Abstract
The genus Beauveria includes important entomopathogenic and endophytic fungi; among them, Beauveria bassiana is the most studied species. However, there is little knowledge regarding their antimicrobial activity. The current research has been conducted to evaluate the in vitro antagonistic activity of B. bassiana and the antimicrobial efficacy of its Exo and Endo metabolites against Bacillus cereus, B. megaterium, Clavibacter michiganensis (Gram positive bacteria, G+ve), Xanthomonas campestris, Pseudomonas aeruginosa and P. fluorescence (Gram negative bacteria, G−ve). In addition, solid-phase microextraction (SPME) was coupled with Gas Chromatography-Mass Spectrometry (GC/MS) to qualitatively measure the volatile organic compounds’ (VOCs) metabolic profile of the most efficient studied isolate of B. bassiana. The obtained results showed that the isolate UniB2439-3 has a promising antibacterial effect against most of the studied target bacteria. An SPME-GC/MS analysis of VOCs revealed the presence of ethanol, butanal,2-methyl, 2,4-dimethyl-1-heptene, octane, 4-methyl and β-elemene as the dominant bioactive compounds. The results demonstrated that the efficient isolate of B. bassiana can be potentially used as a biocontrol agent against several bacteria, especially G+ve ones.
1. Introduction
Genus Beauveria includes entomopathogenic and endophytic fungi, which are widespread in different habitats [1,2,3]. Furthermore, many researchers have reported that fungi in the genus Beauveria can produce enzymes for biotransformation and biodestructors [4,5,6]. Some species of this genus, such as B. bassiana and B. brongniartii, are able to produce mycoinsecticides [7]. B. bassiana is also a beneficial microorganism (BM) and endophytic fungus (EF) in several crops and is commonly known as biological control agent against a variety of agricultural pests [3,8,9,10]. The application of B. bassiana has many advantages, such as being a form of eco-friendly management compared to chemical pesticides, and being harmless to human health [8,10,11,12]. For decades, several scientists have reported the importance of B. bassiana in reducing a range of nuisance insects, where it can induce direct insect mortality [2,13,14] and reach a 90% reduction in life-time fecundity [15].
A recent study, conducted by Barra-Bucarei et al. [16] to evaluate the colonization ability of native endophytes of different strains of B. bassiana and their antifungal effect against Botrytis cinerea in tomato and chili pepper, concluded that all studied strains had significant in vitro antagonism against B. cinerea. The same study reported that the native strains of B. bassiana were able to colonize tomato and chili pepper tissues and provided important levels of antagonism against B. cinerea [16].
Sinno et al. [10] reported that different isolates of B. bassiana have plant-growth-promoting effects (PGP) and are a protective agent for tomato plants against B. cinerea, Alternaria alternata, the pest aphis, and Macrosiphum euphorbiae [10].
The results showed that some studied isolates were able to control the two phytopathogens, and one isolate was also able to promote plant growth [10]. The antibacterial activity of a crude ethyl acetate extract of B. bassiana against some aerobic pathogenic bacteria was tested by Parine et al. [17]. The results explained that the extract of B. bassiana possesses a strong inhibitory activity against many of the tested species, especially Bacillus megaterium, B. subtilis, B. sphaericus and Escherichia coli [17]. It showed a moderate effect against Micrococcus luteus, Pseudomonas aeruginosa and a low effect against Streptococcus pyogenes and Chromobacterium violaceum [17]. In another study, the application of conidia of B. bassiana protected tomato and potato seedlings from the damping-off disease caused by the soil-borne pathogen Rhizoctonia solani [18,19].
Recently, there is a large amount of interest in discovering natural-substances-based plants or those with microbe origins that have an antimicrobial effect [20,21]. However, the newly discovered natural substances should be evaluated for safety to avoid any possible negative health impact [22,23]. In addition, the discovery of possible natural alternatives to the excessive use of synthetic chemicals, decreasing the environmental hazards and avoiding the appearance of new microbial strains that are resistant to common microbicide compounds, should be highly considered [18,24].
There is little information regarding the bioactive metabolites with antimicrobial activity produced by either diffusable or volatile B. bassiana or their mechanism(s) of action regarding either their antimicrobial or plant-growth-promotion effects. A recent study conducted by Wang et al. [25] reported that B. bassiana produces a variety of toxins, such as beauvericin, bassianin, bassianolide, beauverolides, tenellin, oosporein, and oxalic acid, which enable B. bassiana to invade, parasitize, and destroy host tissues. Therefore, the precise chemical characterization and determination of the main bioactive single substances of B. bassiana will certainly aid in understanding its biological importance. In addition, the details of the chemical constituents of B. bassiana will undoubtedly have various applications, such as controlling plant diseases, taking into consideration the heavy reliance on chemicals that are extremely harmful to the environment as well as plants, animals, and human health. Furthermore, the insecticidal effects of B. bassiana have also been extensively studied, while their antifungal or antibacterial effects have received less attention.
Different volatiles are produced in huge quantities by a number of microorganisms. The volatile molecules, which can be both organic (VOCs) and inorganic, are crucial for such an environment, since they have the power to affect both beneficial and harmful microbes [26]. The significance of studying microbial volatile compounds is due to the fact that one of the typical strategies of inter- and intra-organismal communication is due to their production of volatile substances [26].
The main objective of this research is to study the chemical composition of the principal volatile organic compounds (VOCs) of B. bassiana. Hence, the chemical composition of B. bassiana metabolites will aid in the detection and differentiation of this species from others. The full identification of its metabolite profile can aid in its utilization in industrial, agricultural, and pharmaceutical fields. In addition, in this research, we will expand the possible benefits of B. bassiana against new non-reported target phytopathogens. The aims of the current research were to: (i) evaluate the antagonistic activity of five isolates of B. bassiana against some bacteria; (ii) investigate the in vitro antimicrobial activity of diffusible and volatile metabolites produced by the most efficient isolate; and (iii) chemically characterize VOCs produced by the most efficient isolate using SPME-GC/MS analysis.
