Allelopathic Activity of the Invasive Plant Polygonum chinense Linn. and Its Allelopathic Substances
Abstract
1. Introduction
2. Results
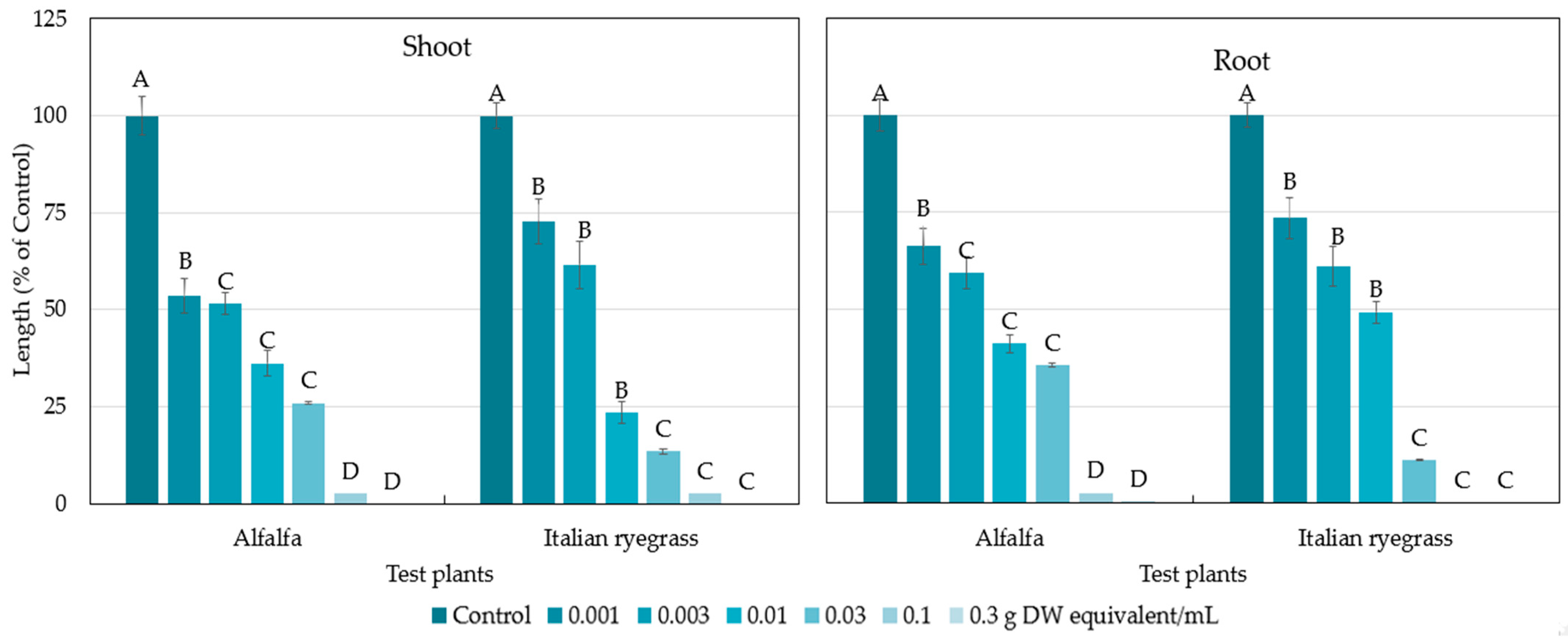
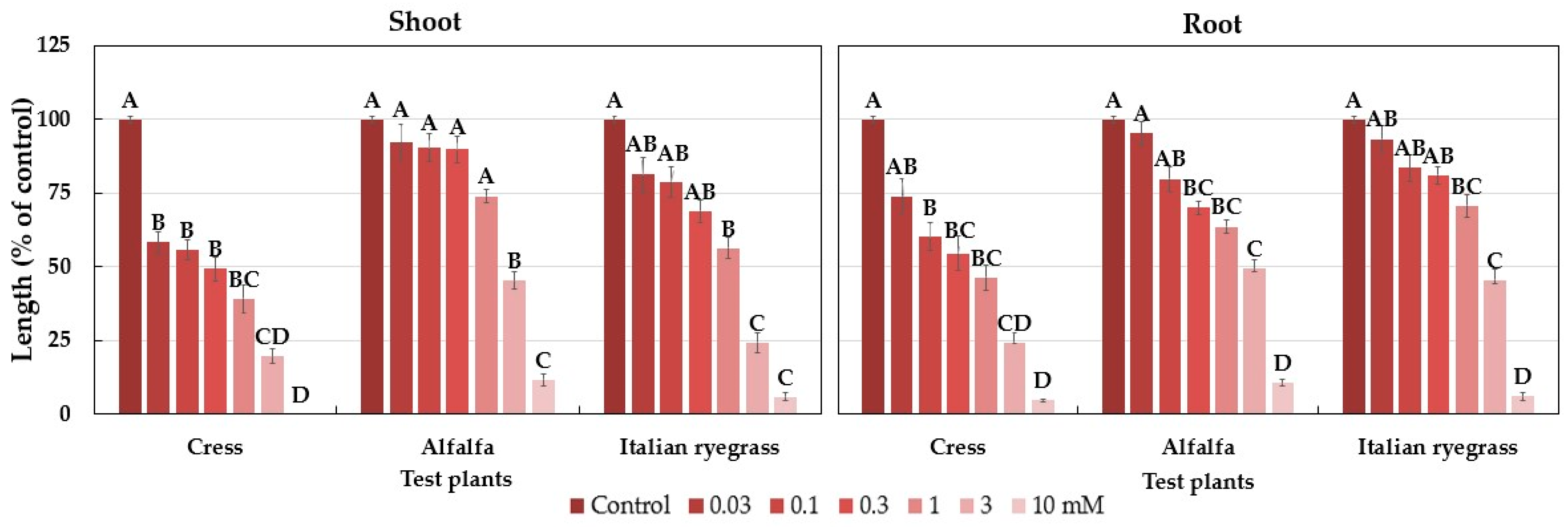
2.1. Allelopathic Activity of the Polygonum chinense Plants
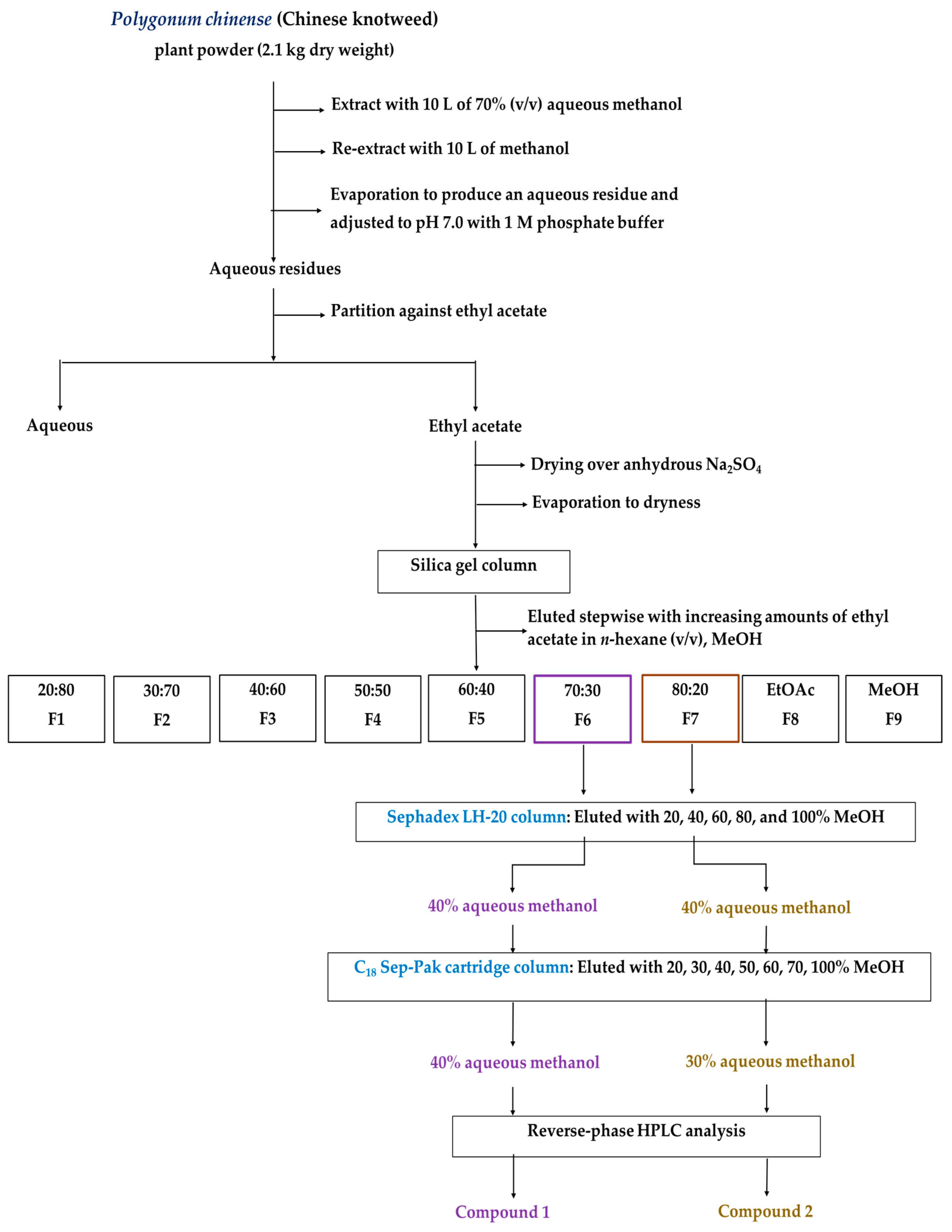
2.2. Isolation and Purification of the Allelopathic Substances
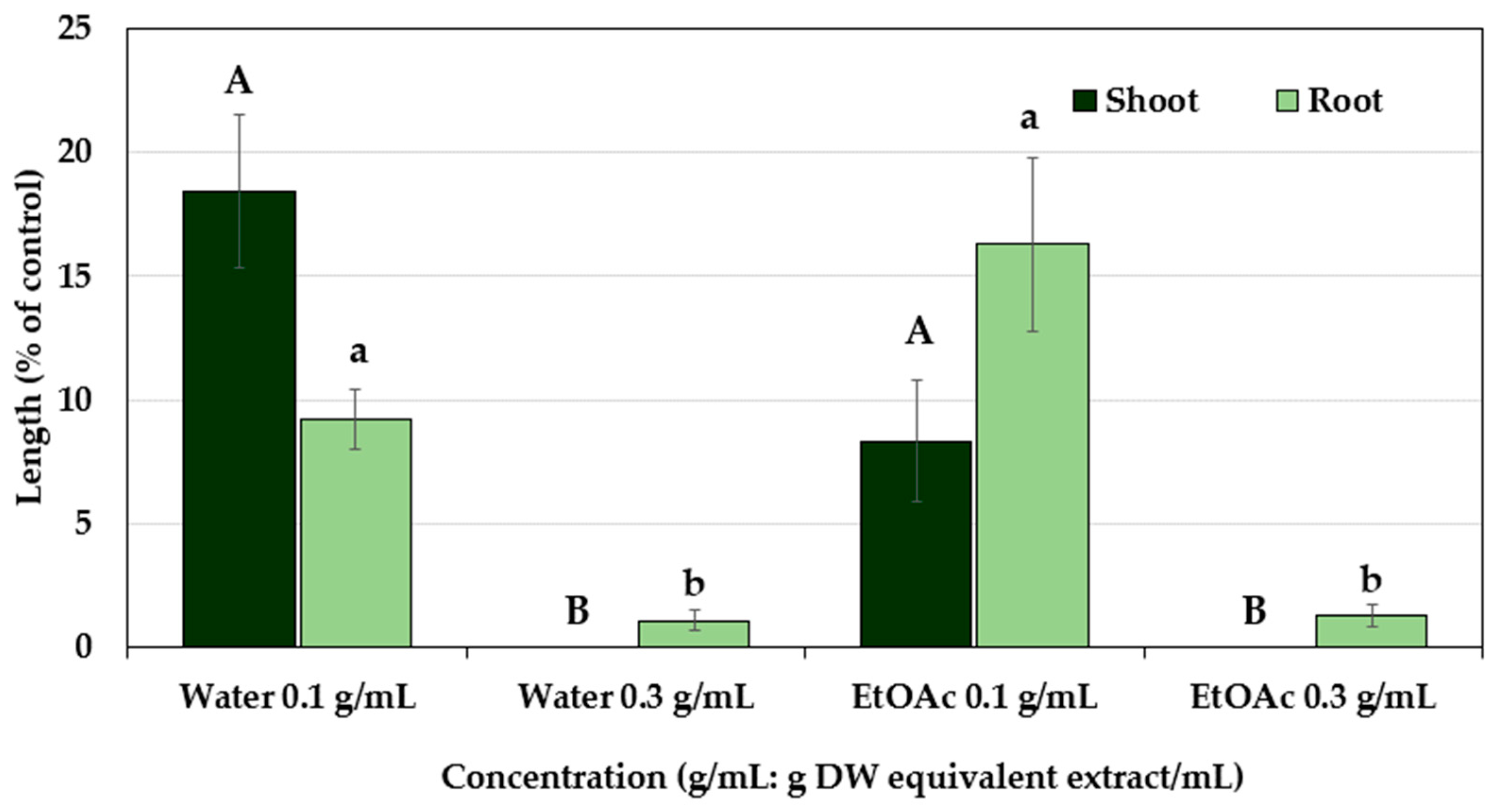
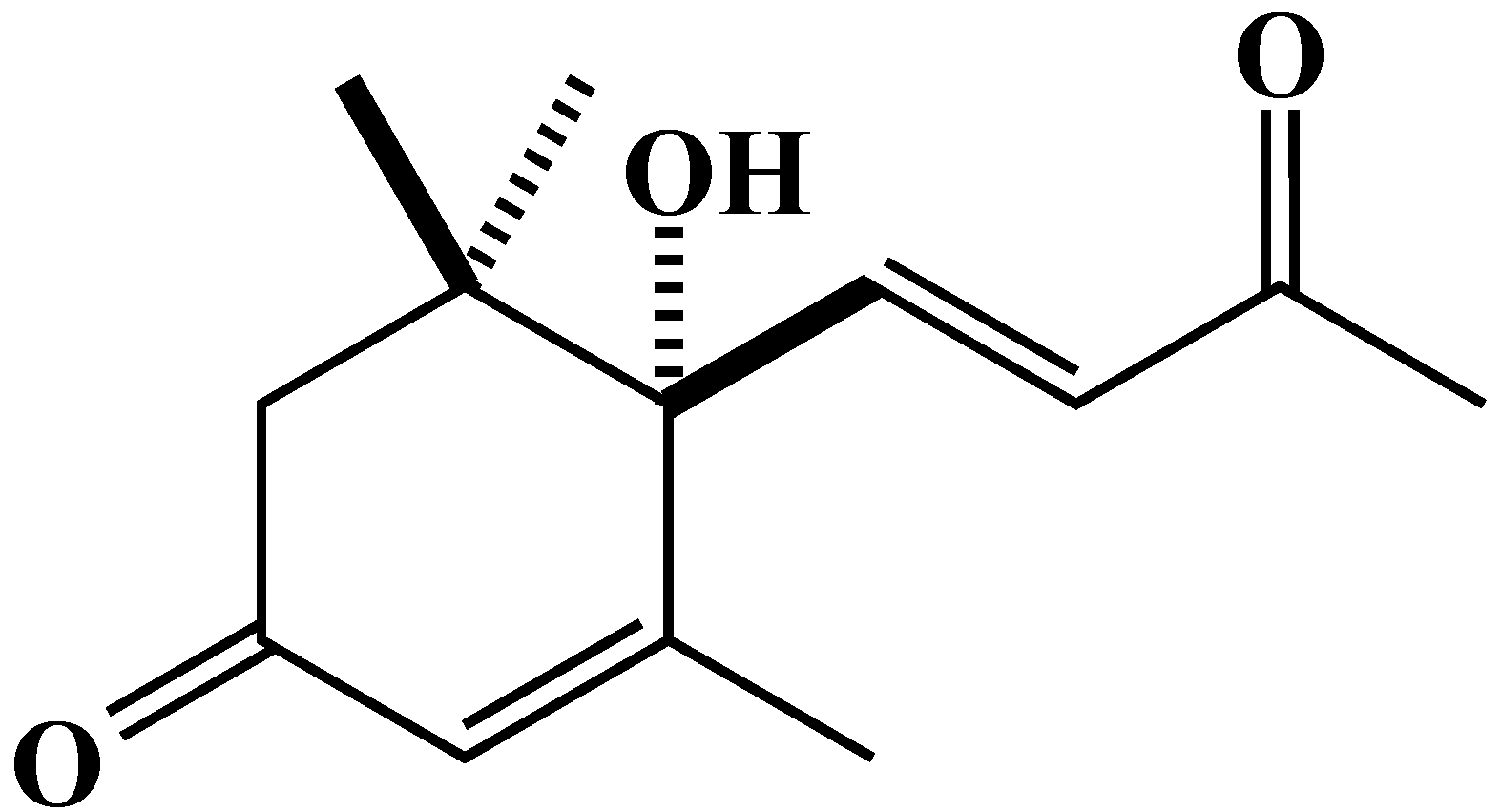
2.3. Characterization and Biological Activity of Compound 1
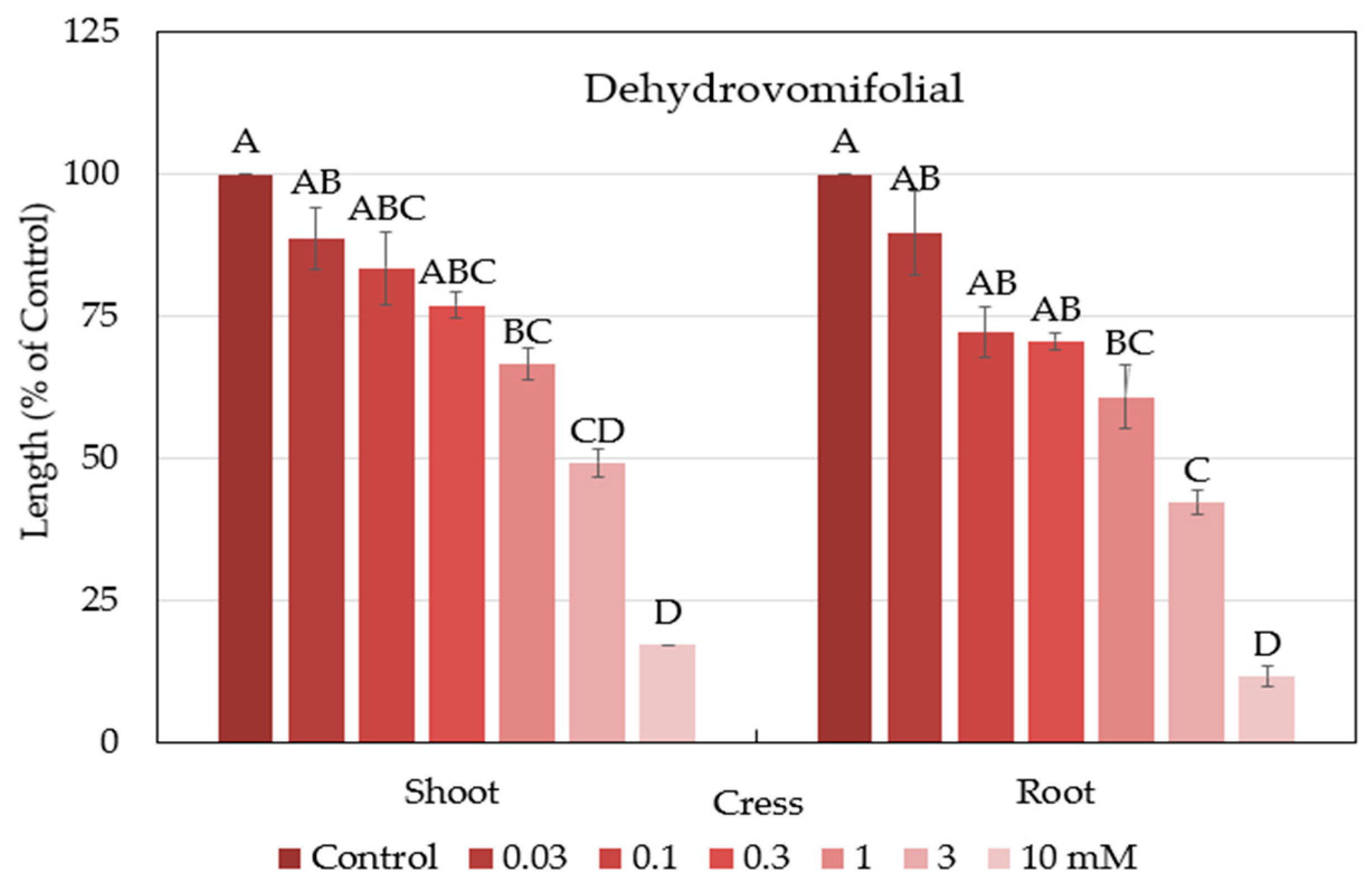
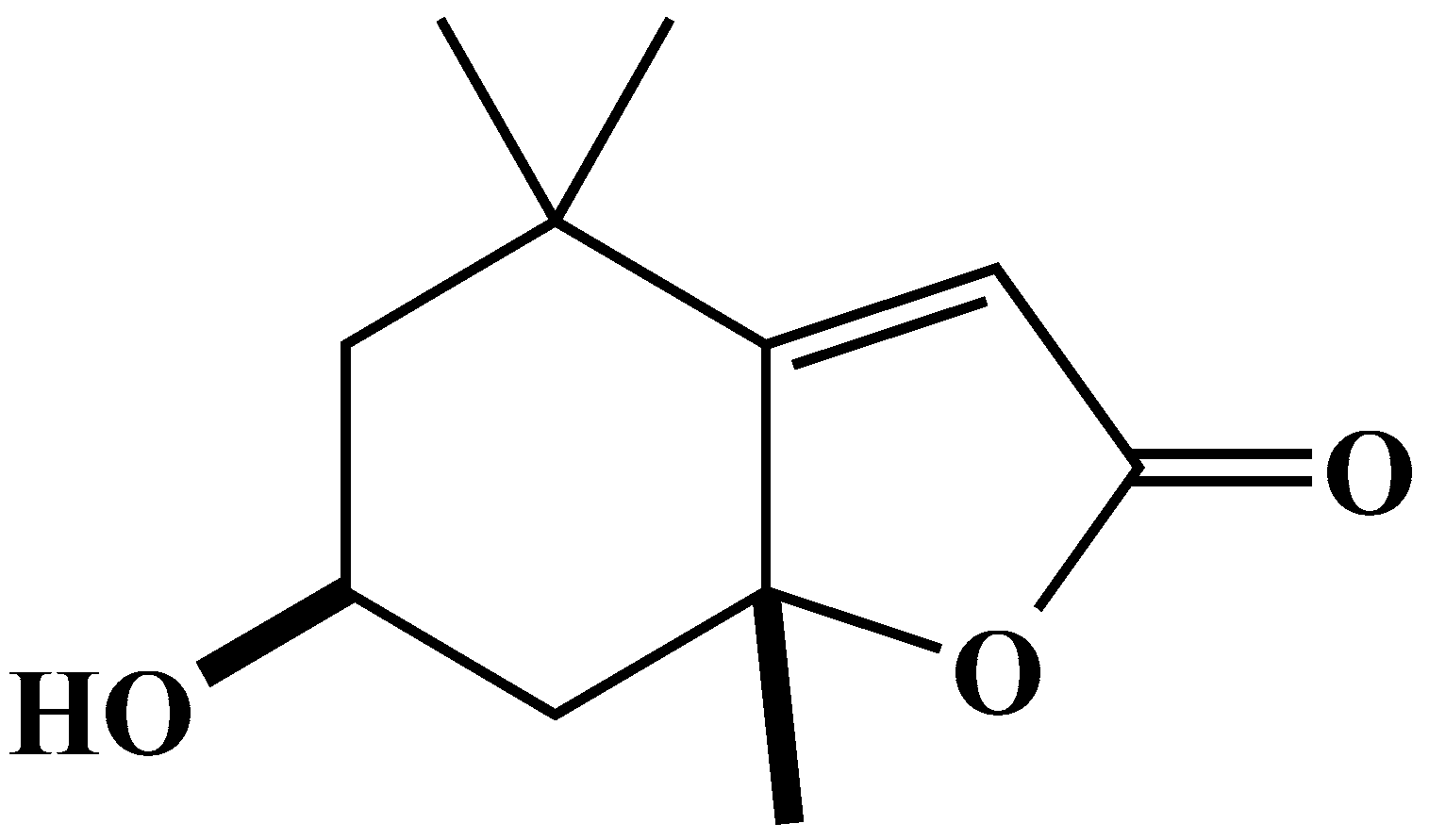
2.4. Characterization and Biological Activity of Compound 2
3. Discussion
4. Materials and Methods
4.1. Extraction and Plant Material
4.2. Growth-Inhibitory Assay
