycyclic tetraterpene | 🌱 | [29] | antihyperlipidemic [175]; |
| 152 | zeaxanthin | oxygenated polycyclic tetraterpene | 🌱 | [29] | n/a |
5.1.2. Phenylpropanoids from the Essential Oils
5.1.3. Diterpenes
5.1.4. Tetraterpenes
5.2. Phenolic Compounds
5.2.1. Flavonoids
5.2.2. Phenolic Acids
| No | Compound | Structure/Class | Presence in | Analytical Reference | Pharmacology |
|---|---|---|---|---|---|
| 1 | Apigenin diglucoside | flavone | 🌱 | [5] | anticancer, anti-inflammatory, antimicrobial, antioxidant [183]; anxiolytic [184]; |
| 2 | Caffeic acid | phenolic acid | 🌱 | [11] | anticancer [185,186,187]; antimutagenic, antihyperglycemic, anti-inflammatory, antioxidant, [188,189]; antmicrobial [186]; cardioprotective [189]; hepatoprotective [190]; neuroprotective [187,191]; |
| 3 | Caftaric acid | phenolic acid | 🌱 | [12] | anticancer, antidiabetic, antihypertensive, anti-inflammatory, antimutagenic, antioxidant, hepatoprotective, [192]; |
| 4 | Cynarin | phenolic acid | 🌱 | [12] | anticancer, antidiabetic antiulcer, antivirus, antioxidant, hepatoprotective, hypocholesterolemic [193]; cardioprotective [194]; |
| 5 | (-)-(Epi)catechin | flavanol | 🌱 | [5,177,180] | neuroprotective [195]; antioxidant [195,196]; antimicrobial [197]; anti-inflammatory, antiallergenic, antivirus, anticancer, skin penetration enhancer, UV-protection [198]; |
| 6 | (-)-(Epi)gallocatechin | 🌱 | [5,177,180] | ||
| 7 | Epigallocatechin-(4β-->8)-gallocatechin-(4α-->8)-gallocatechin trimer | 🌱 | [5,180] | ||
| 8 | Epigallocatechin-(4β-->8)-gallocatechin-(4α-->8)-catechin trimer | 🌱 | [5,180] | ||
| 9 | (-)-(Epi)catechin-(epi)gallocatechin dimer | 🌱 | [5] | ||
| 10 | (-)-(Epi)gallocatechin-(epigallocatechin dimer | 🌱 | [5] | ||
| 11 | (-)-(Epi)gallocatechin gallate | 🌱 | [5] | ||
| 12 | 4,3′,4′-Trimethyl catechin | 🌱 | [12] | ||
| 13 | Ferulic acid oligomer | phenolic acid | 🌱 | [12] | antioxidant [199]; antimicrobial [200]; anti-inflammatory [201]; neuroprotective [202]; antiviral [203]; antiallergic [204]; hepatoprotective [205]; anticancer [206]; antithrombotic [207]; antidiabetic [208]; |
| 14 | Hydroxy-ferulic acid rhamnoside | phenolic acid glycoside | 🌱 | [5] | |
| 15 | Feruoyl quinic glucoside | 🌱 | [12] | n/a | |
| 16 | Gallic acid | phenolic acid | 🌱 | [5,182] | anti-inflammatory [209,210]; antiobesity, antioxidant [211]; hepatoprotective [210]; anticancer [212]; antifungal [213]; |
| 17 | Galloyl glucose | flavanol | 🌱 | [12] | antiviral [214]; |
| 18 | Glucogallin | flavanol | 🌱 | [5,11] | antioxidant, anti-inflammatory, antidiabetic, cataract-preventing, antiglaucoma, UV-protective [215]; |
| 19 | Glycitin 6″-O-malonate | isoflavone (flavonoid glycoside) | 🌱 | [12] | n/a |
| 20 | Hexahydroxydiphenoyl- D-glucose | hydrolysable tannin | [5] | antioxidant [216] | |
| 21 | Kaempferol diglucoside | flavonol | 🌱 | [5] | antidiabetic [217]; anxiolytic, antidepressant, antiepileptic, anti-inflammatory, neuroprotective, analgesic [218]; |
| 22 | Kaempferol 3-O-rutinoside | 🌱 | [5,177] | ||
| 23 | Luteolin-7-O-rutinoside | flavonol | 🌱 | [12] | antioxidant, anti-inflammatory [219]; |
| 24 | Myricetin hexoside | flavonol | 🌱 | [5,177] | anticancer, antidiabetic, antiobesity, cardioprotective, osteoporosis protective, anti-inflammatory, hepatoprotective [220]; |
| 25 | Myricetin glycoside | 🌱 | [11] | ||
| 26 | Myricetin 3-O-rutinoside | 🌱 | [12] | ||
| 27 | Myricetin-O-galloyl-hexoside | 🌱 | [12] | ||
| 28 | Myricitrin | 🌱 | [5] | ||
| 29 | Oenin | anthocyanin (flavonoid glycoside) | 🌱 | [12] | anticancer [221]; neuroprotective [222]; anti-inflammatory [223]; antioxidant [224]; |
| 30 | Pedunculagin | ellagitannin | 🌱 | [5,11] | antioxidant, anti-inflammatory, dermatoprotective [225]; anticancer [226]; antibacterial [227]; |
| 31 | Pelargonidin 3-O-(6″malonylglucoside) | anthocyanin (flavonoid glycoside) | 🌱 | [12] | cardioprotective, neuroprotective [228,229]; |
| 32 | Peonidin 3-O-(6″-p-coumaroyl) glucoside | anthocyanin (flavonoid glycoside) | 🌱 | [12] | cardioprotective, neuroprotective [228,229]; |
| 33 | Petunidin | anthocyanidin (O-methylated flavonoid) | 🌱 | [12] | antioxidant [230]; cardioprotective, neuroprotective [228,229]; |
| 34 | Procyanidin | flavanol | 🌱 | [12] | antioxidant [231]; antibacterial [232]; anticancer [233]; anti-inflammatory [231,234]; |
| 35 | Prunin | flavanone | 🌱 | [12] | antihyperlipidemic, antihyperglycemic, antidiabetic [235]; |
| 36 | Punicalagin | ellagitannin | 🌱 | [11] | anti-inflammatory, antioxidant, neuroprotective, hepatoprotective, cardioprotective, antiviral, antimicrobial, anticancer, antidiabetic, antihyperlipidemic, gastroprotective [236]; |
| 37 | Punicalagin gallate | 🌱 | [11] | ||
| 38 | Quercetin | flavonol | 🌱 | [12] | anticancer, antitumor, anti-inflammatory, antiviral, antihypercholesterolemia, antihyperglycemic, antioxidant, antibacterial, cardioprotective, gastroprotective, hepatoprotective, antihypertensive, nephroprotective, neuroprotective [237]; antidepressant [238]; antimicrobial [239], antifungal [240]; antiallergic [241] antiobesity [242]; |
| 39 | Quercetin glucoside | 🌱 | |||
| 40 | Quercetin 3-O-glucoside | 🌱 | [12] | ||
| 41 | Quercetin 3-O-(2’-cumaroyl)- rutinoside | 🌱 | [12] | ||
| 42 | Quercetin 3,4-diglucoside | 🌱 | [12] | ||
| 43 | Quercetin 3-O-(2’-caffeoyl)-rutinoside | 🌱 | [5] | ||
| 44 | Quercitrin | 🌱 | [5,11] | ||
| 45 | Rutin | 🌱 | [5,11,12,177] | ||
| 46 | Quinic acid | phenolic acid | 🌱 | [12,177] | radioprotective [243]; neuroprotective [244]; anti-inflammatory [245]; 5antiviral [246]; |
| 47 | 3p-Coumaroylquinic acid | 🌱 | [12] | n/a | |
| 48 | 3-Caffeoylquinic acid | 🌱 | [11] | antioxidant, anti-inflammatory [247]; | |
| 49 | Caffeoylquinic glycoside | phenolic acid glycoside | 🌱 | [12] | enzyme inhibition, hepatoprotective [248]; |
| 50 | Isorhamnetin- O-rutinoside | flavonol | 🌱 | [5] | anti-atherosclerosis, cardioprotective, neuroprotective, anticancer, antihypertensive, antioxidant antihyperglycemic, hepatoprotective, anti-inflammatory, anti-osteoporosis, antiobesity, UV-protection [249]; antimicrobial [250]; |
| 51 | 3-O-Methylrosmarinic acid | caffeic acid ester | 🌱 | [12] | anti-inflammatory, antioxidant, antidiabetes, antivirus, antitumor, neuroprotective, hepatoprotective [251]; |
| 52 | Methoxy dihydroferuoyl methyl rosmarinic acid | 🌱 | [12] | ||
| 53 | Dihydroxy-dihydro feruoyl methyl rosmarinic acid | 🌱 | [12] | ||
| 54 | p-Hydroxy benzil rosmarinic acid | 🌱 | [12] | ||
| 55 | Shikimic acid dimer | phenolic acid | 🌱 | [12] | anticoagulant [252]; antithrombotic [253]; |
| 56 | 6′-O-Sinapoyl sucrose | hydroxy-cinnamate sucrose esters | 🌱 | [12] | antioxidant [254]; |
| 57 | Syringyl shikimic acid dimer | phenolic acid | 🌱 | [12] | n/a |
| 58 | Uralenneoside | p-hydroxy- benzoic acid alkyl ester | 🌱 | [5] | n/a |
5.3. Carbonylic Compounds
5.4. Phytohormones and Vitamin E
5.5. Alkanes
5.6. Other Compounds: Fatty and Carboxylic Acids
6. Preparation Methods of the C. albidus Extracts
6.1. Traditional Preparations
6.2. Actual and Alternative Extraction Methods
7. Bioavailability of the Groups of Compounds Found in the C. albidus Extracts
8. Therapeutical Uses
8.1. Traditional Uses
8.2. Scientific Evidence Confirming Traditional Uses
8.2.1. Antimicrobial Activity
8.2.2. Anti-Inflammatory, Antinociceptive, Analgesic, and Sedative Activity
9. Potential Pharmacological Applications of C. albidus and Their Mechanisms
9.1. Prevention of Neurodegenerative Diseases
9.1.1. Free Radical Scavenging
9.1.2. Heavy Metal Chelation
10. Toxicity
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guzmán, B.; Vargas, P. Systematics, Character Evolution, and Biogeography of Cistus L. (Cistaceae) Based on ITS, TrnL-TrnF, and MatK Sequences. Mol. Phylogenet. Evol. 2005, 37, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Wagner, G. Pflanzennamen Und Botanische Fachwörter: Botanisches Lexikon; 8., neubearbeitete und erw. Aufl.; Neumann-Neudamm: Melsungen, Germany, 1984; ISBN 978-3-7888-0421-3. [Google Scholar]
- Schmidt, W. Gehölze für Mediterrane Gärten; Hortus Mediterraneus; Ulmer: Stuttgart, Germany, 1999; ISBN 978-3-8001-6590-2. [Google Scholar]
- Coombes, A.J. Dictionary of Plant Names, New ed.; Hamlyn: London, UK, 1994; ISBN 978-0-600-58187-1. [Google Scholar]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A Systematic Study of the Polyphenolic Composition of Aqueous Extracts Deriving from Several Cistus Genus Species: Evolutionary Relationship: Polyphenolic Characterization of Cistus Aqueous Extracts. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Ormeño, E.; Baldy, V.; Ballini, C.; Fernandez, C. Production and Diversity of Volatile Terpenes from Plants on Calcareous and Siliceous Soils: Effect of Soil Nutrients. J. Chem. Ecol. 2008, 34, 1219–1229. [Google Scholar] [CrossRef]
- Gülz, P.-G.; Herrmann, T.; Hangst, K. Leaf Trichomes in the Genus Cistus. Flora 1996, 191, 85–104. [Google Scholar] [CrossRef]
- Pardo de Santayana, M. Inventario Español de los Conocimientos Tradicionales Relativos de la Biodiversidad: Primera fase: Introducción, Metodología y Fichas; Ministerio para la Transición Ecológica: Madrid, Spain, 2014; ISBN 978-84-491-1401-4. [Google Scholar]
- Verde, A.; Fajardo, J.; Obón, C.; Cebrían, F.; Rivera, D. Guía de las Plantas Medicinales de Castilla-La Mancha; Altabán Ediciones: Albacete, Spain, 2008; ISBN 987-84-96465-53-4. [Google Scholar]
- Mulet Pascual, L. Estudio Etnobotánico de La Provincia de Castellón; Diputación de Castellón: Castellón, Spain, 1991; ISBN 978-84-86895-24-2. [Google Scholar]
- Lukas, B.; Jovanovic, D.; Schmiderer, C.; Kostas, S.; Kanellis, A.; Gómez Navarro, J.; Aytaç, Z.; Koç, A.; Sözen, E.; Novak, J. Intraspecific Genetic Diversity of Cistus creticus L. and Evolutionary Relationships to Cistus albidus L. (Cistaceae): Meeting of the Generations? Plants 2021, 10, 1619. [Google Scholar] [CrossRef]
- Mastino, P.; Marchetti, M.A.; Costa, J.; Juliano, C.; Usai, M. Analytical Profiling of Phenolic Compounds in Extracts of Three Cistus Species from Sardinia and Their Potential Antimicrobial and Antioxidant Activity. Chem. Biodivers. 2021, 18, e2100053. [Google Scholar] [CrossRef]
- Tahiri, O.; Atmani-Kilani, D.; Sanchez-Fidalgo, S.; Aparicio-Soto, M.; Alarcón-de-la-Lastra, C.; Barrajón-Catalán, E.; Micol, V.; Atmani, D. The Flavonol-Enriched Cistus albidus Chloroform Extract Possesses in Vivo Anti-Inflammatory and Anti-Nociceptive Activity. J. Ethnopharmacol. 2017, 209, 210–218. [Google Scholar] [CrossRef]
- Makabe, H.; Fujii, H.; Mori, M.; Matsumoto, K.; Ishihara, C.; Kawaguchi, K.; Kawahara, S.; Hattori, Y. Synthesis of Prodelphinidin Trimer Isolated from Cistus albidus and Its Growth Inhibitory Activity Against Human Prostate Cancer Cell Lines. Heterocycles 2016, 92, 1822. [Google Scholar] [CrossRef]
- Centenaro, G.; de Miguel, S.; Amouzgar, L.; Piñuela, Y.; Son, D.; Bonet, J.A.; de Aragón, J.M.; Dashevskaya, S.; Castaño, C.; Alday, J.G. Silvicultural Management and Altitude Prevail on Soil Properties and Fungal Community in Shaping Understorey Plant Communities in a Mediterranean Pine Forest. Sci. Total Environ. 2023, 858, 159860. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Jing, X.; Van Stan, J.T.; Plaza-Álvarez, P.A.; Gonzalez-Romero, J.; Peña, E.; Moya, D.; Antonio Zema, D.; de las Heras, J. Changes in Soil Functionality Eight Years after Fire and Post-Fire Hillslope Stabilisation in Mediterranean Forest Ecosystems. Geoderma 2022, 409, 115603. [Google Scholar] [CrossRef]
- El Mamoun, I.; Mouna, F.; Mohammed, A.; Najib, B.; Zine-El Abidine, T.; Abdelkarim, G.; Didier, B.; Laurent, L.; Abdelaziz, S. Zinc, Lead, and Cadmium Tolerance and Accumulation in Cistus libanotis, Cistus albidus and Cistus salviifolius: Perspectives on Phytoremediation. Remediat. J. 2020, 30, 73–80. [Google Scholar] [CrossRef]
- Civeyrel, L.; Leclercq, J.; Demoly, J.-P.; Agnan, Y.; Quèbre, N.; Pélissier, C.; Otto, T. Molecular Systematics, Character Evolution, and Pollen Morphology of Cistus and Halimium (Cistaceae). Plant Syst. Evol. 2011, 295, 23–54. [Google Scholar] [CrossRef]
- Brossa, R.; Pintó-Marijuan, M.; Francisco, R.; López-Carbonell, M.; Chaves, M.M.; Alegre, L. Redox Proteomics and Physiological Responses in Cistus albidus Shrubs Subjected to Long-Term Summer Drought Followed by Recovery. Planta 2015, 241, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Casadesús, A.; Bouchikh, R.; Pérez-Llorca, M.; Munné-Bosch, S. Linking Jasmonates with Vitamin E Accumulation in Plants: A Case Study in the Mediterranean Shrub Cistus albidus L. Planta 2021, 253, 36. [Google Scholar] [CrossRef]
- Guzmán, B.; Lledó, M.D.; Vargas, P. Adaptive Radiation in Mediterranean Cistus (Cistaceae). PLoS ONE 2009, 4, e6362. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.; Miret, J.A.; Van Der Kelen, K.; Rombaut, D.; Van Breusegem, F.; Munné-Bosch, S. Zeatin Modulates Flower Bud Development and Tocopherol Levels in Cistus albidus (L.) Plants as They Age. Plant Biol. 2015, 17, 90–96. [Google Scholar] [CrossRef]
