Anatomical and Biomechanical Properties of the Junction between Stem and Aerial Roots of Selenicereus undatus
Abstract
1. Introduction
2. Results
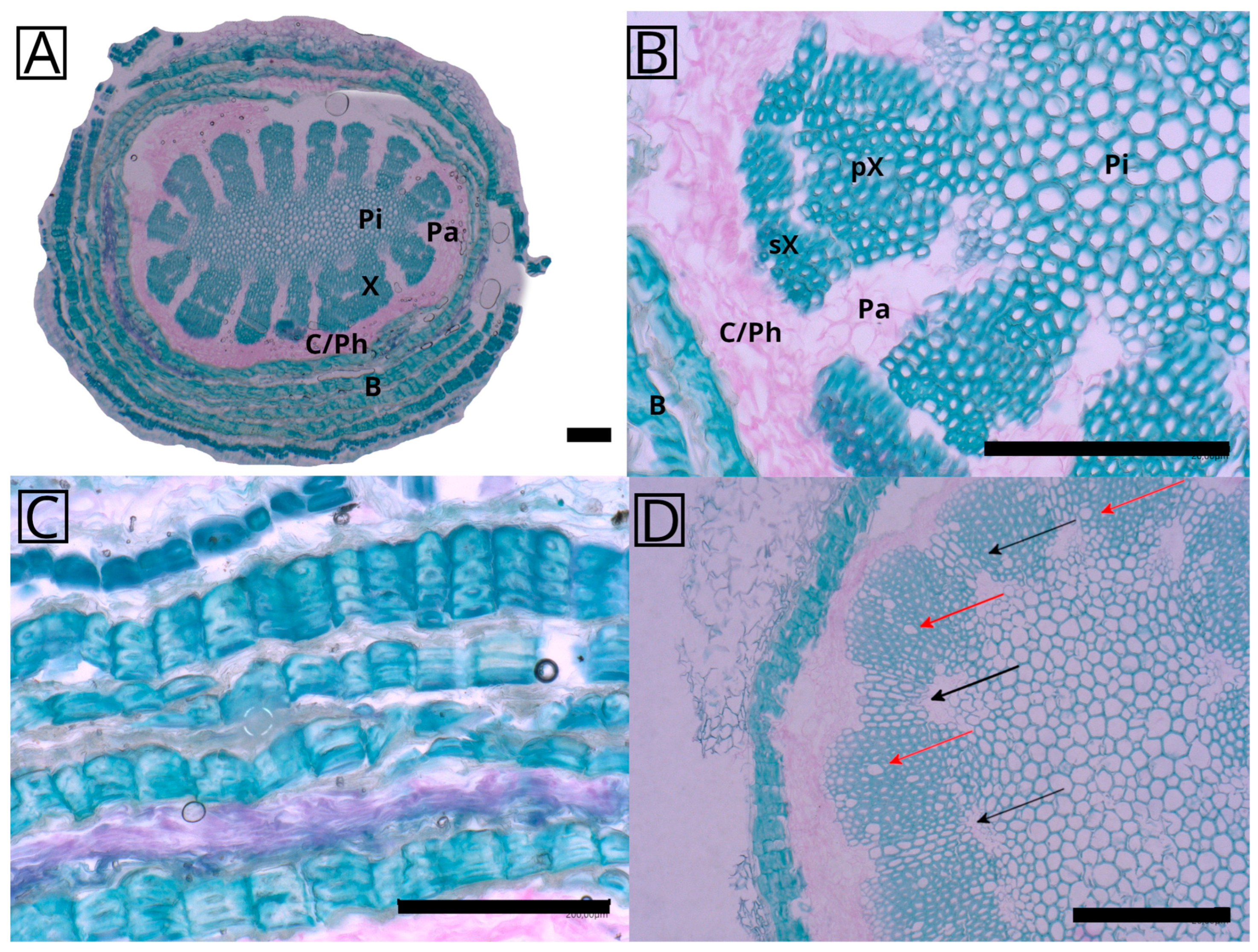
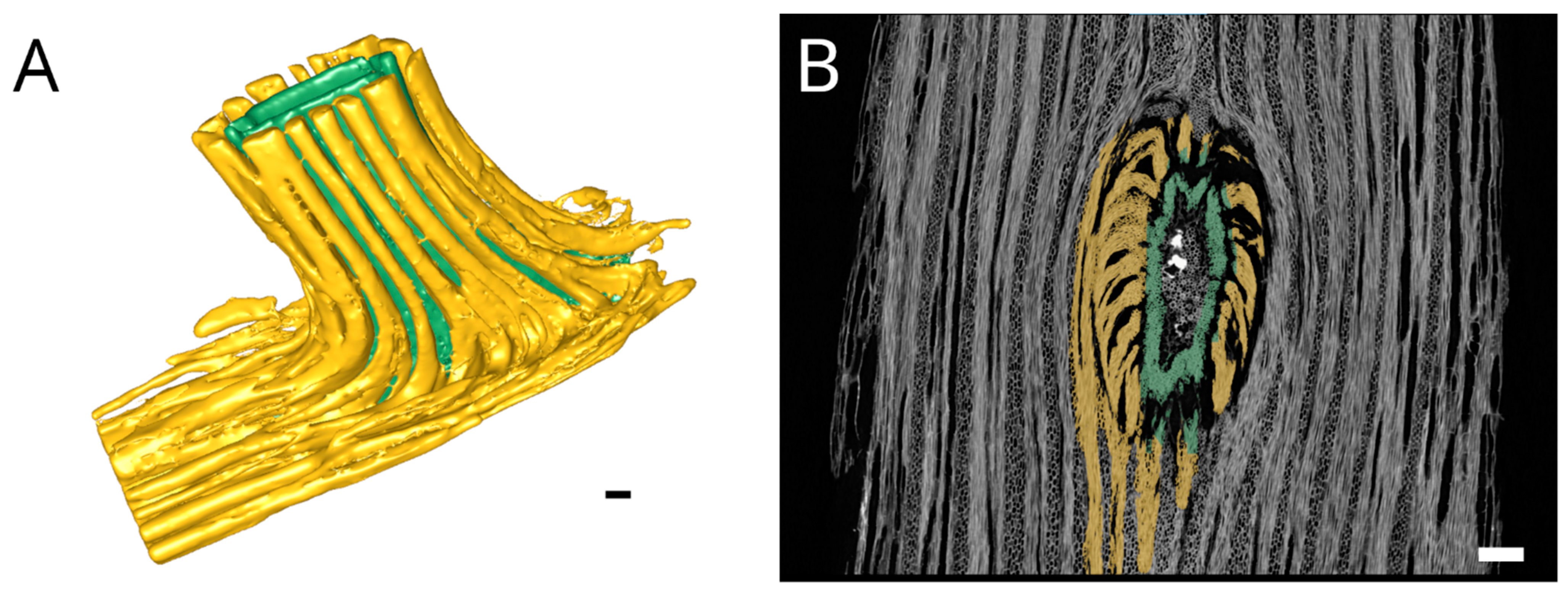
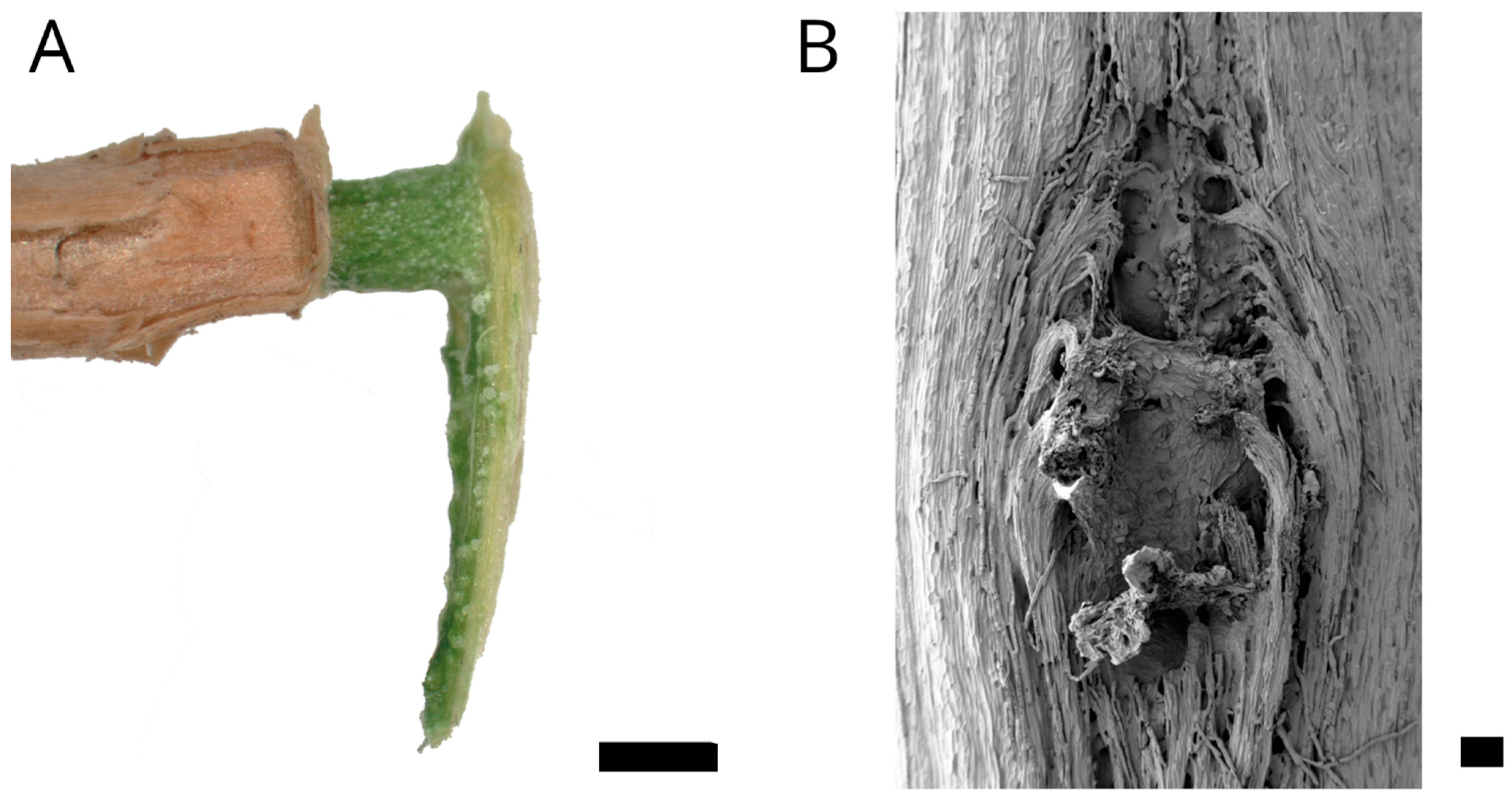
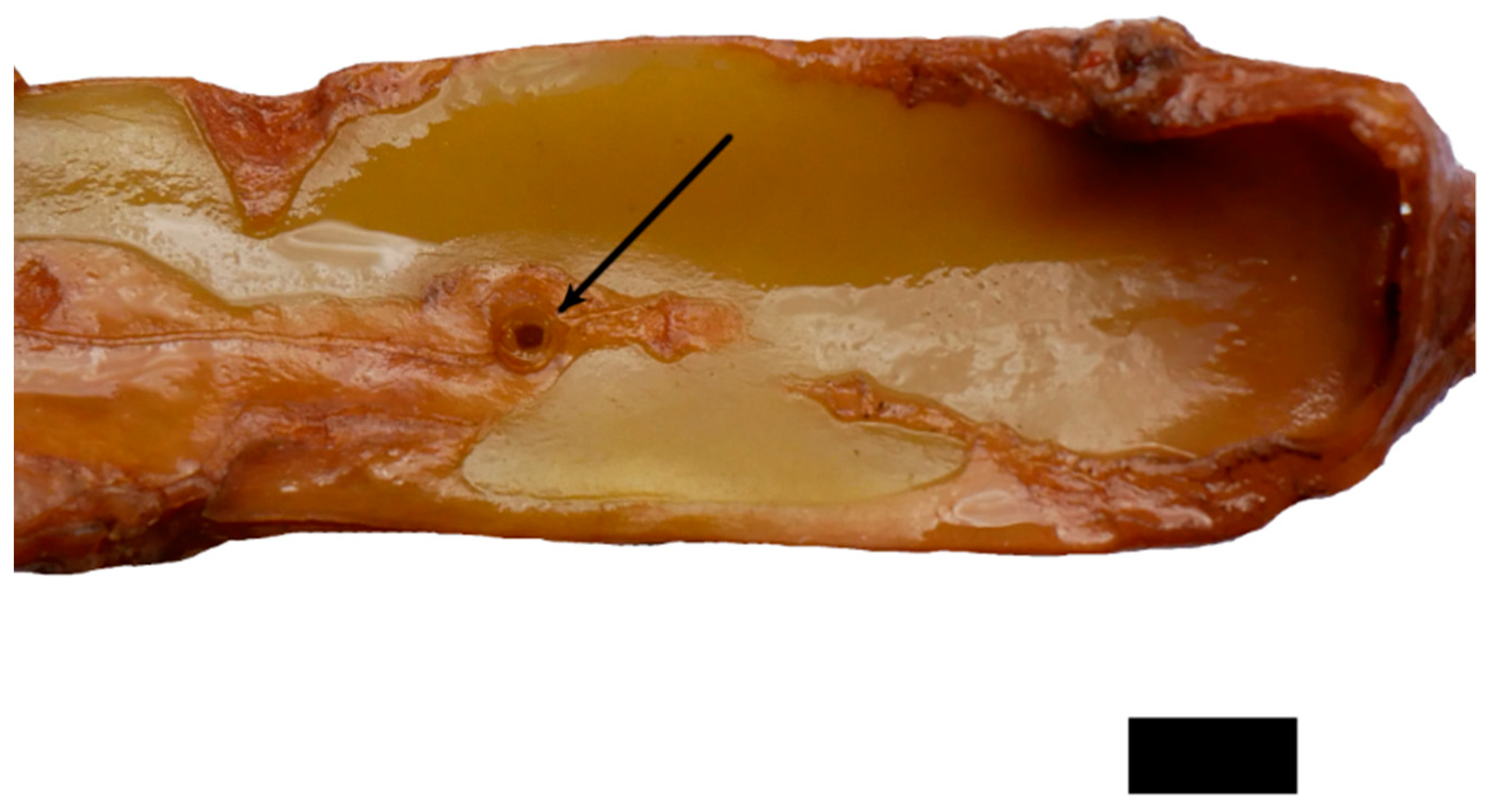
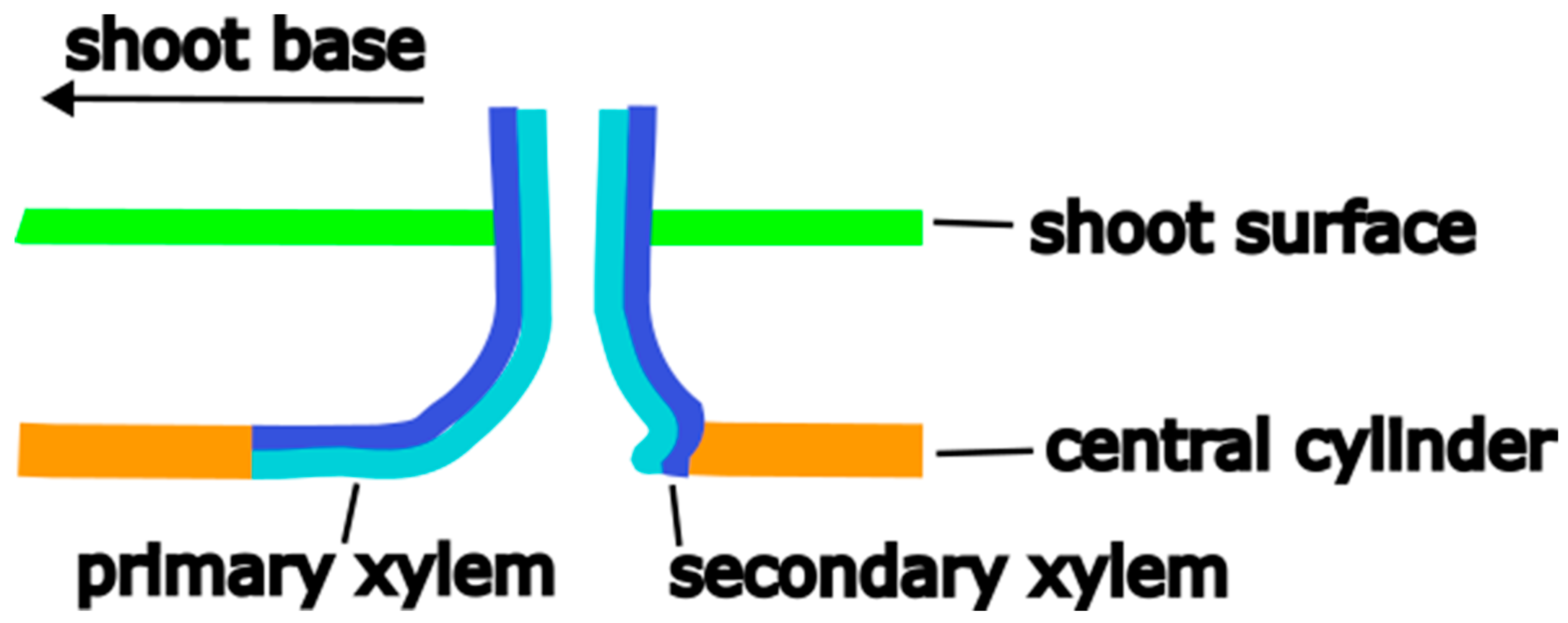
2.1. Anatomy and Morphology
2.2. Biomechanics
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Anatomy and Morphology
4.3. Biomechanical Testing
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zotz, G. Plants on Plants—The Biology of Vascular Epiphytes; Fascinating Life Sciences; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-39236-3. [Google Scholar]
- Melzer, B.; Steinbrecher, T.; Seidel, R.; Kraft, O.; Schwaiger, R.; Speck, T. The Attachment Strategy of English Ivy: A Complex Mechanism Acting on Several Hierarchical Levels. J. R. Soc. Interface 2010, 7, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Chabbert, B.; Monties, B.; Speck, T. Mechanical, Chemical