Abstract
Background: Sainfoin is a forage legume that is widely distributed around the world and is beneficial for animals owing to the characteristics of its condensed tannins (CTs), which, from certain plants, can prolong the aerobic stability of silage. Methods: The present study investigated whether sainfoin CTs can prolong aerobic stability by adding polyethylene glycol (PEG) to inactivate CT activity in the silage system. Results: The results showed that aerobic stability increased under the PEG treatment (p < 0.05). Ammonia nitrogen (0.71 g/kg DM vs. 0.94 g/kg DM; p < 0.05) was higher in the PEG-treated group compared with the control after 3 d of aerobic exposure. BA was detected only in the PEG-treated group upon aerobic exposure. Yeasts were more abundant in the control compared with the PEG-treated group after 7 d of aerobic exposure, after which the relative abundance of Lactobacillus was lower in the PEG-treated group (65.01% vs. 75.01% in the control; p < 0.05), while the relative abundance of Pediococcus was higher in the PEG-treated group compared with the control (10.9% vs. 4.49%, respectively; p < 0.05).The relative abundances of Apiotrichum and Aspergillus were lower in the control than in the PEG-treated group after 7 d of aerobic exposure. Conclusions: The results suggested that sainfoin CTs decreased aerobic stability, but could inhibit certain bacteria and fungi, such as Pediococcus and Apiotrichum, and preserve the protein content during the aerobic exposure of silage.
1. Introduction
Sainfoin (Onobrychis viciifolia) is a forage legume that is widely distributed in Europe, Asia, and North America [1]. Sainfoin has several benefits for the agricultural industry, including its drought tolerance and nitrogen-fixing activity, protein protection in the rumen, methane reduction, and the inhibition of rumen protozoa [2]. The condensed tannins (CTs) from sainfoin are primarily responsible for these effects in ruminants, which are due to the formation of CTs–protein complexes and the stabilization of CTs–protein complexes in certain environments [3]. Many legumes contain CTs, but sainfoin is considered superior because it has the highest capacity to bind protein and causes the least inhibition of cellulose digestion by rumen bacteria [4]. Consequently, sainfoin could be a potential high-quality forage for animals. In the production of animal feed, haymaking and ensiling are common methods used to preserve forage. Among these, ensiling is an important method used to preserve forage [5]. It is well known that the fermentation of forage under anaerobic environments through lactic acid bacteria (LAB) results in the production of organic acid and a decreased pH, which extends the associated feed storage time and improves the feed palatability [6]. During ensiling, changes in the microbial community play a key role in fermentation. Numerous studies have focused on the ensiling of crops, such as corn, alfalfa, barley, and ryegrass, to clarify the microbial community during ensiling through the use of next-generation sequencing techniques (NGS) [7].
The use of legume silage, such as alfalfa, usually results in protein degradation due to its higher protein content and buffering capacity compared with grass silage [8]. Several studies have observed that protein degradation is inhibited by CTs during ensiling [9,10]. This effect still appears after the aerobic exposure of silage, and the aerobic stability is prolonged due to the presence of existing with CTs [9]. However, the addition of CTs from the quebracho plant (Schinopsis lorentzii) showed no effect on the aerobic stability of corn silage, but decreased the aerobic stability when the addition level exceeded 50 g CTs/kg dry matter (DM) [11]. The differences between the findings of the two studies probably occurred because the effect of CTs, which are obtained from different plants, on silage systems is complex [3]. In general, CTs can inhibit some bacteria and fungi [9,12]. Recently, our previous study observed that CTs from sainfoin could inhibit protein degradation via the inhibition of protease and Pediococcus activity, and inhibited some yeast during the early stage of ensiling [13,14]. In general, yeast can initiate aerobic deterioration after silage is exposed to the air. Thus, whether the effects of CTs on fermentation and the microbial community, especially that of yeast, during the aerobic exposure of sainfoin silage have the capacity to prolong aerobic stability needs further investigation. Therefore, the present study was conducted to investigate the effects of CTs on the bacterial and fungal community via NGS techniques and the fermentation characteristics during aerobic exposure of sainfoin silage.
2. Results
2.1. Characteristics of Fermentation during Aerobic Exposure in Sainfoin Silage
The silage characteristics are shown in Table 1. The pH was highest in the control compared with PEG treatment at 7 d of aerobic exposure (p < 0.05). The water-soluble carbohydrate (WSC) content was lower in the polyethylene glycol (PEG) treatment after 60 d of ensiling (p < 0.05), but there was no significant difference in the WSC between the control and the PEG-treated group at 7 d of aerobic exposure (p > 0.05). The lactic acid (LA) and acetic acid (AA) contents decreased in both the control and PEG-treated groups after 3 d of aerobic exposure (p < 0.05), but the LA and AA contents showed no difference between the two groups (p > 0.05). BA was detected in the PEG-treated group but was not detected in the control group after 60 d of ensiling and 7 d of aerobic exposure. The yeast count was lower in the control compared with the PEG-treated group after 60 d of ensiling (p < 0.05) but higher in the control compared with the PEG-treated group after 7 d of aerobic exposure (p < 0.05). The mold count showed the same results as the yeast count. The PEG-treated group showed higher aerobic stability than the control (182 h vs. 156 h, respectively, p < 0.05).

Table 1.
Characteristics of silage during ensiling and aerobic exposure.
