How Can Biological and Chemical Silver Nanoparticles Positively Impact Physio-Chemical and Chloroplast Ultrastructural Characteristics of Vicia faba Seedlings?
Abstract
1. Introduction
2. Results
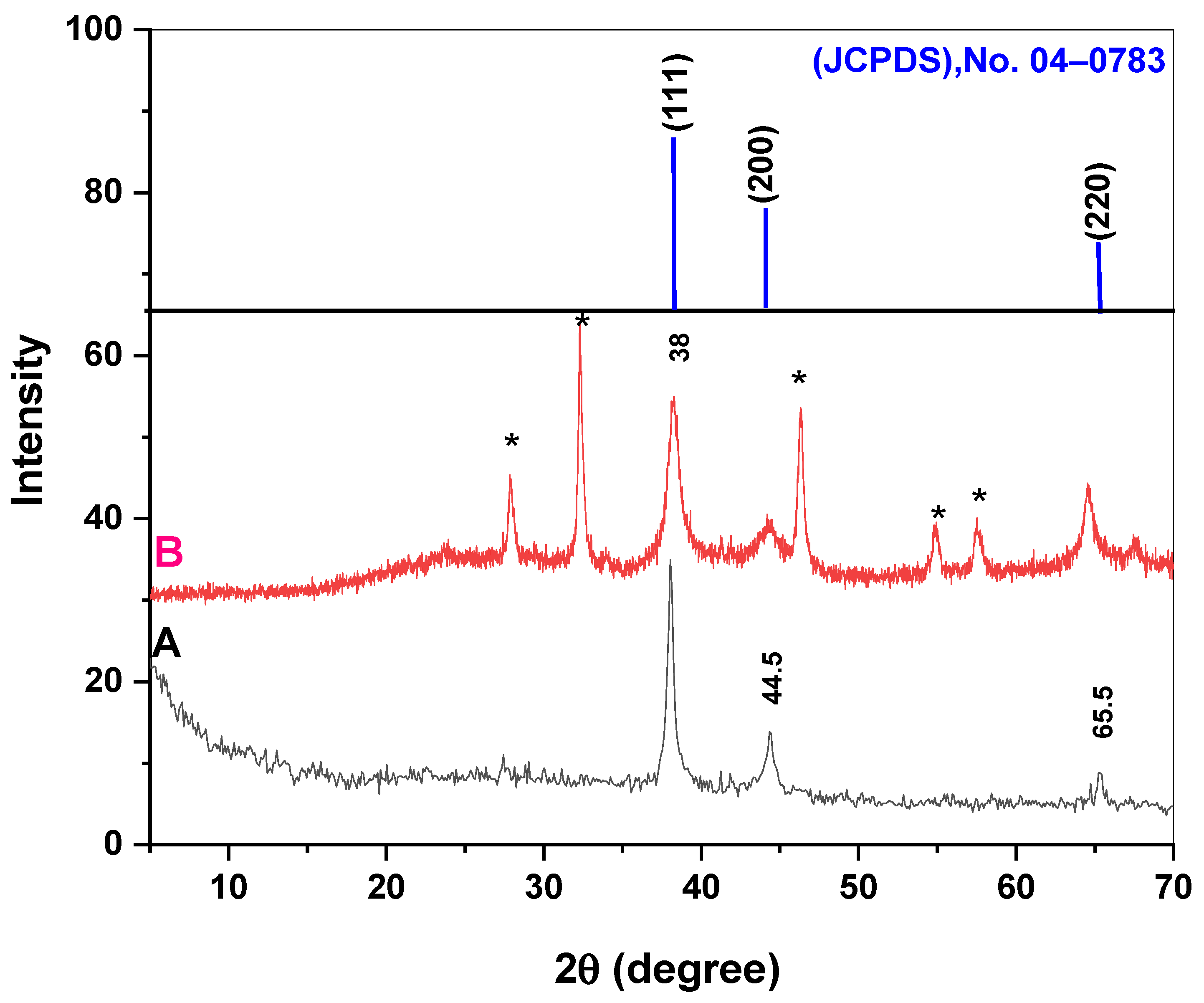
2.1. Characterization of the Prepared Chem-AgNPs and Bio-AgNPs
2.2. Changes in Growth Vigor of Root and Shoot
2.3. Changes in Photosynthetic Pigments, Total Soluble Sugars, Starch, and Total Carbohydrates Content
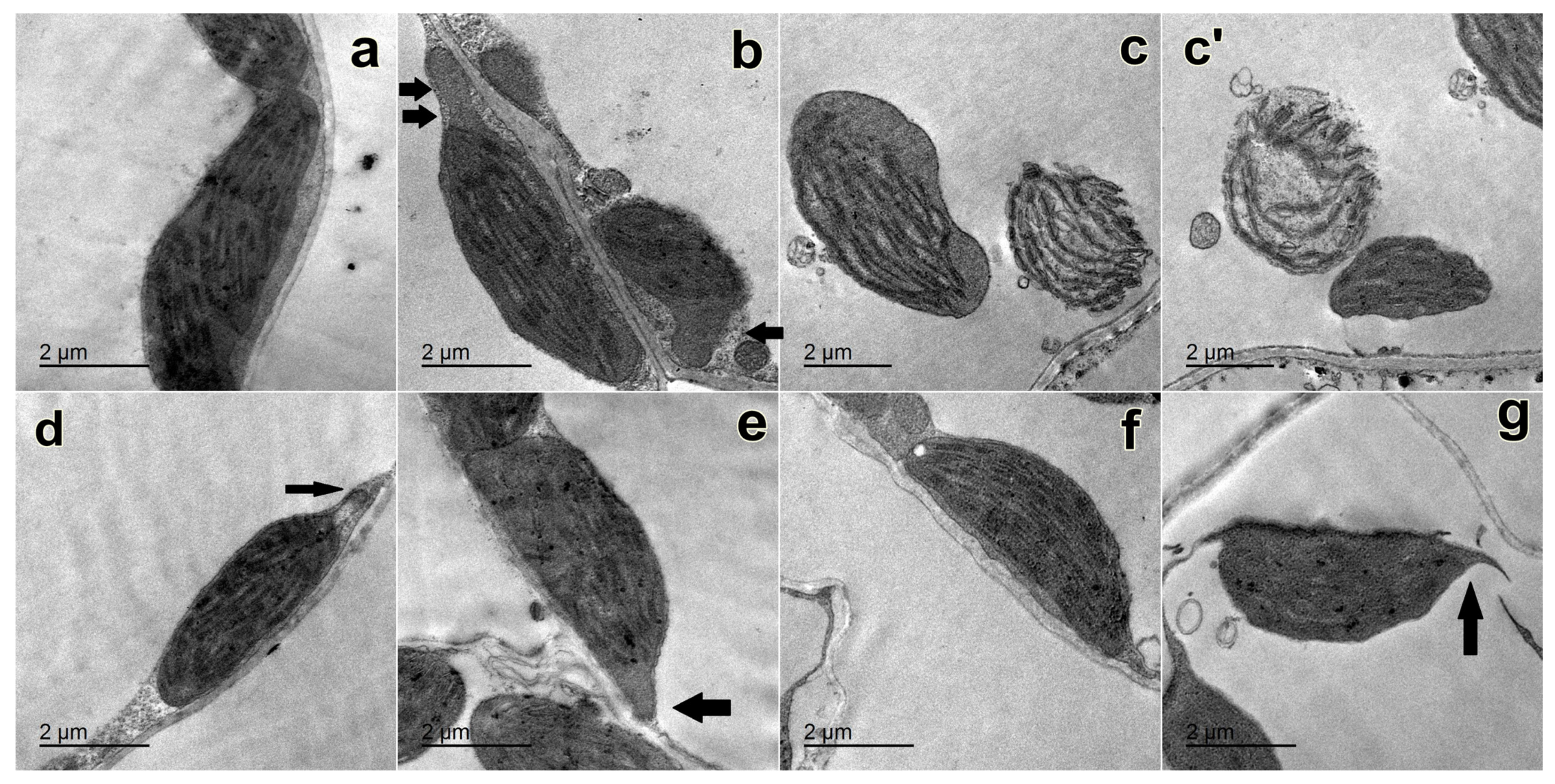
2.4. Changes in the Ultrastructure of Chloroplasts
2.5. Changes in H2O2 Content and Antioxidant Enzymes Activities
2.6. Changes in Silver Content in Shoots and Roots of Faba Bean Seedlings
3. Discussion
4. Materials and Methods
4.1. Materials
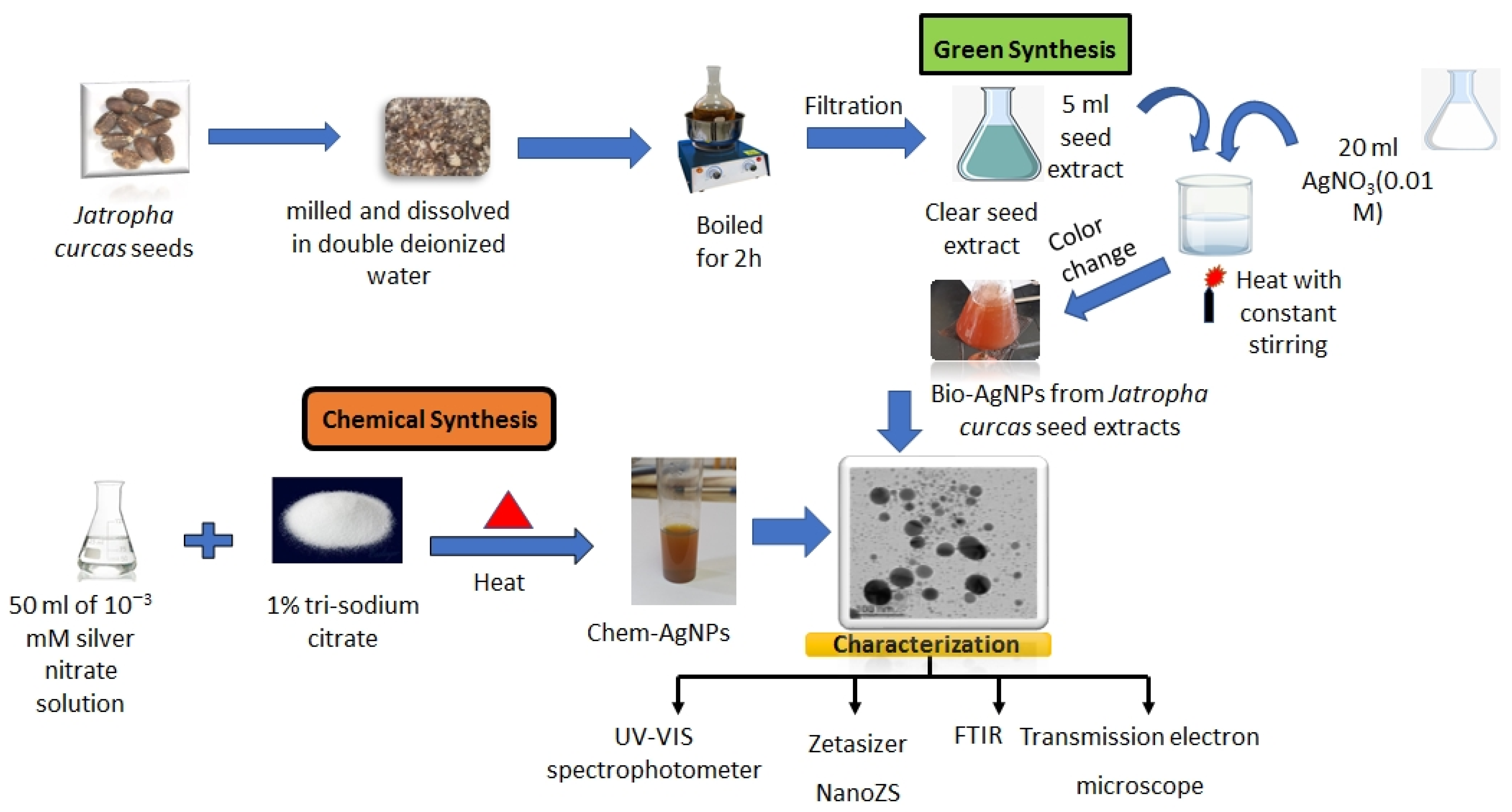
4.2. Synthesis of AgNPs using Trisodium Citrate (Chem-AgNPs)
4.3. Synthesis of AgNPs using Jatropha Seed Extract (Bio-AgNPs)
4.4. Characterization of the Obtained AgNPs (Chem- or Bio-AgNPs)
- Transmission Electron Microscopy (TEM) Analysis of AgNPs
- Confirming AgNPs Synthesis Using UV-Visible Absorbance Spectroscopy Analysis
- Determination of AgNPs Zeta Potential Analysis
- X-Ray diffraction of the AgNPs
4.5. Experimental Setup and Exposure to AgNPs
4.6. Biochemical Analyses
4.6.1. Estimation of Photosynthetic Pigments
4.6.2. Evaluation of Total Soluble Sugars, Total Carbohydrates, and Starch
4.6.3. Measurement of H2O2 Content and Antioxidant Enzyme Activities
4.6.4. Determination of Ag in Plant Samples
4.7. Ultrastructure of Chloroplasts Using the Transmission Electron Microscope
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassanein, R.A.; Hashem, H.A.; Khalil, R.R. Stigmasterol treatment increases salt stress tolerance of faba bean plants by enhancing antioxidant systems. Plant Omics 2012, 5, 476–485. [Google Scholar]
- Mogazy, A.M.; Hanafy, R.S. Foliar spray of biosynthesized zinc oxide nanoparticles alleviate salinity stress effect on Vicia faba plants. J. Soil Sci. Plant Nutr. 2022, 22, 2647–2662. [Google Scholar] [CrossRef]
- Qados, A.M.A. Mechanism of nanosilicon-mediated alleviation of salinity stress in faba bean (Vicia faba L.) plants. Am. J. Exp. Agric. 2015, 7, 78–95. [Google Scholar] [CrossRef]
- Conrath, U. Molecular aspects of defense priming. Trends Plant Sci. 2011, 16, 524–531. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Hosseinzadeh-Mahootchy, A.; Zehtab-Salmasi, S.; Tourchi, M. Improving field performance of aged chickpea seeds by hydro-priming under water stress. Int. J. Plant Animal Environ. Sci. 2012, 2, 168–176. [Google Scholar]
