Flower Size as an Honest Signal in Royal Irises (Iris Section Oncocyclus, Iridaceae)
Abstract
1. Introduction
2. Results
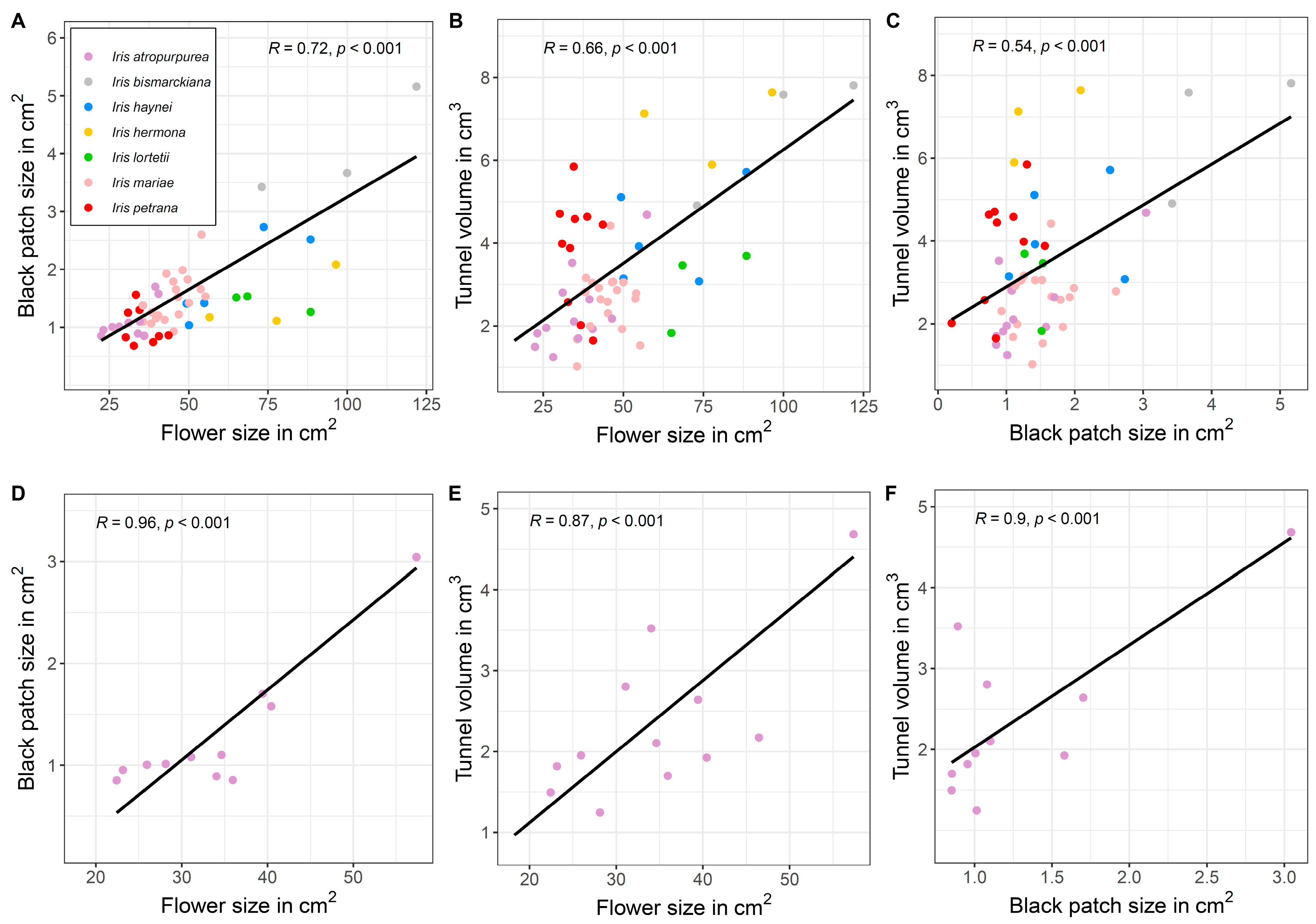
2.1. Controlled Environment (TAUBG)
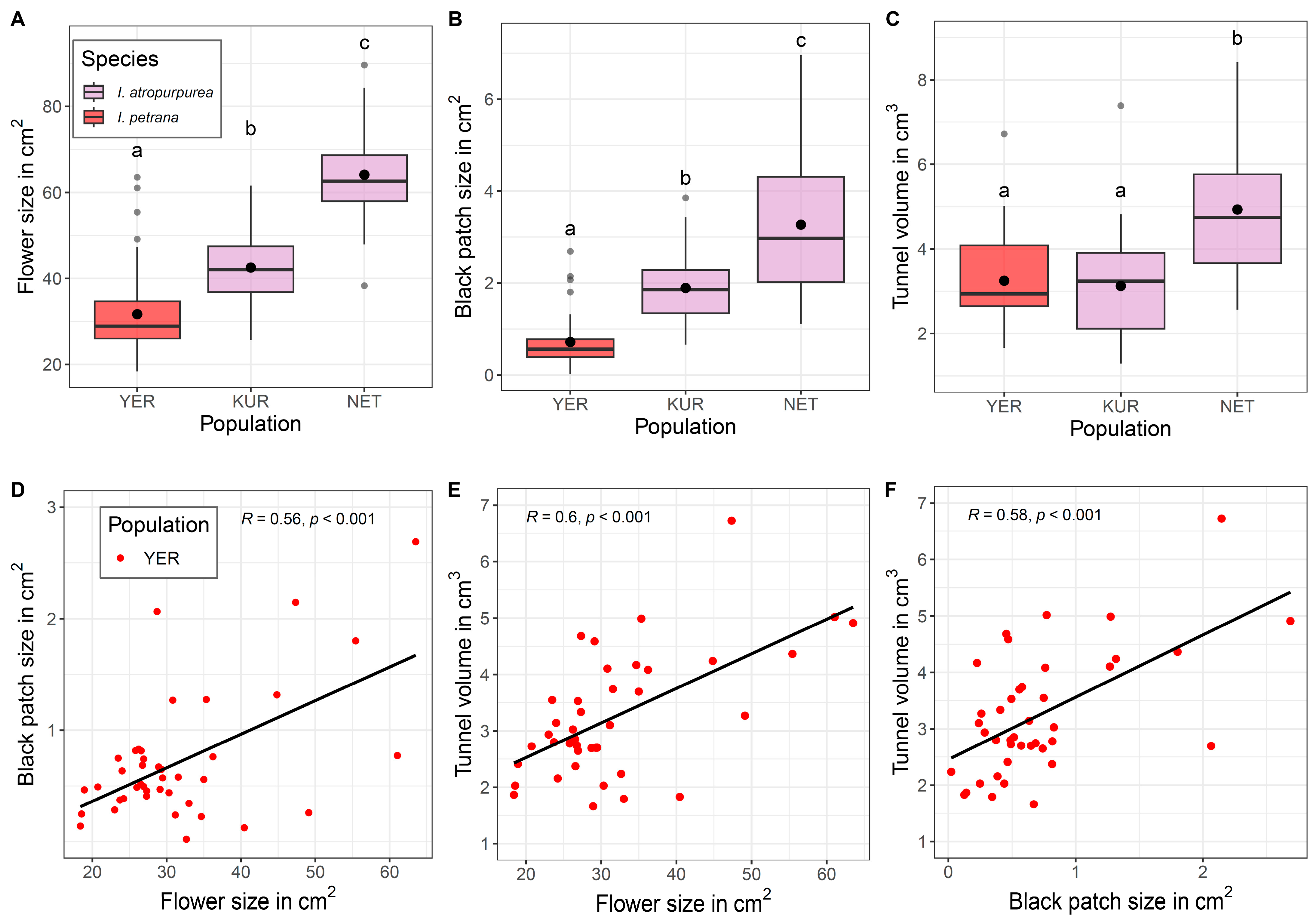
2.2. Natural Populations
2.3. Fitness from Natural Populations
3. Discussion
3.1. Honest Signals of Flower and Black Patch Are Species- and Population-Specific
3.2. Are Larger Shelters Better?
3.3. Abiotic Factors Can Affect the Selection of Flower Size
3.4. Is There an Indirect Selection of Black Patch Size?
4. Materials and Methods
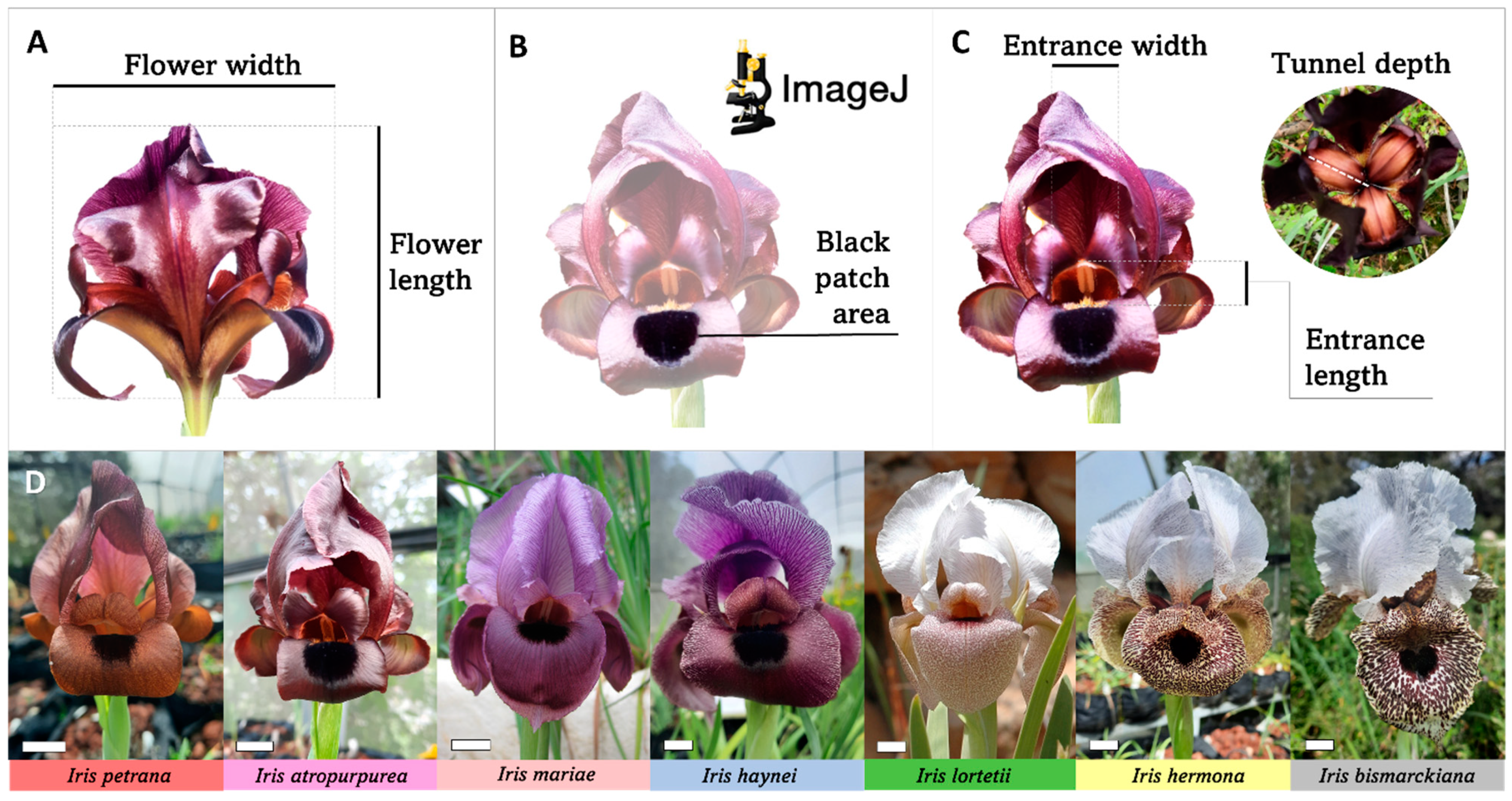
4.1. Flower Size, Black Patch Size, and Tunnel Volume Measurements
4.2. Sampling of Plant Material
4.3. Fitness Estimates in Natural Populations
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chittka, L.; Raine, N.E. Recognition of Flowers by Pollinators. Curr. Opin. Plant Biol. 2006, 9, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kearns, C.A.; Inouye, D.W.; Waser, N.M. Endangered Mutualisms: The Conservation of Plant-Pollinator Interactions. Annu. Rev. Ecol. Syst. 1998, 29, 83–112. [Google Scholar] [CrossRef]
- Schaefer, H.M.; Schaefer, V.; Levey, D.J. How Plant-Animal Interactions Signal New Insights in Communication. Trends Ecol. Evol. 2004, 19, 577–584. [Google Scholar] [CrossRef]
- Knauer, A.C.; Schiestl, F.P. Bees Use Honest Floral Signals as Indicators of Reward When Visiting Flowers. Ecol. Lett. 2015, 18, 135–143. [Google Scholar] [CrossRef]
- van der Kooi, C.J.; Reuvers, L.; Spaethe, J. Honesty, Reliability, and Information Content of Floral Signals. iScience 2023, 26, 107093. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, W.S.; Antonsen, L.; Pélabon, C. Phenotypic Selection on Dalechampia Blossoms: Honest Signaling Affects Pollination Success. Ecology 2005, 86, 3323–3333. [Google Scholar] [CrossRef]