and X-Ray Analysis of Wood in the Two Tropical Lianas Bauhinia Guianensis and Condylocarpon Guianense: Variations during Ontogeny. Planta 2003, 217, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Mylo, M.D.; Hofmann, M.; Balle, F.; Beisel, S.; Speck, T.; Speck, O. Biomechanics of the Parasite–Host Interaction of the European Mistletoe. J. Exp. Bot. 2022, 73, 1204–1221. [Google Scholar] [CrossRef] [PubMed]
- Soffiatti, P.; Rowe, N.P. Mechanical Innovations of a Climbing Cactus: Functional Insights for a New Generation of Growing Robots. Front. Robot. AI 2020, 7, 64. [Google Scholar] [CrossRef]
- Bastola, A.K.; Soffiatti, P.; Behl, M.; Lendlein, A.; Rowe, N.P. Structural Performance of a Climbing Cactus: Making the Most of Softness. J. R. Soc. Interface 2021, 18, 20210040. [Google Scholar] [CrossRef]
- Huggett, B.; Tomlinson, P.B. Aspects of Vessel Dimensions in the Aerial Roots of Epiphytic Araceae. Int. J. Plant Sci. 2010, 171, 362–369. [Google Scholar] [CrossRef]
- Mayo, S.J. A Revision of Philodendron Subgenus Meconostigma (Araceae). Kew Bull. 1991, 46, 601. [Google Scholar] [CrossRef]
- Tenorio, V.; Sakuragui, C.M.; Vieira, R.C. Structures and Functions of Adventitious Roots in Species of the Genus Philodendron Schott (Araceae). Flora Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 547–555. [Google Scholar] [CrossRef]
- Krainz, H. Hylocereus undatus. In Die Kakteen; Krainz, H., Ed.; Franckh’sche Verlagshandlung: Stuttgart, Germany, 1959; Volume 10. [Google Scholar]
- Anderson, E.F.; Barthlott, W.; Eggli, U. Das große Kakteen-Lexikon; Ulmer: Stuttgart, Germany, 2011; ISBN 978-3-8001-5964-2. [Google Scholar]
- Mizrahi, Y.; Nerd, A. Climbing and Columnar Cacti: New Arid Land Fruit Crops. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; p. 9. [Google Scholar]
- Nobel, P.S. (Ed.) Cacti: Biology and Uses; University of California Press: Berkeley, CA, USA, 2002; ISBN 978-0-520-23157-3. [Google Scholar]