- Hassanisaadi, M.; Barani, M.; Rahdar, A.; Heidary, M.; Thysiadou, A.; Kyzas, G.Z. Role of agrochemical-based nanomaterials in plants: Biotic and abiotic stress with germination improvement of seeds. Plant Growth Regul. 2022, 97, 375–418. [Google Scholar] [CrossRef]
- Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual effect of nanomaterials on germination and seedling growth: Stimulation vs. phytotoxicity. Plants 2020, 9, 1745. [Google Scholar] [CrossRef]
- Alshehddi, L.A.A.; Bokhari, N. Influence of gold and silver nanoparticles on the germination and growth of Mimusops laurifolia seeds in the South-Western regions in Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 574–580. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotchnol. 2018, 16, 1–28. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H.M. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef]
- Budhani, S.; Egboluche, N.P.; Arslan, Z.; Yu, H.; Deng, H. Phytotoxic effect of silver nanoparticles on seed germination and growth of terrestrial plants. J. Environ. Sci. Health C 2019, 37, 330–355. [Google Scholar] [CrossRef] [PubMed]
- Jayarambabu, N.; Rao, K.; Park, S.H.; Rajendar, V. Biogenic synthesized Fe3O4 nanoparticles affect on growth parameter of maize (Zea mays L.). Dig. J. Nanomater. Biost. 2018, 13, 903–913. [Google Scholar]
- Youssef, M.S.; Elamawi, R.M. Evaluation of phytotoxicity, cytotoxicity, and genotoxicity of ZnO nanoparticles in Vicia faba. Environ. Sci. Pollut. Res. 2020, 27, 18972–18984. [Google Scholar] [CrossRef] [PubMed]
- Prażak, R.; Święciło, A.; Krzepiłko, A.; Michałek, S.; Arczewska, M. Impact of Ag nanoparticles on seed germination and seedling growth of green beans in normal and chill temperatures. Agriculture 2020, 10, 312. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano Fertilization as an Emerging Fertilization Technique: Why Can Modern Agriculture Benefit from Its Use? Plants 2021, 10, 2. [Google Scholar] [CrossRef]
- Salama, H.M.H. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Savithramma, N.; Ankanna, S.; Bhumi, G. Effect of nanoparticles on seed germination and seedling growth of Boswellia Ovalifoliolata—An endemic and endangered medicinal tree Taxon. Nano Vis. 2012, 2, 61–68. [Google Scholar]
- Parveen, A.; Rao, S.O. Effect of nanosilver on seed germination and seedling growth in Pennisetum glaucum. J. Clust. Sci. 2015, 26, 693–701. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Singh, J.; Liu, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Tripathi, D.K.; Sharma, S. Differential phytotoxic impact of plant mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front. Plant Sci. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Wu, J.; Wang, G.; Vijver, M.G.; Bosker, T.; Peijnenburg, W.J. Foliar versus root exposure of AgNPs to lettuce: Phytotoxicity, antioxidant responses and internal translocation. Environ. Pollut. 2020, 261, 114117. [Google Scholar] [CrossRef]