2. Results
2.1. Molecular Identification of the Studied Isolates of Beauveria
The PCR amplification for β-tubulin genes with Bt2a/Bt2b produced, for each gDNA extracted from the above five isolates (UniB2439-1, UniB2439-2, UniB2439-3, UniB2439-4, and UniB2439-5), amplicons with a nucleotide length of about 330 bp. The DNA extracted from the same five isolates and amplified with ITS5/ITS4 for rRNA produced amplicons with a nucleotide length of about 600 bp. No amplification was observed in the case of the negative control. The amplicons were directly sequenced (BMR Genomics, Padova, Italy), and the obtained sequences were compared with those available in the GenBank nucleotide archive, showing a high similarity percentage (97.29%) with the sequences AB829899, AB829898, and CP045886.1, and those available for B. bassiana in the NCBI database using Basic Local Alignment Search Tool software (BLAST) (Bethesda, Rockville Pike, MD, USA) [27]. The five obtained sequences were deposited in the NCBI GeneBank with accession numbers FR989662–FR989666. The phylogenetic analysis confirmed the identification of the five studied isolates as B. bassiana (Figures S1 and S2).
2.2. Antagonistic Activity of B. bassiana Isolates
The preliminary results showed that all tested isolates of B. bassiana had antagonistic effects against most tested bacterial strains, as illustrated in Table S1. UniB2439-3 was the most efficient isolate. In fact, this isolate showed the most significant effect against Bacillus cereus and Clavibacter michiganensis, a moderate effect against B. megaterium and a low effect against Xanthomonas campestris and Pseudomonas fluorescens. This isolate did not show any activity against P. aeruginosa (Figure 1). Therefore, the UniB2439-3 isolate was selected for further biological and chromatographic analyses.
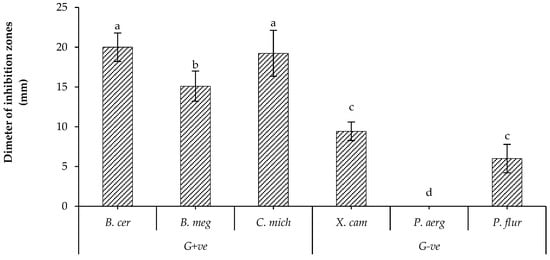
Figure 1.
Antagonistic activity of B. bassiana UniB2439-3. Bars with different letters are significantly different at p < 0.05. Data for each bar are expressed as the mean of three replicates ± SDs.
2.3. Antimicrobial Activity of Exo- and Endo-Diffusible Metabolites
The antimicrobial activity of extracted metabolites was determined following the disc-diffusion method. The obtained results for the metabolites extracted from the selected isolate of B. bassiana UniB2439-3 showed that extracellular metabolites (Exo-ME) were more able to inhibit the growth of most tested bacterial strains than the endocellular (Endo-ME) (Table 1). In particular, Exo-ME showed the most significant activity against C. michiganensis. In addition, both extracts showed equal activity against X. campestris, whereas only Endo-ME showed antibacterial activity against P. aeruginosa. On the other hand, Endo-ME was not active against B. cereus or C. michiganensis.

Table 1.
Antibacterial activity of diffusible metabolites from B. bassiana UniB2439-3.
2.4. Antibacterial Activity of Volatiles Metabolites
The in vitro antibacterial activity of the volatile metabolites eventually emitted by B. bassiana was evaluated against both the grown-visible colonies (GVC) and aqueous suspension (AQS) of each tested bacterial strain. The results of an in vitro bioactivity assay demonstrated that the studied isolate of B. bassiana (UniB2439-3) produced bioactive volatile metabolites that were able to significantly reduce the growth of tested bacterial strains compared to tetracycline (positive control). In particular, the efficacy of the produced volatile substances was high against the AQS of all tested bacterial strains, higher than GVC (Table 2). In addition, the highest antibacterial activity was observed in the case of GVC against B. megaterium (G+ve) and P. fluorescens (G−ve), estimated at 77.5 and 52.5%, respectively. On the other hand, the highest antibacterial activity in the case of AQS was observed against B. megaterium (G+ve) and P. aeruginosa (G−ve), estimated at 92.0 and 87.5%, respectively.

Table 2.
Antibacterial activity of volatile metabolites from B. bassiana UniB2439-3.
2.5. SPME-GC/MS Analysis of VOCs
A GC-MS analysis of the VOCs produced by B. bassiana UniB2439-3 was illustrated in Figure S3. In Table 3, all detected volatile compounds identified from B. bassiana UniB2439-3 are listed. The most dominant principal compounds, followed by their relative area percentage (R.A.%), are as follows: (i) nitrous oxide (27.57%), (ii) ethanol (4.69%), (iii) butanal, 3-methyl (1.32%), (iv) 2,4-dimethyl-1-heptene (0.63%), (v) octane, 4-methyl (1.99%), and (vi) β-elemene (6.98%) (Figure 2a–f). The mass spectra of the most abundant compounds are illustrated in Figures S4–S8. The resulting VOCs were not detected in the PDA culture used as a negative control (not inoculated with the fungus).

Table 3.
SPME-GC/MS analysis of VOCs extracted from B. bassiana UniB2439-3.
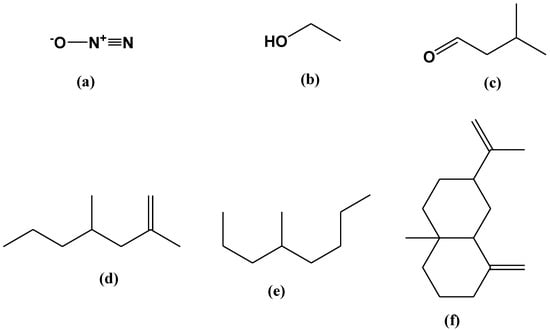
Figure 2.