- Schwager, H.; Neinhuis, C.; Mauseth, J.D. Secondary Growth of the Leaf and Bud Traces in Hylocereus Undatus (Cactaceae) during the Formation of Branches or Flowers. Int. J. Plant Sci. 2015, 176, 762–769. [Google Scholar] [CrossRef]
- Mylo, M.D.; Hesse, L.; Masselter, T.; Leupold, J.; Drozella, K.; Speck, T.; Speck, O. Morphology and Anatomy of Branch–Branch Junctions in Opuntia Ficus-Indica and Cylindropuntia Bigelovii: A Comparative Study Supported by Mechanical Tissue Quantification. Plants 2021, 10, 2313. [Google Scholar] [CrossRef]
- Keller, L. Anatomische Studien über die Luftwurzeln Einiger Dikotyledonen. Ph.D. Thesis, Universitäts-Buchdruckerei, Heidelberg, Germany, 1889. [Google Scholar]
- Haehnel, K. Anatomisch-Biologische Betrachtungen über die Kakteen; Deutsche Schule zu Mexico: Mexico City, Mexico, 1912. [Google Scholar]
- Ewers, F.W.; North, G.B.; Nobelf, P.S. Root—Stem Junctions of a Desert Monocotyledon and a Dicotyledon: Hydraulic Consequences under Wet Conditions and during Drought. New Phytol. 1992, 121, 377–385. [Google Scholar] [CrossRef]