2.2. Bacterial Community during Aerobic Exposure
The alpha diversity of bacteria after 60 d of ensiling and aerobic exposure in sainfoin silage is shown in Table 2. The Good’s coverage (>0.999) results indicated that the degree of sequencing was sufficient for the bacterial community analysis. The results revealed that there was no significant difference in the richness and diversity of the bacteria community between the control and the PEG-treated group during 7 d of aerobic exposure (p > 0.05). The beta analysis of the bacterial community showed that there was no significant difference between the control and the PEG-treated group during 7 d of aerobic exposure (Figure 1, R2 = 0.0810, p = 0.0560).

Table 2.
Alpha diversity of bacteria after 60 d of ensiling and aerobic exposure in sainfoin silage 1.
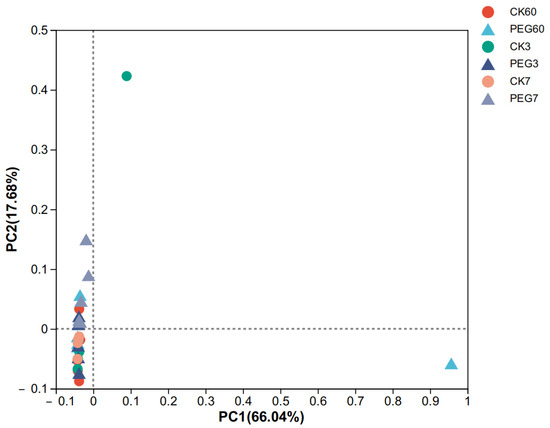
Figure 1.
Principal coordinate analysis (PCoA) plot based on the weighted UniFrac distance for the bacterial community of sainfoin silage. CK60: sainfoin ensiling after 60 d. CK3: sainfoin silage after 3 d of aerobic exposure. CK7: sainfoin silage after 7 d of aerobic exposure. CK: control; PEG: polyethylene glycol (R2 = 0.0810, p = 0.0560).
At the phylum level, as shown in Figure 2a, Firmicutes was dominant during 7 d of aerobic exposure, followed by Actinobacteria and Proteobacteria, with relative abundances of 80.81–96.73%, 1.28–6.60%, and 0.93–2.63%, respectively. There was no difference in the relative abundances of these bacteria between the control and the PEG-treated group after 60 d of ensilage and 7 d of aerobic exposure (p > 0.05). After 3 d of aerobic exposure, the relative abundance of Firmicutes in the PEG treatment was higher than that in the control (96.46 vs. 93.97%; p < 0.05), but the relative abundance of Proteobacteria was lower than that of the control (1.01 vs. 2.27%; p < 0.05).
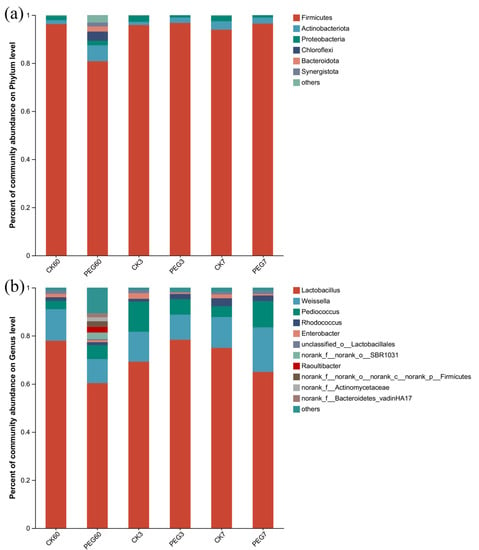
Figure 2.
Bacterial community at the (a) phylum and (b) genus level during the aerobic exposure of sainfoin silage. CK60: sainfoin ensiling after 60 d. CK3: sainfoin silage after 3 d of exposure. CK7: sainfoin silage after 7 d of exposure. CK: control; PEG: polyethylene glycol.
At the genus level, as shown in Figure 2b, Lactobacillus was dominant during 7 d of aerobic exposure, followed by Weissella, Pediococcus, and Rhodococcus, with relative abundances of 60.36–78.42%, 9.98–18.44%, 3.42–12.53%, and 1.22–3.35%, respectively. After 60 d of ensiling, the relative abundances of these four bacteria showed no differences (p > 0.05). The same results were observed after 3 d of aerobic exposure (p > 0.05). After 7 d of aerobic exposure, the relative abundance of Lactobacillus was lower in the PEG-treated group than in the control (65.01 vs. 75.01%, respectively; p < 0.05), while the relative abundance of Pediococcus was higher in the PEG-treated group when compared with the control (10.9 vs. 4.49%, respectively; p < 0.05). Additionally, the relative abundance of Enterobacter in the control was higher than that in the PEG-treated group at 7 d of aerobic exposure (1.54–2.3 vs. 0.46–0.56%; p < 0.05).
2.3. Fungal Community during Aerobic Exposure
The alpha diversity of fungi after 60 d of ensiling and aerobic exposure in sainfoin silage is shown in Table 3. The Good’s coverage results (>0.999) indicated that the degree of sequencing was sufficient for the fungal community analysis. The results showed that there was no difference in the diversity of the fungal community between the control and the PEG-treated group during 7 d of aerobic exposure (p > 0.05). Similarly, the beta analysis of the fungal community indicated that there was no clear difference between the control and the PEG-treated group during 7 d of aerobic exposure (Figure 3, R2 = 0.4715, p = 0.001).

Table 3.
Alpha diversity of fungi after 60 d of ensiling and aerobic exposure in sainfoin silage 1.
