Culturomics and Circular Agronomy: Two Sides of the Same Coin for the Design of a Tailored Biofertilizer for the Semi-Halophyte Mesembryanthemum crystallinum
Abstract
1. Introduction
2. Results
2.1. Soil Characterization
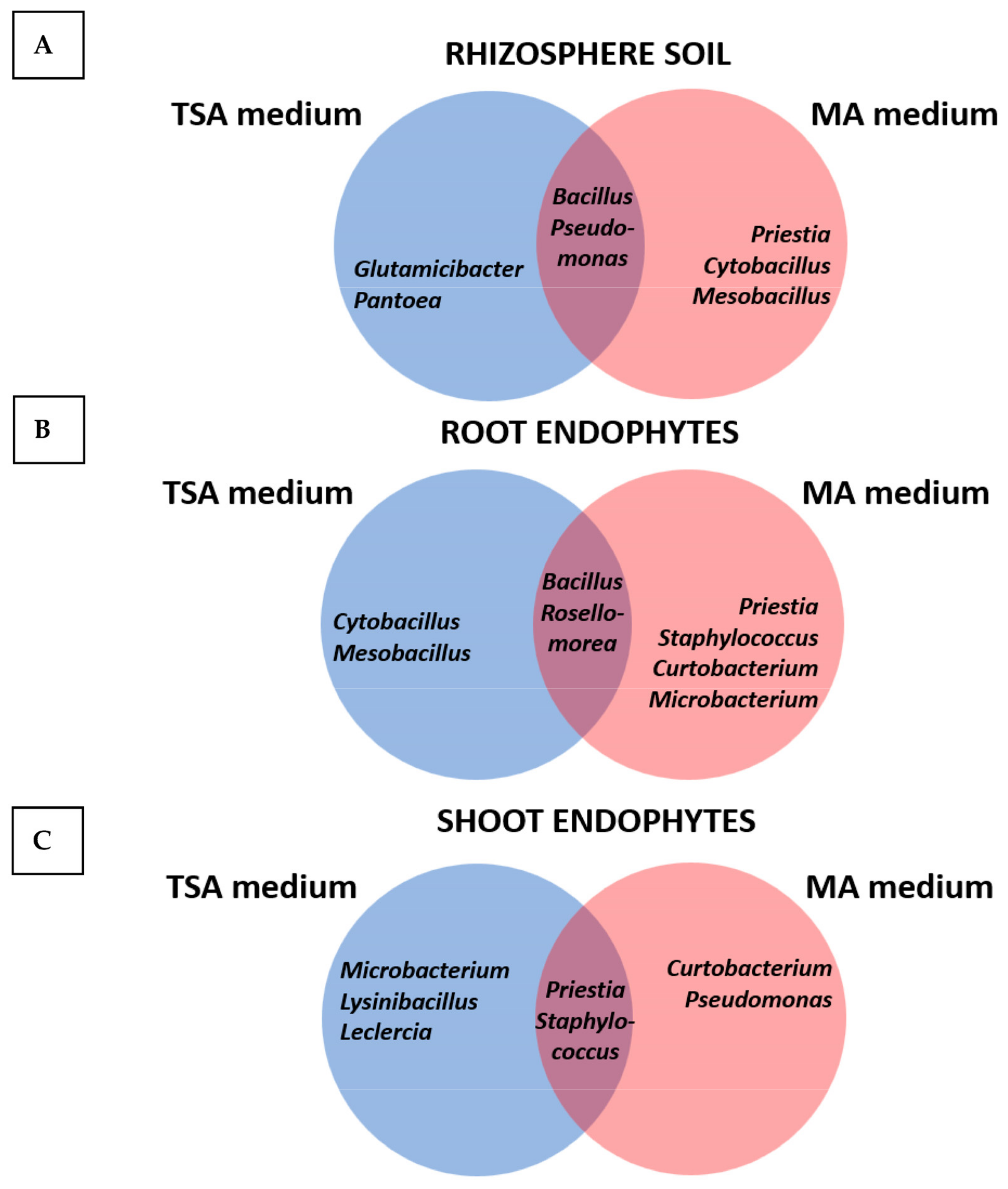
2.2. Culturomics and Standard Approaches for the Isolation of Rhizosphere Bacteria and Endophytes from the Microbiome of Mesembryanthemum crystallinum
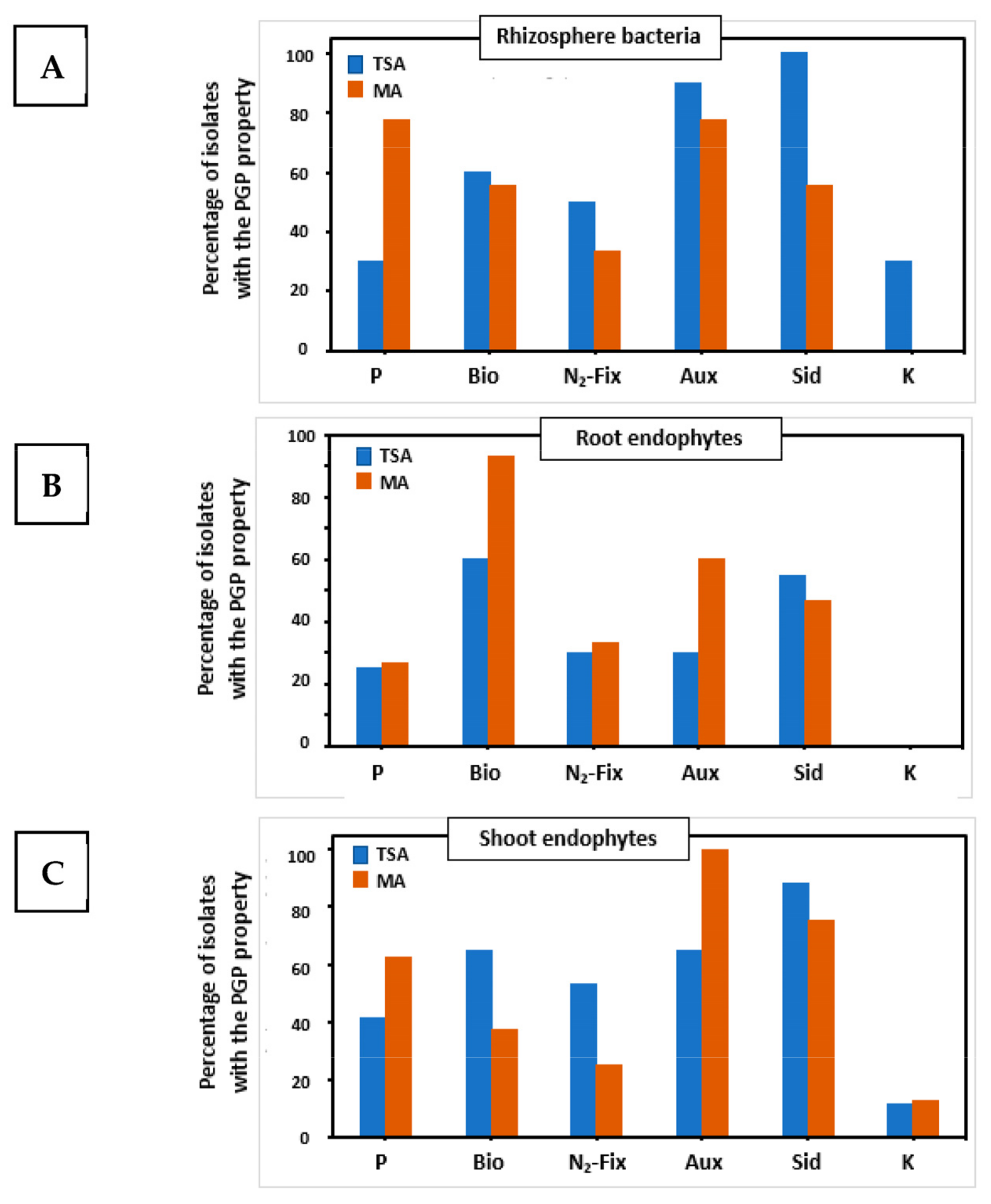
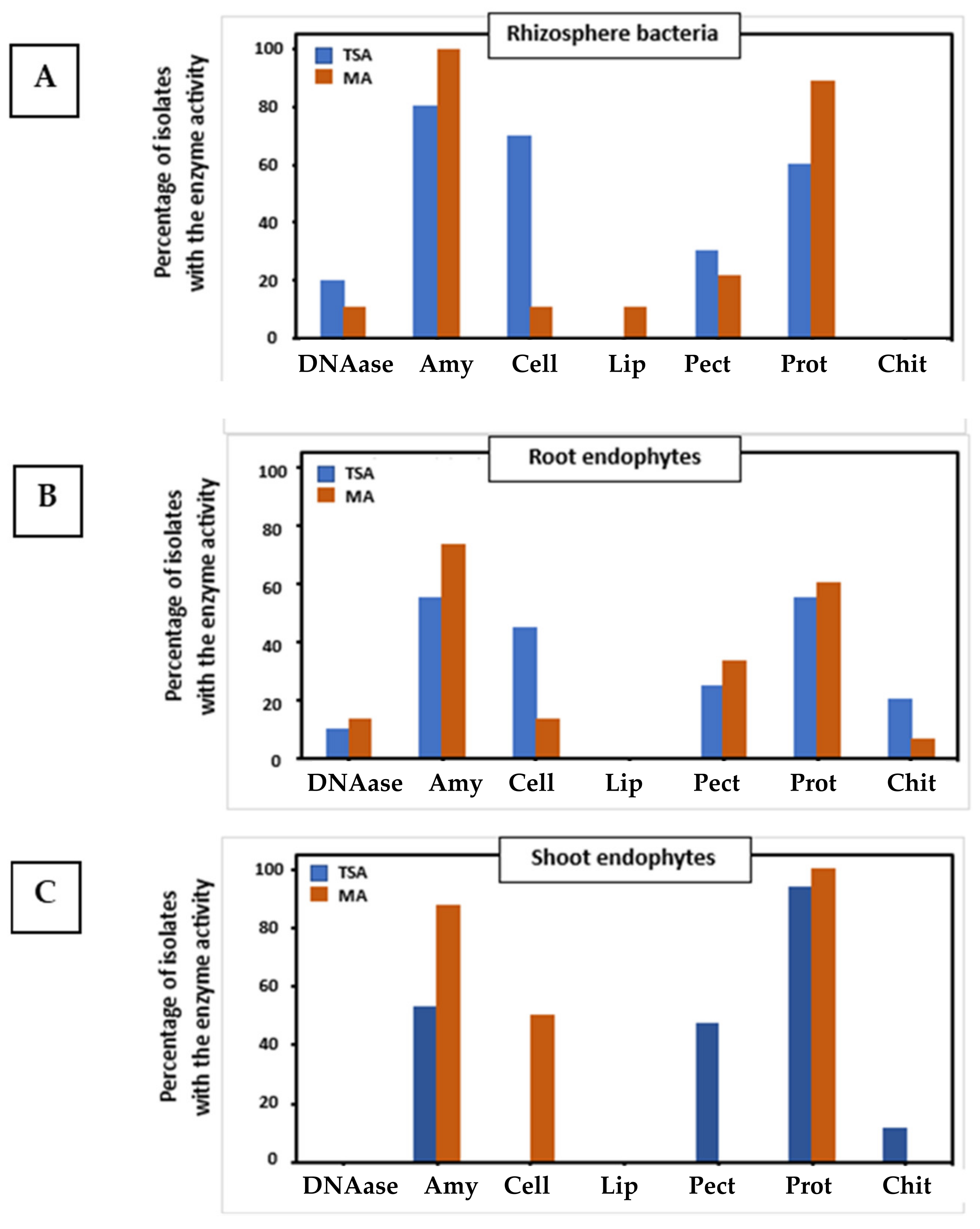
2.3. Analysis of Plant Growth Promoting Properties and Extracellular Enzymatic Activities
2.4. Selection of the Consortia Members
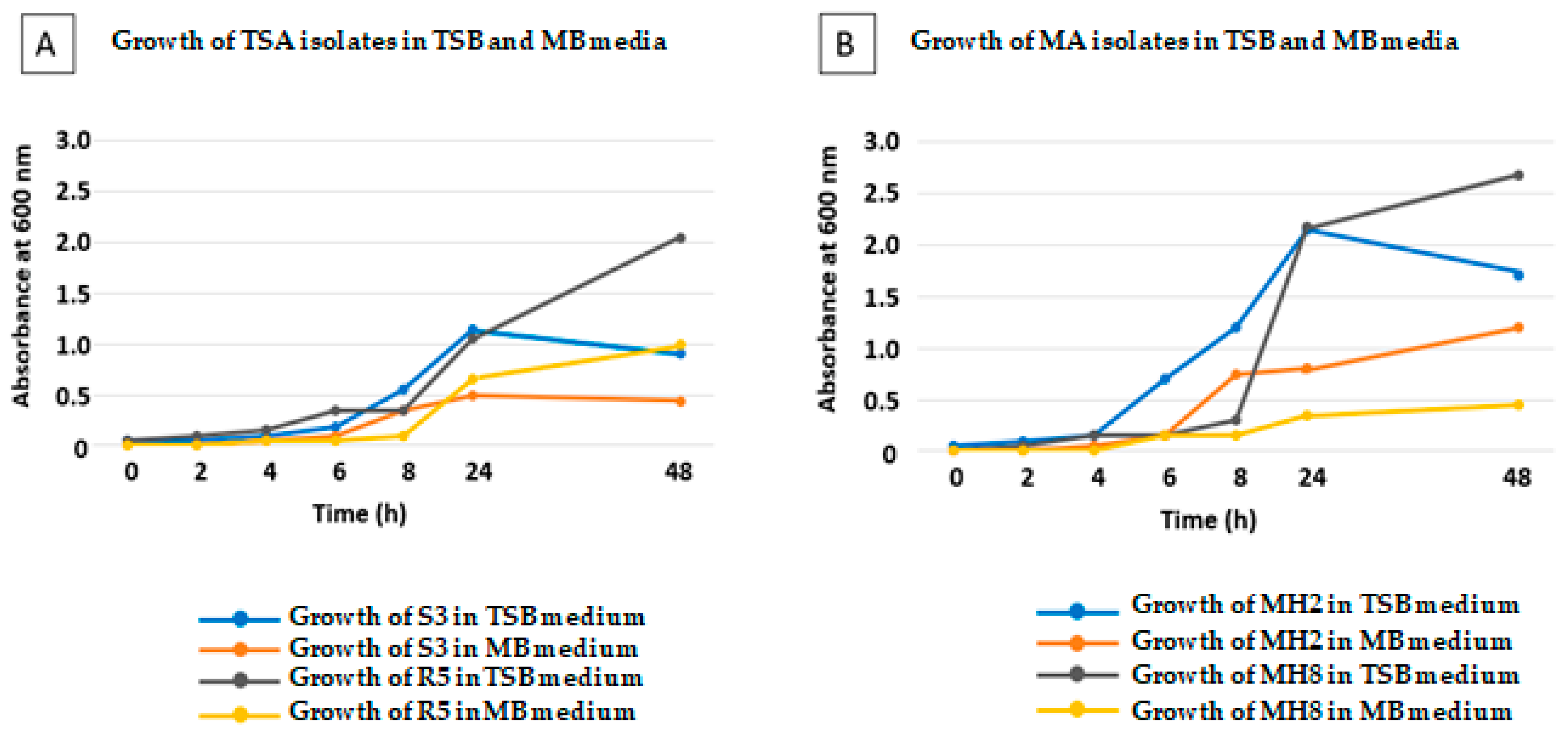
2.5. Growth of the Selected Bacteria in Liquid TSB and Mesem Broth MB Liquid Media
2.6. Effect of Inoculation on the Germination of Mesembryanthemum crystallinum Seeds
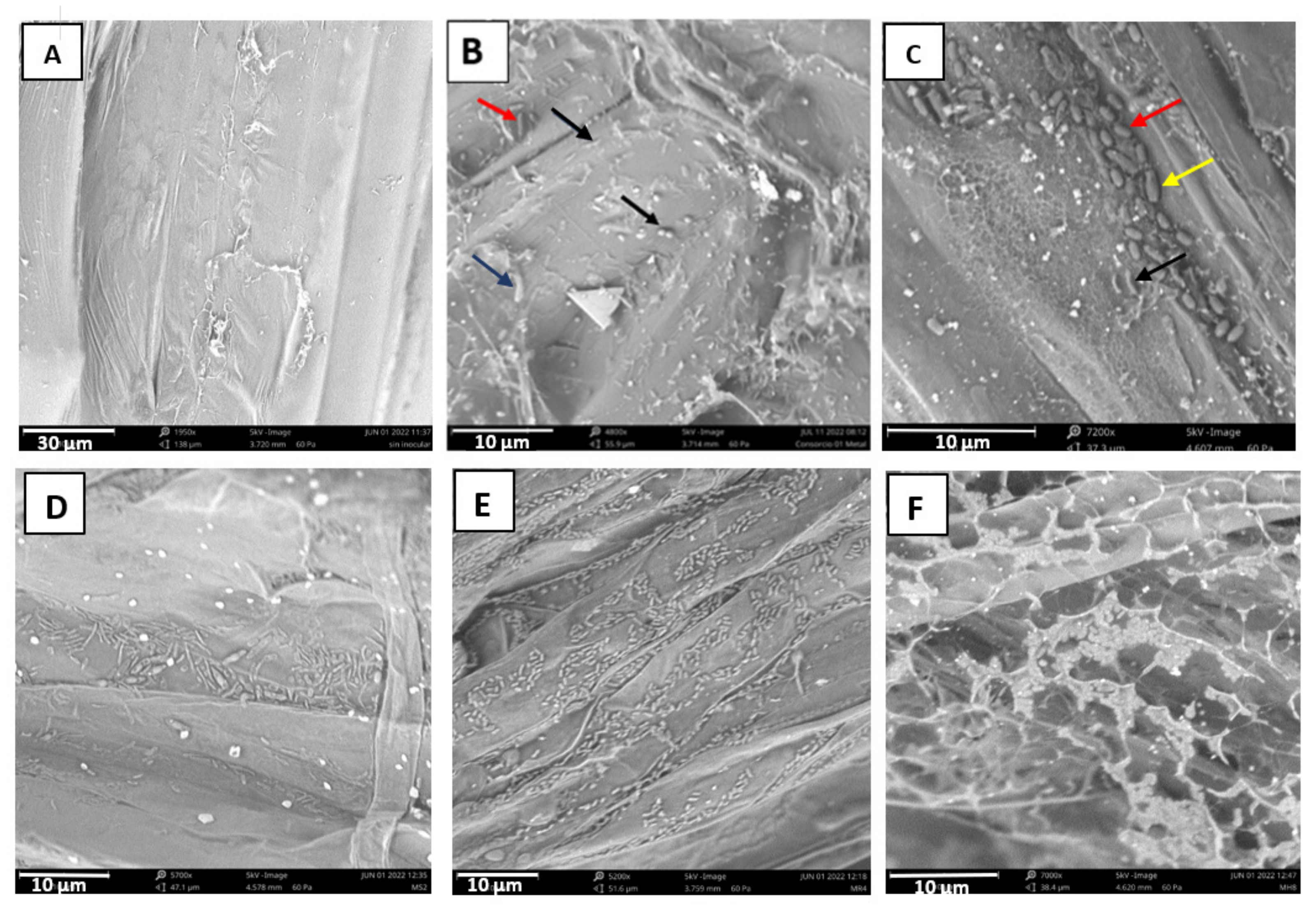
2.7. Root Colonization
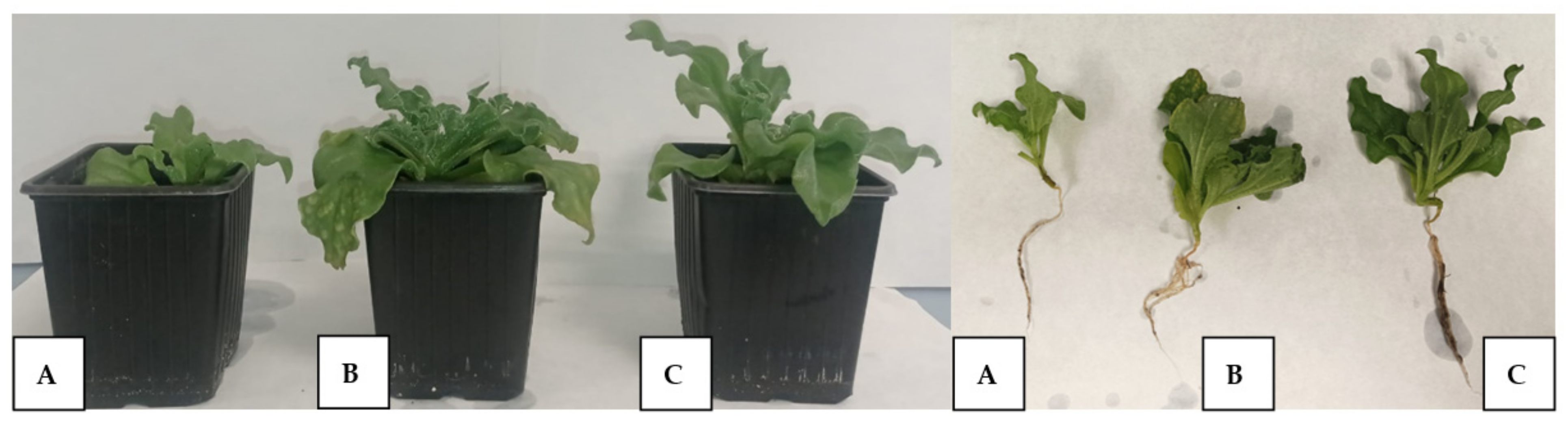
2.8. Effect of Inoculation with Bacterial Consortia on Plant´s Growth and Physiological Status
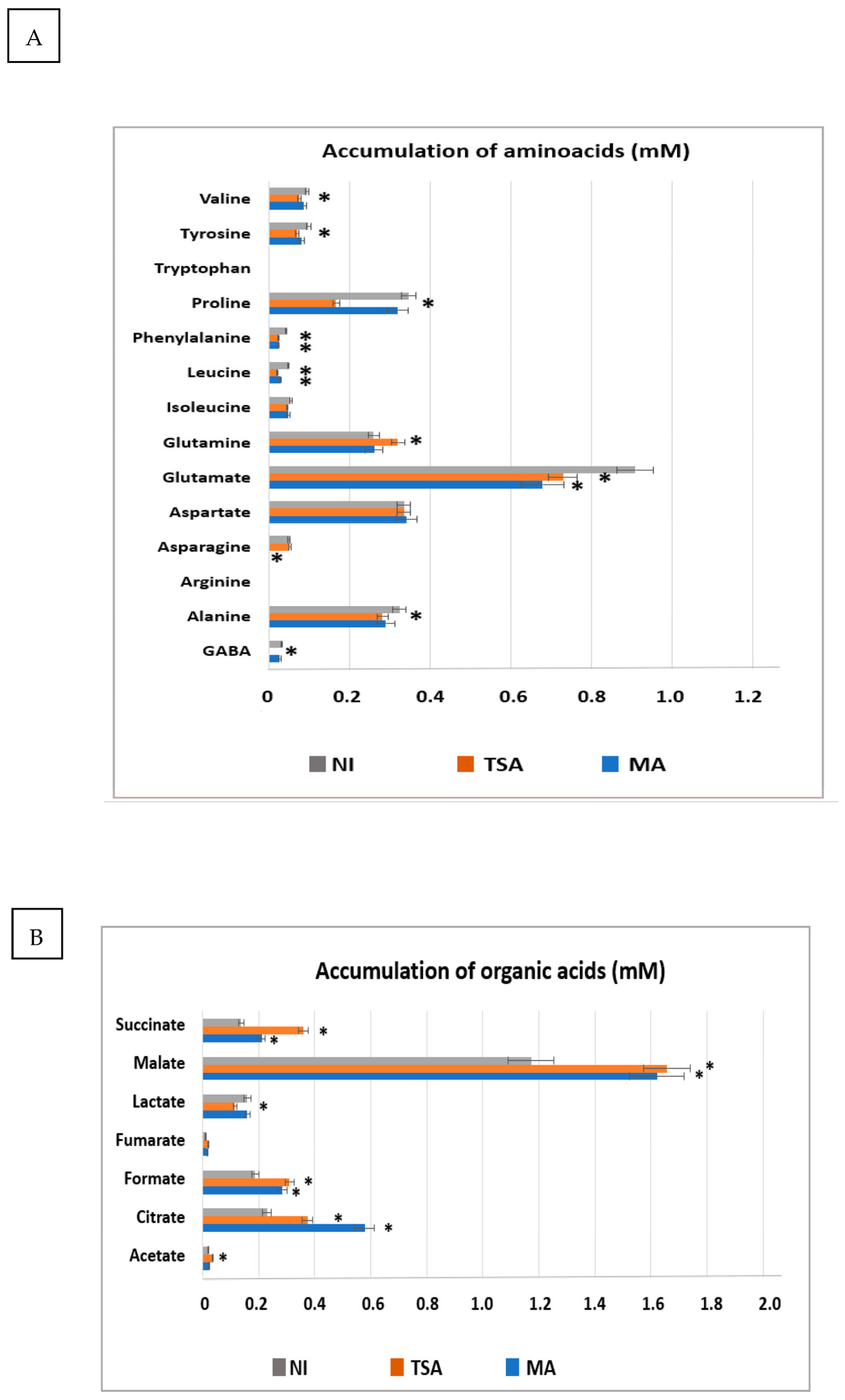
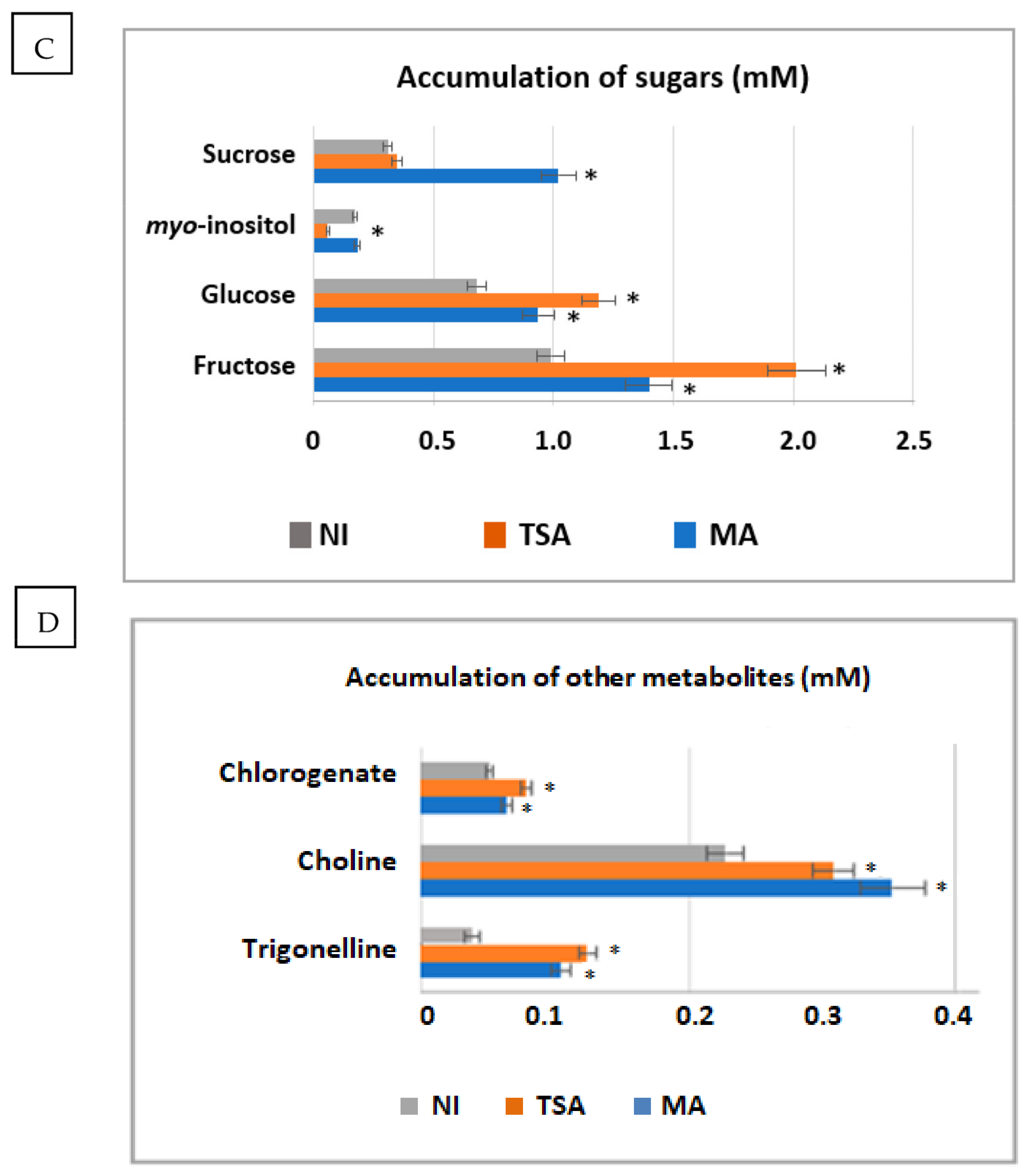
2.9. Modulation of Metabolites Accumulation upon Inoculation with Selected Consortia
3. Discussion
4. Materials and Methods
4.1. Collection of Soil and Plant Samples
4.2. Soil Characterization