- Bertolasi, B.; Zago, L.; Gui, L.; Sitzia, T.; Vanetti, I.; Binelli, G.; Puppi, G.; Buldrini, F.; Pezzi, G. Phenological and Genetic Characterization of Mediterranean Plants at the Peripheral Range: The Case of Cistus albidus near Lake Garda. Flora 2019, 252, 26–35. [Google Scholar] [CrossRef]
- Grant, O.M.; McNeilly, T.; Incoll, L.D. Genetic Diversity of Cistus albidus in South-East Spain Does Not Relate to Mesoclimate. Funct. Plant Biol. 2006, 33, 247. [Google Scholar] [CrossRef]
- Guzmán, B.; Vargas, P. Long-Distance Colonization of the Western Mediterranean by Cistus ladanifer (Cistaceae) despite the Absence of Special Dispersal Mechanisms. J. Biogeogr. 2009, 36, 954–968. [Google Scholar] [CrossRef]
- Arrington, J.M.; Kubitzki, K. Cistaceae. In Flowering Plants Dicotyledons; Kubitzki, K., Bayer, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 62–70. ISBN 978-3-642-07680-0. [Google Scholar]
- Müller, M.; Siles, L.; Cela, J.; Munné-Bosch, S. Perennially Young: Seed Production and Quality in Controlled and Natural Populations of Cistus albidus Reveal Compensatory Mechanisms That Prevent Senescence in Terms of Seed Yield and Viability. J. Exp. Bot. 2014, 65, 287–297. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Casadesús, A.; Müller, M.; Munné-Bosch, S. Leaf Orientation as Part of the Leaf Developmental Program in the Semi-Deciduous Shrub, Cistus albidus L.: Diurnal, Positional, and Photoprotective Effects During Winter. Front. Plant Sci. 2019, 10, 767. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Llorca, M.; Casadesús, A.; Munné-Bosch, S.; Müller, M. Contrasting Patterns of Hormonal and Photoprotective Isoprenoids in Response to Stress in Cistus albidus during a Mediterranean Winter. Planta 2019, 250, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Jubany-Marí, T.; Alegre, L. Enhanced Photo- and Antioxidative Protection, and Hydrogen Peroxide Accumulation in Drought-Stressed Cistus clusii and Cistus albidus Plants. Tree Physiol. 2003, 23, 1–12. [Google Scholar] [CrossRef]
- Roy, J.; Sonie, L. Germination and Population Dynamics of Cistus Species in Relation to Fire. J. Appl. Ecol. 1992, 29, 647. [Google Scholar] [CrossRef]
- Casadesús, A.; Bouchikh, R.; Munné-Bosch, S. Contrasting Seasonal Abiotic Stress and Herbivory Incidence in Cistus albidus L. Plants Growing in Their Natural Habitat on a Mediterranean Mountain. J. Arid Environ. 2022, 206, 104842. [Google Scholar] [CrossRef]
- Cabezudo, B.; Pérez Latorre, A.V.; Navarro, T.; Nieto Caldera, J.M. Estudios Fenomorfológicos En La Vegetación Del Sur de España. II. Alcornocales Mesomediterráneos. (Montes de Málaga, Málaga). Acta Bot. Malacit. 1993, 18, 179–188. [Google Scholar] [CrossRef]
- Blasco, S.; Mateu, I. Flowering and Fruiting Phenology and Breeding System of Cistus albidus L. Acta Bot. Gallica 1995, 142, 245–251. [Google Scholar] [CrossRef]
- Siles, L.; Müller, M.; Cela, J.; Hernández, I.; Alegre, L.; Munné-Bosch, S. Marked Differences in Seed Dormancy in Two Populations of the Mediterranean Shrub, Cistus albidus L. Plant Ecol. Divers. 2017, 10, 231–240. [Google Scholar] [CrossRef]
- Rizzotto, M. Ricerche tassonomiche e corologiche sulle Cistaceae. 1: Il genere Cistus L. in Italia. Webbia 1979, 33, 343–378. [Google Scholar] [CrossRef]
- Robles, C.; Dutoit, T.; Bonin, G. Inhibition Mechanisms and Successional Processes: A Case Study of Cistus albidus L. in Provence. Ecosyst. Sustain. Dev. 1998, 1, 437–446. [Google Scholar]
- Thanos, A.C.; Geroghiou, K.; Kadis, C.; Pantazi, C. Cistaceae: A plant family with hard seeds. Isr. J. Bot. 1993, 41, 251–263. [Google Scholar]
- Trabaud, L.; Oustric, J. Heat Requirements for Seed Germination of Three Cistus Species in the Garrigue of Southern France. Flora 1989, 183, 321–325. [Google Scholar] [CrossRef]
- Trabaud, L.; Renard, P. Do light and litter influence the recruitment of cistus spp. Stands? Isr. J. Plant Sci. 1999, 47, 1–9. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A Classification System for Seed Dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Polunin, O.; Schauer, T.; Everard, B. Pflanzen Europas; BLV-Bestimmungsbuch; BLV(-Verl. Ges.): München, Germany, 1971; ISBN 978-3-405-10929-5. [Google Scholar]
- Robles, C.; Garzino, S. Essential Oil Composition of Cistus albidus Leaves. Phytochemistry 1998, 48, 1341–1345. [Google Scholar] [CrossRef]
- Castells, E.; Peñuelas, J. Is There a Feedback between N Availability in Siliceous and Calcareous Soils and Cistus albidus Leaf Chemical Composition? Oecologia 2003, 136, 183–192. [Google Scholar] [CrossRef]
- ISO Standard No. 9235:2013; International Organization for Standardization Aromatic Natural Raw Materials. ISO: Geneva, Switzerland, 2013.
- Bechlaghem, K.; Allali, H.; Benmehdi, H.; Aissaoui, N.; Flamini, G. Chemical Analysis of the Essential Oils of Three Cistus Species Growing in North- West of Algeria. Agric. Conspec. Sci. 2019, 84, 283–293. [Google Scholar]
- Llusià, J.; Peñuelas, J.; Ogaya, R.; Alessio, G. Annual and Seasonal Changes in Foliar Terpene Content and Emission Rates in Cistus albidus L. Submitted to Soil Drought in Prades Forest (Catalonia, NE Spain). Acta Physiol. Plant. 2010, 32, 387–394. [Google Scholar] [CrossRef]
- Palá-Paúl, J.; Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Sanz, J. Seasonal Variation in Chemical Composition of Cistus albidus L. from Spain. J. Essent. Oil Res. 2005, 17, 19–22. [Google Scholar] [CrossRef]
- Maccioni, S.; Baldini, R.; Cioni, P.L.; Tebano, M.; Flamini, G. In Vivo Volatiles Emission and Essential Oils from Different Organs and Pollen Of Cistus albidus from Caprione (Eastern Liguria, Italy). Flavour Fragr. J. 2007, 22, 61–65. [Google Scholar] [CrossRef]
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile Profile of Spanish Cistus Plants as Sources of Antimicrobials for Industrial Applications. Ind. Crops Prod. 2015, 74, 425–433. [Google Scholar] [CrossRef]
- Ormeño, E.; Mévy, J.P.; Vila, B.; Bousquet-Mélou, A.; Greff, S.; Bonin, G.; Fernandez, C. Water Deficit Stress Induces Different Monoterpene and Sesquiterpene Emission Changes in Mediterranean Species. Relationship between Terpene Emissions and Plant Water Potential. Chemosphere 2007, 67, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Ormeño, E.; Bousquet-Mélou, A.; Mévy, J.-P.; Greff, S.; Robles, C.; Bonin, G.; Fernandez, C. Effect of Intraspecific Competition and Substrate Type on Terpene Emissions from Some Mediterranean Plant Species. J. Chem. Ecol. 2007, 33, 277–286. [Google Scholar] [CrossRef]
- Paolini, J.; Tomi, P.; Bernardini, A.-F.; Bradesi, P.; Casanova, J.; Kaloustian, J. Detailed Analysis of the Essential Oil from Cistus albidus L. by Combination of GC/RI, GC/MS and 13C-NMR Spectroscopy. Nat. Prod. Res. 2008, 22, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Gao, X. Brain Targeted Drug Delivery Systems: A Focus on Nanotechnology and and Nanoparticulates; Academic Press: London, UK, 2019; ISBN 978-0-12-814002-4. [Google Scholar]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor Activates and Strongly Desensitizes the Transient Receptor Potential Vanilloid Subtype 1 Channel in a Vanilloid-Independent Mechanism. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Welsch, C.W.; Rao, A.R. Modulatory Influence of Camphor on the Activities of Hepatic Carcinogen Metabolizing Enzymes and the Levels of Hepatic and Extrahepatic Reduced Glutathione in Mice. Cancer Lett. 1995, 88, 163–169. [Google Scholar] [CrossRef]
- Laude, E.A.; Morice, A.H.; Grattan, T.J. The Antitussive Effects of Menthol, Camphor and Cineole in Conscious Guinea-Pigs. Pulm. Pharmacol. 1994, 7, 179–184. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Y.; Sun, Y.; Sheng, X.; Zhang, J.; Zhang, Z.; Ding, J. Effect of Menthol and Camphor on Permeation of Compound Diphenhydramine Cream in Vitro. Cent South Pharm. 2011, 9, 97–101. [Google Scholar]
- Patra, J.K.; Das, G.; Bose, S.; Banerjee, S.; Vishnuprasad, C.N.; Pilar Rodriguez-Torres, M.; Shin, H. Star Anise (Illicium verum): Chemical Compounds, Antiviral Properties, and Clinical Relevance. Phytother. Res. 2020, 34, 1248–1267. [Google Scholar] [CrossRef]
- Jeong, J.-G.; Kim, Y.S.; Min, Y.K.; Kim, S.H. Low Concentration of 3-Carene Stimulates the Differentiation of Mouse Osteoblastic MC3T3-E1 Subclone 4 Cells. Phytother. Res. 2008, 22, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.L.; Jimenez, J.; Ocete, M.A.; Zarzuelo, A.; Cabo, M.M. Comparative Study of Different Essential Oils of Bupleurum gibraltaricum Lamarck. Pharmazie 1989, 44, 284–287. [Google Scholar] [PubMed]
- Pina-Vaz, C.; Gonçalves Rodrigues, A.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.J.; Martinez-de-Oliveira, J. Antifungal Activity of Thymus Oils and Their Major Compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Aeschbach, R.; Löliger, J.; Scott, B.C.; Murcia, A.; Butler, J.; Halliwell, B.; Aruoma, O.I. Antioxidant Actions of Thymol, Carvacrol, 6-Gingerol, Zingerone and Hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef]
- Jayakumar, S.; Madankumar, A.; Asokkumar, S.; Raghunandhakumar, S.; Gokula dhas, K.; Kamaraj, S.; Divya, M.G.J.; Devaki, T. Potential Preventive Effect of Carvacrol against Diethylnitrosamine-Induced Hepatocellular Carcinoma in Rats. Mol. Cell. Biochem. 2012, 360, 51–60. [Google Scholar] [CrossRef]
- Hotta, M.; Nakata, R.; Katsukawa, M.; Hori, K.; Takahashi, S.; Inoue, H. Carvacrol, a Component of Thyme Oil, Activates PPAR alpha and Gamma and Suppresses COX-2 Expression. J. Lipid Res. 2010, 51, 132–139. [Google Scholar] [CrossRef]
- Cho, S.; Choi, Y.; Park, S.; Park, T. Carvacrol Prevents Diet-Induced Obesity by Modulating Gene Expressions Involved in Adipogenesis and Inflammation in Mice Fed with High-Fat Diet. J. Nutr. Biochem. 2012, 23, 192–201. [Google Scholar] [CrossRef]
- Decker, K.; Keppler, D. Galactosamine Induced Liver Injury. Prog. Liver Dis. 1972, 4, 183–199. [Google Scholar]
- Boskabady, M.H.; Jandaghi, P. Relaxant Effects of Carvacrol on Guinea Pig Tracheal Chains and Its Possible Mechanisms. Pharmazie 2003, 58, 661–663. [Google Scholar]
- Peixoto-Neves, D.; Silva-Alves, K.S.; Gomes, M.D.M.; Lima, F.C.; Lahlou, S.; Magalhães, P.J.C.; Ceccatto, V.M.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Vasorelaxant Effects of the Monoterpenic Phenol Isomers, Carvacrol and Thymol, on Rat Isolated Aorta. Fundam. Clin. Pharmacol. 2010, 24, 341–350. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.; Moreira, J.C.F.; Pasquali, M.A.B.; Rabie, S.M.S.; Pires, A.S.; Schröder, R.; Rabelo, T.K.; Santos, J.P.A.; Lima, P.S.S.; Cavalcanti, S.C.H.; et al. Antinociceptive Activity and Redox Profile of the Monoterpenes (+)-Camphene, p -Cymene, and Geranyl Acetate in Experimental Models. ISRN Toxicol. 2013, 2013, 459530. [Google Scholar] [CrossRef] [PubMed]
- Arabloei Sani, M.; Yaghmaei, P.; Hajebrahimi, Z.; Hayati Roodbari, N. Therapeutic Effect of P-Cymene on Lipid Profile, Liver Enzyme, and Akt/Mtor Pathway in Streptozotocin-Induced Diabetes Mellitus in Wistar Rats. J. Obes. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. D-Limonene: Safety and Clinical Applications. Altern. Med. Rev. J. Clin. Ther. 2007, 12, 259–264. [Google Scholar]
- Lorigooini, Z.; Boroujeni, S.N.; Sayyadi-Shahraki, M.; Rahimi-Madiseh, M.; Bijad, E.; Amini-Khoei, H. Limonene through Attenuation of Neuroinflammation and Nitrite Level Exerts Antidepressant-Like Effect on Mouse Model of Maternal Separation Stress. Behav. Neurol. 2021, 2021, 8817309. [Google Scholar] [CrossRef]
- Pattnaik, S.; Subramanyam, V.R.; Bapaji, M.; Kole, C.R. Antibacterial and Antifungal Activity of Aromatic Constituents of Essential Oils. Microbios 1997, 89, 39–46. [Google Scholar]
- Linck, V.M.; da Silva, A.L.; Figueiró, M.; Caramão, E.B.; Moreno, P.R.H.; Elisabetsky, E. Effects of Inhaled Linalool in Anxiety, Social Interaction and Aggressive Behavior in Mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef]
- Jana, S.; Patra, K.; Sarkar, S.; Jana, J.; Mukherjee, G.; Bhattacharjee, S.; Mandal, D.P. Antitumorigenic Potential of Linalool Is Accompanied by Modulation of Oxidative Stress: An in Vivo Study in Sarcoma-180 Solid Tumor Model. Nutr. Cancer 2014, 66, 835–848. [Google Scholar] [CrossRef]
- Sugawara, Y.; Hara, C.; Aoki, T.; Sugimoto, N.; Masujima, T. Odor Distinctiveness between Enantiomers of Linalool: Difference in Perception and Responses Elicited by Sensory Test and Forehead Surface Potential Wave Measurement. Chem. Senses 2000, 25, 77–84. [Google Scholar] [CrossRef]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. Anti-Inflammatory Activity of Linalool and Linalyl Acetate Constituents of Essential Oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef]