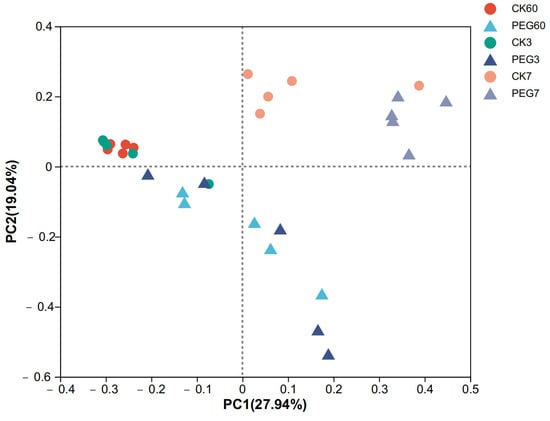
Figure 3.
Principal coordinate analysis (PCoA) plot based on the weighted UniFrac distance for the fungal community of sainfoin silage. CK60: sainfoin ensiling after 60 d. CK3: sainfoin silage after 3 d of exposure. CK7: sainfoin silage after 7 d of exposure. CK: control; PEG: polyethylene glycol (R2 = 0.4715, p = 0.001).
At the phylum level, as shown in Figure 4a, Ascomycota (relative abundance of 48.47–77.76%) was dominant in both the control and PEG-treated silage groups after 60 d of ensiling and during 7 d of aerobic exposure.
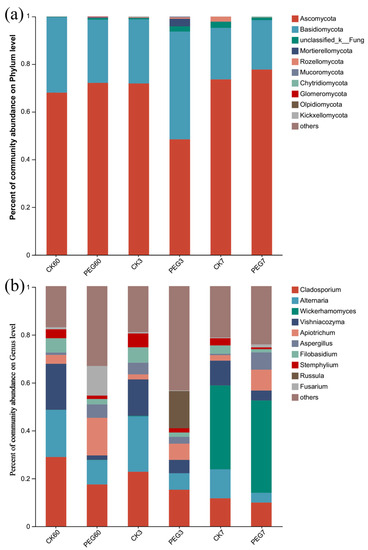
Figure 4.
Fungal community at the (a) phylum and (b) genus level during the aerobic exposure of sainfoin silage. CK60: sainfoin ensiling after 60 d. CK3: sainfoin silage after 3 d of exposure. CK7: sainfoin silage after 7 d of exposure. CK: control; PEG: polyethylene glycol.
At the genus level, as shown in Figure 4b, Cladosporium was dominant in both the control and the PEG-treated group after 60 d of ensiling and 3 d of aerobic exposure, followed by Alternaria and Vishniacozyma. After 3 d of aerobic exposure, the relative abundances of Alternaria and Filobasidium were both higher in the control than in the PEG-treated group (23.02 vs. 6.88% for Alternaria and 6.4 vs. 1.79% for Filobasidium; p < 0.05). After 7 d of aerobic exposure, the relative abundances of Aspergillus and Apiotrichum (0.58 vs. 7.19% for Aspergillus and 2.28 vs. 8.63% for Apiotrhicum in the control vs. the PEG-treated group, respectively) were lower but that of Stemphylium (3.00 vs. 0.78%) was higher in the control than in the PEG-treated group (p < 0.05).
3. Discussion
3.1. Aerobic Stability and Fermentation
The results showed that the aerobic stability of the PEG-treated group was higher than that of the control. In general, the DM, AA, and BA contents and the yeast and mold counts are the most important factors affecting silage aerobic stability [15]. DM usually has a negative effect on temperature rise during aerobic exposure, in which the temperature of crops with higher DM contents (300–500 g/kg fresh weight) rises more rapidly compared to crops with lower DM contents (150–300 g/kg fresh weight) [8]. According to the results of the present study, the DM content was 247.88–264.4 g/kg in both the control and PEG-treated group during aerobic exposure, which indicated that in this situation, the DM had no effect on aerobic stability. The contents of LA and AA were both lower when combined with CTs from purple prairie clover (PPC, Dalea purpurea), but the aerobic stability was prolonged during 14 d of aerobic exposure to silage, indicating that CTs directly inhibited yeast activity [9]. Consequently, the CTs from PPC had a strong capacity to prolong aerobic stability. However, CTs from quebracho had no effect on the aerobic stability of silage, although these CTs had the same effects on LA and AA contents as the CTs from PPC during the aerobic exposure of silage [11]. Thus, CTs and organic acids could be the two most important factors affecting aerobic stability, and the difference between the two silages could be attributed to the different CTs from different materials. The present study showed that CTs could decrease aerobic stability. In addition, the initial organic acid content and water activity determine the relationship between the residual WSC and the aerobic stability of silage [16]. The residual WSC can not only provide the substrate for yeast but can also result in the reduction of water activity [15]. The present study showed that the content of WSC exhibited no difference between the control and the PEG-treated group (p > 0.05), which indicated that the organic acid content was probably the main factor influencing the aerobic stability of silage.
There were two peaks of silage temperature, with the yeast and aerobic AA bacteria causing the first peak, followed by mold development [17]. It is well known that yeasts grow rapidly during the aerobic exposure of silage, thereby initiating the aerobic deterioration process. Previous studies have shown that the inhibitory effect of CTs on yeast occurs during ensiling [13]. However, the present study showed that the count of yeast was 13.67% higher in the control than in the PEG-treated group after 7 d of aerobic exposure. The results suggest that CTs from sainfoin lost their ability to inhibit yeasts during the aerobic exposure of silage.