4.3. Preparation of Plant-Based Culture Medium for Culturomics
4.4. Isolation and Identification of Rhizosphere Bacteria and Endophytes on Standard and Plant-Based Media
4.5. Analysis of Plant Growth Promoting Properties of the Isolates
4.6. Compatibility of Selected Strains and Formulation of Biofertilizers for Mesembryanthemum crystallinum
4.7. Effect of Inoculation on Seed Germination
4.8. Observation of Root Colonization by Low Vacuum Scanning Electron Microscopy
4.9. Cultivation of Plants under Greenhouse Conditions
4.10. Evaluation of the Growth and Physiological Status of Plants
4.11. Effect of Inoculation on the Accumulation of Metabolites in Shoots
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Aslam, R.; Bostan, N.; Amen, N.-E.; Maria, M.; Safdar, W. A critical review on halophytes: Salt tolerant plants. J. Med. Plants Res. 2011, 5, 7108–7118. [Google Scholar] [CrossRef]
- Stevanović, Z.D.; Stanković, M.S.; Stanković, J.; Janaćković, P.; Stanković, M. Use of halophytes as medicinal plants: Phytochemical diversity and biological activity. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; Hasanuzzaman, M., Shabala, S., Fujita, M., Eds.; CABI: Wallingford, UK, 2019; Chapter 21; pp. 343–358. [Google Scholar] [CrossRef]
- Sharma, R.; Wungrampha, S.; Singh, V.; Pareek, A.; Sharma, M.K. Halophytes As Bioenergy Crops. Front. Plant Sci. 2016, 7, 1372. [Google Scholar] [CrossRef]