4.3. Isolation and Purification of the Growth-Inhibitory Substances
4.4. Bioassay of the Identified Compounds
4.5. Spectral Data
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Forest Service. Pacific Island Ecosystems at Risk (PIER): Plant threats to Pacific Ecosystems. United States Forest Service, United States Department of Agriculture. 2010. Available online: http://www.hear.org/pier/index.html (accessed on 2 June 2023).
- Galloway, D.J.; Lepper, V.E. Persicaria chinensis—A New Alien Asian Invader? In Proceedings of the 17th Australasian Weeds Conference, Christchurch, New Zealand, 26–30 September 2010; pp. 174–175. Available online: http://www.caws.org.au/awc/2010/awc201011741.pdf (accessed on 2 June 2023).
- eFloras. Flora of North America; Missouri Botanical Garden: St. Louis, MO, USA; Harvard University Herbaria: Cambridge, MA, USA, 2003; Available online: http://www.efloras.org/flora_page.aspx?flora_id=1 (accessed on 7 April 2023).
- eFloras. Flora of China; Missouri Botanical Garden: St. Louis, MO, USA; Harvard University Herbaria: Cambridge, MA, USA, 2003; Available online: http://www.efloras.org/flora_page.aspx?flora_id=2 (accessed on 7 April 2023).
- Goodland, T.; Healey, J.R. The Invasion of Jamaican montane Rainforests by the Australian Tree Pittosporum undulatum in the Blue Mountains of Jamaica; School of Agricultural and Forest Sciences, University of Wales: Bangor, UK, 1996; 55p. [Google Scholar]
- Tanaka, Y.; Van-Ke, N. Edible Wild Plants of Vietnam: The Bountiful Garden; Orchid Press: Bangkok, Thailand, 2007; p. 121. ISBN 978-9-74524-089-6. [Google Scholar]