- Hajian, M.H.; Ghorbanpour, M.; Abtahi, F.; Hadian, J. Differential effects of biogenic and chemically synthesized silver-nanoparticles application on physiological traits, antioxidative status and californidine content in California poppy (Eschscholzia californica Cham). Environ. Pollut. 2022, 292, 118300. [Google Scholar] [CrossRef] [PubMed]
- Gardea-Torresdey, J.L.; Parsons, J.G.; Gomez, E.; Peralta-Videa, J.; Troiani, H.E.; Santiago, P.; Yacaman, M.J. Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett. 2002, 2, 397–401. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.; Jose-Yacaman, M. Alfalfa sprouts: A natural source for the synthesis of silver nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Vilchis-Nestor, A.R.; Sánchez-Mendieta, V.; Camacho-López, M.A.; Gómez-Espinosa, R.M.; Camacho-López, M.A.; Arenas-Alatorre, J.A. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett. 2008, 62, 3103–3105. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Bakar, N.A.; Ismail, J.; Bakar, M.A. Synthesis and characterization of silver nanoparticles in natural rubber. Mater. Chem. Phys. 2007, 104, 276–283. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Sharma, N.C.; Sahi, S.V.; Nath, S.; Parsons, J.G.; Gardea-Torresde, J.L.; Pal, T. Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environ. Sci. Technol. 2007, 41, 5137–5142. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Riayatsyah, T.M.I.; Sebayang, A.H.; Silitonga, A.S.; Padli, Y.; Fattah, I.M.R.; Kusumo, F.; Ong, H.C.; Mahlia, T.M.I. Current progress of Jatropha curcas commoditisation as biodiesel feedstock: A comprehensive review. Front. Energy Res. 2022, 9, 1019. [Google Scholar] [CrossRef]
- He, S.; Guo, Z.; Zhang, Y.; Zhang, S.; Wang, J.; Gu, N. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater. Lett. 2007, 61, 3984–3987. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H. Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. J. Biotechnol. 2017, 262, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. One-step green synthesis of antibacterial silver nanoparticles embedded in electrospun cyclodextrin nanofibers. Carbohydr. Polym. 2019, 207, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Küünal, S.; Visnapuu, M.; Volubujev, O.; Rosario, M.S.; Rauwel, P.; Rauwel, E. Optimisation of plant mediated synthesis of silver nanoparticles by common weed Plantago major and their antimicrobial properties. IOP Conf. Ser. Mater. Sci. Eng. 2019, 613, 12003. [Google Scholar] [CrossRef]