- Fenster, C.B.; Cheely, G.; Dudash, M.R.; Reynolds, R.J. Nectar Reward and Advertisement in Hummingbird-Pollinated Silene virginica (Caryophyllaceae). Am. J. Bot. 2006, 93, 1800–1807. [Google Scholar] [CrossRef]
- Ortiz, P.L.; Fernández-Díaz, P.; Pareja, D.; Escudero, M.; Arista, M. Do Visual Traits Honestly Signal Floral Rewards at Community Level? Funct. Ecol. 2021, 35, 369–383. [Google Scholar] [CrossRef]
- Ornelas, J.F.; Ordano, M.; De-Nova, A.J.; Quintero, M.E.; Garland, T. Phylogenetic Analysis of Interspecific Variation in Nectar of Hummingbird-Visited Plants. J. Evol. Biol. 2007, 20, 1904–1917. [Google Scholar] [CrossRef]
- Tavares, D.C.; Freitas, L.; Gaglianone, M.C. Nectar Volume Is Positively Correlated with Flower Size in Hummingbird-Visited Flowers in the Brazilian Atlantic Forest. J. Trop. Ecol. 2016, 32, 335–339. [Google Scholar] [CrossRef]
- Parachnowitsch, A.L.; Manson, J.S.; Sletvold, N. Evolutionary Ecology of Nectar. Ann. Bot. 2019, 123, 247–261. [Google Scholar] [CrossRef]
- Choteau, M.; Barabé, D.; Gibernau, M. A Comparative Study of Inflorescence Characters and Pollen—Ovule Ratios among the Genera Philodendron and Anthurium (Araceae). Int. J. Plant Sci. 2006, 167, 817–829. [Google Scholar] [CrossRef][Green Version]
- Stanton, M.L.; Preston, R.E. Ecological Consequences and Phenotypic Correlates of Petal Size Variation in Wild Radish, Raphanus sativus (Brassicaceae). Am. J. Bot. 1988, 75, 528–539. [Google Scholar] [CrossRef]
- Pélabon, C.; Thöne, P.; Hansen, T.F.; Armbruster, W.S. Signal Honesty and Cost of Pollinator Rewards in Dalechampia scandens (Euphorbiaceae). Ann. Bot. 2012, 109, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.R. Floral Colour Changes as Cues for Pollinators. Nature 1991, 354, 227–229. [Google Scholar] [CrossRef]