- Manousaki, E.; Kalogerakis, N. Halophytes—An emerging trend in phytoremediation. Int. J. Phytoremediat. 2011, 13, 959–969. [Google Scholar] [CrossRef] [PubMed]
- García-Caparrós, P.; Llanderal, A.; Lao, M.T. Halophytes as an Option for the Restoration of Degraded Areas and Landscaping. In Handbook of Halophytes: From Molecules to Ecosystems Towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, D.; Murillo-Amador, B.; Cristiano, G.; De Lucia, B. Halophyte Common Ice Plants: A Future Solution to Arable Land Salinization. Sustainability 2019, 11, 6076. [Google Scholar] [CrossRef]
- Lopes, M.; Sanches-Silva, A.; Castilho, M.; Cavaleiro, C.; Ramos, F. Halophytes as source of bioactive phenolic compounds and their potential applications. Crit. Rev. Food Sci. Nutr. 2021, 2, 1078–1101. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Sui, N.; Han, G. Increases of Unsaturated Fatty Acids in Membrane Lipids Protects Photosystem II from Photoinhibition under Salinity in Different Halophytes. J. Agric. Sci. Arch. 2014, 6, 623–629. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Halophytic herbs of the Mediterranean basin: An alternative approach to health. Food Chem. Toxicol. 2018, 114, 155–169. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; Shehata, H.S. Ecology and development of Mesembryanthemum crystallinum L. in the Deltaic Mediterranean coast of Egypt. Egypt. J. Basic Appl. Sci. 2014, 1, 29–37. [Google Scholar] [CrossRef]
- Adams, P.; Nelson, D.E.; Yamada, S.; Chmara, W.; Jensen, R.G.; Bohnert, H.J.; Griffiths, H. Growth and development of Mesembryanthemum crystallinum (Aizoaceae). New Phytol. 1998, 138, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.J.; Cushman, J.C. The Ice Plant Cometh: Lessons in Abiotic Stress Tolerance. J. Plant Growth Regul. 2000, 19, 334–346. [Google Scholar] [CrossRef]
- Foster, S.; Hobbs, C. A Field Guide to Western Medicinal Plants and Herbs; Houghton Mifflin Harcourt: Boston, MA, USA, 2002. [Google Scholar]
- Agarie, S.; Kawaguchi, A.; Kodera, A.; Sunagawa, H.; Kojima, H.; Nose, A.; Nakahara, T. Potential of the Common Ice Plant, Mesembryanthemum crystallinum as a New High-Functional Food as Evaluated by Polyol Accumulation. Plant Prod. Sci. 2009, 12, 37–46. [Google Scholar] [CrossRef]
- Drira, R.; Matsumoto, T.; Agawa, M.; Sakamoto, K. Ice Plant (Mesembryanthemum crystallinum) Extract Promotes Lipolysis in Mouse 3T3-L1 Adipocytes Through Extracellular Signal-Regulated Kinase Activation. J. Med. Food 2016, 19, 274–280. [Google Scholar] [CrossRef]