Fungi can usually be inhibited by undissociated short-chain fatty acids during aerobic exposure to silage [15]. Theoretically, LA should be more dissociated than other short-chain fatty acids because it has the lowest pKa compared with PA and AA [18], that is, it results in rapid, decreased pH but lowers the capacity to inhibit fungal growth. PA has shown the highest capacity to inhibit yeasts and molds, followed by AA and LA [19]. Thus, researchers have observed that well-fermented silage that contains higher levels of LA among the total fermentation acids is prone to instability during aerobic exposure [20,21]. Furthermore, the high positive correlation between the concentration of AA and aerobic stability suggests that AA can improve the aerobic stability of silage [22]. AA is an antifungal agent, and a decreased AA content results in aerobic deterioration during the aerobic exposure of silage [23]. The results of the present study showed that the AA content did not differ between the control and the PEG-treated group after 7 d of aerobic exposure. Thus, the AA content was not a factor in the increased yeast in the control after 7 d of aerobic exposure. BA has also been found to increase the aerobic stability of silage [24,25,26]. The results of the present study showed that the BA content was stabilized in the PEG-treated group after 60 d of ensiling and 7 d of aerobic exposure, while no BA was detected in the control. A meta-analysis showed that CTs had a dose-dependent effect on the inhibition of BA production [5]. The results of the present study suggested that the higher aerobic stability of the PEG-treated group was due to the BA content, while the CTs from sainfoin probably inhibited BA production, which indirectly caused the yeast to grow faster upon the aerobic exposure of sainfoin silage.
The crude protein content decreased by 5.93% with the addition of PEG during the aerobic exposure of silage. Consequently, the AN content increased to 24.46% in the PEG-treated group compared with the control after 3 d of aerobic exposure. Microbial activity, such as the activity of Entrobacter and Clostridium, is the main contributor to AN production during ensiling, and the activity of both Entrobacter and Clostridium is inhibited at pH values below 4.5 [8]. The results of the present study showed that the pH value was above 4.5 in both the control and PEG-treated group during 7 d of aerobic exposure, indicating that CTs could be the main reason for this difference. The formation of protein–tannin complexes composed of sainfoin CTs and lucerne fraction 1 protein was found to be stable in the pH range of 4.0–6.5 [27]. The findings of the present study suggested that CTs inhibited protein degradation during the aerobic exposure of silage.
3.2. Bacterial Community
At the phylum level, Firmicutes was dominant in both the control and the PEG-treated group during 7 d of aerobic exposure. Several studies have observed that the dominant bacteria at the phylum level shifted from Firmicutes to Proteobacteria after aerobic exposure to silage. The results of the present study suggested that silage quality probably stabilized during 7 d of aerobic exposure. The addition of PEG decreased Proteobacteria but increased Firmicutes when compared with the control after 3 d of aerobic exposure. The increased Firmicutes activity following the addition of PEG was likely related to the decreased pH [28]. This impact should have persisted after 7 d of aerobic exposure as the pH of the PEG-treated group was the lowest. However, the impact of PEG on Firmicutes disappeared when the aerobic exposure time was prolonged (7 d). The present results suggested that the change in bacterial community composition was probably complicated due to the presence of CTs during the aerobic exposure of sainfoin silage.
At the genus level, the bacterial community of the PEG-treated group was mainly consistent with that of the control after 60 d of ensiling. The same results were observed after 3 d of aerobic exposure, except for the finding that Enterobacter showed higher activity in the control compared with the PEG-treated group. Consistently, Enterobacter still showed higher activity in the control after 7 d of aerobic exposure. Enterobacter shows less activity at pH values below 4.5 [29]. In the present study, the pH was over 4.5 in the control group during 7 d of aerobic exposure. CTs from sainfoin have shown the ability to inhibit some strains of Enterobacter [12]. However, combined with the findings of our previous study, this ability probably disappeared in the silage system in both the anaerobic fermentation and aerobic exposure stages [13]. Thus, the pH could be the main factor stimulating Enterobacter activity.
Lactobacillus was dominant in both the control and the PEG-treated group during 7 d of aerobic exposure. Similar results were observed in corn and wheat silage after exposure to the air [21,30]. After 7 d of aerobic exposure, the Lactobacillus activity in the PEG-treated group was lower than that of the control, while the Pediococcus activity exhibited the opposite trend. The pH was 4.5–4.6 in both the control and the PEG-treated group during 7 d of aerobic exposure, and the pH in the PEG-treated group was lower compared with control. Pediococcus can grow well under pH values ranging from 4.3–4.9 during ensiling [31]. The pH, in the present study, could not influence Pediococcus activity during 7 d of aerobic exposure. In our previous study, CTs from sainfoin showed the ability to inhibit Pediococcus but had no impact on Lactobacillus during ensiling [13]. Additionally, there were antagonistic effects between Lactobacillus and Pediococcus during ensiling [14]. The results of the present study suggested that Lactobacillus activity increased due to the indirect effect of the inhibition of Pediococcus activity by CTs during the aerobic exposure of silage.
Notably, the PEG-treated group contained BA during the aerobic exposure of silage, but only some Clostridium genera were detected, such as Clostridium_sensu_stricto_1. The relative abundance of this bacteria did not differ between the control and the PEG-treated group (p > 0.05), and the relative abundance of this bacteria was far below 1%. Other research did not detect BA during sainfoin ensiling, and the relative abundance of Clostridium tyrobutricum was 27.89% [32]. The results suggested that the CTs from sainfoin inhibited BA production, but the mechanism needs further study.