- Zhang, C.; Vereecken, N.J.; Wang, L.; Tian, B.; Dafni, A.; Yang, Y.; Duan, Y. Are Nectar Guide Colour Changes a Reliable Signal to Pollinators That Enhances Reproductive Success? Plant Ecol. Divers. 2017, 10, 89–96. [Google Scholar] [CrossRef]
- Peach, K.; Liu, J.W.; Klitgaard, K.N.; Mazer, S.J. Sex-Specific Floral Attraction Traits in a Sequentially Hermaphroditic Species. Ecol. Evol. 2020, 10, 1856–1875. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhang, C.; Tian, B.; Sun, X.D.; Guo, W.; Zhang, T.F.; Yang, Y.P.; Duan, Y.W. Reproductive Isolation Is Mediated by Pollen Incompatibility in Sympatric Populations of Two Arnebia Species. Ecol. Evol. 2015, 5, 5838–5846. [Google Scholar] [CrossRef][Green Version]
- Medel, R.; Botto-Mahan, C.; Kalin-Arroyo, M. Pollinator-Mediated Selection on the Nectar Guide Phenotype in the Andean Monkey Flower, Mimulus Luteus. Ecology 2003, 84, 1721–1732. [Google Scholar] [CrossRef]
- Vereecken, N.J.; Dorchin, A.; Dafni, A.; Hötling, S.; Schulz, S.; Watts, S. A Pollinators’ Eye View of a Shelter Mimicry System. Ann. Bot. 2013, 111, 1155–1165. [Google Scholar] [CrossRef]
- Sapir, Y.; Shmida, A.; Ne’eman, G. Pollination of Oncocyclus irises (Iris: Iridaceae) by Night-Sheltering Male Bees. Plant Biol. 2005, 7, 417–424. [Google Scholar] [CrossRef]
- Lozada-Gobilard, S.; Motter, A.; Sapir, Y. Among-years Rain Variation Is Associated with Flower Size, but Not with Signal Patch Size in Iris Petrana. Ecology 2023, 104, e3839. [Google Scholar] [CrossRef] [PubMed]
- Galen, C. Measuring Pollinator-Mediated Selection on Morphometric Floral Traits: Bumblebees and the Alpine Sky Pilot, Polemonium viscosum. Evolution 1989, 43, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Spaethe, J.; Tautz, J.; Chittka, L. Visual Constraints in Foraging Bumblebees: Flower Size and Color Affect Search Time and Flight Behavior. Proc. Natl. Acad. Sci. USA 2001, 98, 3898–3903. [Google Scholar] [CrossRef] [PubMed]