- Deters, A.M.; Meyer, U.; Stintzing, F.C. Time-dependent bioactivity of preparations from cactus pear (Opuntia ficus indica) and ice plant (Mesembryanthemum crystallinum) on human skin fibroblasts and keratinocytes. J. Ethnopharmacol. 2012, 142, 438–444. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jo, S.-G.; Jung, S.-K.; Park, W.-T.; Kim, K.-Y.; Park, Y.-W.; Park, J.-H. Immunomodulatory effects of ethanol extract of germinated ice plant (Mesembryanthemum crystallinum). Lab. Anim. Res. 2017, 33, 32–39. [Google Scholar] [CrossRef]
- Bouftira, I.; Abdelly, C.; Sfar, S. Characterization of cosmetic cream with Mesembryanthemum crystallinum plant extract: Influence of formulation composition on physical stability and anti-oxidant activity. Int. J. Cosmet. Sci. 2008, 30, 443–452. [Google Scholar] [CrossRef]
- Kim, D.E.; Chang, B.Y.; Hwang, Y.S.; Park, J.H.; Kim, K.Y.; Jung, S.K.; Kim, S.Y. Effects of Mesembryanthemum crystallinum on the anti-oxidant, anti-wrinkle and whitening skin agents. In Proceedings of the 5th International Conference and Exhibition on Pharmacognosy, Phytochemistry & Natural Products, Melbourne, Australia, 24–25 July 2017; Available online: https://www.iomcworld.com/proceedings/effects-of-mesembryanthemum-crystallinum-on-the-antioxidant-antiwrinkle-and-whitening-skin-agents-38146.html (accessed on 1 May 2023).