Chemical structures of the main VOCs identified by the SPME analysis: (a) nitrous oxide; (b) ethanol; (c) butanal,3-methyl; (d) 2,4-dimethyl-1-heptene; (e) octane, 4-methyl and (f) β-elemene.
The eventual fragmentation of the acquired volatile metabolites, as described here, is also shown in Table 3. Beauvericin was fragmented into diethyl phthalate with 90%, the major dominant constituent, carbon dioxide, and nitrous oxide. Bassianolide was converted to butanal, 3-methyl- with 81% or 1-butanol, 3-methyl- with 83%. Regarding bassianin, GC-MS analysis showed that this compound was converted into 2,4-Dimethyl-1-heptene with 90%. Beauveriolide was fragmented into butanal, 3-methyl with 81%, carbon dioxide and nitrous oxide. Regarding cyclosporine, the results demonstrated that this compound was fragmented into butanal, 2-methyl with 90%, butanal, 3-methyl with 81%, 1-butanol, 3-methyl with 83%, carbon dioxide and nitrous oxide.
3. Discussion
Research has been conducted recently to overcome the multi-drug-resistant (MDR) microorganisms to different antibiotics and chemotherapeutic agents [29]. Hence, the search for new active and natural agents has attracted great interest, particularly for human health and environmental protection [30]. Beauveria, one of the most studied genera among entomopathogenic fungi, has various biological applications as a growth-promoting agent or insecticide [9,10,31,32].
The results revealed that both extracts are less effective than the control (tetracycline); nevertheless, they can be regarded as hopeful and prospective antimicrobial agents or as alternatives for synthetic pesticides. On the other hand, considering the higher activity of Exo-ME against C. michiganensis and the equal activity of both extracts against X. campestris, it would be beneficial to consider the potential synergistic effects of combining the two extracts in future studies.
On the other hand, Barra-Bucarei et al. [16] studied the antifungal activity of 10 native strains of B. bassiana, an endophyte for tomato and chili pepper, and observed that the majority of the studied native strains were able to colonize tomato and chili pepper tissues and showed a promising antagonistic effect against B. cinerea.
The capacity of B. bassiana to produce several volatile metabolites with possible antimicrobial effects is in agreement with previous bibliographic research investigating its antagonistic effect against several phytopathogens [9,17,33]. In fact, the bibliographic research revealed that the genus Beauveria produced some interesting metabolites, such as oosporein, beauvericin, bassianolide, bassianin, beauveriolide, bassiacridin and cyclosporine, with notable insecticide and antimicrobial actions [34,35,36,37,38,39,40].
Among the different bioactive metabolites produced by B. bassiana, several studies revealed that beauvericin and oosporein evidenced remarkable antibiotic and antifungal properties [39,40], which are probably involved in the microbial growth-inhibition observed in the bioassay presented in this study. Furthermore, Wang and Xu [41] reported that beauvericin was one of the active constituents of B. bassiana and confirmed to have antimicrobial activity and anti-tumor effects, especially against human leukemia. In another study, conducted by Manning and Wyatt [42], the results demonstrated that oosporein, extracted from the broth cultures of Beauveria and Chaetomium, has been identified as a toxic substance for plants and poultry.
Our findings from the SPME-GC/MS analysis showed that B. bassiana produces a variety of important VOCs, such as: (i) butanal, 3-methyl; (ii) 2,4-dimethyl-1-heptene; and (iii) octane, 4-methyl. These findings are in agreement with those of Chiron and Michelot [43], who explained that the main chemical groups released by fungi are alcohols (isomers of butanol, pentanol, and octanol), hydrocarbons, ketones, and terpenes [44].
The possible mechanism of volatile antimicrobial effects, in general, may be explained by the potential of volatiles to flow across a structure of soil gaps since they are active in both gaseous and liquid phases and have the potential of revolatization after flowing through water-saturated pores [26]. However, because of their high vapor pressure, volatiles mostly traverse through vapor diffusion. However, this process is regulated by the intrinsic chemical characteristics of each VOC and also the physicochemical characteristics of adjacent soil, which affect adsorption, desorption, and degradation.
In particular, 2,4-dimethyl-1-heptene showed antimicrobial activity, as reported by Mannaa and Kim [45]. In addition, 2,3,3-trimethyl-Octane, which is close to octane, 4-methyl, showed a higher docking energy than the commercial anti-inflammatory drug, as reported by Saravanakumar et al. [46]. Methyl-1-butanol was identified as one of the primary volatile chemicals released from active cultures of Enterobacter agglomerans [47]. Salih et al. [48] also reported that butanol, among the major constituents detected in Coccoloba peltate, showed notable antioxidant and cytotoxic effects.
Our obtained results also detected the presence of an important sesquiterpene compound identified as β-elemene (cyclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl), 1S-(1.alpha.,2.beta.,4. beta.) among the detected VOC substances from the studied Beauveria isolate. β-elemene was identified for the first time in 1994 in a dry rhizome extract from Curcuma phaeocaulis, C. kwangsinensis, and C. wenyujing [35]. In addition, β-elemene is also one of the common sesquiterpenes of several aromatic essential oils extracted from Proteus vulgaris [49]. β-elemene was also found in wild hops from Lithuania at levels up to 14% [50], and in notable amounts in the medical cannabis cultivar ‘bedropuur’ [51]. The same compound has notable antimicrobial activity against different pathogens, including Mycobacterium tuberculosis, as reported by Sieniawska et al. [52].
Generally, the mechanism of the antimicrobial activity of several terpenes is highly related to their lipophilic properties, which enable them to dissolve in the phospholipid layers of a microbial cell membrane [53]. Particularly, natural sesquiterpenes such as β-elemene, which originate from plants and microorganisms, showed promising antimicrobial activity [54,55]. A recent study conducted by Monga and Sharma [56] reported that β-elemene and R-limonene play an essential role in degrading the microbial cell wall, altering the expressions of dprE1 and clgR genes, which are responsible for cell wall synthesis and cell membrane preservation, respectively.