- Barbosa, A.S.; Sá, R.D.; Silva, F.C.L.; Randau, K.P.; Andrade, A.P.; Medeiros, R.L.S. Anatomical Characterization of Pilosocereus Pachycladus, F. Ritter Roots. Acta Hortic. 2015, 1087, 239–242. [Google Scholar] [CrossRef]
- Freeman, T.P. The developmental anatomy of opuntia basilaris. i. embryo, root, and transition zone. Am. J. Bot. 1969, 56, 1067–1074. [Google Scholar] [CrossRef]
- Garcia, J.D.S.; Scremin-Dias, E.; Soffiatti, P. Stem and Root Anatomy of Two Species of Echinopsis (Trichocereeae, Cactaceae). Rev. Mex. Biodiv. 2012, 83, 1036–1044. [Google Scholar] [CrossRef]
- Seidel, M. Tensile Surface Structures. A Practical Guide to Cable and Membrane Construction: Materials, Design, Assembly and Erection, 1st ed.; Wiley: Hoboken, NJ, USA, 2009; ISBN 978-3-433-02922-0. [Google Scholar]
- Rowe, N.P.; Speck, T. Biomechanical Characteristics of the Ontogeny and Growth Habit of the Tropical Liana Condylocarpon Guianense (Apocynaceae). Int. J. Plant Sci. 1996, 157, 406–417. [Google Scholar] [CrossRef]