- Bouftira, I.; Abdelly, C.; Sfar, S. Antioxidant and Antibacterial Properties of Mesembryanthemum crystallinum and Carpobrotus edulis Extracts. Adv. Chem. Eng. Sci. 2012, 2, 359–365. [Google Scholar] [CrossRef]
- Rodríguez-Llorente, I.; Pajuelo, E.; Navarro-Torre, S.; Mesa-Marín, J.; Caviedes, M.A. Bacterial Endophytes from Halophytes: How Do They Help Plants to Alleviate Salt Stress? In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Kumar, M., Etesami, H., Kumar, V., Eds.; Springer: Singapore, 2019; Chapter 6; pp. 147–160. [Google Scholar] [CrossRef]
- Christakis, C.A.; Daskalogiannis, G.; Chatzaki, A.; Markakis, E.A.; Mermigka, G.; Sagia, A.; Rizzo, G.F.; Catara, V.; Lagkouvardos, I.; Studholme, D.J.; et al. Endophytic Bacterial Isolates From Halophytes Demonstrate Phytopathogen Biocontrol and Plant Growth Promotion Under High Salinity. Front. Microbiol. 2021, 12, 681567. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Vaseva, I.I.; Qudeimat, E.; Potuschak, T.; Du, Y.; Genschik, P.; Vandenbussche, F.; Van Der Straeten, D. The plant hormone ethylene restricts Arabidopsis growth via the epidermis. Proc. Natl. Acad. Sci. USA 2018, 115, E4130–E4139. [Google Scholar] [CrossRef] [PubMed]
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O. Plant Growth Promoting Rhizobacterial Mitigation of Drought Stress in Crop Plants: Implications for Sustainable Agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Mahmood, A.; Amaya, R.; Turgay, O.C.; Yaprak, A.E.; Taniguchi, T.; Kataoka, R. High salt tolerant plant growth promoting rhizobacteria from the Common ice-plant Mesembryanthemum crystallinum L. Rhizosphere 2019, 9, 10–17. [Google Scholar] [CrossRef]
- Supel, P.; Śliwa-Cebula, M.; Miszalski, Z.; Kaszycki, P. Cadmium-Tolerant Rhizospheric Bacteria of the C3/CAM Intermediate Semi-Halophytic Common Ice Plant (Mesembryanthemum crystallinum L.) Grown in Contaminated Soils. Front. Plant Sci. 2022, 13, 820097. [Google Scholar] [CrossRef]
- Mahmood, A.; Kataoka, R. Metabolite profiling reveals a complex response of plants to application of plant growth-promoting endophytic bacteria. Microbiol. Res. 2020, 234, 126421. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernández, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Greub, G. Culturomics: A new approach to study the human microbiome. Clin. Microbiol. Infect. 2012, 18, 1157–1159. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.C.; Armougom, F.; Million, M.; Hugon, P.; Pagnier, I.; Robert, C.; Bittar, F.; Fournous, G.; Giménez, G.; Maraninchi, M.; et al. Microbial culturomics: Paradigm shift in the human gut microbiome study. Clin. Microbiol. Infect. 2012, 18, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, M.S.; Hamza, M.A.; Youssef, H.H.; Patz, S.; Becker, M.; El Sawey, H.; Nemr, R.; Daanaa, H.-S.A.; Mourad, E.F.; Morsi, A.T.; et al. Culturomics of the plant prokaryotic microbiome and the dawn of plant-based culture media—A review. J. Adv. Res. 2019, 19, 15–27. [Google Scholar] [CrossRef]
- Nemr, R.A.; Mohab, K.; Sarhan, M.S.; Abbas, M.T.; Elsawey, H.; Youssef, H.H.; Hamza, M.A.; Morsi, A.T.; El-Tahan, M.; Fayez, M.; et al. “In situ similis” Culturing of Plant Microbiota: A Novel Simulated Environmental Method Based on Plant Leaf Blades as Nutritional Pads. Front. Microbiol. 2020, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Sierra Aragón, M. Niveles de Metales Pesados y Elementos Asociados en Suelos de la Provincia de Almería. Parámetros Que los Afectan y Riesgo de Contaminación. Ph.D. Thesis, University of Granada, Granada, Spain, 2005. (In Spanish). [Google Scholar]
- Jung, B.K.; Ibal, J.C.; Pham, H.Q.; Kim, M.C.; Park, G.S.; Hong, S.J.; Jo, H.W.; Park, C.E.; Choi, S.D.; Jung, Y.; et al. Quorum Sensing System Affects the Plant Growth Promotion Traits of Serratia fonticola GS2. Front. Microbiol. 2020, 11, 536865. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Ke, J.; Wang, B.; Yoshikuni, Y. Microbiome Engineering: Synthetic Biology of Plant-Associated Microbiomes in Sustainable Agriculture. Trends Biotechnol. 2021, 39, 244–261. [Google Scholar] [CrossRef]
- Biosca, E.G.; Flores, R.; Santander, R.D.; Díez-Gil, J.L.; Barreno, E. Innovative Approaches Using Lichen Enriched Media to Improve Isolation and Culturability of Lichen Associated Bacteria. PLoS ONE 2016, 11, e0160328. [Google Scholar] [CrossRef]
- Youssef, H.H.; Hamza, M.A.; Fayez, M.; Mourad, E.F.; Saleh, M.Y.; Sarhan, M.S.; Suker, R.M.; Eltahlawy, A.A.; Nemr, R.A.; El-Tahan, M.; et al. Plant-based culture media: Efficiently support culturing rhizobacteria and correctly mirror their in-situ diversity. J. Adv. Res. 2016, 7, 305–316. [Google Scholar] [CrossRef]
- Munene, L.; Mugweru, J.; Mwirichia, R. Management of bacterial wilt caused by Curtobacterium flaccumfaciens pv. flaccumfaciens in common bean (Phaseolus vulgaris) using rhizobacterial biocontrol agents. Lett. Appl. Microbiol. 2023, 76, ovac011. [Google Scholar] [CrossRef]