- Devi, T.B.; Ahmaruzzaman, M.; Begum, S. A rapid, facile and green synthesis of Ag/AgCl nanoparticles for the effective reduction of 2,4-dinitrophenyl hydrazine. New J. Chem. 2016, 40, 1497. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef]
- Cvjetko, P.; Milošić, A.; Domijan, A.M.; Vrček, I.V.; Tolić, S.; Štefanić, P.P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef]
- Štefanić, P.P.; Cvjetko, P.; Biba, R.; Domijan, A.M.; Letofsky-Papst, I.; Tkalec, M.; Šikić, S.; Cindrić, M.; Balen, B. Physiological, ultrastructural and proteomic responses of tobacco seedlings exposed to silver nanoparticles and silver nitrate. Chemosphere 2018, 209, 640–653. [Google Scholar] [CrossRef]
- Harris, A.T.; Bali, R. On the formation and extent of uptake of silver nanoparticles by live plants. J. Nanoparticle Res. 2008, 10, 691–695. [Google Scholar] [CrossRef]
- Fayez, K.A.; El-Deeb, B.A.; Mostafa, N.Y. Toxicity of biosynthetic silver nanoparticles on the growth, cell ultrastructure and physiological activities of barley plant. Acta Physiol. Plant. 2017, 39, 1–13. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Gruyer, N.; Dorais, M.; Bastien, C.; Dassylva, N.; Triffault-Bouchet, G. Interaction between silver nanoparticles and plant growth. Acta Hortic. 2014, 1037, 795–800. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Molecular processes induced in primed seeds—Increasing the potential to stabilize crop yields under drought conditions. J. Plant Physiol. 2016, 203, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using photosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in plant science: To make a long story short. Front. Bioeng. Biotechnol. 2019, 7, 120. [Google Scholar] [CrossRef]
- Kaveh, R.; Li, Y.S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Van Aken, B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013, 47, 10637–10644. [Google Scholar] [CrossRef]
- Farghaly, F.A.; Nafady, N.A. Green synthesis of silver nanoparticles using leaf extract of Rosmarinus officinalis and its effect on tomato and wheat plants. J. Agric. Sci. 2015, 7, 277. [Google Scholar] [CrossRef]
- Hatami, M.; Kariman, K.; Ghorbanpour, M. Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants. Sci. Total Environ. 2016, 571, 275–291. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Ren, B.; Ding, L.; Gao, C.; Shen, Q.; Guo, S. Drought-induced root aerenchyma formation restricts water uptake in rice seedlings supplied with nitrate. Plant Cell Physiol. 2012, 53, 495–504. [Google Scholar] [CrossRef]
- Hatami, M.; Naghdi Badi, H.; Ghorbanpour, M. Nano-elicitation of secondary pharmaceutical metabolites in plant cells: A review. J. Med. Plants 2019, 3, 6–36. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrowicz-Trzcińska, M.; Bederska-Błaszczyk, M.; Szaniawski, A.; Olchowik, J.; Studnicki, M. The effects of copper and silver nanoparticles on container-grown Scots Pine (Pinus sylvestris L.) and Pedunculate Oak (Quercus robur L.) seedlings. Forests 2019, 10, 269. [Google Scholar] [CrossRef]