3.3. Fungal Community
The fungal richness decreased during aerobic exposure, in accordance with the findings of previous research [28]. High fungal diversity can improve the aerobic stability of whole-plant corn silage [33]. In the present study, the fungal diversity showed no difference between treatments or between days of aerobic exposure (p > 0.05), indicating that the CTs did not prolong the aerobic stability of sainfoin silage. The fungal community composition was the same between the control and the PEG-treated group during 7 d of aerobic exposure (Figure 4). The most abundant fungal genera during 7 d of aerobic exposure were Cladosporium, Alternaria, Wickerhamomyces, Vishniacozyma, and Apiotrichum, among which Wickerhamomyces, Vishniacozyma, and Apiotrichum belonged to the yeasts.
Wickerhamomyces showed overall competitiveness due to its high tolerance for stressful environments, such as low pH levels, water activity, and the presence of LAB, as well as its ability to metabolize a large number of carbon and nitrogen sources and produce toxins [34]. The relative abundance of Wickerhamomyces was below 1% after 3 d but increased rapidly, resulting in dominance after 7 d of aerobic exposure in both the control and PEG-treated group. Additionally, some strains of Wickerhamomyces have shown the ability to produce substantial amounts of AA through the fermentation of WSC [35]. The present findings suggested that, although the growth of Wickerhamomyces probably slowed, it could become the predominant fungi in the silage system upon aerobic exposure. Notably, CTs showed no effect on Wickerhamomyces activity during the aerobic exposure of silage.
Apiotrichum is an anamorphic basidiomycetous yeast genus that is widely distributed around the world [36]. Some strains of Apiotrichum have shown good AA tolerance and assimilation capability, and grow well in different ratios of AA-, PA-, and BA-based mixtures through the use of these acids as substrates to produce lipids [37]. This capability remains functional in silage systems. Researchers observed that Apiotrichum was the dominant fungi during ensiling when the AA content was the highest (2.48% DM) when compared with other treatments [38]. In the present study, the activity of Apiotrichum in the PEG-treated group was higher than that of the control after 7 d of aerobic exposure (the relative abundance of Apiotrichum was 8.63 vs. 2.28%). The present results showed that the AA content was below 1.65% DM and showed no difference between the control and the PEG-treated group after 7 d of aerobic exposure, indicating that AA probably did not affect Apiotrichum activity. Thus, the results probably suggested that CTs had a strong ability to inhibit Apiotrichum growth in the silage system.
4. Materials and Methods
4.1. Silage Preparation
Sainfoin (Onobrychis viciifolia socp. cv. Qi-Tai, BY2020-003, grassland farming, Xinjiang, China) seeds were sown in an experimental field of Shihezi University on 1 May 2022 (N 44.21; E 85.57, Xinjiang, China). The study area has a temperate desert climate and the average annual rainfall is 128 mm. Whole sainfoin plants were harvested in July 2022 at the early flower stage. Samples were wilted until DM content approximated 250 g/kg fresh weight, then the plants were chopped into 1–2 cm pieces. Polyethylene glycol (PEG) (Sigma-Aldrich, Shanghai, China; molecular weight, 6000) was used to inactivate CT activity (the ratio of PEG:CT was 2:1, based on DM). The PEG water solution (640 g/L) was prepared and sprayed on samples, and the CT concentration was approximately 50 g/kg DM [13]. After treatment, 1000 g samples were packed into polyethylene plastic bags (30 cm × 50 cm), then compacted and sealed using a vacuum sealer. Five replicates were prepared for the control and PEG treatment. All bags were stored indoors at 20 °C.
4.2. Aerobic Exposure of Silage
After 60 d of fermentation, 3 kg samples of each treatment were transferred to plastic jars (5 L volume, diameter 20.40 cm, height 15.3 cm). Each jar was covered with five layers of cheesecloth to avoid the introduction of impurities and stored at 20 °C for 8 d. A temperature monitoring instrument (i500-E8T, Yuhuan Zhituo Instrument Technology Co., Ltd., Hangzhou, China) was used to record the internal temperature (the temperature probes were embedded in the middle layers of silage) and the ambient temperature (temperature probes were placed outside of the jar) of the silage every 15 min during 8 d of aerobic exposure. The silage was spoiled when the internal temperature was 2 °C higher than the ambient temperature [39].
Samples were taken from each treatment after 60 d of ensiling and at 3 and 7 d of the aerobic exposure of sainfoin silage. The samples were stored at −20 °C and −80 °C for the determination of their characteristics and microbial community analysis, respectively.
4.3. Characteristics Analysis of Silage
Two-hundred-gram silage samples were dried at 65 °C for 48 h and ground to obtain the DM content through the use of a 1.00 mm griddle. The water-soluble carbohydrate content was determined via extract samples through water solution at 100 °C for 30 min, then analysis was conducted according to the anthrone method [40]. An automatic Kjeldahl nitrogen analyzer (K9840, Hanon Co., Ltd., Qingdao, China) was used to determine the nitrogen content of samples according to the guidelines of the Association of Official Agricultural Chemists.