- Jansen, C.; Shimoda, L.M.N.; Kawakami, J.K.; Ang, L.; Bacani, A.J.; Baker, J.D.; Badowski, C.; Speck, M.; Stokes, A.J.; Small-Howard, A.L.; et al. Myrcene and Terpene Regulation of TRPV1. Channels 2019, 13, 344–366. [Google Scholar] [CrossRef]
- Bomfim, L.M.; Menezes, L.R.A.; Rodrigues, A.C.B.C.; Dias, R.B.; Gurgel Rocha, C.A.; Soares, M.B.P.; Neto, A.F.S.; Nascimento, M.P.; Campos, A.F.; Silva, L.C.R.C.e.; et al. Antitumour Activity of the Microencapsulation of Annona vepretorum Essential Oil. Basic Clin. Pharmacol. Toxicol. 2016, 118, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Sayyah, M.; Nadjafnia, L.; Kamalinejad, M. Anticonvulsant Activity and Chemical Composition of Artemisia dracunculus L. Essential Oil. J. Ethnopharmacol. 2004, 94, 283–287. [Google Scholar] [CrossRef]
- Pulaj, B.; Mustafa, B.; Nelson, K.; Quave, C.L.; Hajdari, A. Chemical Composition and in Vitro Antibacterial Activity of Pistacia terebinthus Essential Oils Derived from Wild Populations in Kosovo. BMC Complement. Altern. Med. 2016, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, H.; Chen, S.; Zeng, L.; Wang, T. Anti-Fungal Activity, Mechanism Studies on α-Phellandrene and Nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 13. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, A.C.; Santos, J.A.; Konkiewitz, E.C.; Oesterreich, S.A.; Formagio, A.S.N.; Croda, J.; Ziff, E.B.; Kassuya, C.A.L. Antihyperalgesic and Antidepressive Actions of (R)-(+)-Limonene, α-Phellandrene, and Essential Oil from Schinus terebinthifolius Fruits in a Neuropathic Pain Model. Nutr. Neurosci. 2015, 18, 217–224. [Google Scholar] [CrossRef]
- Lima, D.F.; Brandão, M.S.; Moura, J.B.; Leitão, J.M.R.S.; Carvalho, F.A.A.; Miúra, L.M.C.V.; Leite, J.R.S.A.; Sousa, D.P.; Almeida, F.R.C. Antinociceptive Activity of the Monoterpene α-Phellandrene in Rodents: Possible Mechanisms of Action. J. Pharm. Pharmacol. 2012, 64, 283–292. [Google Scholar] [CrossRef]
- Lin, J.-J.; Lu, K.-W.; Ma, Y.-S.; Tang, N.-Y.; Wu, P.-P.; Wu, C.-C.; Lu, H.-F.; Lin, J.-G.; Chung, J.-G. Alpha-Phellandrene, a Natural Active Monoterpene, Influences a Murine WEHI-3 Leukemia Model in Vivo by Enhancing Macrophague Phagocytosis and Natural Killer Cell Activity. In Vivo 2014, 28, 583–588. [Google Scholar]
- Karthikeyan, R.; Kanimozhi, G.; Prasad, N.R.; Agilan, B.; Ganesan, M.; Srithar, G. Alpha Pinene Modulates UVA-Induced Oxidative Stress, DNA Damage and Apoptosis in Human Skin Epidermal Keratinocytes. Life Sci. 2018, 212, 150–158. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Y.; Zhu, Y.; Zhou, B.; Ren, C.; Liang, S.; Guo, Y. α-Pinene Induces Apoptotic Cell Death via Caspase Activation in Human Ovarian Cancer Cells. Med. Sci. Monit. 2019, 25, 6631–6638. [Google Scholar] [CrossRef]
- Rodrigues, K.A.d.F.; Amorim, L.V.; Dias, C.N.; Moraes, D.F.C.; Carneiro, S.M.P.; Carvalho, F.A.d.A. Syzygium Cumini (L.) Skeels Essential Oil and Its Major Constituent α-Pinene Exhibit Anti-Leishmania Activity through Immunomodulation in Vitro. J. Ethnopharmacol. 2015, 160, 32–40. [Google Scholar] [CrossRef]
- Pinheiro, M.A.; Magalhães, R.; Torres, D.; Cavalcante, R.; Mota, F.X.; Oliveira Coelho, E.A.; Moreira, H.; Lima, G.; da Costa Araújo, P.; Cardoso, J.L.; et al. Gastroprotective Effect of Alpha-Pinene and Its Correlation with Antiulcerogenic Activity of Essential Oils Obtained from Hyptis Species. Pharmacogn. Mag. 2015, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Matthews Jucá, D.; da Silva, M.; Palheta Junior, R.; de Lima, F.; Okoba, W.; Lahlou, S.; de Oliveira, R.; dos Santos, A.; Magalhães, P. The Essential Oil of Eucalyptus tereticornis and Its Constituents, α- and β-Pinene, Show Accelerative Properties on Rat Gastrointestinal Transit. Planta Med. 2011, 77, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Utegenova, G.; Pallister, K.; Kushnarenko, S.; Özek, G.; Özek, T.; Abidkulova, K.; Kirpotina, L.; Schepetkin, I.; Quinn, M.; Voyich, J. Chemical Composition and Antibacterial Activity of Essential Oils from Ferula L. Species against Methicillin-Resistant Staphylococcus aureus. Molecules 2018, 23, 1679. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, N.; Zu, Y.; Fu, Y. Comparative Anti-Infectious Bronchitis Virus (IBV) Activity of (-)-Pinene: Effect on Nucleocapsid (N) Protein. Molecules 2011, 16, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Zamyad, M.; Abbasnejad, M.; Esmaeili-Mahani, S.; Mostafavi, A.; Sheibani, V. The Anticonvulsant Effects of Ducrosia anethifolia (Boiss) Essential Oil Are Produced by Its Main Component Alpha-Pinene in Rats. Arq. Neuropsiquiatr. 2019, 77, 106–114. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Zhang, Q.; Shan, Y.; Gu, W.; Wang, S. Design, Synthesis and Biological Evaluation of Novel β-Pinene-Based Thiazole Derivatives as Potential Anticancer Agents via Mitochondrial-Mediated Apoptosis Pathway. Bioorg. Chem. 2019, 84, 468–477. [Google Scholar] [CrossRef]
- Liao, S.; Shang, S.; Shen, M.; Rao, X.; Si, H.; Song, J.; Song, Z. One-Pot Synthesis and Antimicrobial Evaluation of Novel 3-Cyanopyridine Derivatives of (−)-β-Pinene. Bioorg. Med. Chem. Lett. 2016, 26, 1512–1515. [Google Scholar] [CrossRef]
- Felipe, C.F.B.; Albuquerque, A.M.S.; de Pontes, J.L.X.; de Melo, J.Í.V.; Rodrigues, T.C.M.L.; de Sousa, A.M.P.; Monteiro, Á.B.; Ribeiro, A.E.d.S.; Lopes, J.P.; de Menezes, I.R.A.; et al. Comparative Study of Alpha- and Beta-Pinene Effect on PTZ-Induced Convulsions in Mice. Fundam. Clin. Pharmacol. 2019, 33, 181–190. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Sinakos, Z.; Papageorgiou, V.P. Radical Scavenging Activity of Crocus sativus L. Extract and Its Bioactive Constituents. Phytother. Res. 2005, 19, 997–1000. [Google Scholar] [CrossRef]
- Pintado, C.; de Miguel, A.; Acevedo, O.; Nozal, L.; Novella, J.L.; Rotger, R. Bactericidal Effect of Saffron (Crocus sativus L.) on Salmonella enterica during Storage. Food Control 2011, 22, 638–642. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Talebzadeh, F. Anticonvulsant Evaluation of Safranal and Crocin from Crocus sativus in Mice. Fitoterapia 2005, 76, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Noraei, N.B. Anxiolytic and Hypnotic Effect of Crocus sativus Aqueous Extract and Its Constituents, Crocin and Safranal, in Mice. Phytother. Res. 2009, 23, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Kianbakht, S.; Mozaffari, K. Effects of Saffron and Its Active Constituents, Crocin and Safranal, on Prevention of Indomethacin Induced Gastric Ulcers in Diabetic and Nondiabetic Rats. JMPIR 2009, 8, 30–38. [Google Scholar]
- Rudbäck, J.; Bergström, M.A.; Börje, A.; Nilsson, U.; Karlberg, A.-T. α-Terpinene, an Antioxidant in Tea Tree Oil, Autoxidizes Rapidly to Skin Allergens on Air Exposure. Chem. Res. Toxicol. 2012, 25, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Grando, T.H.; Souza, C.F.; Gressler, L.T.; Stefani, L.M.; da Silva, A.S.; Monteiro, S.G. In Vitro and in Vivo Action of Terpinen-4-Ol, γ-Terpinene, and α-Terpinene against Trypanosoma evansi. Exp. Parasitol. 2016, 162, 43–48. [Google Scholar] [CrossRef]
- Fadel, H.; Kebbi, S.; Chalchat, J.-C.; Figueredo, G.; Chalard, P.; Benayache, F.; Ghedadba, N.; Benayache, S. Identification of Volatile Components and Antioxidant Assessment of the Aerial Part Extracts from an Algerian Cistus albidus L. of the Aures Region. J. New Technol. Mater. 2020, 10, 38–46. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a Natural Monoterpene: A Review of Its Biological Properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Couladis, M.; Chinou, I.B.; Tzakou, O.; Petrakis, P.V. Composition and Antimicrobial Activity of the Essential Oil Of Hypericum rumeliacum Subsp.Apollinis (Boiss. & Heldr.). Phytother. Res. 2003, 17, 152–154. [Google Scholar] [CrossRef]
- Souza, R.; Cardoso, M.; Menezes, C.; Silva, J.; De Sousa, D.; Batista, J. Gastroprotective Activity of α-Terpineol in Two Experimental Models of Gastric Ulcer in Rats. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2011, 19, 277–281. [Google Scholar]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Jagadeesh, G.S.; Selvaraj, P. Thymol, a Dietary Monoterpene Phenol Abrogates Mitochondrial Dysfunction in β-Adrenergic Agonist Induced Myocardial Infarcted Rats by Inhibiting Oxidative Stress. Chem. Biol. Interact. 2016, 244, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Pari, L. Role of Thymol on Hyperglycemia and Hyperlipidemia in High Fat Diet-Induced Type 2 Diabetic C57BL/6J Mice. Eur. J. Pharmacol. 2015, 761, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.S.; Bomfim, R.R.; Jesus, H.C.R.; Alves, P.B.; Blank, A.F.; Estevam, C.S.; Antoniolli, A.R.; Thomazzi, S.M. Evaluation of the Analgesic and Anti-Inflammatory Effects of the Essential Oil of Lippia gracilis Leaves. J. Ethnopharmacol. 2010, 129, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Bukovská, A.; Cikoš, Š.; Juhás, Š.; Il’ková, G.; Rehák, P.; Koppel, J. Effects of a Combination of Thyme and Oregano Essential Oils on TNBS-Induced Colitis in Mice. Mediators Inflamm. 2007, 1–9. [Google Scholar] [CrossRef]
- Bhandari, S.S.; Kabra, M.P. To Evaluate Anti-Anxiety Activity of Thymol. J. Acute Dis. 2014, 3, 136–140. [Google Scholar] [CrossRef]
- López-Carbonell, M.; Gabasa, M.; Jáuregui, O. Enhanced Determination of Abscisic Acid (ABA) and Abscisic Acid Glucose Ester (ABA-GE) in Cistus albidus Plants by Liquid Chromatography-Mass Spectrometry in Tandem Mode. Plant Physiol. Biochem. 2009, 47, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Llorca, M.; Caselles, V.; Müller, M.; Munné-Bosch, S. The Threshold between Life and Death in Cistus albidus L. Seedlings: Mechanisms Underlying Drought Tolerance and Resilience. Tree Physiol. 2021, 41, 1861–1876. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Skoneczka, J.; Kingston, D.G.J.; Krishnan, A.; Misyak, S.A.; Guri, A.J.; Pereira, A.; Carter, A.B.; Minorsky, P.; Tumarkin, R.; et al. Mechanisms of Action and Medicinal Applications of Abscisic Acid. Curr. Med. Chem. 2010, 17, 467–478. [Google Scholar] [CrossRef]
- Mollashahi, M.; Abbasnejad, M.; Esmaeili-Mahani, S. Phytohormone Abscisic Acid Elicits Antinociceptive Effects in Rats through the Activation of Opioid and Peroxisome Proliferator-Activated Receptors β/δ. Eur. J. Pharmacol. 2018, 832, 75–80. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic Properties of the Terpenoids Aromadendrene and 1,8-Cineole from the Essential Oil of Eucalyptus globulus against Antibiotic-Susceptible and Antibiotic-Resistant Pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Yeo, S.K.; Ali, A.Y.; Hayward, O.A.; Turnham, D.; Jackson, T.; Bowen, I.D.; Clarkson, R. β-Bisabolene, a Sesquiterpene from the Essential Oil Extract of Opoponax (Commiphora guidottii), Exhibits Cytotoxicity in Breast Cancer Cell Lines: Anti-tumour properties of β-bisabolene. Phytother. Res. 2016, 30, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Singh, M.; Dubey, V.; Srivastava, S.; Luqman, S.; Bawankule, D.U. α-(-)-Bisabolol Reduces pro-Inflammatory Cytokine Production and Ameliorates Skin Inflammation. Curr. Pharm. Biotechnol. 2014, 15, 173–181. [Google Scholar] [CrossRef] [PubMed]
- van Zyl, R.L.; Seatlholo, S.T.; van Vuuren, S.F.; Viljoen, A.M. The Biological Activities of 20 Nature Identical Essential Oil Constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Seki, T.; Kokuryo, T.; Yokoyama, Y.; Suzuki, H.; Itatsu, K.; Nakagawa, A.; Mizutani, T.; Miyake, T.; Uno, M.; Yamauchi, K.; et al. Antitumor Effects of α-Bisabolol against Pancreatic Cancer. Cancer Sci. 2011, 102, 2199–2205. [Google Scholar] [CrossRef]
- Ho, C.-L.; Liao, P.-C.; Wang, E.I.-C.; Su, Y.-C. Composition and Antifungal Activities of the Leaf Essential Oil of Neolitsea parvigemma from Taiwan. Nat. Prod. Commun. 2011, 6, 1357–1360. [Google Scholar] [CrossRef]
- Mastino, P.M.; Marchetti, M.; Costa, J.; Usai, M. Comparison of Essential Oils from Cistus Species Growing in Sardinia. Nat. Prod. Res. 2017, 31, 299–307. [Google Scholar] [CrossRef]
- Takei, M.; Umeyama, A.; Arihara, S. T-Cadinol and Calamenene Induce Dendritic Cells from Human Monocytes and Drive Th1 Polarization. Eur. J. Pharmacol. 2006, 537, 190–199. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a Phytocannabinoid Attenuates Oxidative Stress, Neuroinflammation, Glial Activation, and Salvages Dopaminergic Neurons in a Rat Model of Parkinson Disease. Mol. Cell. Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef]
- Bahi, A.; Al Mansouri, S.; Al Memari, E.; Al Ameri, M.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 Receptor Agonist Produces Multiple Behavioral Changes Relevant to Anxiety and Depression in Mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef]