Some recent studies reported on the promising cytotoxic effects of β-elemene, which can inhibit cell proliferation, arrest cell cycle, and induce cell apoptosis or autophagy [57]. Β-elemene is one of the most promising inhibitors of the glycolysis rate-limiting enzyme, especially (PKM2), through its interference with tumor glycolysis, which is considered one of the most important recent strategies for treating tumors [58,59]. In fact, research has reported that inhibition of tumor growth and proliferation can be achieved by downregulating the expression of the PKM2 enzyme [60]. In addition, Pan et al. [61] pointed to the role of β-elemene in inhibiting breast cancer cell migration by converting dimer and tetramer forms of PKM2, inhibiting aerobic glycolysis, and reducing the utilization of glucose and the production of lactic acid for tumor cell growth.
4. Materials and Methods
4.1. Isolation, Culturing and Identification
Five strains of Beauveria bassiana (UniB2439-1; UniB2439-2, UniB2439-3, UniB2439-4, UniB2439-5) were isolated from different rhizospheric soil samples of Actinidia spp. and identified based on their morphological features and molecular basis. For molecular identification, the total gDNA was extracted, and a partial part of β-tubulin and ribosomal DNA genes were amplified using the universal primers Bt2a (5′-GGTAACCAAATCGGTGCTGCTTTC-3′)/Bt2b (5′-ACCCTCAGTGTAGTGACCCTTGGC-3′) [62], and ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′)/ITS4 (5′-TCCTCCGCTTATTGATATGC-3′), which was used for amplifying the ribosomal DNA [63]. The obtained amplicons were sequenced and then analyzed using Basic Local Alignment Search Tool software (BLAST-USA). A partial phylogenetic analysis was carried out for the two amplified genes. The studied isolates were maintained as lyophils at 4 °C in the fungal collection of the School of Agricultural, Forestry, Food and Environmental Sciences (SAFE), University of Basilicata, Potenza, Italy. The subcultures were carried out on Sabouraud Dextrose Agar plus 1% yeast-extract (SDAY) nutrient media [5] and incubated at 22 ± 2 °C for 96 h [64].
4.2. Antagonistic Activity
The antagonistic activity of the five studied isolates of B. bassiana was evaluated against some pathogenic bacteria. All tested isolates were obtained from the pure cultures conserved in the collection of SAFE and identified using morphological and molecular methods. The tested bacteria strains are listed in Table 4.

Table 4.
The tested bacterial strains in the current study.
An antibacterial assay was carried out as described by Elshafie et al. [65]. A fungal disc of approximately 0.5 cm from the fresh PDA culture (96 h) of each studied isolate of B. bassiana was deposited in the center of the King B nutrient media (KB) Petri dish and incubated for 16 h at 22 ± 2 °C. Successively, a suspension of soft-agar (0.7%) of each tested bacteria at 108 CFU/mL was sprayed over the plates using Eco-Spray Ecological Aerosol (Seidden Identification, Madrid, Spain). All plates were incubated at 30 °C for 24 h. Two KB plates inoculated only with each tested bacteria were used as a negative control. The experiment was run in triplicate, and the diameter of the inhibition zone was measured with a caliber and recorded as the mean ± SD (n = 3). The antagonistic bacterial activity percentage (ABP%) was calculated using Equation (1):
where ABP is the antagonistic bacterial activity; D.iz: is the diameter of inhibition zones in cm; D.ct: is the diameter of control plates in cm.
ABP (%) = D.iz/D.ct × 100
4.3. Extraction of Secondary Metabolites
On the basis of the preliminary antagonistic assay, the most efficient isolate (UniB2439-3) of B. bassiana was selected for successive studies. For this purpose, 2 mL of the fungal suspension (106 spores/mL) of the above isolate was used to inoculate 500 mL SDY broth nutrient media and then incubated for 7 days at 25 °C in agitation (180 rpm). Both Exo-ME and Endo-ME were extracted from the broth culture after the incubation period.
For Endo-ME, the incubated broth culture was centrifuged at 40,000× g for 15 min, and the pellet (2 g) was collected, resuspended in 50 mL of Limonene (CAS 138-86-3- Aldrich, Steinheim, Germany) and shaken for 2 h; after this, the solvent was evaporated using the rotary-evaporator (Heidolph WB2000, Schwabach, Germany). The residue was resuspended in 2 mL of sterile distilled water (SDW), following the Solid Phase Extraction (SPE) method using a C-18 column (Thermo Scientific, Rockwood, TN, USA), and recovered using 1 mL methanol to reach the final original concentration of (20 mg/mL) [24].
For Exo-ME, the supernatant (250 mL) obtained from the above centrifugation step was filtered using 0.22 µm (syringe filter—hydrophilic, Minisart, Goettingen, Germany) and extracted using a separator funnel containing 250 mL ethyl acetate/ethanol (70:30; v/v) and shacked for 15 min. The organic phase was filtered through a filter paper (Whatman, Ø. 25 mm, Merck KGaA, Darmstadt, Germany) and evaporated using the rotary-evaporator. The dry residue (50 mg) was resuspended in 2 mL SDW, extracted through SPE using a C-18 column, and recovered using 1 mL methanol to reach the final original concentration of (16 mg/mL) [24].
4.4. Antibacterial Activity of Diffusible Metabolites
The antibacterial activity of both metabolite extracts, compared with the same bacteria strains used for the initial antagonistic assay, is listed in Table 4.