- Dobias, J.; Bernier-Latmani, R. Silver release from silver nanoparticles in natural waters. Environ. Sci. Technol. 2013, 47, 4140–4146. [Google Scholar] [CrossRef] [PubMed]
- Leela, A.; Vivekanandan, M. Tapping the unexploited plant resources for the synthesis of silver nanoparticles. Afr. J. Biotechnol. 2008, 7, 3162–3165. [Google Scholar]
- Moulton, M.C.; Braydich-Stolle, L.K.; Nadagouda, M.N.; Kunzelman, S.; Hussain, S.M.; Varma, R.S. Synthesis, characterization and biocompatibility of “green” synthesized silver nanoparticles using tea polyphenols. Nanoscale 2010, 2, 763–770. [Google Scholar] [CrossRef]
- Ushahra, J.; Bhati-Kushwaha, H.; Malik, C.P. Biogenic nanoparticle-mediated augmentation of seed germination, growth, and antioxidant level of Eruca sativa Mill. varieties. Appl. Biochem. Biotechnol. 2014, 174, 729–738. [Google Scholar] [CrossRef]
- Baskar, V.; Venkatesh, J.; Park, S.W. Impact of biologically synthesized silver nanoparticles on the growth and physiological responses in Brassica rapa ssp. pekinensis. Environ. Sci. Pollut. Res. 2015, 22, 17672–17682. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Ramachandran, R.; Abirami, S.M.; Mohan, N.; Kalaichelvan, P.T. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem. 2012, 47, 651–658. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P.T. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectroch. Acta A Mol. Biomol. Spectrosc. 2012, 93, 95–99. [Google Scholar] [CrossRef]
- Wang, J.; An, C.; Zhang, M.; Qin, C.; Ming, X.; Zhang, Q. Photochemical conversion of AgCl nanocubes to hybrid AgCl–Ag nanoparticles with high activity and long-term stability towards photocatalytic degradation of organic dyes. Can. J. Chem. 2012, 90, 858–864. [Google Scholar] [CrossRef]
- El-Kader, F.H.A.; Hakeem, N.A.; Osman, W.H.; Menazea, A.A.; Abdelghany, A.A. Nanosecond laser irradiation as new route for silver nanoparticles precipitation in glassy matrix. Silicon 2019, 11, 377–381. [Google Scholar] [CrossRef]
- Falco, W.F.; Queiroz, A.M.; Fernandes, J.; Botero, E.R.; Falcão, E.A.; Guimarães, F.E.G.; M’Peko, J.C.; Oliveira, S.L.; Colbeck, I.; Caires, A.R.L. Interaction between chlorophyll and silver nanoparticles: A close analysis of chlorophyll fluorescence quenching. J. Photochem. Photobiol. A Chem. 2015, 299, 203–209. [Google Scholar] [CrossRef]