- de Oliveira, C.C.; de Oliveira, C.V.; Grigoletto, J.; Ribeiro, L.R.; Funck, V.R.; Grauncke, A.C.B.; de Souza, T.L.; Souto, N.S.; Furian, A.F.; Menezes, I.R.A.; et al. Anticonvulsant Activity of β-Caryophyllene against Pentylenetetrazol-Induced Seizures. Epilepsy Behav. 2016, 56, 26–31. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-Caryophyllene and β-Caryophyllene Oxide-Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Türkez, H.; Çelik, K.; Toğar, B. Effects of Copaene, a Tricyclic Sesquiterpene, on Human Lymphocytes Cells in Vitro. Cytotechnology 2014, 66, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Togar, B.; Tatar, A. Tricyclic Sesquiterpene Copaene Prevents H2O2-Induced Neurotoxicity. J. Intercult. Ethnopharmacol. 2014, 3, 21. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, S.J.; Park, N.J.; Joo, W.H.; Lee, S.-J.; Choi, Y.-W. α-Iso-Cubebene Exerts Neuroprotective Effects in Amyloid Beta Stimulated Microglia Activation. Neurosci. Lett. 2013, 555, 143–148. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, S.D.; Lee, H.Y.; Baek, S.-H.; Ko, M.J.; Son, B.G.; Park, S.; Choi, Y.W.; Bae, Y.-S. α-Iso-Cubebene, a Natural Compound Isolated from Schisandra chinensis Fruit, Has Therapeutic Benefit against Polymicrobial Sepsis. Biochem. Biophys. Res. Commun. 2012, 426, 226–231. [Google Scholar] [CrossRef]
- Baek, S.E.; Jang, E.J.; Choi, J.M.; Choi, Y.W.; Kim, C.D. α-Iso-Cubebene Attenuates Neointima Formation by Inhibiting HMGB1-Induced Monocyte to Macrophage Differentiation via Suppressing ROS Production. Int. Immunopharmacol. 2022, 111, 109121. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, G.; Almanza, G.R.; Cheng, Y.; Peng, J.; Hamann, M.; Duan, R.-D.; Åkesson, B. Antiproliferative Effects of Curcuphenol, a Sesquiterpene Phenol. Fitoterapia 2010, 81, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Yao, C.; Zhu, J.; Xie, Y.; Ye, X.-Y.; Bai, R.; Xie, T. Anti-Tumor Drug Discovery Based on Natural Product β-Elemene: Anti-Tumor Mechanisms and Structural Modification. Molecules 2021, 26, 1499. [Google Scholar] [CrossRef]
- Asakura, K.; Matsuo, Y.; Oshima, T.; Kihara, T.; Minagawa, K.; Araki, Y.; Kagawa, K.; Kanemasa, T.; Ninomiya, M. ω-Agatoxin IVA-Sensitive Ca2+ Channel Blocker, α-Eudesmol, Protects against Brain Injury after Focal Ischemia in Rats. Eur. J. Pharmacol. 2000, 394, 57–65. [Google Scholar] [CrossRef]
- Moon, P.-D.; Han, N.-R.; Lee, J.S.; Kim, H.-Y.; Hong, S.; Kim, H.-J.; Yoo, M.-S.; Kim, H.-M.; Jeong, H.-J. β-Eudesmol Inhibits Thymic Stromal Lymphopoietin through Blockade of Caspase-1/NF-ΚB Signal Cascade in Allergic Rhinitis Murine Model. Chem. Biol. Interact. 2018, 294, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Narahara, C.; Saeheng, T.; Chaijaroenkul, W.; Dumre, S.P.; Na-Bangchang, K.; Karbwang, J. β-Eudesmol Induces the Expression of Apoptosis Pathway Proteins in Cholangiocarcinoma Cell Lines. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2020, 25, 7. [Google Scholar] [CrossRef]
- Dhyani, P.; Sati, P.; Sharma, E.; Attri, D.C.; Bahukhandi, A.; Tynybekov, B.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Suleria, H.A.R.; et al. Sesquiterpenoid Lactones as Potential Anti-Cancer Agents: An Update on Molecular Mechanisms and Recent Studies. Cancer Cell Int. 2022, 22, 305. [Google Scholar] [CrossRef] [PubMed]
- Guedes, J.B.; do Nascimento, A.L.; Costa, W.K.; de Veras, B.O.; de Aguiar, J.C.R.d.O.F.; Navarro, D.M.d.A.F.; Napoleão, T.H.; da Silva, M.V.; de Oliveira, A.M.; Correia, M.T.d.S. Eugenia gracillima Essential Oil Has Pharmaceutical Applications in Pain and Inflammation without Toxic Effects in Mice. J. Ethnopharmacol. 2023, 303, 115941. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, S.; Bruno, M.; Bramucci, M.; Quassinti, L.; Lupidi, G.; Fiorini, D.; Maggi, F. Kundmannia sicula (L.) DC: A Rich Source of Germacrene D. J. Essent. Oil Res. 2017, 29, 437–442. [Google Scholar] [CrossRef]
- Mendes De Lacerda Leite, G.; De Oliveira Barbosa, M.; Pereira Lopes, M.J.; De Araújo Delmondes, G.; Bezerra, D.S.; Araújo, I.M.; Carvalho De Alencar, C.D.; Melo Coutinho, H.D.; Peixoto, L.R.; Barbosa-Filho, J.M.; et al. Pharmacological and Toxicological Activities of α-Humulene and Its Isomers: A Systematic Review. Trends Food Sci. Technol. 2021, 115, 255–274. [Google Scholar] [CrossRef]
- Sabir, U.; Irfan, H.M.; Alamgeer Ullah, A.; Althobaiti, Y.S.; Alshehri, F.S.; Niazi, Z.R. Downregulation of Hepatic Fat Accumulation, Inflammation and Fibrosis by Nerolidol in Purpose Built Western-Diet-Induced Multiple-Hit Pathogenesis of NASH Animal Model. Biomed. Pharmacother. 2022, 150, 112956. [Google Scholar] [CrossRef]
- Dong, J.-R.; Chang, W.-W.; Chen, S.-M. Nerolidol Inhibits Proliferation of Leiomyoma Cells via Reactive Oxygen Species-Induced DNA Damage and Downregulation of the ATM/Akt Pathway. Phytochemistry 2021, 191, 112901. [Google Scholar] [CrossRef]
- Misra, B.B.; Dey, S. Evaluation of in Vivo Anti-Hyperglycemic and Antioxidant Potentials of α-Santalol and Sandalwood Oil. Phytomedicine 2013, 20, 409–416. [Google Scholar] [CrossRef]
- Garlet, Q.I.; Souza, C.F.; Rodrigues, P.; Descovi, S.N.; Martinez-Rodríguez, G.; Baldisserotto, B.; Heinzmann, B.M. GABAa Receptor Subunits Expression in Silver Catfish (Rhamdia Quelen) Brain and Its Modulation by Nectandra grandiflora Nees Essential Oil and Isolated Compounds. Behav. Brain Res. 2019, 376, 112178. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Prasad, S.; Yuan, W.; Li, S.; Aggarwal, B.B. Identification of a Novel Compound (β-Sesquiphellandrene) from Turmeric (Curcuma longa) with Anticancer Potential: Comparison with Curcumin. Investig. New Drugs 2015, 33, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, J.; Yang, B.; Lv, G.-P.; Li, S.-P. Free Radical Scavenging Activity and Characterization of Sesquiterpenoids in Four Species of Curcuma Using a TLC Bioautography Assay and GC-MS Analysis. Molecules 2010, 15, 7547–7557. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.G.; Xavier-Júnior, F.H.; Barros, M.R.; Menezes, T.M.; de Assis, C.R.D.; de Melo, A.C.G.R.; Veras, B.O.; Ferraz, V.P.; Filho, A.A.M.; Yogui, G.T.; et al. Impact on Cholinesterase-Inhibition and in Silico Investigations of Sesquiterpenoids from Amazonian Siparuna guianensis Aubl. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 252, 119511. [Google Scholar] [CrossRef] [PubMed]
- Manjima, R.B.; Ramya, S.; Kavithaa, K.; Paulpandi, M.; Saranya, T.; Harysh Winster, S.B.; Balachandar, V.; Arul, N. Spathulenol Attenuates 6-Hydroxydopamine Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Gene Rep. 2021, 25, 101396. [Google Scholar] [CrossRef]
- do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.d.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, Anti-Inflammatory, Antiproliferative and Antimycobacterial Activities of the Essential Oil of Psidium guineense Sw. and Spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- de Matos Balsalobre, N.; dos Santos, E.; Mariano dos Santos, S.; Arena, A.C.; Konkiewitz, E.C.; Ziff, E.B.; Nazari Formagio, A.S.; Leite Kassuya, C.A. Potential Anti-Arthritic and Analgesic Properties of Essential Oil and Viridiflorol Obtained from Allophylus edulis Leaves in Mice. J. Ethnopharmacol. 2023, 301, 115785. [Google Scholar] [CrossRef]
- Akiel, M.A.; Alshehri, O.Y.; Aljihani, S.A.; Almuaysib, A.; Bader, A.; Al-Asmari, A.I.; Alamri, H.S.; Alrfaei, B.M.; Halwani, M.A. Viridiflorol Induces Anti-Neoplastic Effects on Breast, Lung, and Brain Cancer Cells through Apoptosis. Saudi J. Biol. Sci. 2022, 29, 816–821. [Google Scholar] [CrossRef]
- Trevizan, L.N.F.; Nascimento, K.F.d.; Santos, J.A.; Kassuya, C.A.L.; Cardoso, C.A.L.; Vieira, M.d.C.; Moreira, F.M.F.; Croda, J.; Formagio, A.S.N. Anti-Inflammatory, Antioxidant and Anti-Mycobacterium tuberculosis Activity of Viridiflorol: The Major Constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. J. Ethnopharmacol. 2016, 192, 510–515. [Google Scholar] [CrossRef]
- Oon, S.F.; Nallappan, M.; Tee, T.T.; Shohaimi, S.; Kassim, N.K.; Sa’ariwijaya, M.S.F.; Cheah, Y.H. Xanthorrhizol: A Review of Its Pharmacological Activities and Anticancer Properties. Cancer Cell Int. 2015, 15, 100. [Google Scholar] [CrossRef]
- Mordukhova, E.A.; Kim, D.; Kim, W.-G.; Hwang, J.-K.; Pan, J.-G. The Food-Grade Antimicrobial Xanthorrhizol Targets the Enoyl-ACP Reductase (FabI) in Escherichia coli. Bioorg. Med. Chem. Lett. 2020, 30, 127651. [Google Scholar] [CrossRef]
- Oon, S.F.; Nallappan, M.; Kassim, N.K.; Shohaimi, S.; Sa’ariwijaya, M.S.F.; Tee, T.T.; Cheah, Y.H. Hypolipidemic Activities of Xanthorrhizol Purified from Centrifugal TLC. Biochem. Biophys. Res. Commun. 2016, 478, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Hwang, J.-K.; Chun, H.S. Xanthorrhizol Attenuates Dextran Sulfate Sodium-Induced Colitis via the Modulation of the Expression of Inflammatory Genes in Mice. Life Sci. 2011, 88, 864–870. [Google Scholar] [CrossRef]
- Borgonetti, V.; Governa, P.; Manetti, F.; Galeotti, N. Zingiberene, a Non-Zinc-Binding Class I HDAC Inhibitor: A Novel Strategy for the Management of Neuropathic Pain. Phytomedicine 2023, 111, 154670. [Google Scholar] [CrossRef]
- Bou, D.; Lago, J.; Figueiredo, C.; Matsuo, A.; Guadagnin, R.; Soares, M.; Sartorelli, P. Chemical Composition and Cytotoxicity Evaluation of Essential Oil from Leaves of Casearia sylvestris, Its Main Compound α-Zingiberene and Derivatives. Molecules 2013, 18, 9477–9487. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.A.; Silva, R.F.; de Moura, F.B.R.; Narduchi, C.T.; Deconte, S.R.; Sartorelli, P.; Tomiosso, T.C.; Lago, J.H.G.; Araújo, F. de A. α-Zingiberene, a Sesquiterpene from Essential Oil from Leaves of Casearia sylvestris, Suppresses Inflammatory Angiogenesis and Stimulates Collagen Deposition in Subcutaneous Implants in Mice. Nat. Prod. Res. 2022, 36, 5858–5862. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Esposito, A.; Degan, P.; Caicci, F.; Manni, L.; Liguori, A.; Bisio, A.; Iobbi, V.; Schito, A.; Traverso, C.E.; et al. The diterpene manool extracted from Salvia tingitana lowers free radical production in retinal rod outer segments by inhibiting the extramitochondrial F1 F0 ATP synthase. Cell Biochem. Funct. 2021, 39, 528–535. [Google Scholar] [CrossRef]
- Nicolella, H.D.; de Oliveira, P.F.; Munari, C.C.; Costa, G.F.D.; Moreira, M.R.; Veneziani, R.C.S.; Tavares, D.C. Differential Effect of Manool—A Diterpene from Salvia officinalis, on Genotoxicity Induced by Methyl Methanesulfonate in V79 and HepG2 Cells. Food Chem. Toxicol. 2014, 72, 8–12. [Google Scholar] [CrossRef]
- Monteiro, A.S.e.N.; Campos, D.R.; Albuquerque, A.A.S.; Evora, P.R.B.; Ferreira, L.G.; Celotto, A.C. Efeito Do Diterpeno Manool Sobre a Pressão Arterial e Reatividade Vascular Em Ratos Normotensos e Hipertensos. Arq. Bras. Cardiol. 2020, 115, 669–677. [Google Scholar] [CrossRef]
- Castro, C.H.; Pontes, C.N.R. Efeitos Cardiovasculares Do Diterpeno Manool Em Ratos Normotensos e Hipertensos. Arq. Bras. Cardiol. 2020, 115, 678–679. [Google Scholar] [CrossRef]
- Angelopoulou, D.; Demetzos, C.; Dimas, C.; Perdetzoglou, D.; Loukis, A. Essential Oils and Hexane Extracts from Leaves and Fruits of Cistus monspeliensis. Cytotoxic Activity of Ent-13-Epi-Manoyl Oxide and Its Isomers. Planta Med. 2001, 67, 168–171. [Google Scholar] [CrossRef]
- Kim, J.-H.; Na, H.-J.; Kim, C.-K.; Kim, J.-Y.; Ha, K.-S.; Lee, H.; Chung, H.-T.; Kwon, H.J.; Kwon, Y.-G.; Kim, Y.-M. The Non-Provitamin A Carotenoid, Lutein, Inhibits NF-KappaB-Dependent Gene Expression through Redox-Based Regulation of the Phosphatidylinositol 3-Kinase/PTEN/Akt and NF-KappaB-Inducing Kinase Pathways: Role of H(2)O(2) in NF-KappaB Activation. Free Radic. Biol. Med. 2008, 45, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-L.; Zhao, Y.-N.; Shi, Z.-Z.; Cong, D.; Bai, Y.-S. Lutein Inhibits Cell Growth and Activates Apoptosis via the PI3K/AKT/MTOR Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells. J. Environ. Pathol. Toxicol. Oncol. Off. 2018, 37, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, Y.; Niu, Q.; Xu, S.; Pang, L.; Ma, R.; Jing, M.; Feng, G.; Tang, J.X.; Zhang, Q.; et al. Lutein Has a Protective Effect on Hepatotoxicity Induced by Arsenic via Nrf2 Signaling. BioMed Res. Int. 2015, 2015, 315205. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Li, Z.; Ji, X.; Huang, J.; Zhang, H.; Jiang, C. The Protective Role of Lutein on Isoproterenol-Induced Cardiac Failure Rat Model through Improving Cardiac Morphology, Antioxidant Status via Positively Regulating Nrf2/HO-1 Signalling Pathway. Pharm. Biol. 2019, 57, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Nakai, M.; Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Suppressive Effect of Neoxanthin on the Differentiation of 3T3-L1 Adipose Cells. J. Oleo Sci. 2008, 57, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Teuscher, E. Biogene Arzneimittel: Lehrbuch der Pharmazeutischen Biologie; 2020; ISBN 978-3-8047-3607-8. Available online: https://www.amazon.de/Biogene-Arzneimittel-Lehrbuch-Pharmazeutischen-Biologie/dp/3804736076 (accessed on 10 August 2023).