Disc diffusion assay. An antibacterial test of both metabolite extracts produced by the most bioactive isolate UniB2439-3 was carried out following the disc-diffusion method, as described by Elshafie et al. [66] and Sofo et al. [67]. A bacterial suspension of each tested bacteria was prepared in sterile distilled water adjusted at 106 CFU/mL (OD ≈ 0.2 nm) using UV-Spectrophotometer (Amersham, Ultraspec 1100 pro/500 pro, UK). A total of 4 mL of bacterial suspension mixed with soft agar 0.7% (9:1; v/v) was poured over each KB plate (Ø 9 cm). Blank discs of 6 mm (OXOID, Milan, Italy) were then placed over the plates and 15 µL from each tested metabolite extract (Exo-ME 16 mg/mL and Endo-ME 20 mg/mL) were carefully applied to the discs. Tetracycline (1600 µg/mL) was used as a positive control. The experiment was performed in triplicate, and the antibacterial activity was estimated by measuring the diameter of the inhibition zone in mm ± SDs compared to the positive control.
4.5. Antibacterial Activity of Volatiles Metabolites
The tested bacterial strains were initially subcultured on 14 mL KB medium in Petri dishes and incubated at 37 °C for 24 h. The most efficient isolate of B. bassiana (UniB2439-3) was cultured on PDA media (14 mL Petri dishes) and incubated at 22 °C for 96 h. The test was performed according to Wan et al. [68] using a double-dish chamber containing the studied strain of B. bassiana in one downward dish of KB (Ø 90 mm), and the tested bacterial strains were singularly inoculated on the upward dish, either by direct colony inoculation or the spread of 50 μL of an aqueous suspension (107 CFU/mL). In brief, the direct inoculation of colonies was carried out using a sterile swab to homogenize the colonies over the KB surface. The concentration of the aqueous bacterial suspension was adjusted using turbidimetry. The chamber was sealed with Parafilm™ and incubated at 37 °C in darkness for 48 h. The antibacterial activity of the eventually produced volatile metabolites was evaluated by measuring the inhibition percentages of GVC and AQS of each tested bacterial strain. The experiment was carried out twice with three replicates.
4.6. SPME-GC/MS of VOCs
The fresh culture (96 h) of the selected Beauveria isolate was inoculated in a glass tube of 10 mL PDA nutrient media and incubated at 22 °C for 5 days in darkness to collect the volatile organic compounds (VOCs) [69]. The eventually produced VOCs were qualitatively analyzed using the Solid Phase Micro Extraction method (SPME), as discussed below.
The SPME fiber coated with 100 μm of non-grafted poly (dimethylsiloxane) phase (Supelco 57300-U, mounted on a Supelco 57330 support; Merck KGaA, Darmstadt, Germany), was conditioned for 1 h at 250 °C in a stream of helium. A blank run was performed after each analysis to confirm that no residual compounds were polluting the fiber or the column. The fiber was later introduced to the injection port of an HP6890 plus gas chromatograph equipped with a Phenomenex Zebron ZB-5 MS capillary column (30 m × 0.25 mm ID × 0.25 μm film thickness). An HP 5973 mass-selective (mass range: 15–800 mAU; scan rate: 1.9 scan/s; EM voltage: 1435) was used as a detector, whereas helium at 0.8 mL/min was used as a carrier gas. The injection port, equipped with a glass insert (internal diameter 0.75 mm), was split at 250 °C. A desorption time of 1.0 min was used. The detector was maintained at 230 °C. Oven was maintained at 80 °C for 3 min, then the temperature was increased to 250 °C (20 °C/min) for 10 min. All the analyses were performed in triplicate. The chromatograms obtained from the total ion current were integrated without any correction for coelutions, and the results were expressed as a percent of the total area of peaks [70]. All peaks were identified from their mass spectra by comparison with those present in Wiley 275 and NIST 02 libraries [28]. PDA media (not inoculated with the fungus) was used as a negative control. The analysis was carried out twice with three replicates (different injections).
4.7. Statistical Analysis
The obtained results for the biological assays were statistically analyzed, applying one-way ANOVA using the Package for the Social Sciences (SPSS) version 13.0 (Prentice Hall: Chicago, IL, USA, 2004). Tukey B Post-Hoc multiple comparison test was used to evaluate the significance level with a probability of p < 0.05.
5. Conclusions
B. bassiana, apart from being a notable entomopathogenic fungi or biocontrol agent against some phytopathogens, by itself or through its bioactive metabolites. In particular, B. bassiana or its bioactive metabolites could also be used efficiently to control several bacteria in the agronomic field, where the use of antibiotics is forbidden, especially in organic farming. In addition, B. bassiana could also be a useful biocontrol agent against MDR microorganisms to different antibiotics, which are considered a dominant medical problem worldwide. The obtained results from the current research concluded that B. bassiana UniB2439-3 was able to produce some interesting VOCs, such as β-elemene, which has been reported previously to have a strong antimicrobial effect against several pathogenic microorganisms. The ability of B. bassiana to produce the above-mentioned metabolites can underline its antagonistic activity against several phytopathogens, as reported previously in the bibliographic research. Future studies remain necessary to evaluate the in vivo antimicrobial activity of each identified bioactive VOC from B. bassiana against serious phytopathogens, considering that the use of antibiotics is forbidden in agriculture in many countries. Therefore, the search for possible natural alternatives as efficient antimicrobial agents remains necessary.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12152854/s1, Figure S1: Evolutionary relationships of Beauveria bassiana and related species nucleotide sequences based on ITS region (567 bp); Figure S2: Evolutionary relationships of Beauveria bassiana and related species nucleotide sequences based on beta tubulin 2 gene (340 bp); Figure S3: Chromatogram of VOCs extracted from B. bassiana UniB2439-3; Figure S4: Mass spectra of ethanol; Figure S5: Mass spectra of Butanal, 2-methyl; Figure S6: Mass spectra of 2,4-Dimethyl-1-heptene; Figure S7: Mass spectra of Octane, 4-methyl; Figure S8: Mass spectra of β-elemene. Table S1: Antagonistic antibacterial activity of the five studied isolates of Beauveria sp.