- Noori, A.; Donnelly, T.; Colbert, J.; Cai, W.; Newman, L.A.; White, J.C. Exposure of tomato (Lycopersicon esculentum) to silver nanoparticles and silver nitrate: Physiological and molecular response. Int. J. Phytoremediation 2020, 22, 40–51. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Rani, P.U.; Yasur, J.; Loke, K.S.; Dutta, D. Effect of synthetic and biosynthesized silver nanoparticles on growth, physiology and oxidative stress of water hyacinth: Eichhornia crassipes (Mart) Solms. Acta Physiol. Plant. 2016, 38, 58. [Google Scholar] [CrossRef]
- Pavlovic, D.; Nikolic, B.; Djurovic, S.; Waisi, H.; Andjelkovic, A.; Marisavljevic, D. Chlorophyll as a measure of plant health: Agroecological aspects. Pestic. Phytomed. (Belgrade) 2014, 29, 21–34. [Google Scholar] [CrossRef]
- Gao, F.; Hong, F.; Liu, C.; Zheng, L.; Su, M.; Wu, X.; Yang, F.; Wu, C.; Yang, P. Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach: Inducing complex of rubisco-rubisco activase. Biol. Trace Elem. Res. 2006, 111, 239–253. [Google Scholar] [CrossRef]
- Racuciu, M.; Creanga, D. TMA-OH coated magnetic nanoparticles internalized in vegetal tissue. Rom. J. Phys. 2007, 52, 395. [Google Scholar]
- Qian, H.; Peng, X.; Han, X.; Ren, J.; Sun, L.; Fu, Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 2013, 25, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.S.; Qiu, X.N.; Li, G.B.; Li, W.; Yin, L.Y. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ. Toxicol. Chem. 2014, 33, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Venzhik, J.V.; Titov, D.F.; Talanova, V.V.; Miroslavov, E.D.; Koteeva, N.K. Structural and functional reorganization of photosynthetic apparatus in cold adaptation of wheat plants. Tsitologiia 2012, 54, 916–924. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Wise, R.R. The ultrastructure of chilling stress. Plant Cell Environ. 2000, 23, 337–350. [Google Scholar] [CrossRef]
- Paramonova, N.V.; Shevyakova, N.I.; Kuznetsov, V.V. Ultrastructure of chloroplasts and their storage inclusions in the primary leaves of Mesembryanthemum crystallinum affected by putrescine and NaCl. Russ. J. Plant Physiol. 2004, 51, 86–96. [Google Scholar] [CrossRef]
- Kohler, R.H.; Schwille, P.; Webb, W.W.; Hanson, M.R. Active protein transport through plastid tubules: Velocity quantified by fluorescence correlation spectroscopy. J. Cell Sci. 2000, 113, 3921–3930. [Google Scholar] [CrossRef]
- Gray, J.C.; Hansen, M.R.; Shaw, D.J.; Graham, K.; Dale, R.; Smallman, P.; Natesan, S.K.; Newell, C.A. Plastid stromules are induced by stress treatments acting through abscisic acid. Plant J. 2012, 69, 387–398. [Google Scholar] [CrossRef]