- Patel, M.; Patel, K.; Al-Keridis, L.A.; Alshammari, N.; Badraoui, R.; Elasbali, A.M.; Al-Soud, W.A.; Hassan, M.I.; Yadav, D.K.; Adnan, M. Cadmium-Tolerant Plant Growth-Promoting Bacteria Curtobacterium oceanosedimentum Improves Growth Attributes and Strengthens Antioxidant System in Chili (Capsicum frutescens). Sustainability 2022, 14, 4335. [Google Scholar] [CrossRef]
- Díez-Méndez, A.; Rivas, R. Improvement of saffron production using Curtobacterium herbarum as a bioinoculant under greenhouse conditions. AIMS Microbiol. 2017, 3, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Gupta, R.S. A phylogenomic and comparative genomic framework for resolving the polyphyly of the genus Bacillus: Proposal for six new genera of Bacillus species, Peribacillus gen. nov., Cytobacillus gen. nov., Mesobacillus gen. nov., Neobacillus gen. nov., Metabacillus gen. nov. and Alkalihalobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 406–438. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.G.; Lee, K.E.; Asaf, S.; Khan, M.A.; Lee, I.J. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, J.; Mackiewicz, B.; Kinga Lemieszek, M.; Golec, M.; Milanowski, J. Pantoea agglomerans: A mysterious bacterium of evil and good. Part III. Deleterious effects: Infections of humans, animals and plants. Ann. Agric. Environ. Med. 2016, 23, 197–205. [Google Scholar] [CrossRef]
- Chen, C.; Xin, K.; Liu, H.; Cheng, J.; Shen, X.; Wang, Y.; Zhang, L. Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci. Rep. 2017, 7, 41564, Erratum in Sci. Rep. 2021, 11, 8160. [Google Scholar] [CrossRef]
- Ji, J.; Yuan, D.; Jin, C.; Wang, G.; Li, X.; Guan, C. Enhancement of growth and salt tolerance of rice seedlings (Oryza sativa L.) by regulating ethylene production with a novel halotolerant PGPR strain Glutamicibacter sp. YD01 containing ACC deaminase activity. Acta Physiol. Plant. 2020, 42, 42. [Google Scholar] [CrossRef]
- Paredes-Páliz, K.I.; Pajuelo, E.; Doukkali, B.; Caviedes, M.A.; Rodríguez-Llorente, I.D.; Mateos-Naranjo, E. Bacterial inoculants for enhanced seed germination of Spartina densiflora: Implications for restoration of metal polluted areas. Mar. Pollut. Bull. 2016, 110, 396–400. [Google Scholar] [CrossRef]
- Kaneko, M.; Itoh, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Matsuoka, M. The alpha-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol. 2002, 128, 1264–1270. [Google Scholar] [CrossRef]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Rhizosphere Colonization Determinants by Plant Growth-Promoting Rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef]
- Ngo, N.G.; Yamada, T.; Higuma, S.; Ueno, N.; Saito, K.; Kojima, K.; Maeda, M.; Yamaya-Ito, H.; Ohkama-Ohtsu, N.; Kanekatsu, M.; et al. Spore inoculation of Bacillus pumilus TUAT1 strain, a biofertilizer microorganism, enhances seedling growth by promoting root system development in rice. Soil Sci. Plant Nutr. 2019, 65, 598–604. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.-P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Al-Mushhin, A.A.M.; Qari, S.H.; Fakhr, M.A.; Alnusairi, G.S.H.; Alnusaire, T.S.; ALrashidi, A.A.; Latef, A.A.H.A.; Ali, O.M.; Khan, A.A.; Soliman, M.H. Exogenous Myo-Inositol Alleviates Salt Stress by Enhancing Antioxidants and Membrane Stability via the Upregulation of Stress Responsive Genes in Chenopodium quinoa L. Plants 2021, 10, 2416. [Google Scholar] [CrossRef] [PubMed]
- Flores-Duarte, N.J.; Pajuelo, E.; Mateos-Naranjo, E.; Navarro-Torre, S.; Rodríguez-Llorente, I.D.; Redondo-Gómez, S.; Carrasco López, J.A. A Culturomics-Based Bacterial Synthetic Community for Improving Resilience towards Arsenic and Heavy Metals in the Nutraceutical Plant Mesembryanthemum crystallinum. Int. J. Mol. Sci. 2023, 24, 7003. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Hollenbeck, C.B. An introduction to the nutrition and metabolism of choline. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 100–113. [Google Scholar] [CrossRef]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Directions for making mechanical analyses of soil by the hydrometer method. Soil Sci. 1936, 42, 225–229. [Google Scholar] [CrossRef]
- Walkley, A.; Black, C.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Standard Operating Procedure for Soil Total Nitrogen. Dumas Dry Combustion Method; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/3/cb3646en/cb3646en.pdf (accessed on 1 May 2023).
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorous in Soil by Extraction with Sodium Bicarbonate; USDA Circ. 939; US Gov Print Office: Washington, DC, USA, 1954. Available online: https://ia803207.us.archive.org/21/items/estimationofavai939olse/estimationofavai939olse.pdf (accessed on 11 May 2023).