- Tomás-Menor, L.; Morales-Soto, A.; Barrajón-Catalán, E.; Roldán-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the Antibacterial Activity and the Composition of Extracts Derived from Various Spanish Cistus Species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef]
- Sticher, O.; Heilmann, J.; Zündorf, I.; Hänsel, R.; Steinegger, E. Pharmakognosie—Phytopharmazie; 10., völlig neu Bearbeitete Auflage.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2015; ISBN 978-3-8047-3144-8. [Google Scholar]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie; Springer-Lehrbuch; Sechste, Vollständig Überarbeitete Auflage; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-73201-3. [Google Scholar]
- Qa’dan, F.; Petereit, F.; Nahrstedt, A. Prodelphinidin Trimers and Characterization of a Proanthocyanidin Oligomer from Cistus albidus. Pharmazie 2003, 58, 416–419. [Google Scholar]
- Wiesner, J.V. Die Rohstoffe des Pflanzenreiches; Verlag W. Engelmann: Leipzig, Berlin, Germany, 1921. [Google Scholar]
- Gonçalves, S.; Gomes, D.; Costa, P.; Romano, A. The Phenolic Content and Antioxidant Activity of Infusions from Mediterranean Medicinal Plants. Ind. Crops Prod. 2013, 43, 465–471. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Salgueiro, J.B.; Ardenghi, P.; Dias, M.; Ferreira, M.B.; Izquierdo, I.; Medina, J.H. Anxiolytic Natural and Synthetic Flavonoid Ligands of the Central Benzodiazepine Receptor Have No Effect on Memory Tasks in Rats. Pharmacol. Biochem. Behav. 1997, 58, 887–891. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Konieczny, P.; Majka, M. Caffeic Acid Expands Anti-Tumor Effect of Metformin in Human Metastatic Cervical Carcinoma HTB-34 Cells: Implications of AMPK Activation and Impairment of Fatty Acids De Novo Biosynthesis. Int. J. Mol. Sci. 2017, 18, 462. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic Acid: A Review of Its Potential Use in Medications and Cosmetics. Anal Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Lopes, R.; Costa, M.; Ferreira, M.; Gameiro, P.; Fernandes, S.; Catarino, C.; Santos-Silva, A.; Paiva-Martins, F. Caffeic Acid Phenolipids in the Protection of Cell Membranes from Oxidative Injuries. Interaction with the Membrane Phospholipid Bilayer. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183727. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-Protective and Antioxidant Properties of Caffeic Acid and Chlorogenic Acid: Mechanistic Role of Angiotensin Converting Enzyme, Cholinesterase and Arginase Activities in Cyclosporine Induced Hypertensive Rats. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Hong, C.-O.; Lee, G.P.; Kim, C.-T.; Lee, K.-W. The Hepatoprotection of Caffeic Acid and Rosmarinic Acid, Major Compounds of Perilla frutescens, against t-BHP-Induced Oxidative Liver Damage. Food Chem. Toxicol. 2013, 55, 92–99. [Google Scholar] [CrossRef]
- Kulkarni, N.P.; Vaidya, B.; Narula, A.S.; Sharma, S.S. Neuroprotective Potential of Caffeic Acid Phenethyl Ester (CAPE) in CNS Disorders: Mechanistic and Therapeutic Insights. Curr. Neuropharmacol. 2021, 19, 1401–1415. [Google Scholar] [CrossRef]
- Khaled, M.; Koriem, M. Caftaric Acid: An Overview on Its Structure, Daily Consumption, Bioavailability and Pharmacological Effects. Biointerface Res. Appl. Chem. 2020, 10, 5616–5623. [Google Scholar] [CrossRef]
- Gezer, C. Potential Health Effects of the Popular Compound of Artichoke: Cynarin (CYN). Prog. Nutr. 2017, 19. [Google Scholar] [CrossRef]
- Xia, N.; Pautz, A.; Wollscheid, U.; Reifenberg, G.; Förstermann, U.; Li, H. Artichoke, Cynarin and Cyanidin Downregulate the Expression of Inducible Nitric Oxide Synthase in Human Coronary Smooth Muscle Cells. Molecules 2014, 19, 3654–3668. [Google Scholar] [CrossRef]
- Sebastiani, G.; Almeida-Toledano, L.; Serra-Delgado, M.; Navarro-Tapia, E.; Sailer, S.; Valverde, O.; Garcia-Algar, O.; Andreu-Fernández, V. Therapeutic Effects of Catechins in Less Common Neurological and Neurodegenerative Disorders. Nutrients 2021, 13, 2232. [Google Scholar] [CrossRef] [PubMed]
- Jakubek, P.; Rajić, J.; Kuczyńska, M.; Suliborska, K.; Heldt, M.; Dziedziul, K.; Vidaković, M.; Namieśnik, J.; Bartoszek, A. Beyond Antioxidant Activity: Redox Properties of Catechins May Affect Changes in the DNA Methylation Profile—The Example of SRXN1 Gene. Antioxidants 2023, 12, 754. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Caleare, A.; Hensel, A.; Mello, J.C.P.; Pinha, A.B.; Panizzon, G.P.; Lechtenberg, M.; Petereit, F.; Nakamura, C.V. Flavan-3-Ols and Proanthocyanidins from Limonium brasiliense Inhibit the Adhesion of Porphyromonas gingivalis to Epithelial Host Cells by Interaction with Gingipains. Fitoterapia 2017, 118, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Pinheiro, P.G.; Santiago, G.M.P.; da Silva, F.E.F.; de Araújo, A.C.J.; de Oliveira, C.R.T.; Freitas, P.R.; Rocha, J.E.; Neto, J.B.d.A.; da Silva, M.M.C.; Tintino, S.R.; et al. Ferulic Acid Derivatives Inhibiting Staphylococcus aureus TetK and MsrA Efflux Pumps. Biotechnol. Rep. 2022, 34, e00717. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Qiu, W.; Shi, Y. Ferulic Acid Exhibits Anti-Inflammatory Effects by Inducing Autophagy and Blocking NLRP3 Inflammasome Activation. Mol. Cell. Toxicol. 2022, 18, 509–519. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic Acid Exerts Neuroprotective Effects against Cerebral Ischemia/Reperfusion-Induced Injury via Antioxidant and Anti-Apoptotic Mechanisms in Vitro and in Vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Ferulic Acid From Plant Biomass: A Phytochemical With Promising Antiviral Properties. Front. Nutr. 2021, 8, 777576. [Google Scholar] [CrossRef]
- Lee, C.-C.; Wang, C.-C.; Huang, H.-M.; Lin, C.-L.; Leu, S.-J.; Lee, Y.-L. Ferulic Acid Induces Th1 Responses by Modulating the Function of Dendritic Cells and Ameliorates Th2-Mediated Allergic Airway Inflammation in Mice. Evid.-Based Complement. Altern. Med. 2015, 2015, 678487. [Google Scholar] [CrossRef]
- Esmat, M.A.; Osman, A.; Hassan, R.E.; Hagag, S.A.; El-Maghraby, T.K. Hepatoprotective Effect of Ferulic Acid and/or Low Doses of γ-Irradiation against Cisplatin-Induced Liver Injury in Rats. Hum. Exp. Toxicol. 2022, 41, 9603271221136204. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yu, H.; Guo, W.; Kong, Y.; Gu, L.; Li, Q.; Yang, S.; Zhang, Y.; Wang, Y. The Anticancer Effects of Ferulic Acid Is Associated with Induction of Cell Cycle Arrest and Autophagy in Cervical Cancer Cells. Cancer Cell Int. 2018, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Park, J.-K.; Kim, K.-M.; Lee, H.-J.; Kim, S. In Vitro and in Vivo Antithrombotic and Cytotoxicity Effects of Ferulic Acid. J. Biochem. Mol. Toxicol. 2018, 32, e22004. [Google Scholar] [CrossRef]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic Acid Exerts Its Antidiabetic Effect by Modulating Insulin-Signalling Molecules in the Liver of High-Fat Diet and Fructose-Induced Type-2 Diabetic Adult Male Rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef]
- Kroes, B.H.; van den Berg, A.J.; Quarles van Ufford, H.C.; van Dijk, H.; Labadie, R.P. Anti-Inflammatory Activity of Gallic Acid. Planta Med. 1992, 58, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Ojeaburu, S.I.; Oriakhi, K. Hepatoprotective, Antioxidant and, Anti-Inflammatory Potentials of Gallic Acid in Carbon Tetrachloride-Induced Hepatic Damage in Wistar Rats. Toxicol. Rep. 2021, 8, 177–185. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, L.; Wu, P.; Li, W.; Li, T.; Gu, R.; Dan, X.; Li, Z.; Fan, X.; Xiao, Z. Gallic Acid Has Anticancer Activity and Enhances the Anticancer Effects of Cisplatin in Non-small Cell Lung Cancer A549 Cells via the JAK/STAT3 Signaling Pathway. Oncol. Rep. 2019, 41, 1779–1788. [Google Scholar] [CrossRef]
- Li, Z.-J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.-G.; Aibai, S. Antifungal Activity of Gallic Acid In Vitro and In Vivo. Phytother. Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Kim, C.Y.; Lee, J.S.; Kim, T.G.; Kim, S.H.; Lee, C.-K.; Lee, B.-B.; Shin, C.-G.; Huh, H.; Kim, J. Inhibition of HIV-1 Integrase by Galloyl Glucoses from Terminalia chebula and Flavonol Glycoside Gallates from Euphorbia pekinensis. Planta Med. 2002, 68, 457–459. [Google Scholar] [CrossRef]
- Khan, A.N.; Singh, R.; Bhattacharya, A.; Chakravarti, R.; Roy, S.; Ravichandiran, V.; Ghosh, D. A Short Review on Glucogallin and Its Pharmacological Activities. Mini-Rev. Med. Chem. 2022, 22, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Lakshmanapermalsamy, P.; Illuri, R.; Bhosle, D.; Sangli, G.K.; Mundkinajeddu, D. In Vitro Evaluation of Antioxidant Potential of Isolated Compounds and Various Extracts of Peel of Punica Granatum L. Pharmacogn. Res. 2018, 10, 44–48. [Google Scholar]
- Habtemariam, S. α-Glucosidase Inhibitory Activity of Kaempferol-3-O-Rutinoside. Nat. Prod. Commun. 2011, 6, 1934578X1100600. [Google Scholar] [CrossRef]
- Silva dos Santos, J.; Gonçalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the Anti-Inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A Comprehensive Review on Its Biological Potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Oliveira, H.; Wu, N.; Zhang, Q.; Wang, J.; Oliveira, J.; de Freitas, V.; Mateus, N.; He, J.; Fernandes, I. Bioavailability Studies and Anticancer Properties of Malvidin Based Anthocyanins, Pyranoanthocyanins and Non-Oxonium Derivatives. Food Funct. 2016, 7, 2462–2468. [Google Scholar] [CrossRef]
- Shih, P.-H.; Wu, C.-H.; Yeh, C.-T.; Yen, G.-C. Protective Effects of Anthocyanins against Amyloid β-Peptide-Induced Damage in Neuro-2A Cells. J. Agric. Food Chem. 2011, 59, 1683–1689. [Google Scholar] [CrossRef]