- Aung, A.T.; Ohn, T.M. Morphological characters, histological characters and nutritional values of Polygonum chinense L. J. Myanmar Acad. Arts Sci. 2018, 16, 4. [Google Scholar]
- Xiao, H.; Tsang, S.; Qin, H.; Choi, F.F.K.; Yang, Z.; Han, Q.; Chen, H.; Xu, H.; Shen, H.; Lu, A.; et al. A bioactivity-guided study on the anti-diarrheal activity of Polygonum chinense Linn. J. Ethnopharmacol. 2013, 149, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Kim, M.; Jang, Y.; Lee, H.W.; Nguyen, H.T.; Nguyen, T.N.; Park, H.W.; Le Dang, Q.; Kim, J.C. Characterization and mechanisms of anti-influenza virus metabolites isolated from the Vietnamese medicinal plant Polygonum chinense. BMC Complement. Altern. Med. 2017, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Chen, D.; Chunli, L.; Qin, K.; Zhou, Q.; Pu, N.; Song, S.; Wang, X. Antimicrobial and anti-biofilm activity of Polygonum chinense L. aqueous extract against Staphylococcus aureus. Sci. Rep. 2022, 12, 21988. [Google Scholar] [CrossRef]
- PPQ. Weed Risk Assessment for Persicaria chinensis (L.) H. Gross (Polygonaceae)—Chinese Knotweed; United States Department of Agriculture, Animal and Plant Health Inspection Service, Plant Protection and Quarantine (PPQ): Raleigh, NC, USA, 2017; p. 25. [Google Scholar]
- Randall, R.P. A Global Compendium of Weeds. Hawaiian Ecosystems at Risk and Department of Agriculture of Western Australia. 2007. Available online: http://www.hear.org/gcw/ (accessed on 16 September 2022).