- Rowe, N.; Speck, T. Plant Growth Forms: An Ecological and Evolutionary Perspective. New Phytol. 2005, 166, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Köhler, L.; Speck, T.; Spatz, H.-C. Micromechanics and Anatomical Changes during Early Ontogeny of Two Lianescent Aristolochia Species. Planta 2000, 210, 691–700. [Google Scholar] [CrossRef]
- Rowe, N.; Isnard, S.; Speck, T. Diversity of Mechanical Architectures in Climbing Plants: An Evolutionary Perspective. J. Plant Growth Regul. 2004, 23, 108–128. [Google Scholar] [CrossRef]
- Niklas, K.J. An Engineering Approach to Plant Form and Function; University of Chicago Press: Chicago, IL, USA, 1992. [Google Scholar]
- Barbosa, A.C.F.; Pace, M.R.; Witovisk, L.; Angyalossy, V. A New Method to Obtain Good Anatomical Slides of Heterogeneous Plant Parts. IAWA J. 2010, 31, 373–383. [Google Scholar] [CrossRef]
- Mozzi, G.; Romero, E.; Martínez-Quezada, D.M.; Hultine, K.R.; Crivellaro, A. PEG Infiltration: An Alternative Method to Obtain Thin Sections of Cacti Tissues. IAWA J. 2021, 42, 204–208. [Google Scholar] [CrossRef]