- Arista, M.; Ortiz, P.L. Differential Gender Selection on Floral Size: An Experimental Approach Using Cistus Salvifolius. J. Ecol. 2007, 95, 973–982. [Google Scholar] [CrossRef]
- Hempel De Ibarra, N.; Langridge, K.V.; Vorobyev, M. More than Colour Attraction: Behavioural Functions of Flower Patterns. Curr. Opin. Insect Sci. 2015, 12, 64–70. [Google Scholar] [CrossRef]
- Galen, C.; Newport, M.E.A. Bumble Bee Behavior and Selection on Flower Size in the Sky Pilot, Polemonium viscosum. Oecologia 1987, 74, 20–23. [Google Scholar] [CrossRef]
- Parachnowitsch, A.L.; Kessler, A. Pollinators Exert Natural Selection on Flower Size and Floral Display in Penstemon Digitalis. New Phytol. 2010, 188, 393–402. [Google Scholar] [CrossRef]
- Lebel, M.; Obolski, U.; Hadany, L.; Sapir, Y. Pollinator-Mediated Selection on Floral Size and Tube Color in Linum Pubescens: Can Differential Behavior and Preference in Different Times of the Day Maintain Dimorphism? Ecol. Evol. 2018, 8, 1096–1106. [Google Scholar] [CrossRef]
- Ne’eman, G.; Ne’eman, R. Factors Determining Visual Detection Distance to Real. J. Pollinat. Ecol. 2017, 20, 1–12. [Google Scholar] [CrossRef]
- Blarer, A.; Keasar, T.; Shmida, A. Possible Mechanisms for the Formation of Flower Size Preferences by Foraging Bumblebees. Isr. J. Zool. 2002, 46, 159. [Google Scholar] [CrossRef]
- Biernaskie, J.M.; Walker, S.C.; Gegear, R.J. Bumblebees Learn to Forage like Bayesians. Am. Nat. 2009, 174, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Monty, A.; Saad, L.; Mahy, G. Bimodal Pollination System in Rare Endemic Oncocyclus Irises (Iridaceae) of Lebanon. Can. J. Bot. 2006, 84, 1327–1338. [Google Scholar] [CrossRef]