Author Contributions
Conceptualization, I.C. and H.S.E.; formal analysis, R.R.; funding acquisition, I.C.; investigation, I.C., S.A.S., R.R. and H.S.E.; methodology, S.A.S., R.R. and H.S.E.; writing—original draft, S.A.S. and H.S.E.; writing—review and editing, I.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basilicata Region-Phytosanitary Office (Matera, Italy), under the project “Epidemiological studies regarding the presence and spread in Basilicata of pathogens of agricultural and forestry plants with particular focus on those of quarantine. Molecular characterization and possibilities of control”, REP. n. 600 of 21.12.2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monz´on, A.; Ownley, B.H.; et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Barbarin, A.M.; Jenkins, N.E.; Rajotte, E.G.; Thomas, M.B. A preliminary evaluation of the potential of Beauveria bassiana for bed bug control. J. Invertebrate Pathol. 2012, 111, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Wraight, S.P.; Lopes, R.B.; Faria, M. Chapter 16: Microbial Control of Mite and Insect Pests of Greenhouse Crops. In Microbial Control of Insect and Mite Pests; Academic Press: Yakima, WA, USA, 2017; pp. 237–252. [Google Scholar]
- Yuan, W.; Wang, P.; Zhang, Z.; Li, S. Glycosylation of (–)-maackiain by Beauveria bassiana and Cunninghamella echinulata var. elegans. Biocatal. Biotransform. 2010, 28, 117–121. [Google Scholar] [CrossRef]
- Berestetskiya, A.O.; Ivanovaa, A.N.; Petrovaa, M.O.; Prokof’evab, D.S.; Stepanychevaa, E.A.; Uspanovc, A.M.; Lednev, G.R. Comparative analysis of the biological activity and chromatographic profiles of the extracts of Beauveria bassiana and B. pseudobassiana cultures grown on different nutrient substrates. Microbiology 2018, 87, 200–214. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Y.; Yang, X.; Pei, X.; Guo, S.; Pei, Y. Expression of a Beauveria bassiana chitinase (Bbchit1) in Escherichia coli and Pichia pastoris. Protein Expr. Purif. 2007, 56, 93–99. [Google Scholar] [CrossRef]
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef]
- Fabrice, D.H.; Elie, D.A.; Kobi, D.O.; Valerien, Z.A.; Thomas, H.A.; Joëlle, T.; Maurille, E.I.A.T.; Dénis, O.B.; Manuele, T. Toward the efficient use of Beauveria bassiana in integrated cotton insect pest management. J. Cotton Res. 2020, 3, 24. [Google Scholar]
- Wang, Y.; Tang, D.; Duan, D.; Wang, Y.; Yu, H. Morphology, molecular characterization, and virulence of Beauveria pseudobassiana isolated from different hosts. J. Inverteb. Pathol. 2020, 72, 107333. [Google Scholar] [CrossRef]
- Sinno, M.; Ranesi, M.; Di Lelio, I.; Iacomino, G.; Becchimanzi, A.; Barra, E.; Molisso, D.; Pennacchio, F.; Digilio, M.C.; Vitale, S.; et al. Selection of endophytic Beauveria bassiana as a dual biocontrol agent of tomato pathogens and pests. Pathogens 2021, 10, 1242. [Google Scholar] [CrossRef]
- Faria, M.R.; Wraight, S.P. Biological control of Bemisia tabaci with fungi. Crop Prot. 2001, 20, 767–778. [Google Scholar] [CrossRef]
- Faria, M.R.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Fargues, J.; Remaudiere, G. Consideration on the specificity of entomopathogenic fungi. Mycopathologia 1977, 62, 31–37. [Google Scholar] [CrossRef]
- Hasaballah, A.I.; Fouda, M.A.; Hassan, M.I.; Omar, G.M. Pathogenicity of Beauveria bassiana and Metarhizium anisopliae on the adult housefly, Musca domestica L. Egypt. Acad. J. Biolog. Sci. A Entomol. 2017, 10, 79–86. [Google Scholar]
- Acharya, N.; Rajotte, E.G.; Jenkins, N.E.; Thomas, M.B. Potential for biocontrol of house flies, Musca domestica, using fungal biopesticides. Biocontrol Sci. Technol. 2015, 25, 513–524. [Google Scholar] [CrossRef]
- Barra-Bucarei, L.; Iglesias, A.F.; González, M.G.; Aguayo, G.S.; Carrasco-Fernández, J.; Castro, J.F.; Campos, J.O. Antifungal activity of Beauveria bassiana Endophyte against Botrytis cinerea in Two Solanaceae Crops. Microorganisms 2020, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Parine, N.R.; Pathan, A.K.; Sarayu, B.; Nishanth, V.S.; Bobbarala, V. Antibacterial efficacy of secondary metabolites from entomopathogenic fungi Beauveria bassiana. Int. J. Chem. Anal. Sci. 2010, 1, 94–96. [Google Scholar]
- Ownley, B.H.; Bishop, D.G.; Pereira, R.M. Biocontrol of Rhizoctonia damping off of tomato with Beauveria bassiana. Phytopathology 2000, 90, S58. [Google Scholar]
- Tomilova, O.G.; Shaldyaeva, E.M.; Kryukova, N.A.; Pilipova, Y.V.; Schmidt, N.S.; Danilov, V.P.; Kryukov, V.Y.; Glupov, V.V. Entomopathogenic fungi decrease Rhizoctonia disease in potato in field conditions. PeerJ 2020, 8, e9895. [Google Scholar] [CrossRef]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.R.; Caputo, L. Antimicrobial and phytotoxic activity of Origanum heracleoticum and O. majorana essential oils growing in Cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Mancini, E.; Camele, I.; Martino, L.D.; De Feo, V. In vivo antifungal activity of two essential oils from Mediterranean plants against postharvest brown rot disease of peach fruit. Ind. Crops Prod. 2015, 66, 11–15. [Google Scholar] [CrossRef]