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Sumner, M.E.; Miller, W.P. Cation Exchange Capacity and Exchange Coefficients. In Methods of Soil Analysis Part 3: Chemical Methods; Sparks, D.L., Ed.; SSSA Book Series 5; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1201–1230. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Lee, J.-H.; Jung, Y.; Kim, M.; Kim, S.; Kim, B.K.; Lim, Y.-W. EzTaxon: A web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2007, 57 Pt 10, 2259–2261. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Hu, X.; Chen, J.; Guo, J. Two Phosphate- and Potassium-solubilizing Bacteria Isolated from Tianmu Mountain, Zhejiang, China. World J. Microbiol. Biotechnol. 2006, 22, 983–990. [Google Scholar] [CrossRef]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of Indole-3-acetic Acid (IAA) Production in Klebsiella by LC-MS/MS and the Salkowski Method. Bio Protoc. 2019, 9, e3230. [Google Scholar] [CrossRef]
- Louden, B.C.; Haarmann, D.; Lynne, A.M. Use of Blue Agar CAS Assay for Siderophore Detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef]
- Flores-Duarte, N.J.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Pajuelo, E.; Rodriguez-Llorente, I.D.; Navarro-Torre, S. Role of Nodulation-Enhancing Rhizobacteria in the Promotion of Medicago sativa Development in Nutrient-Poor Soils. Plants 2022, 11, 1164. [Google Scholar] [CrossRef]
- Libik-Konieczny, M.; Kozieradzka-Kiszkurno, M.; Michalec-Warzecha Miszalski, Z.; Bizan, J.; Konieczny, R. Influence of anti- and prooxidants on rhizogenesis from hypocotyls of Mesembryanthemum crystallinum L. cultured in vitro. Acta Physiol. Plant. 2017, 39, 166. [Google Scholar] [CrossRef]
- Drakopoulou, S.; Orfanakis, E.; Karagiannaki, I.; Gaitis, F.; Skoulika, S.; Papaioannou, A.; Boukouvalas, G.; Petropoulos, G.; Katsoudas, V.; Kontzedaki, R.; et al. Comparative Evaluation of Different Targeted and Untargeted Analytical Approaches to Assess Greek Extra Virgin Olive Oil Quality and Authentication. Molecules 2022, 27, 1350. [Google Scholar] [CrossRef]

| Soil Characteristics | |
|---|---|
| pH | 7.24 ± 0.01 |
| Electric conductivity (mS∙cm−1) | 0.983 ± 0.027 |
| Oxidable organic matter (%) | 2.37 ± 0.07 |
| Organic Carbon (%) | 1.37 ± 0.04 |
| Nitrogen Content (%) | 0.26 ± 0.01 |
| Available P (mg∙Kg−1) | 49.70 ± 0.60 |
| Content of macro- and micronutrients | |
| Ca (cmol∙Kg−1) | 9.04 ± 0.57 |
| Mg (cmol∙Kg−1) | 3.60 ± 0.06 |
| K (cmol∙Kg−1) | 2.11 ± 0.07 |
| Na (cmol∙Kg−1) | 3.76 ± 0.11 |
| Fe (mg∙Kg−1) | 33. 93 ± 0.69 |
| Mn (mg∙Kg−1) | 18.14 ± 0.38 |
| Zn (mg∙Kg−1) | 20.97 ± 1.43 |
| Cu (mg∙Kg−1) | 5.21 ± 0.07 |
| Cd (mg∙Kg−1) | ND |
| Pb (mg∙Kg−1) | ND |
| Texture | |
| Clay (%) | 17.0 ± 1.8 |
| Silt (%) | 43.0 ± 1.0 |
| Sand (%) | 40.0 ± 1.4 |
| Culture Medium | Compartment | Number of Isolates | Gram Staining | |
|---|---|---|---|---|
| % Gram-Positive | % Gram-Negative | |||
| TSA | ||||
| Rhizosphere soil | 10 | 40 (4 strains) | 60 (6 strains) | |
| Root | 26 | 100 (26 strains) | 0 (0 strains) | |
| Shoots | 11 | 82 (9 strains) | 18 (2 strains) | |
| Mesem Agar | ||||
| Rhizosphere soil | 11 | 82 (9 strains) | 18 (2 strains) | |
| Root | 15 | 100 (16 strains) | 0 (0 strains) | |
| Shoots | 11 | 91 (10 strains) | 9 (1 strain) | |
| Isolation Medium | TSA | MA | ||||
|---|---|---|---|---|---|---|
| Strain | S3 | R5 | H4 | MS2 | MR4 | MH8 |
| Identification | Pantoea agglomerans | Bacillus velezensis | Pseudomonas chlororaphis | Priestia megaterium | Bacillus subtilis | Pseudomonas gessardii |
| PGP properties | ||||||
| P solubilization (Ø) | + (0.3 cm) | − | + (0.5 cm) | + (0.3 cm) | − | + (0.3 cm) |
| Biofilm formation (A575nm) | + (0.150) | + (0.156) | − | + (0.122) | ++ (0.463) | − |
| N2-fixation | + | + | − | − | + | − |
| Auxin production (µg mL−1) | +++ (17.62 µg mL−1) | − | ++ (2.03 µg mL−1) | ++ (2.0 µg mL−1) | − | ++ (13.15 µg mL−1) |
| Siderophores production (Ø) | ++ (1.4 cm) | − | + (1 cm) | ++ (1.5 cm) | + (1.0 cm) | +++ (1.9 cm) |
| K solubilization (Ø) | − | − | + (0.3 mm) | − | − | ++ (0.6 cm) |
| Extracellular enzymes | ||||||
| DNAase | − | + | − | − | + | − |
| Amylase | − | + | + | + | + | + |
| Cellulase | + | + | − | − | − | − |
| Lipase | − | − | − | − | − | − |
| Pectinase | − | +++ | ++ | − | +++ | − |
| Protease | − | + | + | + | + | + |
| Chitinase | − | − | − | − | − | − |
| Parameter | Non-Inoculated | TSA | MA |
|---|---|---|---|
| Shoot fresh weight (g) | 6.57 ± 3.39 (a) | 16.32± 7.67 (b) | 16.35 ± 1.68 (b) |
| Shoot dry matter (g) | 0.11 ± 0.03 (a) | 0.30 ± 0.10 (b) | 0.34 ± 0.02 (b) |
| Root fresh weight (g) | 0.13 ± 0.08 (a) | 0.26 ± 0.04 (b) | 0.36 ± 0.05 (c) |
| Root dry matter (g) | 0.012 ± 0.005 (a) | 0.024 ± 0.004(b) | 0.034 ± 0.010 (c) |
| Maximum quantum efficiency of PSII photochemistry (Fv/Fm) | 0.75 ± 0.03 (a) | 0.76 ± 0.06 (a) | 0.71 ± 0.02 (a) |
| Actual quantum efficiency of PSII (ɸPSII) | 0.37 ± 0.02 (a) | 0.39 ± 0.04 (a) | 0.37 ± 0.03 (a) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajuelo, E.; Flores-Duarte, N.J.; Navarro-Torre, S.; Rodríguez-Llorente, I.D.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Carrasco López, J.A. Culturomics and Circular Agronomy: Two Sides of the Same Coin for the Design of a Tailored Biofertilizer for the Semi-Halophyte Mesembryanthemum crystallinum. Plants 2023, 12, 2545. https://doi.org/10.3390/plants12132545
Pajuelo E, Flores-Duarte NJ, Navarro-Torre S, Rodríguez-Llorente ID, Mateos-Naranjo E, Redondo-Gómez S, Carrasco López JA. Culturomics and Circular Agronomy: Two Sides of the Same Coin for the Design of a Tailored Biofertilizer for the Semi-Halophyte Mesembryanthemum crystallinum. Plants. 2023; 12(13):2545. https://doi.org/10.3390/plants12132545
Chicago/Turabian StylePajuelo, Eloísa, Noris J. Flores-Duarte, Salvadora Navarro-Torre, Ignacio D. Rodríguez-Llorente, Enrique Mateos-Naranjo, Susana Redondo-Gómez, and José A. Carrasco López. 2023. "Culturomics and Circular Agronomy: Two Sides of the Same Coin for the Design of a Tailored Biofertilizer for the Semi-Halophyte Mesembryanthemum crystallinum" Plants 12, no. 13: 2545. https://doi.org/10.3390/plants12132545
APA StylePajuelo, E., Flores-Duarte, N. J., Navarro-Torre, S., Rodríguez-Llorente, I. D., Mateos-Naranjo, E., Redondo-Gómez, S., & Carrasco López, J. A. (2023). Culturomics and Circular Agronomy: Two Sides of the Same Coin for the Design of a Tailored Biofertilizer for the Semi-Halophyte Mesembryanthemum crystallinum. Plants, 12(13), 2545. https://doi.org/10.3390/plants12132545