- Waugh, J. Trade Related Pathways to the Introduction of Terrestrial Invasive Species in the Insular Caribbean (Report to International Programs, US Forest Service) (Draft); International Union for Conservation of Nature: Gland, Switzerland, 2008; p. 37. [Google Scholar]
- Rao, V.S.; Rahman, F.; Sharma, S.N.; Singh, H.S. Control of persistent weeds of tea. In Proceedings of the 28th Tocklai Biennial Conference, Jorhat, India, 24–26 November 1977; pp. 61–64. [Google Scholar]
- Chakravartee, J. Weed control in tea. Two Bud 1994, 41, 2–11. [Google Scholar]
- Haridas, P.; Sharma, V.S. Some common weeds of South Indian tea fields. 14. Polygonum chinense L. and Calceolaria mexicana Benth. Plant. Chron. 1974, 69, 379–380. [Google Scholar]
- Biosecurity New Zealand. Chinese Knotweed, Persicaria chinensis. New Zealand Government, Biosecurity New Zealand. Available online: https://www.biosecurity.govt.nz/pests/chinese-knotweed (accessed on 14 October 2011).
- Farooq, N.; Abbas, T.; Tanveer, A.; Jabran, K. Allelopathy for weed management. In Co-Evolution of Secondary Metabolites; Merillon, J.M., Ramawat, K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–16. [Google Scholar]
- Singh, V.V.; Singh, S.K.; Pratap, T. Tank mix herbicide combination effect on weed and yield of wheat in North-Eastern plain zone. Pharma Innov. J. 2020, 11, 1359–1362. [Google Scholar]
- Bufford, J.L.; Hulme, P.E. Increased adaptive phenotypic plasticity in the introduced range in alien weeds under drought and flooding. Biol. Invasions 2021, 23, 2675–2688. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Y.; Chen, J.; Ji, J.; He, H. Identification and comparison of allelopathic effects from leaf and flower volatiles of the invasive plants’ Mikania micrantha. Chemoecology 2021, 31, 355–365. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy of knotweeds as invasive plants. Plants 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Ridenour, W.M.; Callaway, R.M. The relative importance of allelopathy in interference: The effects of an invasive weed on a native bunchgrass. Oecologia 2001, 126, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Stinson, K.A.; Campbell, S.A.; Powell, J.R.; Wolfe, B.E.; Callaway, R.M.; Thelen, G.C.; Hallett, S.G.; Prati, D.; Klironomos, J.N. Invasive plants suppress the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol. 2006, 4, e140. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Cipollini, D.; Barto, K.; Thelen, G.C.; Hallett, S.G.; Prati, D.; Stinson, K.; Klironomos, J. Novel weapons: Invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 2008, 89, 1043–1055. [Google Scholar] [CrossRef]
- Yang, R.Y.; Mei, L.X.; Tang, J.J.; Chen, X. Allelopathic effects of invasive Solidago canadensis L. on germination and growth of native Chinese plant species. Allelopath. J. 2007, 19, 241–247. [Google Scholar]
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do allelopathic compounds in invasive Solidago canadensis restrain the native European flora? J. Ecol. 2008, 96, 993–1001. [Google Scholar] [CrossRef]
- Li, Y.-P.; Feng, Y.-L.; Kang, Z.-L.; Zheng, Y.-L.; Zhang, J.-L.; Chen, Y.-J. Changes in soil microbial communities due to biological invasions can reduce allelopathic effects. J. Appl. Ecol. 2017, 54, 1281–1290. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Feng, Y.L.; Zhang, L.K.; Callaway, R.M.; Valiente-Banuet, A.; Luo, D.Q.; Liao, Z.Y.; Lei, Y.B.; Barclay, G.F.; Silva-Pereyra, C. Integrating novel chemical weapons and evolutionarily increased competitive ability in success of a tropical invader. New Phytol. 2015, 205, 1350–1359. [Google Scholar] [CrossRef]
- Bourchier, R.S.; Van Hezewijk, B.H. Distribution and potential spread of Japanese knotweed (Polygonum cuspidatum) in Canada relative to climatic thresholds. Invasive Plant Sci. Manag. 2010, 3, 32–39. [Google Scholar] [CrossRef]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1379. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Liu, W.; Guo, J.; Qiang, S.; Li, B.; Wang, J.; Yang, G.; Niu, H.; Gui, F.; Huang, W.; et al. Invasive mechanism and control strategy of Ageratina adenophora (Sprengel). Sci. China Life Sci. 2010, 53, 1291–1298. [Google Scholar] [CrossRef]
- Inoue, M.; Nishimura, H.; Li, H.H.; Mizutani, J. Allelochemicals from Polygonum sachalinense Fr. Schm. (Polygonaceae). J. Chem. Ecol. 1992, 18, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Murrell, C.; Gerber, E.; Krebs, C.; Parepa, M.; Schaffner, U.; Bossdorf, O. Invasive knotweed affects native plants through allelopathy. Am. J. Bot. 2011, 98, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Lun, T.L.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of plant-growth inhibitory constituents from Polygonum chinense Linn and evaluation of their bioherbicidal potential. Plants 2023, 12, 1577. [Google Scholar] [CrossRef] [PubMed]