To determine the fermentation characteristics, 20 g silage samples were combined with distilled water and thoroughly blended in a homogenizer (L-1BA, Kuansons Biotechnology Co., Ltd., Shanghai, China). After filtering the mixture, the supernatant was used for the analysis of organic acids and ammonia nitrogen (AN). The AN was determined according to the phenol-hypochlorite colorimetric methods [41]. The lactic acid (LA), acetic acid (AA), propionic acid (PA), and butyric acid (BA) were analyzed following the methods [13].
For the microbial count, 10 g samples were homogenized in 90 mL sterilized saline (0.8%) and the supernatant was serially diluted (102–108). The microbial incubation was accomplished according to the methods described in a previous study [42]. Yeasts and molds were counted after incubation on Rose–Bengal agar at 25 °C for 78–120 h. LABs were counted after incubation on de Man–Rogosa–Sharpe (MRS) agar at 30 °C for 24 h. Aerobic bacteria were counted after incubation on nutrient agar at 30 °C for 24 h. The number of colony-forming units (CFUs) was expressed per gram of fresh forage, and the microbial count was presented as log-transformed before statistical analysis.
4.4. DNA Extraction and Sequence Analysis of Bacteria and Fungi
The total DNA of each sample was extracted with a commercial DNA Kit (FastDNA® Spin Kit for Soil, MP Biomedicals, New York, NY, USA). Primers targeted the V3–V4 (338F: ACTCCTACGGGAGGCAGCAG; 806R: GGACTACHVGGGTWTCTAAT) regions of bacterial 16S rDNA and the ITS1 regions (ITS1F: 5′-CTTGGTCATTTAGAGGAAGTAA-3′; ITS2R: 5′-GCTGCGTTCTTCATCGATGC-3′) of fungi. The amplicons were extracted, purified, and analyzed according to methods described in [43]. The Quantitative Insights Into Microbial Ecology (QIIME, v1.8.0) pipeline was employed to process the sequencing data according to methods described in [44]. The operational taxonomic unit classification was conducted by using BLAST to search the representative sequences set against the Greengenes Database using the best hit method [45]. The raw sequences of all samples were deposited in the National Center for Biotechnology (NCBI) Sequence Read Archive (SRA) under accession numbers “PRJNA948115” and “PRJNA953494” for bacteria and fungi, respectively (accessed on 1 June 2023).
4.5. Statistical Analysis
The characteristics of sainfoin silage were subjected to a two-way analysis of variance, 2 × 3 factorial complete randomized design (PEG and control groups × 60 fermentation d, and aerobic exposure for 3 and 7 d). Data were analyzed using IBM SPSS 22 Statistics (IBM Corp., Armonk, NY, USA). Significant differences between treatments were determined using Tukey’s test at p < 0.05.
5. Conclusions
In this study, CTs inhibited BA production in the silage system, and the PEG treatment increased the aerobic stability of silage due to BA production. The crude protein content was stabilized and the AN decreased with the presence of CTs. Lactobacillus was dominant during 7 d of the aerobic exposure to silage. Wickerhamomyces grew slowly but would become dominant with prolonged aerobic exposure time, and its activity was not affected by CTs. CTs could inhibit some bacteria and fungi, such as Pediococcus and Apiotrichum, during the aerobic exposure of silage, which probably had no effect on the aerobic stability of silage.
Author Contributions
R.H.: conceptualization, methodology, writing—review and editing. X.W.: resources, validation, supervision, review and editing. C.M.: resources, validation, supervision, review and editing. F.Z.: resources, investigation, validation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the China Agriculture Research System of MOF and MARA [grant number CARS], the Innovation and Entrepreneurship Plan Project for the Xinjiang Production and Construction Corps’ Science and Technology Commissioner [grant number 2022CB022], and the South Xinjiang Key Industry Innovation and Development Support Plan Project [grant number 2022DB017].
Data Availability Statement
The datasets supporting the conclusions of this article are available in the National Center for Biotechnology (NCBI) Sequence Read Archive (SRA) under accession numbers “PRJNA948115” and “PRJNA953494” for bacteria and fungi, respectively. http://www.ncbi.nlm.nih.gov (accessed on 1 August 2023).