- Decendit, A.; Mamani-Matsuda, M.; Aumont, V.; Waffo-Teguo, P.; Moynet, D.; Boniface, K.; Richard, E.; Krisa, S.; Rambert, J.; Mérillon, J.-M.; et al. Malvidin-3-O-β Glucoside, Major Grape Anthocyanin, Inhibits Human Macrophage-Derived Inflammatory Mediators and Decreases Clinical Scores in Arthritic Rats. Biochem. Pharmacol. 2013, 86, 1461–1467. [Google Scholar] [CrossRef]
- Rossetto, M.; Vanzani, P.; Mattivi, F.; Lunelli, M.; Scarpa, M.; Rigo, A. Synergistic Antioxidant Effect of Catechin and Malvidin 3-Glucoside on Free Radical-Initiated Peroxidation of Linoleic Acid in Micelles. Arch. Biochem. Biophys. 2002, 408, 239–245. [Google Scholar] [CrossRef]
- Kim, M.; Yin, J.; Hwang, I.H.; Park, D.H.; Lee, E.K.; Kim, M.J.; Lee, M.W. Anti-Acne Vulgaris Effects of Pedunculagin from the Leaves of Quercus mongolica by Anti-Inflammatory Activity and 5α-Reductase Inhibition. Molecules. 2020, 25, 2154. [Google Scholar] [CrossRef]
- Chang, J.H.; Cho, J.H.; Kim, H.H.; Lee, K.P.; Lee, M.W.; Han, S.S.; Lee, D.I. Antitumor Activity of Pedunculagin, One of the Ellagitannin. Arch. Pharm. Res. 1995, 18, 396–401. [Google Scholar] [CrossRef]
- Silva Fernandes, A.; Hollanda Véras, J.; Silva, L.S.; Puga, S.C.; Luiz Cardoso Bailão, E.F.; de Oliveira, M.G.; Cardoso, C.G.; Carneiro, C.C.; Costa Santos, S.D.; Chen-Chen, L. Pedunculagin Isolated from Plinia cauliflora Seeds Exhibits Genotoxic, Antigenotoxic and Cytotoxic Effects in Bacteria and Human Lymphocytes. J. Toxicol. Environ. Health A 2022, 85, 353–363. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations. Int. J. Mol. Sci. 2021, 22, 11076. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Deng, Z.; Chen, F.; Zheng, L.; Pan, Y.; Xing, Q.; Tsao, R.; Li, H. Synergistic Antioxidant Effects of Petunidin and Lycopene in H9c2 Cells Submitted to Hydrogen Peroxide: Role of Akt/Nrf2 Pathway. J. Food Sci. 2020, 85, 1752–1763. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Gao, H.; Yuan, R.; Han, S.; Li, X.-X.; Tang, M.; Dong, B.; Li, J.-X.; Zhao, L.-C.; Feng, J.; et al. Procyanidin A2, a Polyphenolic Compound, Exerts Anti-Inflammatory and Anti-Oxidative Activity in Lipopolysaccharide-Stimulated RAW264.7 Cells. PLoS ONE 2020, 15, e0237017. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Matkowski, A.; Hadzik, J.; Dobrowolska-Czopor, B.; Olchowy, C.; Dominiak, M.; Kubasiewicz-Ross, P. Proanthocyanidins and Flavan-3-Ols in the Prevention and Treatment of Periodontitis-Antibacterial Effects. Nutrients 2021, 13, 165. [Google Scholar] [CrossRef]
- Choy, Y.Y.; Fraga, M.; Mackenzie, G.G.; Waterhouse, A.L.; Cremonini, E.; Oteiza, P.I. The PI3K/Akt Pathway Is Involved in Procyanidin-Mediated Suppression of Human Colorectal Cancer Cell Growth. Mol. Carcinog. 2016, 55, 2196–2209. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, C.; Yao, Q.; Qian, L.; Liu, J.; Xie, X.; Ma, W.; Nie, X.; Lai, B.; Xiao, L.; et al. Procyanidin B2 Activates PPARγ to Induce M2 Polarization in Mouse Macrophages. Front. Immunol. 2019, 10, 1895. [Google Scholar] [CrossRef]
- Jung, H.A.; Ali, M.Y.; Bhakta, H.K.; Min, B.-S.; Choi, J.S. Prunin Is a Highly Potent Flavonoid from Prunus davidiana Stems That Inhibits Protein Tyrosine Phosphatase 1B and Stimulates Glucose Uptake in Insulin-Resistant HepG2 Cells. Arch. Pharm. Res. 2017, 40, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Rozadi, N.; Oktavia, S.; Fauziah, F. Pharmacological Activities of Punicalagin: A Review. J. Drug Deliv. Ther. 2022, 12, 148–155. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Quercetin and Its Role in Biological Functions: An Updated Review. EXCLI J. 2018, 17, 856–863. [Google Scholar] [CrossRef]
- Chimenti, F.; Cottiglia, F.; Bonsignore, L.; Casu, L.; Casu, M.; Floris, C.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A.; et al. Quercetin as the Active Principle of Hypericum hircinum Exerts a Selective Inhibitory Activity against MAO-A: Extraction, Biological Analysis, and Computational Study. J. Nat. Prod. 2006, 69, 945–949. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Rocha, M.F.G.; Sales, J.A.; da Rocha, M.G.; Galdino, L.M.; de Aguiar, L.; Pereira-Neto, W. de A.; de Aguiar Cordeiro, R.; Castelo-Branco, D. de S.C.M.; Sidrim, J.J.C.; Brilhante, R.S.N. Antifungal Effects of the Flavonoids Kaempferol and Quercetin: A Possible Alternative for the Control of Fungal Biofilms. Biofouling 2019, 35, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols Inhibit Proinflammatory Mediator Release, Intracellular Calcium Ion Levels and Protein Kinase C Theta Phosphorylation in Human Mast Cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A Combination of Quercetin and Resveratrol Reduces Obesity in High-Fat Diet-Fed Rats by Modulation of Gut Microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- Cinkilic, N.; Cetintas, S.K.; Zorlu, T.; Vatan, O.; Yilmaz, D.; Cavas, T.; Tunc, S.; Ozkan, L.; Bilaloglu, R. Radioprotection by Two Phenolic Compounds: Chlorogenic and Quinic Acid, on X-Ray Induced DNA Damage in Human Blood Lymphocytes in Vitro. Food Chem. Toxicol. 2013, 53, 359–363. [Google Scholar] [CrossRef]
- Hur, J.Y.; Soh, Y.; Kim, B.H.; Suk, K.; Sohn, N.W.; Kim, H.C.; Kwon, H.C.; Lee, K.R.; Kim, S.Y. Neuroprotective and Neurotrophic Effects of Quinic Acids from Aster scaber in PC12 Cells. Biol. Pharm. Bull. 2001, 24, 921–924. [Google Scholar] [CrossRef]
- Jang, S.-A.; Park, D.W.; Kwon, J.E.; Song, H.S.; Park, B.; Jeon, H.; Sohn, E.-H.; Koo, H.J.; Kang, S.C. Quinic Acid Inhibits Vascular Inflammation in TNF-α-Stimulated Vascular Smooth Muscle Cells. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 96, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Zanello, P.R.; Koishi, A.C.; Rezende Júnior, C.d.O.; Oliveira, L.A.; Pereira, A.A.; de Almeida, M.V.; Duarte dos Santos, C.N.; Bordignon, J. Quinic Acid Derivatives Inhibit Dengue Virus Replication in Vitro. Virol. J. 2015, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic Acids: Chemistry, Biosynthesis, Occurrence, Analytical Challenges, and Bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yu, T.; Han, S.; Chen, X.; Wang, K.; Gao, P.; Li, L. Caffeoylquinic Acids Protect against Alcoholic Liver Injury and Rare C10 Acetylenic Acids from Erigeron Breviscapus. Nat. Prod. Res. 2023, 1–6. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Jnawali, H.N.; Jeon, D.; Jeong, M.-C.; Lee, E.; Jin, B.; Ryoo, S.; Yoo, J.; Jung, I.D.; Lee, S.J.; Park, Y.-M.; et al. Antituberculosis Activity of a Naturally Occurring Flavonoid, Isorhamnetin. J. Nat. Prod. 2016, 79, 961–969. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Tang, L.; Xiang, H.; Sun, Y.; Qiu, L.; Chen, D.; Deng, C.; Chen, W. Monopalmityloxy Shikimic Acid: Enzymatic Synthesis and Anticoagulation Activity Evaluation. Appl. Biochem. Biotechnol. 2009, 158, 408–415. [Google Scholar] [CrossRef]
- Veach, D.; Hosking, H.; Thompson, K.; Santhakumar, A.B. Anti-Platelet and Anti-Thrombogenic Effects of Shikimic Acid in Sedentary Population. Food Funct. 2016, 7, 3609–3616. [Google Scholar] [CrossRef]
- Fabre, N.; Urizzi, P.; Souchard, J.P.; Fréchard, A.; Claparols, C.; Fourasté, I.; Moulis, C. An Antioxidant Sinapic Acid Ester Isolated from Iberis Amara. Fitoterapia 2000, 71, 425–428. [Google Scholar] [CrossRef]
- Legrum, W. Riechstoffe, Zwischen Gestank und Duft: Vorkommen, Eigenschaften und Anwendung von Riechstoffen und deren Gemischen; Studienbücher Chemie; 1. Aufl.; Vieweg + Teubner: Wiesbaden, Germany, 2011; ISBN 978-3-8348-1245-2. [Google Scholar]
- Fahlbusch, K.-G.; Hammerschmidt, F.-J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. Flavors and Fragrances. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA, Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; p. a11_141. ISBN 978-3-527-30673-2. [Google Scholar]
- Fenaroli, G.; Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 4th ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-0-8493-0946-5. [Google Scholar]
- Oñate, M.; Munné-Bosch, S. Loss of Flower Bud Vigour in the Mediterranean Shrub, Cistus albidus L. at Advanced Developmental Stages. Plant Biol. 2010, 12, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Jubany-Mari, T.; Munne-Bosch, S.; Lopez-Carbonell, M.; Alegre, L. Hydrogen Peroxide Is Involved in the Acclimation of the Mediterranean Shrub, Cistus albidus L., to Summer Drought. J. Exp. Bot. 2008, 60, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Epimerisation of Catechins in Green Tea Infusions. Food Chem. 2000, 70, 337–344. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Jiang, X. Reaction Kinetics of Degradation and Epimerization of Epigallocatechin Gallate (EGCG) in Aqueous System over a Wide Temperature Range. J. Agric. Food Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Saklar, S.; Ertas, E.; Ozdemir, I.S.; Karadeniz, B. Effects of Different Brewing Conditions on Catechin Content and Sensory Acceptance in Turkish Green Tea Infusions. J. Food Sci. Technol. 2015, 52, 6639–6646. [Google Scholar] [CrossRef]
- Alami Merrouni, I.; Kharchoufa, L.; Bencheikh, N.; Elachouri, M. Ethnobotanical Profile of Medicinal Plants Used by People of North-Eastern Morocco: Cross-Cultural and Historical Approach (Part I). Ethnobot. Res. Appl. 2021, 21, 1–45. [Google Scholar]
- Las Plantas En La Cultura Popular de La Provincia de Albacete; Fajardo, J.; Verde, A.; Rivera, D.; Obón, C. (Eds.) Serie I—Estudios; Instituto de Estudios Albacetenses “Don Juan Manuel” de la Excma; Diputacioń de Albacete: Albacete, Spain, 2000; ISBN 978-84-95394-08-8. [Google Scholar]
- Ajjoun, M.; Kharchoufa, L.; Alami Merrouni, I.; Elachouri, M. Moroccan Medicinal Plants Traditionally Used for the Treatment of Skin Diseases: From Ethnobotany to Clinical Trials. J. Ethnopharmacol. 2022, 297, 115532. [Google Scholar] [CrossRef]
- Fernández Ocaña, A.M. Estudio Etnobotánico En El Parque Natural de Las Sierras de Cazorla, Segura y Las Villas; Investigación Química de Un Grupo de Especies Interesantes, Departamento de Biología Animal, Vegetal y Ecología, Facultad de Ciencias Experimentales, Universidad de Jaén: Jáen, Spain, 2000. [Google Scholar]