- Hisahiro, K.; Masaki, B.; Toru, O. Two new megastigmanes from the leaves of Cucumis sativus. Chem. Pharm. Bull. 2007, 55, 133–136. [Google Scholar]
- Mi-Ran, K.; Seung-Kyu, L.; Chang-Soo, K.; Kyung-Soon, K.; Dong-Cheul, M. Phytochemical constituents of Carpesium macrocephalum FR- et SAV-. Arch. Pharm. Res. 2004, 27, 1029–1033. [Google Scholar]
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Rośliny Obcegopochodzenia w Polsce ze Szczególnym Uwzględnieniem Gatunków Inwazyjnych (Plants of Foreign Origin in Poland, with Particular Emphasis on Invasive Species); Generalna Dyrekcja Ochrony Środowiska: Warszawa, Poland, 2012; p. 196. [Google Scholar]
- Yuan, Y.G.; Wang, B.; Zhang, S.S.; Tang, J.J.; Tu, C.; Hu, S.J.; Chen, X. Enhanced allelopathy and competitive ability of invasive plant Solidago canadensis in its introduced range. J. Plant Ecol. 2013, 6, 253–263. [Google Scholar] [CrossRef]
- Suzuki, M.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic property of the invasive plant Tithonia diversifolia and a phytotoxic substance. Acta Biol. Hung. 2017, 68, 187–195. [Google Scholar] [CrossRef][Green Version]
- Kyaw, E.H.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of the phytotoxic potential of Dregea volubilis (L.f.) Benth. ex Hook. f. and identification of its phytotoxic substances for weed control. Agriculture 2022, 12, 1826. [Google Scholar] [CrossRef]
- Moh, S.M.; Kurisawa, N.; Suenaga, K.; Kato-Noguchi, H. Allelopathic potential of Marsdenia tenacissima (Roxb.) Moon against four test plants and the biological activity of its allelopathic novel compound, 8-dehydroxy-11β-O-acetyl-12β-O-tigloyl-17β-marsdenin. Plants 2023, 12, 1663. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, A.; Afshari, R.T.; Asadi, A.; Gharineh, M.H. Allelopathic effects of aqueous extract of leaf stem and root of Sorghum bicolor on seed germination and seedling growth of Vigna radiata L. Not. Sci. Biol. 2011, 3, 114–118. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Alami-Saeid, K.H.; Moshatati, A. Effect of leaf, stem and root extract of alfalfa (Melilotus indicus) on seed germination and seedling growth of wheat (Triticum aestivum). Int. J. Agric. Sci. 2013, 5, 44–49. [Google Scholar]
- Sodaeizadeh, H.; Rafieiolhossaini, M.; Havlík, J.; van Damme, P. Allelopathic Activity of Different Plant Parts of Peganum harmala L. and Identification of Their Growth Inhibitors Substances. Plant Growth Regul. 2009, 59, 227–236. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; De Luna, R.D.; Hofilena, J.G. Antimicrobial terpenoids from Pterocarpus indicus. Nat. Prod. Res. 2005, 19, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xie, H.; Gong, Y.; Wang, Q.; Yang, Y. Secondary metabolites from the seaweed Gracilaria lemaneiformis and their allelopathic effects on Skeletonema costatum. Biochem. Syst. Ecol. 2011, 39, 397–400. [Google Scholar] [CrossRef]
- Takasugi, M.; Anetai, M.; Katsui, N.; Masamune, T. The occurrence of vomifoliol, dehydrovomifoliol and dihydrophaseic acid in the roots of “kidney bean” (Phaseolus vulgaris L.). Chem. Lett. 1973, 2, 245–248. [Google Scholar] [CrossRef]
- Macías, F.A.; Oliva, R.M.; Varela, R.M.; Torres, A.; Molinillo, J.M. Allelochemicals from sunflower leaves cv. Peredovick. Phytochem. 1999, 52, 613–621. [Google Scholar] [CrossRef]
- Kim, I.; Chin, Y.W.; Lim, S.W.; Kim, Y.C.; Kim, J. Norisoprenoids and hepatoprotective flavone glycosides from the aerial parts of Beta vulgaris var. cicla. Arch. Pharm. Res. 2004, 27, 600–603. [Google Scholar] [CrossRef]
- Cutillo, F.; D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Zarrelli, A. Terpenoids and phenol derivatives from Malva silvestris. Phytochemistry 2006, 67, 481–485. [Google Scholar] [CrossRef]
- Machado, F.B.; Yamamoto, R.E.; Zanoli, K.; Nocchi, S.R.; Novello, C.R.; Schuquel, I.T.A.; Sakuragui, C.M.; Luftmann, H.; Ueda-Nakamura, T.; Nakamura, C.V.; et al. Evaluation of the antiproliferative activity of the leaves from Arctium lappa by a bioassay-guided fractionation. Molecules 2012, 17, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, L.; Mantovanelli, G.C.; Bernardi, D.I.; Silva, A.A.; De Oliveira, R.S., Jr.; Ishii-Iwamoto, E.L.; Sarragiotto, M.H.; Baldoqui, D.C. Isolation of the constituents and evaluation of allelopathic potential of Raphanus sativus L. (Brassicaceae). Planta Med. 2014, 80, P1L61. [Google Scholar] [CrossRef]
- Mayer, H. Synthesis of optically active carotenoids and related compounds. Pure Appl. Chem. 1979, 51, 535–564. [Google Scholar] [CrossRef]