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Carbonero, C.H.; Mueller-Harvey, I.; Brown, T.A.; Smith, L. Sainfoin (Onobrychis viciifolia): A Beneficial Forage Legume. Plant Genet. Resour. -Charact. Util. 2011, 9, 70–85. [Google Scholar] [CrossRef]
- Mora-Ortiz, M.; Smith, L.M.J. Onobrychis viciifolia; A Comprehensive Literature Review of Its History, Etymology, Taxonomy, Genetics, Agronomy and Botany. Plant Genet. Resour. 2018, 16, 403–418. [Google Scholar] [CrossRef]
- Zeller, W.E. Activity, Purification, and Analysis of Condensed Tannins: Current State of Affairs and Future Endeavors. Crop Sci. 2019, 59, 886–904. [Google Scholar] [CrossRef]
- McAllister, T.A.; Martinez, T.; Bae, H.; Muir, A.; Yanke, L.; Jones, G. Characterization of Condensed Tannins Purified from Legume Forages: Chromophore Production, Protein Precipitation, and Inhibitory Effects on Cellulose Digestion. J. Chem. Ecol. 2005, 31, 2049–2068. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Sujarnoko, T.U.P.; Ridla, M.; Kondo, M.; Kreuzer, M. Silage Quality as Influenced by Concentration and Type of Tannins Present in the Material Ensiled: A Meta-Analysis. J. Anim. Physiol. Anim. Nutr. 2019, 103, 456–465. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y. Influence of Lactobacillus spp. from an Inoculant and of Weissella and Leuconostoc spp. from Forage Crops on Silage Fermentation. Appled Environ. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef]
- Guo, X.S.; Xu, D.M.; Li, F.H.; Bai, J.; Su, R.A. Current Approaches on the Roles of Lactic Acid Bacteria in Crop Silage. Microb. Biotechnol. 2023, 16, 67–87. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Herson, S. The Biochemistry of Silage; Chalcombe Publications: Kingston, UK; Kent, UK, 1991. [Google Scholar]
- Peng, K.; Jin, L.; Niu, Y.D.; Huang, Q.; McAllister, T.A.; Yang, H.E.; Denise, H.; Xu, Z.; Acharya, S.; Wang, S.; et al. Condensed Tannins Affect Bacterial and Fungal Microbiomes and Mycotoxin Production during Ensiling and upon Aerobic Exposure. Appl. Environ. Microbiol. 2018, 84, e02274. [Google Scholar] [CrossRef]
- He, L.; Lv, H.; Xing, Y.; Chen, X.; Zhang, Q. Intrinsic Tannins Affect Ensiling Characteristics and Proteolysis of Neolamarckia cadamba Leaf Silage by Largely Altering Bacterial Community. Bioresour. Technol. 2020, 311, 123496. [Google Scholar] [CrossRef]
- Bueno, A.V.I.; Jobim, C.C.; Daniel, J.L.P.; Gierus, M. Fermentation Profile and Hygienic Quality of Rehydrated Corn Grains Treated with Condensed Tannins from Quebracho Plant Extract. Anim. Feed. Sci. Technol. 2020, 267, 114559. [Google Scholar] [CrossRef]
- Liu, X.L.; Hao, Y.Q.; Jin, L.; Xu, Z.J.; McAllister, T.A. Anti-Escherichia Coli O157:H7 Properties of Purple Prairie Clover and Sainfoin Condensed Tannins. Molecules 2013, 18, 2183–2199. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Z.; Zhang, F.F.; Wang, T.; Zhang, Y.L.; Li, X.; Chen, Y.C.; Ma, C.H. Effect of Intrinsic Tannins on the Fermentation Quality and Associated with the Bacterial and Fungal Community of Sainfoin Silage. Microorganisms 2022, 10, 844. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Z.; Wang, X.Z.; Ma, C.H.; Zhang, F.F. Effects of Intrinsic Tannins on Proteolysis Dynamics, Protease Activity, and Metabolome during Sainfoin Ensiling. Front. Microbiol. 2022, 13, 976118. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Davies, D.R. The Aerobic Stability of Silage: Key Findings and Recent Developments. Grass Forage Sci. 2013, 68, 1–19. [Google Scholar] [CrossRef]
- Niu, D.Z.; Zheng, M.L.; Zuo, S.S.; Jiang, D.; Xu, C.C. Effects of Maize Meal and Limestone on the Fermentation Profile and Aerobic Stability of Smooth Bromegrass (Bromus inermis Leyss) Silage. Grass Forage Sci. 2018, 73, 622–629. [Google Scholar] [CrossRef]
- Merry, R.J.; Davies, D.R. Propionibacteria and Their Role in the Biological Control of Aerobic Spoilage in Silage. Lait 1999, 79, 149–164. [Google Scholar] [CrossRef]
- Partanen, J.I. Calculation of Stoichiometric Dissociation Constants of Monoprotic Carboxylic Acids in Dilute Aqueous Sodium or Potassium Chloride Solutions and p[m(H+)] Values for Acetate and Formate Buffers at 25 °C. Talanta 2000, 52, 863–871. [Google Scholar] [CrossRef]
- Woolford, M.K. Microbiological Screening of the Straight Chain Fatty Acids (C1–C12) as Potential Silage Additives. J. Sci. Food Agric. 1975, 26, 219–228. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Ashbell, G.; Hen, Y.; Azrieli, A. The Effect of Applying Lactic Acid Bacteria at Ensiling on the Aerobic Stability of Silages. J. Appl. Bacteriol. 1993, 75, 512–518. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.R.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Saldinger, S.S. Bacterial Dynamics of Wheat Silage. Front. Microbiol. 2019, 10, 1532. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Kung, L. The Effects of Lactobacillus Buchneri with or without a Homolactic Bacterium on the Fermentation and Aerobic Stability of Corn Silages Made at Different Locations. J. Dairy Sci. 2010, 93, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.S.; Wang, C.; Sun, L.; Xu, H.W.; Jiang, Y.; Na, N.; Yin, G.M.; Liu, S.B.; Xue, Y.L. Dynamics of Bacterial and Fungal Communities and Metabolites during Aerobic Exposure in Whole-Plant Corn Silages with Two Different Moisture Levels. Front. Microbiol. 