- Rupp, P. Polyglykol als Einbettungsmedium zum Schneiden botanischer Präparate. Mikrokosmos 1964, 53, 123–128. [Google Scholar]
- Mondolot, L.; Roussel, J.-L.; Andary, C. New Applications for an Old Lignified Element Staining Reagent. Histochem. J. 2001, 33, 379–385. [Google Scholar] [CrossRef] [PubMed]


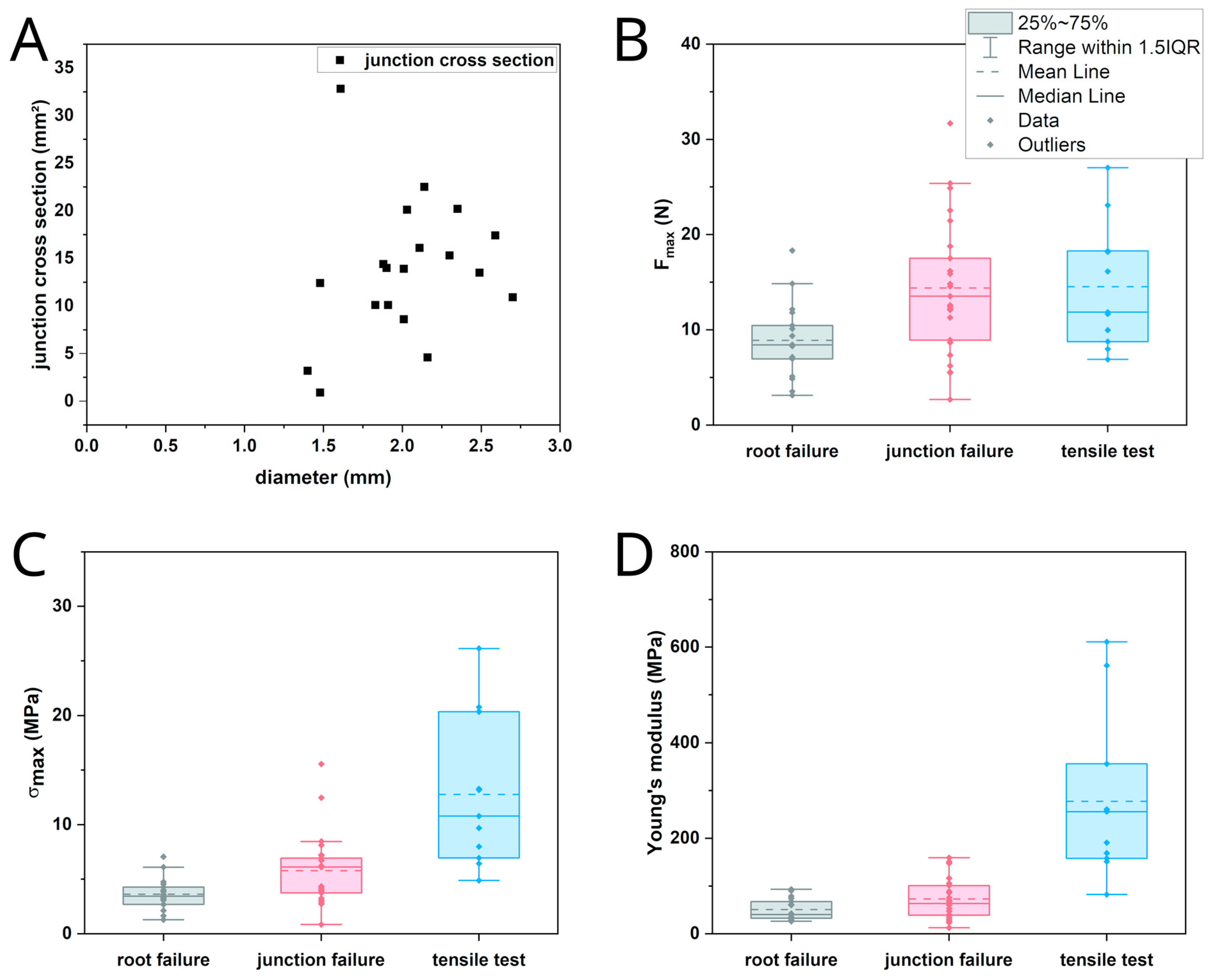
| Breaking Force (N) | Breaking Stress (MPa) | Young’s Modulus (MPa) | |
|---|---|---|---|
| root failure | 8.42 (5.11, n = 17) | 3.45 (1.74, n = 17) | 40.31 (38.42, n = 17) |
| junction failure | 13.52 (9.35, n = 25) | 6.11 (3.55, n = 25) | 63.09 (64.39, n = 25) |
| tensile test | 11.85 (9.96, n = 11) | 10.78 (13.41, n = 11) | 255.86 (196.25, n = 11) |
| Breaking Force (N) | Breaking Stress (MPa) | Young’s Modulus (MPa) | |
|---|---|---|---|
| root failure–junction failure | 0.01693 * | 0.06606 | 0.54207 |
| root failure–tensile test | 0.07513 | 6.55195 × 10−6 * | 4.04988 × 10−6 * |
| junction failure–tensile test | 1 | 0.00632 * | 1.86102 × 10−4 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauls, B.; Lautenschläger, T.; Neinhuis, C. Anatomical and Biomechanical Properties of the Junction between Stem and Aerial Roots of Selenicereus undatus. Plants 2023, 12, 2544. https://doi.org/10.3390/plants12132544
Pauls B, Lautenschläger T, Neinhuis C. Anatomical and Biomechanical Properties of the Junction between Stem and Aerial Roots of Selenicereus undatus. Plants. 2023; 12(13):2544. https://doi.org/10.3390/plants12132544
Chicago/Turabian StylePauls, Bennett, Thea Lautenschläger, and Christoph Neinhuis. 2023. "Anatomical and Biomechanical Properties of the Junction between Stem and Aerial Roots of Selenicereus undatus" Plants 12, no. 13: 2544. https://doi.org/10.3390/plants12132544
APA StylePauls, B., Lautenschläger, T., & Neinhuis, C. (2023). Anatomical and Biomechanical Properties of the Junction between Stem and Aerial Roots of Selenicereus undatus. Plants, 12(13), 2544. https://doi.org/10.3390/plants12132544