- Ren, Y.; Shen, L.; Zhang, D.; Dai, S. Two new sesquiterpenoids from Solanum lyratum with cytotoxic activities. Chem. Pharm. Bull. 2009, 57, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kang, M.-C.; Lee, K.-W.; Kang, S.M.; Lee, W.W.; Jeon, Y.J. Antioxidant activity and cell protective effect of loliolide isolated from Sargassum ringgoldianum subsp. coreanum. Algae 2011, 26, 201–208. [Google Scholar] [CrossRef]
- Okada, N.; Shirata, K.; Niwano, M.; Koshino, H.; Uramoto, M. Immunosuppressive activity of a monoterpene from Eucommia ulmoides. Phytochemistry 1994, 37, 281–282. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Liu, C.-H.; Burnouf, T.; Wang, G.H.; Chang, S.P.; Jassey, A.; Tai, C.; Tai, C.; Huang, C.; Richardson, C.; et al. Activity-based and fraction-guided analysis of Phyllanthus urinaria identifies loliolide as a potent inhibitor of hepatitis C virus entry. Antivir. Res. 2016, 130, 58–68. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Winska, K.; Maczka, W.; Potaniec, B.; Anioł, M. Loliolide—The most ubiquitous lactone. Folia Biol. Oecol. 2015, 11, 1–8. [Google Scholar] [CrossRef]
- Dietz, H.; Winterhalter, P. Phytotoxic constituents from Bunias orientalis leaves. Phytochemistry 1996, 42, 1005–1010. [Google Scholar] [CrossRef]
- Bich, T.T.N.; Kato-Noguchi, H. Isolation and identification of a phytotoxic substance from the emergent macrophyte Centrostachys aquatica. Bot. Stud. 2014, 55, 59. [Google Scholar] [CrossRef]
- Zhou, B.; Kong, C.H.; Li, Y.H.; Wang, P.; Xu, X.H. Crabgrass (Digitaria sanguinalis) allelochemicals that interfere with crop growth and the soil microbial community. J. Agric. Food Chem. 2013, 61, 5310–5317. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of two potential phytotoxic substances from the aquatic fern Marsilea crenata. J. Plant Biol. 2017, 60, 75–81. [Google Scholar] [CrossRef]
- Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity and growth inhibitory substances from Albizia richardiana (Voigt.) King & Prain. Appl. Sci. 2021, 11, 1455. [Google Scholar]
- DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Temussi, F.; Zarrelli, A. New dimeric phenanthrenoids from the rhizomes of Juncus acutus. Structure determination and antialgal activity. Tetrahedron 2003, 59, 2317–2324. [Google Scholar] [CrossRef]
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia. Eng. 2011, 18, 240–246. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Simberloff, D.; McKinney, M.L.; Holle, B.V. How many, and which, plants will invade natural areas. Biol. Invasions 2001, 3, 1–8. [Google Scholar] [CrossRef]
- Meiners, S.J.; Kong, C.H.; Ladwig, L.M.; Pisula, N.L.; Lang, K.A. Developing an ecological context for allelopathy. Plant Ecol. 2012, 213, 1861–1867. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ueda, M.; Furumoto, T.; Kawanami, Y. Retarding activity of 6-O-acyl-D-allose against plant growth. Biosci. Biotechnol. Biochem. 2010, 74, 216–217. [Google Scholar] [CrossRef][Green Version]
- Cueva, C.; Moreno-Arribas, M.V.; Martinez-Alvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Lopez Rivas, C.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 16, 372–382. [Google Scholar] [CrossRef]
- Sanchez-Maldonado, A.F.; Schieber, A.; Ganzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef] [PubMed]


| Test Plant | I50 Value (g DW Equivalent Extract/mL) | |
|---|---|---|
| Shoot | Root | |
| Alfalfa | 0.0035 | 0.0049 |
| Italian ryegrass | 0.0043 | 0.0052 |
| Test Plant | I50 Value (mM) | |
|---|---|---|
| Shoot | Root | |
| Cress | 2 | 1.2 |
| Test Plant | I50 Value (mM) | |
|---|---|---|
| Shoot | Root | |
| Cress | 0.15 | 0.33 |
| Alfalfa | 2.33 | 2.23 |
| Italian ryegrass | 0.80 | 1.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lun, T.L.; Tojo, S.; Teruya, T.; Kato-Noguchi, H. Allelopathic Activity of the Invasive Plant Polygonum chinense Linn. and Its Allelopathic Substances. Plants 2023, 12, 2968. https://doi.org/10.3390/plants12162968
Lun TL, Tojo S, Teruya T, Kato-Noguchi H. Allelopathic Activity of the Invasive Plant Polygonum chinense Linn. and Its Allelopathic Substances. Plants. 2023; 12(16):2968. https://doi.org/10.3390/plants12162968
Chicago/Turabian StyleLun, Thang Lam, Shunya Tojo, Toshiaki Teruya, and Hisashi Kato-Noguchi. 2023. "Allelopathic Activity of the Invasive Plant Polygonum chinense Linn. and Its Allelopathic Substances" Plants 12, no. 16: 2968. https://doi.org/10.3390/plants12162968
APA StyleLun, T. L., Tojo, S., Teruya, T., & Kato-Noguchi, H. (2023). Allelopathic Activity of the Invasive Plant Polygonum chinense Linn. and Its Allelopathic Substances. Plants, 12(16), 2968. https://doi.org/10.3390/plants12162968