- Fiorini, D.; Molle, A.; Nabissi, M.; Santini, G.; Benelli, G.; Maggi, F. Valorizing Industrial Hemp (Cannabis sativa L.) by-Products: Cannabidiol Enrichment in the Inflorescence Essential Oil Optimizing Sample Pre-Treatment Prior to Distillation. Ind. Crops Prod. 2019, 128, 581–589. [Google Scholar] [CrossRef]
- Ross, S.A.; ElSohly, M.A. The Volatile Oil Composition of Fresh and Air-Dried Buds of Cannabis sativa. J. Nat. Prod. 1996, 59, 49–51. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Ghafoor, K. The Effect of Drying Temperatures on Antioxidant Activity, Phenolic Compounds, Fatty Acid Composition and Tocopherol Contents in Citrus Seed and Oils. J. Food Sci. Technol. 2018, 55, 190–197. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Sánchez-Moreno, C.; Saura-Calixto, F. Effect of Temperature on the Free Radical Scavenging Capacity of Extracts from Red and White Grape Pomace Peels. J. Agric. Food Chem. 1998, 46, 2694–2697. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A. Determination of Phenol Content and Antibacterial Activity of Five Medicinal Plants Ethanolic Extracts from North-West of Morocco. J. Plant Pathol. Microbiol. 2016, 7, 4. [Google Scholar] [CrossRef]
- Reverchon, E.; Senatore, F. Isolation of Rosemary Oil: Comparison between Hydrodistillation and Supercritical CO2 Extraction. Flavour Fragr. J. 1992, 7, 227–230. [Google Scholar] [CrossRef]
- He, X.; Yang, J.; Huang, Y.; Zhang, Y.; Wan, H.; Li, C. Green and Efficient Ultrasonic-Assisted Extraction of Bioactive Components from Salvia miltiorrhiza by Natural Deep Eutectic Solvents. Molecules 2019, 25, 140. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and Metabolism of Polyphenols in the Gut and Impact on Health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Thilakarathna, S.; Rupasinghe, H. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Failla, M.L.; Chitchumronchokchai, C.; Ferruzzi, M.G.; Goltz, S.R.; Campbell, W.W. Unsaturated Fatty Acids Promote Bioaccessibility and Basolateral Secretion of Carotenoids and α-Tocopherol by Caco-2 Cells. Food Funct. 2014, 5, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Ferri, P.; Angelino, D.; Gennari, L.; Benedetti, S.; Ambrogini, P.; Del Grande, P.; Ninfali, P. Enhancement of Flavonoid Ability to Cross the Blood–Brain Barrier of Rats by Co-Administration with α-Tocopherol. Food Funct. 2015, 6, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Z.; Lu, Y.; Du, S.-Y.; Shang, K.-X.; Cai, C.-B. Influence of Borneol and Muscone on Geniposide Transport through MDCK and MDCK-MDR1 Cells as Blood–Brain Barrier in Vitro Model. Int. J. Pharm. 2013, 456, 73–79. [Google Scholar] [CrossRef]
- Yin, Y.; Cao, L.; Ge, H.; Duanmu, W.; Tan, L.; Yuan, J.; Tunan, C.; Li, F.; Hu, R.; Gao, F.; et al. L-Borneol Induces Transient Opening of the Blood–Brain Barrier and Enhances the Therapeutic Effect of Cisplatin. NeuroReport 2017, 28, 506–513. [Google Scholar] [CrossRef]
- Yu, B.; Ruan, M.; Dong, X.; Yu, Y.; Cheng, H. The Mechanism of the Opening of the Blood–Brain Barrier by Borneol: A Pharmacodynamics and Pharmacokinetics Combination Study. J. Ethnopharmacol. 2013, 150, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, W.; Chen, L.; Ma, S.; Ping, L.; Yang, Z. Enhancement of Intestinal Absorption of Akebia Saponin D by Borneol and Probenecid in Situ and in Vitro. Environ. Toxicol. Pharmacol. 2010, 29, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz Ariza, F.J.; Le Houérou, H.-N. Flora Básica de la Región de Murcia; Sociedad Cooperativa de Enseñanza Severo Ochoa: Murcia, Spain, 2002; ISBN 978-84-600-9378-7. [Google Scholar]
- Stübing, G.; Peris, J.B. Plantas Medicinales de la Comunidad Valenciana; Generalitat Valenciana, Conselleria de Medio Ambiete: Valencia, Spain, 1998; ISBN 978-84-482-1805-8. [Google Scholar]
- Segarra i Durà, E. Etnobotànica Farmacèutica de Gàtova; Universitat de València: València, Spain, 2008; ISBN 978-84-370-6976-0. [Google Scholar]
- Ledesma, J. Estudio Del Uso Tradicional de Las Plantas Silvestres En La Sierra de Montsant; Trabajo Fin de Carrera, Escuela de Ingeniería Forestal de Lleida: Lleida, Spain, 2004. [Google Scholar]
- Carrió Cabrer, M.E. Contribució a l’etnobotànica de Mallorca; La Biodiversitat Vegetal i La Seva Gestió En Una Illa Mediterrània, Universidad de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Carrió, E.; Vallès, J. Ethnobotany of Medicinal Plants Used in Eastern Mallorca (Balearic Islands, Mediterranean Sea). J. Ethnopharmacol. 2012, 141, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Belda, A.; Zaragozí, B.; Belda, I.; Martínez, J.; Seva, E. Traditional Knowledge of Medicinal Plants in the Serra de Mariola Natural Park, South-Eastern Spain. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 299–309. [Google Scholar] [CrossRef]
- Martínez-Lirola, M.J.; González-Tejero, M.R.; Molero-Mesa, J. Ethnobotanical Resources in the Province of Almeria, Spain: Campos de Nijar. Econ. Bot. 1996, 50, 40–56. [Google Scholar] [CrossRef]
- Torres-Montes, F. Nombres y Usos Tradicionales de Las Plantas Silvestres En Almería (Estudio Linguistico y Etnografico); Diputación de Almería e Instituto de Estudios Almerienses: Almería, Spain, 2004; ISBN 978-84-8108-313-2. [Google Scholar]
- Alarcόn, R.; Pardo-de-Santayana, M.; Priestley, C.; Morales, R.; Heinrich, M. Medicinal and Local Food Plants in the South of Alava (Basque Country, Spain). J. Ethnopharmacol. 2015, 176, 207–224. [Google Scholar] [CrossRef]
- Ortuño Moya, I.M. Etnobotánica de Los Villares y Valdepeñas de Jaén (Sur de La Península Ibérica); Universidad de Jaén—Departamento de Biología Animal, Vegetal y Ecología, Facultad de Ciencias Experimentales: Jaén, Spain, 2004. [Google Scholar]
- Goicoetxea Marcaida, A. Etnobotánica y Medicina Popular en el País Vasco; Pastor: Madrid, Spain, 2017; ISBN 978-84-95461-61-2. [Google Scholar]
- González-Tejero García, M.R. Investigaciones Etnobotánicas En La Provincia de Granada; Departamento de Biología Vegetal, Facultad de Farmacia, Universidad de Granada: Granada, Spain, 1989. [Google Scholar]
- Rivera, D.; Alcaraz, F.; Verde, A.; Fajardo, J.; Obón, C. Las Plantas en la Cultura Popular; SOMEHN (Sociedad Mediterránea de Historia Natural): Murcia, Spain, 2008; ISBN 978-84-931786-0-4. [Google Scholar]
- Guzmán, A. Aproximación a La Etnobotánica de La Provincia de Jaén; Departamento de Biología Vegetal, Universidad de Granada: Granada, Spain, 1997. [Google Scholar]
- Atzei, A.D. Le Piante Nella Tradizione Popolare Della Sardegna: Documentazione Sugli Usi Alimentari, Aromatizzanti, Profumieri, Artigianali, Cosmetici, Medicinali, Veterinari, Magici, Ornamentali, Rituali, Religiosi, Tintori, Antiparassitari e Vari, Delle Piante; C. Delfino: Sassari, Italy, 2003; ISBN 978-88-7138-298-2. [Google Scholar]
- Rivera, D.; Robleda, A.; Obón, C.; Cano, F. Introducción al Mundo de Las Plantas Medicinales En Murcia, 1st ed.; Ayuntamiento de Murcia: Murcia, Spain, 1994; ISBN 978-84-606-1922-2. [Google Scholar]
- Pellicer, J. Costumari Botànic: Recerques Etnobotàniques a Les Comarques Centrals Valencianes; Farga monogràfica, 2nd ed.; Edicions del Bullent: Picanya, Spain, 2000; ISBN 978-84-96187-13-9. [Google Scholar]
- Ascari, J.; de Oliveira, M.S.; Nunes, D.S.; Granato, D.; Scharf, D.R.; Simionatto, E.; Otuki, M.; Soley, B.; Heiden, G. Chemical Composition, Antioxidant and Anti-Inflammatory Activities of the Essential Oils from Male and Female Specimens of Baccharis punctulata (Asteraceae). J. Ethnopharmacol. 2019, 234, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yatoo, M.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H.M.N. Anti-Inflammatory Drugs and Herbs with Special Emphasis on Herbal Medicines for Countering Inflammatory Diseases and Disorders—A Review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Viola, H.; Wasowski, C.; Levi de Stein, M.; Wolfman, C.; Silveira, R.; Dajas, F.; Medina, J.; Paladini, A. Apigenin, a Component of Matricaria recutita Flowers, Is a Central Benzodiazepine Receptors-Ligand with Anxiolytic Effects. Planta Med. 1995, 61, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Vissiennon, C.; Nieber, K.; Kelber, O.; Butterweck, V. Route of Administration Determines the Anxiolytic Activity of the Flavonols Kaempferol, Quercetin and Myricetin—Are They Prodrugs? J. Nutr. Biochem. 2012, 23, 733–740. [Google Scholar] [CrossRef]
- Sloley, B.D.; Urichuk, L.J.; Morley, P.; Durkin, J.; Shan, J.J.; Pang, P.K.; Coutts, R.T. Identification of Kaempferol as a Monoamine Oxidase Inhibitor and Potential Neuroprotectant in Extracts of Ginkgo biloba Leaves. J. Pharm. Pharmacol. 2000, 52, 451–459. [Google Scholar] [CrossRef]
- Coppin, J.P.; Xu, Y.; Chen, H.; Pan, M.-H.; Ho, C.-T.; Juliani, R.; Simon, J.E.; Wu, Q. Determination of Flavonoids by LC/MS and Anti-Inflammatory Activity in Moringa oleifera. J. Funct. Foods 2013, 5, 1892–1899. [Google Scholar] [CrossRef]
- Kuchta, K.; Tung, N.H.; Ohta, T.; Uto, T.; Raekiansyah, M.; Grötzinger, K.; Rausch, H.; Shoyama, Y.; Rauwald, H.W.; Morita, K. The Old Pharmaceutical Oleoresin Labdanum of Cistus creticus L. Exerts Pronounced in Vitro Anti-Dengue Virus Activity. J. Ethnopharmacol. 2020, 257, 112316. [Google Scholar] [CrossRef]
- Dimas, K.; Kokkinopoulos, D.; Demetzos, C.; Vaos, B.; Marselos, M.; Malamas, M.; Tzavaras, T. The Effect of Sclareol on Growth and Cell Cycle Progression of Human Leukemic Cell Lines. Leuk. Res. 1999, 23, 217–234. [Google Scholar] [CrossRef]
- Dimas, K.; Demetzos, C.; Marsellos, M.; Sotiriadou, R.; Malamas, M.; Kokkinopoulos, D. Cytotoxic Activity of Labdane Type Diterpenes against Human Leukemic Cell Lines in Vitro. Planta Med. 1998, 64, 208–211. [Google Scholar] [CrossRef]