- Sapir, Y.; Shmida, A.; Fragman, O.; Comes, H.P. Morphological Variation of the Oncocyclus Irises (Iris: Iridaceae) in the Southern Levant. Bot. J. Linn. Soc. 2002, 139, 369–382. [Google Scholar] [CrossRef][Green Version]
- Sapir, Y.; Shmida, A. Species Concepts and Ecogeographical Divergence of Oncocyclus Irises. Isr. J. Plant Sci. 2002, 50, 119–127. [Google Scholar] [CrossRef]
- Volis, S.; Zhang, Y.H.; Deng, T.; Dorman, M.; Blecher, M.; Abbott, R.J. Divergence and Reproductive Isolation between Two Closely Related Allopatric Iris Species. Biol. J. Linn. Soc. 2019, 127, 377–389. [Google Scholar] [CrossRef]
- Saad, L.; Mahy, G. Molecular and Morphological Variation of Rare Endemic Oncocyclus Irises (Iridaceae) of Lebanon. Bot. J. Linn. Soc. 2009, 159, 123–135. [Google Scholar] [CrossRef]
- Lavi, R.; Sapir, Y. Are Pollinators the Agents of Selection for the Extreme Large Size and Dark Color in Oncocyclus Irises? New Phytol. 2015, 205, 369–377. [Google Scholar] [CrossRef]
- Sapir, Y.; Mazzucco, R. Post-Zygotic Reproductive Isolation among Populations of Iris Atropurpurea: The Effect of Spatial Distance among Crosses and the Role of Inbreeding and Outbreeding Depression in Determining Niche Width. Evol. Ecol. Res. 2012, 14, 425–445. [Google Scholar]
- Sapir, Y.; Shmida, A.; Ne’eman, G. Morning Floral Heat as a Reward to the Pollinators of the Oncocyclus Irises. Oecologia 2006, 147, 53–59. [Google Scholar] [CrossRef]
- Boberg, E.; Ågren, J. Despite Their Apparent Integration, Spur Length but Not Perianth Size Affects Reproductive Success in the Moth-Pollinated Orchid Platanthera Bifolia. Funct. Ecol. 2009, 23, 1022–1028. [Google Scholar] [CrossRef]
- Chapurlat, E.; Ågren, J.; Sletvold, N. Spatial Variation in Pollinator-Mediated Selection on Phenology, Floral Display and Spur Length in the Orchid Gymnadenia Conopsea. New Phytol. 2015, 208, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Sletvold, N.; Ågren, J. Pollinator-Mediated Selection on Floral Display and Spur Length in the Orchid Gymnadenia Conopsea. Int. J. Plant Sci. 2010, 171, 999–1009. [Google Scholar] [CrossRef]