- Anyanwu, M.U.; Okoye, R.C. Antimicrobial Activity of Nigerian Medicinal Plants. J. Intercult. Ethnopharmacol. 2017, 6, 240–259. [Google Scholar] [CrossRef]
- Keifer, M.C.; Firestone, J. Neurotoxicity of pesticides. J. Agromed. 2007, 12, 17–25. [Google Scholar] [CrossRef]
- Camele, I.; Elshafie, H.S.; Caputo, L.; Sakr, S.H.; De Feo, V. Bacillus mojavensis: Biofilm formation and biochemical investigation of its bioactive metabolites. J. Biol. Res. 2019, 92, 39–45. [Google Scholar] [CrossRef]
- Wang, H.; Peng, H.; Li, W.; Cheng, P.; Gong, M. The toxins of Beauveria bassiana and the strategies to improve their virulence to insects. Front. Microbiol. 2021, 12, 705343. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liua, X.; Keyhanib, N.O.; Tanga, G.; Peia, Y.; Zhanga, W.; Tonga, S. Regulatory cascade and biological activity of Beauveria bassiana oosporein that limits bacterial growth after host death. Proc. Natl. Acad. Sci. USA 2017, 114, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- McLafferty, F.W. Wiley Registry of Mass Spectral Data, with NIST Spectral Data CD Rom, 7th ed.; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Yang, X.; Ye, W.; Qi, Y.; Ying, Y.; Xia, Z. Overcoming Multidrug Resistance in Bacteria Through Antibiotics Delivery in Surface-Engineered Nano-Cargos: Recent Developments for Future Nano-Antibiotics. Front. Bioeng. Biotechnol. 2021, 9, 696514. [Google Scholar] [CrossRef]
- Soothill, G.; Hu, Y.; Coates, A. Can we prevent antimicrobial resistance by using antimicrobials better? Pathogens 2013, 2, 422–435. [Google Scholar] [CrossRef]
- Zimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol. 2007, 17, 553–596. [Google Scholar] [CrossRef]
- Chen, W.H.; Liu, M.; Huang, Z.X.; Yang, G.M.; Han, Y.F.; Liang, J.D.; Liang, Z.Q. Beauveria majiangensis, a new entomopathogenic fungus from Guizhou, China. Phytotaxa 2018, 333, 243–250. [Google Scholar] [CrossRef]
- Ownley, B.H.; Pereira, R.M.; Klingeman, W.E.; Quigley, N.B.; Leckie, B.M. Beauveria bassiana, a dual-purpose biological control with activity against insect pests and plant pathogens. Emerg. Concepts Plant Health Manag. 2004, 2004, 255–269. [Google Scholar]
- Suzuki, A.; Kanaoka, M.; Isogai, A.; Tamura, S.; Murakoshi, S.; Ichinoe, M. Bassianolide, a new insecticidal cyclodepsipeptide from Beauveria bassiana and Verticillium lecanii. Tetrahedron Lett. 1977, 18, 2167–2170. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Z.; Yun-Ting, S.; Zeng, Z.; Zhan, X.; Li, C.; Xie, T. A review of medicinal plant species with elemene in China. Afr. J. Pharm. Pharmacol. 2012, 6, 3032–3040. [Google Scholar] [CrossRef]
- El Kichaoui, A.; Elnabris, K.; Shafie, A.; Fayyad, N.; Arafa, M.; El Hindi, M. Development of Beauveria bassiana based bio-fungicide against Fusarium wilt pathogens for Capsicum annuum, a promising approach toward vital biocontrol industry in Gaza strip. IUG J. Nat. Stud. 2017, 25, 183–190. [Google Scholar]
- Vining, L.C.; Kelleher, W.J.; Schwarting, A.E. Oosporein production by a strain of Beauveria bassiana originally identified as Amanita muscaria. Can. J. Microbiol. 1962, 8, 931–933. [Google Scholar] [CrossRef]
- Hamill, R.L.; Higgens, C.E.; Boaz, H.E.; Gorman, M. The structure of beauvericin, a new depsipeptide antibiotic toxic to Artemia salina. Tetrahedron Lett. 1969, 10, 4255–4258. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Castella, G.; Kostecki, M.; Golinski, P.; Ritieni, A.; Chelkowski, J. Beauvericin production by Fusarium species. Appl. Environ. Microbiol. 1998, 64, 3084–3088. [Google Scholar] [CrossRef]
- Nagaoka, T.; Nakata, K.; Kouno, K. Antifungal activity of oosporein from an antagonistic fungus against Phytophthora infestans. Z. Naturforschung C 2004, 59, 302–304. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.; Xu, L. Beauvericin, a bioactive compound produced by fungi: A short review. Molecules 2012, 17, 2367–2377. [Google Scholar] [CrossRef]
- Manning, R.O.; Wyatt, R.D. Comparative toxicity of Chaetomium contaminated corn and various chemical forms of oosporein in broiler chicks. Poult. Sci. 1984, 63, 251–259. [Google Scholar] [CrossRef]
- Chiron, N.; Michelot, D. Mushrooms odors, chemistry and role in the biotic interactions—A review. Cryptogr. Mycol. 2005, 26, 299–365. [Google Scholar]
- Effmert, U.; Kalderás, J.; Warnke, R.; Piechulla, B. Volatile Mediated Interactions Between Bacteria and Fungi in the Soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Kim, K.D. Biocontrol Activity of Volatile-Producing Bacillus megaterium and Pseudomonas protegens Against Aspergillus and Penicillium spp. Predominant in Stored Rice Grains: Study II. Mycobiology 2018, 27, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Chelliah, R.; Hu, X.; Oh, D.-H.; Kathiresan, K.; Wang, M.-H. Antioxidant, Anti-Lung Cancer, and Anti-Bacterial Activities of Toxicodendron vernicifluum. Biomolecules 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Epsky, N.D.; Heath, R.R.; Dueben, B.D.; Lauzon, C.R.; Proveaux, A.T.; Maccollom, G.B. Attraction of 3-methyl-l-butanol and ammonia identified from Enterobacter agglomerans to Anastrepha suspensa. J. Chem. Ecol. 1998, 24, 1867–1880. [Google Scholar] [CrossRef]