2021, 12, 663895. [Google Scholar] [CrossRef] [PubMed]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic Acid Increases Stability of Silage under Aerobic Conditions. Appl. Environ. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Xu, W.B.; Yang, J.S.; Zhao, H.B.; Xin, H.S.; Zhang, Y.G. Effect of Different Levels of Corn Steep Liquor Addition on Fermentation Characteristics and Aerobic Stability of Fresh Rice Straw Silage. Anim. Nutr. 2016, 2, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, Z.; Na, R.S. Effects of Different Additives on Fermentation Quality and Aerobic Stability of Leymus chinensis Silage. Grass Forage Sci. 2018, 73, 413–419. [Google Scholar] [CrossRef]
- Jones, W.T.; Mangan, J.L. Complexes of the Condensed Tannins of Sainfoin (Onobrychis viciifolia Scop.) with Fraction 1 Leaf Protein and with Submaxillary Mucoprotein, and Their Reversal by Polyethylene Glycol and PH. J. Sci. Food Agric. 1977, 28, 126–136. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, X.K.; Gu, Q.C.; Liang, M.Z.; Mu, S.L.; Zhou, B.; Huang, F.; Lin, B.; Zou, C.X. Analysis of the Correlation between Bacteria and Fungi in Sugarcane Tops Silage Prior to and after Aerobic Exposure. Bioresour. Technol. 2019, 291, 121835. [Google Scholar] [CrossRef]
- Muck, R.E. Silage Microbiology and Its Control through Additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.R.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela, S. Microbiome Dynamics during Ensiling of Corn with and without Lactobacillus plantarum Inoculant. Appl. Microbiol. Biotechnol. 2018, 102, 4025–4037. [Google Scholar] [CrossRef]
- Wang, M.S.; Chen, M.Y.; Bai, J.; Zhang, J.Y.; Su, R.N.; Franco, M.; Ding, Z.T.; Zhang, X.; Zhang, Y.; Guo, X.S. Ensiling Characteristics, in Vitro Rumen Fermentation Profile, Methane Emission and Archaeal and Protozoal Community of Silage Prepared with Alfalfa, Sainfoin and Their Mixture. Anim. Feed. Sci. Technol. 2021, 284, 115154. [Google Scholar] [CrossRef]
- Xu, D.M.; Ding, Z.T.; Wang, M.S.; Bai, J.; Ke, W.C.; Zhang, Y.X.; Guo, X.S. Characterization of the Microbial Community, Metabolome and Biotransformation of Phenolic Compounds of Sainfoin (Onobrychis viciifolia) Silage Ensiled with or without Inoculation of Lactobacillus plantarum. Bioresour. Technol. 2020, 316, 123910. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Tan, Z.F.; Gu, L.B.; Ma, H.; Wang, Z.Y.; Wang, L.; Wu, G.F.; Qin, G.Y.; Wang, Y.P. Pang Variation of Microbial Community and Fermentation Quality in Corn Silage Treated with Lactic Acid Bacteria and Artemisia Argyi during Aerobic Exposure. Toxins 2022, 14, 349. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.M.; Moons, M.C.; Huret, S.; Vrancken, G.; De Vuyst, L. Wickerhamomyces Anomalus in the Sourdough Microbial Ecosystem. Antonie Van Leeuwenhoek 2011, 99, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Ravasio, D.; Carlin, S.; Boekhout, T.; Groenewald, M.; Vrhovsek, U.; Walther, A.; Wendland, J. Adding Flavor to Beverages with Non-Conventional Yeasts. Fermentation 2018, 4, 15. [Google Scholar] [CrossRef]
- James, S.A.; Bond, C.J.; Stanley, R.; Ravella, S.R.; Peter, G.; Dlauchy, D.; Roberts, I.N. Apiotrichum terrigenum Sp Nov., a Soil-Associated Yeast Found in Both the UK and Mainland Europe. Int. J. Syst. Evol. Microbiol. 2016, 66, 5046–5050. [Google Scholar] [CrossRef]
- Qian, X.J.; Zhou, X.H.; Chen, L.; Zhang, X.Y.; Xin, F.X.; Dong, W.L.; Zhang, W.M.; Oshsenreither, K.; Jiang, M. Bioconversion of Volatile Fatty Acids into Lipids by the Oleaginous Yeast Apiotrichum porosum DSM27194. Fuel 2021, 290, 119811. [Google Scholar] [CrossRef]
- Xiao, Y.Z.; Sun, L.; Wang, Z.J.; Wang, W.; Xin, X.P.; Xu, L.J.; Du, S. Fermentation Characteristics, Microbial Compositions, and Predicted Functional Profiles of Forage Oat Ensiled with Lactiplantibacillus plantarum or Lentilactobacillus buchneri. Fermentation 2022, 8, 707. [Google Scholar] [CrossRef]
- Ranjit, N.K.; Kung, L. The Effect of Lactobacillus buchneri, Lactobacillus plantarum, or a Chemical Preservative on the Fermentation and Aerobic Stability of Corn Silage. J. Dairy Sci. 2000, 83, 526–535. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R. Determination of Water-Soluble Carbohydrates in Grass. J. Sci. Food Agric. 1964, 15, 395–398. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-Hypochlorite Reaction for Determination of Ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Zahiroddini, H.; Baah, J.; Absalom, W.; McAllister, T.A. Effect of an Inoculant and Hydrolytic Enzymes on Fermentation and Nutritive Value of Whole Crop Barley Silage. Anim. Feed. Sci. Technol. 2004, 117, 317–330. [Google Scholar] [CrossRef]
- Su, R.N.; Ni, K.K.; Wang, T.W.; Yang, X.P.; Zhang, J.; Liu, Y.Y.; Shi, W.X.; Yan, L.; Jie, C.; Zhong, J. Effects of Ferulic Acid Esterase-Producing Lactobacillus fermentum and Cellulase Additives on the Fermentation Quality and Microbial Community of Alfalfa Silage. PeerJ 2019, 7, e7712. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.H.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).