- Boberg, E.; Alexandersson, R.; Jonsson, M.; Maad, J.; Ågren, J.; Nilsson, L.A. Pollinator Shifts and the Evolution of Spur Length in the Moth-Pollinated Orchid Platanthera Bifolia. Ann. Bot. 2014, 113, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.L.; Wyatt, R. Evidence for Pollination Ecotypes in the Yellow-Fringed Orchid, Platanthera Ciliaris. Evolution 1990, 44, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Mahlmann, T.; Hipólito, J.; de Oliveira, F.F. Male Sleeping Aggregation of Multiple Eucerini Bee Genera (Hymenoptera: Apidae) in Chapada Diamantina, Bahia, Brazil. Biodivers. Data J. 2014, 2, e1556. [Google Scholar] [CrossRef]
- Shimron, O.; Hefetz, A. Mating Behavior and Sex Attraction of Eucera Palestinae Friese (Hymenoptera: Anthophoridae). J. Kansas Entomol. Soc. 1985, 58, 526–531. [Google Scholar]
- Alcock, J. Sleeping Aggregations of the Bee Idiomelissodes Duplocincta (Cockerell) (Hymenoptera: Anthophorini) and Their Possible Function. J. Kansas Entomol. Soc. 1998, 71, 74–84. [Google Scholar]
- Linsley, E.G.; Cazier, M. Diurnal and Seasonal Behavior Patterns among Adults of Protoxaea Gloriosa (Hymenoptera, Oxaeidae). Am. Museum Novit. 1972, 25, 1–25. [Google Scholar]
- Burd, M. Bateman’s Principle and Plant Reproduction: The Role of Pollen Limitation in Fruit and Seed Set. Bot. Rev. 1994, 60, 373–425. [Google Scholar] [CrossRef]
- Wesselingh, R.A. Pollen Limitation Meets Resource Allocation: Towards a Comprehensive Methodology: Research Review. New Phytol. 2007, 174, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Sletvold, N.; Agren, J. There Is More to Pollinator-Mediated Selection than Polllen Limitation. Evolution 2014, 68, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.M.; Eisen, K.E.; Martin, R.A.; Sletvold, N. A Meta-Analysis of the Agents of Selection on Floral Traits. Evolution 2018, 73, 4–14. [Google Scholar] [CrossRef]