- Salih, L.M.; Eid, F.A.; Elhaw, M.H.; Hamed, A. In vitro cytotoxic, antioxidant, antimicrobial activity and volatile constituents of Coccoloba peltata Schott cultivated in Egypt. Egypt. J. Chem. 2021, 64, 7157–7163. [Google Scholar]
- Golembiovska, O.; Tsurkan, A.; Vynogradov, B. Components of Prunella vulgaris L. Grown in Ukraine. J. Pharmacogn. Phytochem. 2014, 2, 140–146. [Google Scholar]
- Bernotienë, G.; Nivinskienë, O.; Butkienë, R.; Mockutë, D. Chemical composition of essential oils of hops (Humulus lupulus L.) growing wild in Aukštaitija. Chemija 2004, 15, 31–36. [Google Scholar]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar] [CrossRef]
- Sieniawska, E.; Sawicki, R.; Golus, J.; Swatko-Ossor, M.; Ginalska, G.; Skalicka-Wozniak, K. Nigella damascena L. essential Oil—A valuable source of β-Elemene for antimicrobial testing. Molecules 2018, 23, 256. [Google Scholar] [CrossRef]
- Mendanha, S.A.; Alonso, A. Effects of terpenes on fluidity and lipid extraction in phospholipid membranes. Biophys. Chem. 2015, 198, 45–54. [Google Scholar] [CrossRef]
- Barrero, A.F.; Quilez del Moral, J.F.; Lara, A.; Herrador, M.M. Antimicrobial activity of sesquiterpenes from the essential oil of Juniperus thurifera Wood. Planta Med. 2005, 71, 67–71. [Google Scholar] [CrossRef]
- Drage, S.; Mitter, B.; Tröls, C.; Muchugi, A.; Jamnadass, R.H.; Sessitsch, A.; Hadacek, F. Antimicrobial drimane sesquiterpenes and their effect on endophyte communities in the medical tree Warburgiau gandensis. Front. Microbiol. 2014, 5, 13. [Google Scholar] [CrossRef]
- Monga, A.; Sharma, A. Chapter 9—Natural products encompassing antituberculosis activities. Stud. Nat. Prod. Chem. 2020, 64, 263–301. [Google Scholar]
- Zhai, B.; Zhang, N.; Han, X.; Li, Q.; Zhang, M.; Chen, X.; Li, G.; Zhang, R.; Chen, P.; Wang, W.; et al. Molecular targets of β-elemene, a herbal extract used in traditional Chinese medicine, and its potential role in cancer therapy: A review. Biomed. Pharmacoth. 2019, 14, 108812. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Yan, Y.; Chai, H.; Chen, S.; Xiong, X.; Sun, D.; Yu, Y.; Deng, L.; Cheng, F. Pyruvate kinase M2 affects liver cancer cell behavior through up-regulation of HIF-1α and Bcl-xL in culture. Biomed. Pharmacother. 2015, 69, 277–284. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, G.; Engelbrecht, A.M. Role of PKM2 in directing the metabolic fate of glucose in cancer: A potential therapeutic target. Cell. Oncol. 2018, 41, 343–351. [Google Scholar] [CrossRef]
- Sun, X.; Peng, Y.; Zhao, J.; Xie, Z.; Lei, X.; Tang, G. Discovery and development of tumor glycolysis rate-limiting enzyme inhibitors. Bioorg. Chem. 2021, 112, 104891. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, W.; Huang, S.; Ni, W.; Wei, Z.; Cao, Y.; Yu, S.; Jia, Q.; Wu, Y.; Chai, C.; et al. Beta-elemene inhibits breast cancer metastasis through blocking pyruvate kinase M2 dimerization and nuclear translocation. J. Cell. Mol. Med. 2019, 23, 6846–6858. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Johnson, D.M.; White, R.L.; Pereira, R.M.; Geden, C.J. Beauveria bassiana culturing and harvesting for bioassays with house flies. J. Insect Sci. 2020, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Racioppi, R.; Bufo, S.A.; Camele, I. In vitro study of biological activity of four strains of Burkholderia gladioli pv. agaricicola and identification of their bioactive metabolites using GC–MS. Saudia J. Biol. Sci. 2017, 24, 295–301. [Google Scholar]
- Elshafie, H.S.; Viggiani, L.; Mostafa, M.S.; El-Hashash, M.A.; Bufo, S.A.; Camele, I. Biological activity and chemical identification of ornithine lipid produced by Burkholderia gladioli pv. agaricicola ICMP 11096 using LC-MS and NMR analyses. J. Biol. Res. 2017, 90, 96–103. [Google Scholar]
- Sofo, A.; Elshafie, H.S.; Scopa, A.; Mang, S.M.; Camele, I. Impact of airborne zinc pollution on the antimicrobial activity of olive oil and the microbial metabolic profiles of Zn-contaminated soils in an Italian olive orchard. J. Trace Elem. Med. Biol. 2018, 49, 276–284. [Google Scholar] [CrossRef]
- Wan, M.G.; Li, G.Q.; Zhang, J.B.; Jiang, D.H.; Huang, H.C. Effect of volatile substances of Streptomyces platensis F-1 on control of plant fungal diseases. Biol. Control 2008, 46, 552–559. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Bufo, S.A.; Racioppi, R.; Camele, I. Biochemical Characterization of Volatile Secondary Metabolites Produced by Burkholderia gladioli pv. agaricicola. Int. J. Drug Discov. 2013, 5, 181–184. [Google Scholar]
- Elshafie, H.S.; Camele, I.; Racioppi, R.; Scrano, L.; Iacobellis, N.S.; Bufo, S.A. In vitro antifungal activity of Burkholderia gladioli pv. agaricicola against some Phytopathogenic fungi. Int. J. Mol. Sci. 2012, 13, 16291–16302. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).