- Dimas, K.; Demetzos, C.; Mitaku, S.; Vaos, B.; Marselos, M.; Tzavaras, T.; Kokkinopoulos, D. Cytotoxic Activity and Antiproliferative Effects of a New Semi-Synthetic Derivative of Ent-3 Beta-Hydroxy-13-Epi-Manoyl Oxide on Human Leukemic Cell Lines. Anticancer Res. 1999, 19, 4065–4072. [Google Scholar] [PubMed]
- Fujii, W.; Toda, K.; Matsumoto, K.; Kawaguchi, K.; Kawahara, S.; Hattori, Y.; Fujii, H.; Makabe, H. Syntheses of Prodelphinidin B1, B2, and B4 and Their Antitumor Activities against Human PC-3 Prostate Cancer Cell Lines. Tetrahedron Lett. 2013, 54, 7188–7192. [Google Scholar] [CrossRef]
- Kawahara, S.-I.; Ishihara, C.; Matsumoto, K.; Senga, S.; Kawaguchi, K.; Yamamoto, A.; Suwannachot, J.; Hamauzu, Y.; Makabe, H.; Fujii, H. Identification and Characterization of Oligomeric Proanthocyanidins with Significant Anti-Cancer Activity in Adzuki Beans (Vigna angularis). Heliyon 2019, 5, e02610. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P.C. Reversals of Age-Related Declines in Neuronal Signal Transduction, Cognitive, and Motor Behavioral Deficits with Blueberry, Spinach, or Strawberry Dietary Supplementation. J. Neurosci. 1999, 19, 8114–8121. [Google Scholar] [CrossRef]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The Effect of Flavanol-Rich Cocoa on the FMRI Response to a Cognitive Task in Healthy Young People. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl. S2), S215–S220. [Google Scholar] [CrossRef]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D.L. Cerebral Blood Flow Response to Flavanol-Rich Cocoa in Healthy Elderly Humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433–440. [Google Scholar]
- Gage, F.H. Adult Neurogenesis in Mammals. Science 2019, 364, 827–828. [Google Scholar] [CrossRef]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Aouadhi, C.; Kaddour, R.; Gruber, M.; Zargouni, H.; Zaouali, W.; Hamida, N.B.; Nasri, M.B.; Ouerghi, Z.; Hosni, K. Comparison of Antioxidant and Antimicrobial Activities of Two Cultivated Cistus Species from Tunisia. Biosci. J. 2016, 32, 226–237. [Google Scholar] [CrossRef]
- Limasset, B.; le Doucen, C.; Dore, J.C.; Ojasoo, T.; Damon, M.; Crastes de Paulet, A. Effects of Flavonoids on the Release of Reactive Oxygen Species by Stimulated Human Neutrophils. Multivariate Analysis of Structure-Activity Relationships (SAR). Biochem. Pharmacol. 1993, 46, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wu, L.-J.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Elevated Levels of DNA Repair Enzymes and Antioxidative Enzymes by (+)-Catechin in Murine Microglia Cells after Oxidative Stress. J. Asian Nat. Prod. Res. 2006, 8, 61–71. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A Review on Anti-Cancer Effect of Green Tea Catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Lavanchy, D. Evolving Epidemiology of Hepatitis C Virus. Clin. Microbiol. Infect. 2011, 17, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Hsu, C.-C.; Huang, H.-J.; Chang, C.-J.; Sun, S.-H.; Lin, A.M.-Y. Gallic Acid Attenuated LPS-Induced Neuroinflammation: Protein Aggregation and Necroptosis. Mol. Neurobiol. 2020, 57, 96–104. [Google Scholar] [CrossRef]
- Benito, C.; Tolón, R.M.; Pazos, M.R.; Núñez, E.; Castillo, A.I.; Romero, J. Cannabinoid CB2 Receptors in Human Brain Inflammation. Br. J. Pharmacol. 2008, 153, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Walter, L.; Franklin, A.; Witting, A.; Wade, C.; Xie, Y.; Kunos, G.; Mackie, K.; Stella, N. Nonpsychotropic Cannabinoid Receptors Regulate Microglial Cell Migration. J. Neurosci. 2003, 23, 1398–1405. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene Compounds in Nature: A Review of Their Potential Antioxidant Activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.-S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Braga, P.C.; Dal Sasso, M.; Fonti, E.; Culici, M. Antioxidant Activity of Bisabolol: Inhibitory Effects on Chemiluminescence of Human Neutrophil Bursts and Cell-Free Systems. Pharmacology 2009, 83, 110–115. [Google Scholar] [CrossRef]
- d’Alessio, P.A.; Bisson, J.-F.; Béné, M.C. Anti-Stress Effects of d-Limonene and Its Metabolite Perillyl Alcohol. Rejuvenation Res. 2014, 17, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.A.; de Paula Werner, M.F.; Oliveira, E.C.; Burgos, L.; Pereira, P.; da Silva Brum, L.F.; Story, G.M.; Santos, A.R.S. The Antinociceptive Effect of (-)-Linalool in Models of Chronic Inflammatory and Neuropathic Hypersensitivity in Mice. J. Pain 2010, 11, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, O.; Zhou, F.; Li, Q.; Wu, Z.; Zheng, Y. Linalool Inhibits LPS-Induced Inflammation in BV2 Microglia Cells by Activating Nrf2. Neurochem. Res. 2015, 40, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorelli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and Neurodegenerative Diseases. A Systematic Review. Environ. Res. 2017, 159, 82–94. [Google Scholar] [CrossRef]
- Bakulski, K.M.; Seo, Y.A.; Hickman, R.C.; Brandt, D.; Vadari, H.S.; Hu, H.; Park, S.K. Heavy Metals Exposure and Alzheimer’s Disease and Related Dementias. J. Alzheimers Dis. 2020, 76, 1215–1242. [Google Scholar] [CrossRef]
- Pyatha, S.; Kim, H.; Lee, D.; Kim, K. Association between Heavy Metal Exposure and Parkinson’s Disease: A Review of the Mechanisms Related to Oxidative Stress. Antioxidants 2022, 11, 2467. [Google Scholar] [CrossRef]
- Adams, J.B.; Baral, M.; Geis, E.; Mitchell, J.; Ingram, J.; Hensley, A.; Zappia, I.; Newmark, S.; Gehn, E.; Rubin, R.A.; et al. The Severity of Autism Is Associated with Toxic Metal Body Burden and Red Blood Cell Glutathione Levels. J. Toxicol. 2009, 2009, 532640. [Google Scholar] [CrossRef]
- Arora, M.; Reichenberg, A.; Willfors, C.; Austin, C.; Gennings, C.; Berggren, S.; Lichtenstein, P.; Anckarsäter, H.; Tammimies, K.; Bölte, S. Fetal and Postnatal Metal Dysregulation in Autism. Nat. Commun. 2017, 8, 15493. [Google Scholar] [CrossRef]
- Grove, J.; Ripke, S.; Als, T.D.; Mattheisen, M.; Walters, R.; Won, H.; Pallesen, J.; Agerbo, E.; Andreassen, O.A.; Anney, R.; et al. Common Risk Variants Identified in Autism Spectrum Disorder; Common Risk Variants Identified in Autism Spectrum Disorder. bioRXiv 2017. [Google Scholar] [CrossRef]
- Skogheim, T.S.; Weyde, K.V.F.; Engel, S.M.; Aase, H.; Surén, P.; Øie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Caspersen, I.H.; Hornig, M.; et al. Metal and Essential Element Concentrations during Pregnancy and Associations with Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder in Children. Environ. Int. 2021, 152, 106468. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal Chelation of Polyphenols. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 335, pp. 190–203. ISBN 978-0-12-182236-1. [Google Scholar]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic Biochemical Mechanisms behind the Health Benefits of Polyphenols. Mol. Aspects Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Lawson, M.; Drostinova, L.; Lauro, P.; Poprac, P.; Brezova, V.; Michalik, M.; Lukes, V.; Valko, M. Protective Role of Quercetin against Copper(II)-Induced Oxidative Stress: A Spectroscopic, Theoretical and DNA Damage Study. Food Chem. Toxicol. 2017, 110, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef]
- Savelieff, M.G.; DeToma, A.S.; Derrick, J.S.; Lim, M.H. The Ongoing Search for Small Molecules to Study Metal-Associated Amyloid-β Species in Alzheimer’s Disease. Acc. Chem. Res. 2014, 47, 2475–2482. [Google Scholar] [CrossRef]
- Fernandez, M.T.; Mira, M.L.; Florêncio, M.H.; Jennings, K.R. Iron and Copper Chelation by Flavonoids: An Electrospray Mass Spectrometry Study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef]
- Mignacca, S.A.; Mucciarelli, M.; Colombino, E.; Biasibetti, E.; Muscia, S.; Amato, B.; Presti, V.D.M.L.; Vazzana, I.; Galbo, A.; Capucchio, M.T. Cistus salviifolius Toxicity in Cattle. Vet. Pathol. 2020, 57, 115–121. [Google Scholar] [CrossRef]
- Yeruham, I.; Orgad, U.; Avidar, Y.; Perl, S.; Liberboim, M.; Adler, H.; Shlosberg, A. A Urinary Retention Syndrome in Beef Cows Probably Caused by Ingestion of Cistus. Rev. Méd Vét 2002, 153, 627–632. [Google Scholar]
- Bruno-Soares, A.M.; Abreu, J.M.; Soler, F. Preliminary in Vitro Studies of Cistus salvifolius Leaves in Relation to Metabolic Disorders in Sheep. In Poisonous Plants and Related Toxins; Acamovic, T., Stewart, C.S., Pennycott, T.W., Eds.; CABI: Wallingford, UK, 2004; pp. 56–62. ISBN 978-0-85199-614-1. [Google Scholar]
- Chaachouay, N.; Azeroual, A.; Douira, A.; Zidane, L. Ethnoveterinary Practices of Medicinal Plants among the Zemmour and Zayane Tribes, Middle Atlas, Morocco. S. Afr. J. Bot. 2022, 151, 826–840. [Google Scholar] [CrossRef]

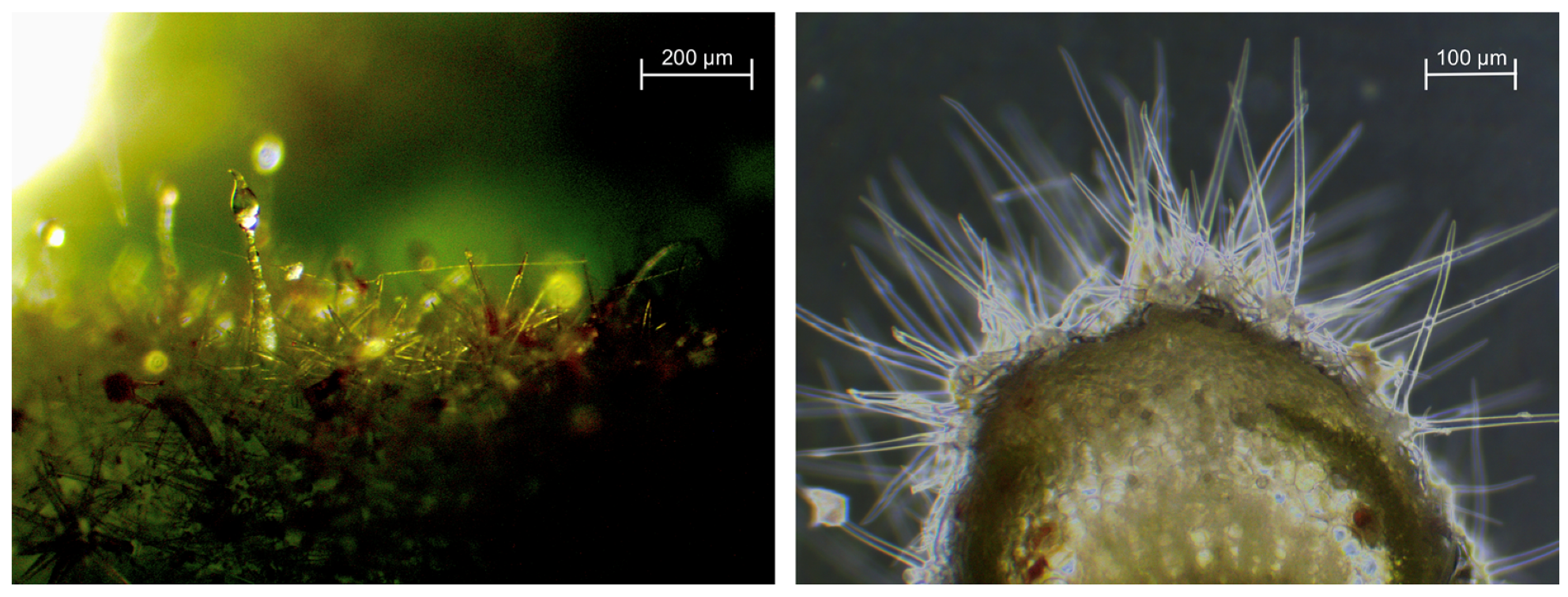
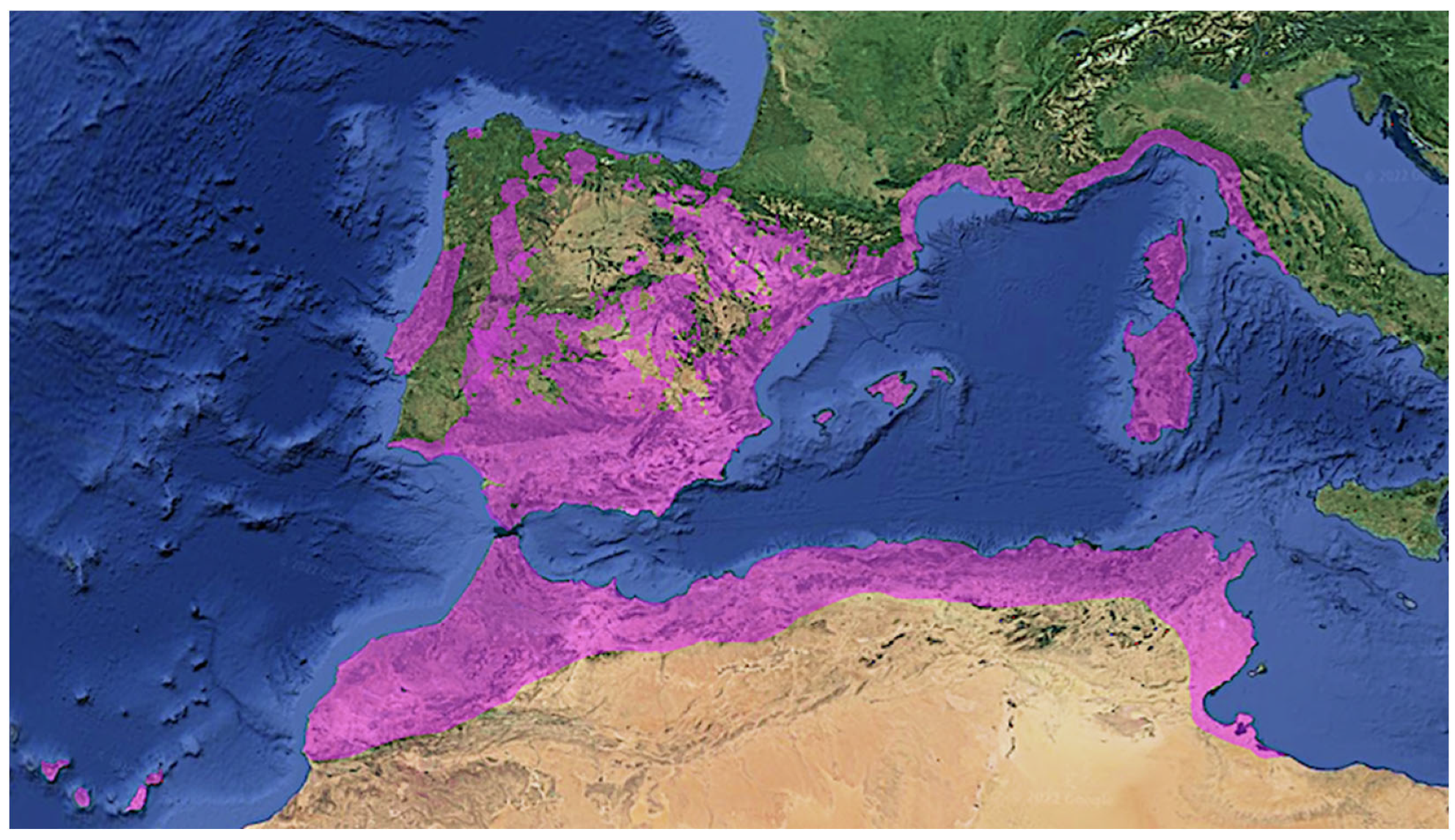

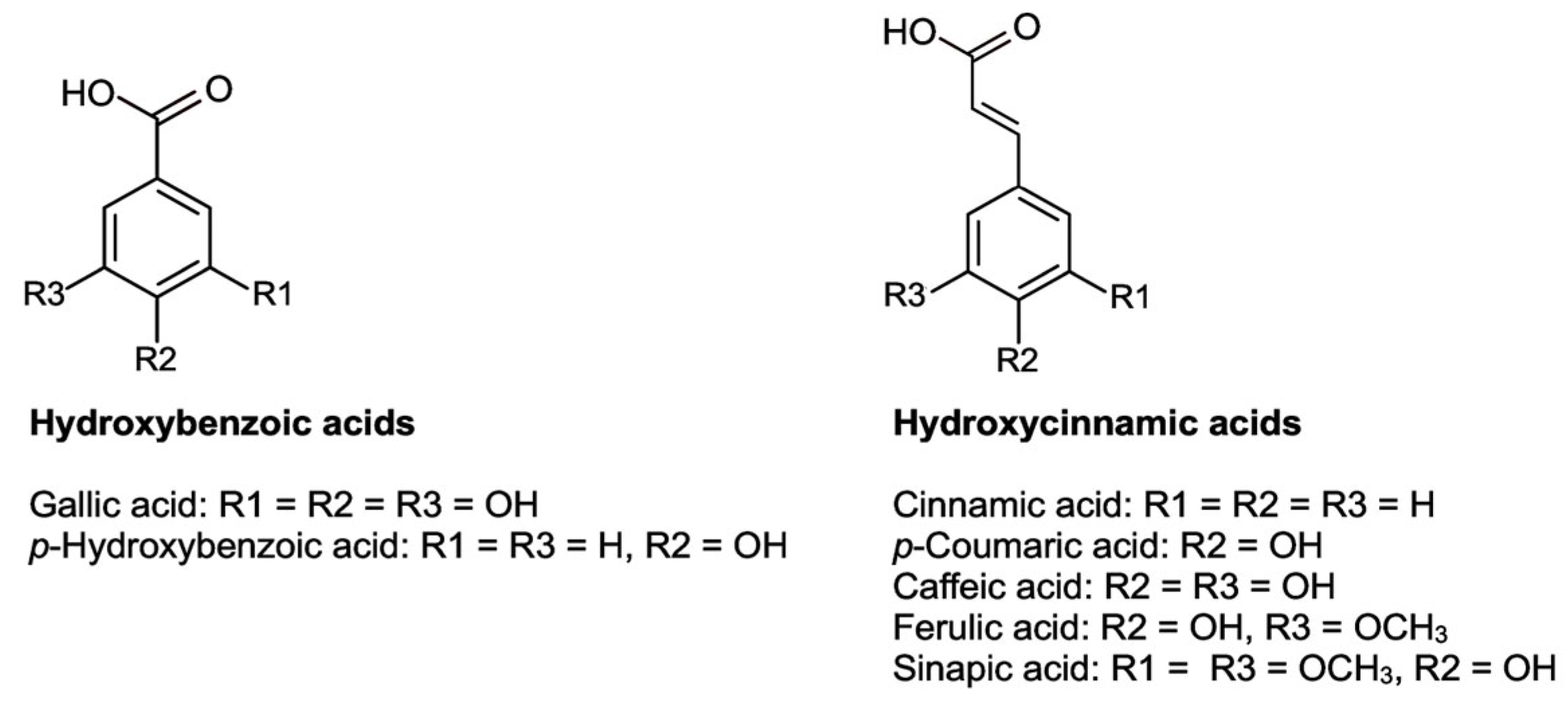
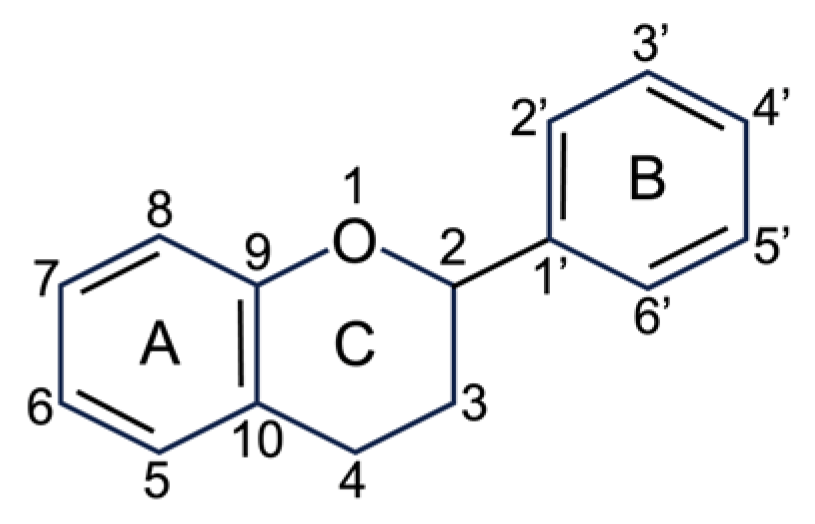
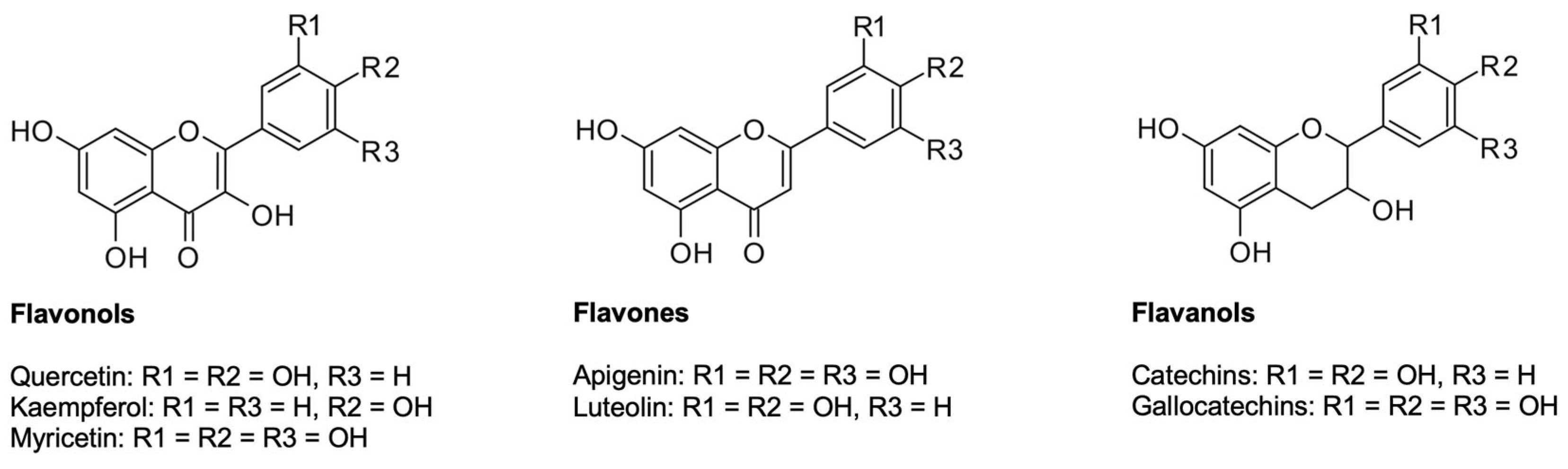
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raus de Baviera, D.; Ruiz-Canales, A.; Barrajón-Catalán, E. Cistus albidus L.—Review of a Traditional Mediterranean Medicinal Plant with Pharmacological Potential. Plants 2023, 12, 2988. https://doi.org/10.3390/plants12162988
Raus de Baviera D, Ruiz-Canales A, Barrajón-Catalán E. Cistus albidus L.—Review of a Traditional Mediterranean Medicinal Plant with Pharmacological Potential. Plants. 2023; 12(16):2988. https://doi.org/10.3390/plants12162988
Chicago/Turabian StyleRaus de Baviera, Daniel, Antonio Ruiz-Canales, and Enrique Barrajón-Catalán. 2023. "Cistus albidus L.—Review of a Traditional Mediterranean Medicinal Plant with Pharmacological Potential" Plants 12, no. 16: 2988. https://doi.org/10.3390/plants12162988
APA StyleRaus de Baviera, D., Ruiz-Canales, A., & Barrajón-Catalán, E. (2023). Cistus albidus L.—Review of a Traditional Mediterranean Medicinal Plant with Pharmacological Potential. Plants, 12(16), 2988. https://doi.org/10.3390/plants12162988