- Galen, C. High and Dry: Drought Stress, Sex-Allocation Trade-Offs, and Selection on Flower Size in the Alpine Wildflower Polemonium viscosum (Polemoniaceae). Am. Nat. 2000, 156, 72–83. [Google Scholar] [CrossRef]
- Teixido, A.L.; Barrio, M.; Valladares, F. Size Matters: Understanding the Conflict Faced by Large Flowers in Mediterranean Environments. Bot. Rev. 2016, 82, 204–228. [Google Scholar] [CrossRef]
- Gallagher, M.K.; Campbell, D.R. Shifts in Water Availability Mediate Plant–Pollinator Interactions. New Phytol. 2017, 215, 792–802. [Google Scholar] [CrossRef]
- Phillips, B.B.; Shaw, R.F.; Holland, M.J.; Fry, E.L.; Bardgett, R.D.; Bullock, J.M.; Osborne, J.L. Drought Reduces Floral Resources for Pollinators. Glob. Chang. Biol. 2018, 24, 3226–3235. [Google Scholar] [CrossRef]
- Osmolovsky, I.; Shifrin, M.; Gamliel, I.; Belmaker, J.; Sapir, Y. Eco-Geography and Phenology Are the Major Drivers of Reproductive Isolation in the Royal Irises, a Species Complex in the Course of Speciation. Plants 2022, 11, 3306. [Google Scholar] [CrossRef]
- Sapir, Y.; Ghara, M. The (Relative) Importance of Pollinator-Mediated Selection for Evolution of Flowers. Am. J. Bot. 2017, 104, 1787–1789. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Volis, S.; Zhang, Y.H.; Dorman, M.; Abbott, R.J. Incipient Speciation in Oncocyclus Irises: Eco-Geographic Isolation and Genetic Divergence with No Reproductive Isolation? Flora Morphol. Distrib. Funct. Ecol. Plants 2021, 275, 151746. [Google Scholar] [CrossRef]
- R Core Team R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http//www.R-project.org (accessed on 28 June 2023).