- Venzhik, Y.; Talanova, V.; Titov, A. The effect of abscisic acid on cold tolerance and chloroplasts ultrastructure in wheat under optimal and cold stress conditions. Acta Physiol. Plant. 2016, 38, 63. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Gurunathan, S.; Chung, I.M. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma 2015, 252, 1031–1046. [Google Scholar] [CrossRef]
- Zare, Z.; Pishkar, L.; Iranbakhsh, A.; Talei, D. Physiological and molecular effects of silver nanoparticles exposure on purslane (Portulaca oleracea L.). Russ. J. Plant Physiol. 2020, 67, 521–528. [Google Scholar] [CrossRef]
- Khan, I.; Raza, M.A.; Khalid, M.H.B.; Awan, S.A.; Raja, N.I.; Zhang, X.; Min, S.; Wu, B.C.; Hassan, M.J.; Huang, L. Physiological and biochemical responses of pearl millet (Pennisetum glaucum L.) seedlings exposed to silver nitrate (AgNO3) and silver nanoparticles (AgNPs). Int. J. Environ. Res. Public Health 2019, 16, 2261. [Google Scholar] [CrossRef]
- Anna, B.; Barbara, K.; Magdalena, O. How the surface properties affect the nano cytotoxicity of silver? Study of the influence of three types of nanosilver on two wheat varieties. Acta Physiol. Plant. 2018, 40, 31. [Google Scholar] [CrossRef]
- Iannone, M.F.; Groppa, M.D.; de Sousa, M.E.; van Raap, M.B.F.; Benavides, M.P. Impact of magnetite iron oxide nanoparticles on wheat (Triticum aestivum L.) development: Evaluation of oxidative damage. Environ. Exp. Bot. 2016, 131, 77–88. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Tripathi, D.K.; Yadav, S.; Kalaji, H.M.; Brestic, M. Phytotoxic effect of silver nanoparticles in Triticum aestivum: Improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica 2019, 57, 209–216. [Google Scholar] [CrossRef]
- Oukarroum, A.; Barhoumi, L.; Pirastru, L.; Dewez, D. Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemna gibba. Environ. Toxicol. Chem. 2013, 32, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Li, P.; Huang, Q.; Zhang, H. 2016. The different response mechanisms of Wolffia globosa: Light-induced silver nanoparticle toxicity. Aquat. Toxicol. 2016, 176, 97–105. [Google Scholar] [CrossRef]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef]
- Van Hoonacker, A.; Englebienne, P. Revisiting silver nanoparticle chemical synthesis and stability by optical spectroscopy. Curr. Nanosci. 2006, 2, 359–371. [Google Scholar] [CrossRef]
- Müller, A.; Behsnilian, D.; Walz, E.; Gräf, V.; Hogekamp, L.; Greiner, R. Effect of culture medium on the extracellular synthesis of silver nanoparticles using Klebsiella pneumoniae, Escherichia coli and Pseudomonas jessinii. Biocatal. Agric. Biotechnol. 2016, 6, 107–115. [Google Scholar] [CrossRef]
- Rauwel, E.; Galeckas, A.; Rauwel, P.; Sunding, M.F.; Fjellvåg, H. Precursor-dependent blue-green photoluminescence emission of ZnO nanoparticles. J. Phys. Chem. C 2011, 115, 25227–25233. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 2231, 606X. [Google Scholar]