| Pop | N Plants | Flowers Measured for Traits | Flowers Marked for Fitness | Flowers That Became Fruits (%) | Fruits with Seeds (%) | Seeds per Fruit (Mean ± SD) |
|---|---|---|---|---|---|---|
| KUR | 15 | 48 | 35 | 16 (46%) | 10 (62%) | 17.6 ± 2.5 |
| NET | 13 | 30 | 27 | 15 (56%) | 6 (40%) | 4.6 ± 1.3 |
| YER | 23 | 41 | 31 | 20 (65%) | 15 (75%) | 18.2 ± 3.2 |
| Species | Pop | Predictors | Fruit Set | Seed Set | |||
|---|---|---|---|---|---|---|---|
| Df | F Value | p Value | F Value | p Value | |||
| I. atropurpurea | KUR | Flower size | 1 | 0.16 | 0.686 | 0.33 | 0.568 |
| Black patch size | 1 | 0.00 | 0.980 | 0.07 | 0.786 | ||
| Tunnel volume | 1 | 0.10 | 0.752 | 0.45 | 0.511 | ||
| Flower size × Black patch size | 1 | 0.66 | 0.428 | 0.62 | 0.441 | ||
| Flower size × Tunnel volume | 1 | 0.86 | 0.368 | 0.55 | 0.465 | ||
| Black patch size × Tunnel volume | 1 | 0.57 | 0.458 | 0.13 | 0.715 | ||
| NET | Flower size | 1 | 1.15 | 0.301 | 1.13 | 0.030 | |
| Black patch size | 1 | 1.49 | 0.240 | 2.26 | 0.154 | ||
| Tunnel volume | 1 | 2.24 | 0.156 | 0.07 | 0.793 | ||
| Flower size × Black patch size | 1 | 1.08 | 0.314 | 10.6 | <0.01 ** | ||
| Flower size × Tunnel volume | 1 | 1.80 | 0.200 | 7.87 | <0.05 * | ||
| Black patch size × Tunnel volume | 1 | 3.71 | 0.07 (*) | 5.40 | <0.08 (*) | ||
| I. petrana | YER | Flower size | 1 | 8.62 | <0.01 ** | 8.48 | <0.01 ** |
| Black patch size | 1 | 0.30 | 0.586 | 0.23 | 0.636 | ||
| Tunnel volume | 1 | 3.41 | 0.07 (*) | 5.01 | <0.05 * | ||
| Flower size × Black patch size | 1 | 1.78 | 0.193 | 0.40 | 0.532 | ||
| Flower size × Tunnel volume | 1 | 0.64 | 0.429 | 0.54 | 0.470 | ||
| Black patch size × Tunnel volume | 1 | 1.17 | 0.289 | 5.12 | <0.05 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozada-Gobilard, S.; Nielsen, N.; Sapir, Y. Flower Size as an Honest Signal in Royal Irises (Iris Section Oncocyclus, Iridaceae). Plants 2023, 12, 2978. https://doi.org/10.3390/plants12162978
Lozada-Gobilard S, Nielsen N, Sapir Y. Flower Size as an Honest Signal in Royal Irises (Iris Section Oncocyclus, Iridaceae). Plants. 2023; 12(16):2978. https://doi.org/10.3390/plants12162978
Chicago/Turabian StyleLozada-Gobilard, Sissi, Nadine Nielsen, and Yuval Sapir. 2023. "Flower Size as an Honest Signal in Royal Irises (Iris Section Oncocyclus, Iridaceae)" Plants 12, no. 16: 2978. https://doi.org/10.3390/plants12162978
APA StyleLozada-Gobilard, S., Nielsen, N., & Sapir, Y. (2023). Flower Size as an Honest Signal in Royal Irises (Iris Section Oncocyclus, Iridaceae). Plants, 12(16), 2978. https://doi.org/10.3390/plants12162978