- Yemm, E.W.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef]
- Hedge, J.E.; Hofreiter, B.T. Carbohydrate Chemistry, 17; Whistler, R.L., Be Miller, J.N., Eds.; Academic Press: New York, NY, USA, 1962. [Google Scholar]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Agarwal, S.; Shaheen, R. Stimulation of antioxidant system and lipid peroxidation by abiotic stresses in leaves of Momordica charantia. Braz. J. Plant Physiol. 2007, 19, 149–161. [Google Scholar] [CrossRef]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Devi, P. Principles and Methods in Plant Molecular Biology, Biochemistry and Genetics; Agrobios: Rajasthan, India, 2000. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis, Pentice Hall of India Pvt; New Delhi Indian Ltd.: New Delhi, Indian, 1973; Volume 498, pp. 151–154. [Google Scholar]
- Reynolds, E.S. The use of lead citrate at a high pH as an electron opaque stain in electron microscopy. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef]
- Juniper, B.E.; Cox, G.C.; Gilchrist, A.J.; Williams, P.K. Techniques for Plant Electron Microscopy; Blackwell Sci. Publ.: Oxford, UK, 1970. [Google Scholar]




| Treatments | Root Growth Vigor | Shoot Growth Vigor | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Root Length (cm/ Seedling) | Root FW (g/Seedling) | Root DW (g/ Seedling) | Root Water Content (g/ Seedling) | Shoot Length (cm/Seedling) | Shoot Fwt (g/ Seedling) | Shoot DW (g/ Seedling) | Shoot Water Content (g/ Seedling) | No. of Leaves/ Seedling | Total Leaf Area (cm2/ Seedling) | |
| Control | 9.17 ± 0.17 bc | 2.54 ± 0.23 a | 0.19 ± 0.03 b | 2.35 ± 0.22 a | 27.67 ± 1.20 a | 3.95 ± 0.21 ab | 0.31 ± 0.01 a | 3.64 ± 0.19 ab | 7 ± 0.58 b | 0.27 ± 0.03 ab |
| 10 ppm AgNO3 | 8.33 ± 0.60 bc | 1.98 ± 0.11 b | 0.13 ± 0.03 b | 1.85 ± 0.24 ab | 20.00 ± 1.15 b | 3.13 ± 0.18 ab | 0.24 ± 0.04 ab | 2.89 ± 0.35 b | 6 ± 0.00 c | 0.25 ± 0.05 b |
| 50 ppm AgNO3 | 7.17 ± 0.17 c | 1.44 ± 0.24 b | 0.11 ± 0.01 b | 1.33 ± 0.10 b | 19.33 ± 0.73 b | 2.68 ± 0.39 b | 0.19 ± 0.04 b | 2.49 ± 0.14 b | 5 ± 0.00 c | 0.19 ± 0.01 b |
| 10 ppm cAgNPs | 10.33 ± 0.83 b | 2.31 ± 0.42 ab | 0.14 ± 0.05 b | 2.17 ± 0.49 a | 26.33 ± 2.33 a | 4.25 ± 0.21 a | 0.35 ± 0.04 a | 3.90 ± 0.31 ab | 8 ± 0.00 a | 0.31 ± 0.04 a |
| 50 ppm cAgNPs | 9.50 ± 0.58 bc | 2.05 ± 0.55 ab | 0.12 ± 0.02 b | 1.93 ± 0.40 ab | 25.67 ± 1.76 a | 3.99 ± 0.73 ab | 0.29 ± 0.04 ab | 3.70 ± 0.56 ab | 6 ± 0.00 a | 0.29 ± 0.11 ab |
| 10 ppm bAgNPs | 13.33 ± 1.67 a | 2.91 ± 0.08 a | 0.25 ± 0.09 a | 2.66 ± 0.20 a | 28.33 ± 0.33 a | 4.48 ± 0.35 a | 0.36 ± 0.06 a | 4.12 ± 0.66 a | 8 ± 0.00 c | 0.33 ± 0.09 a |
| 50 ppm bAgNPs | 11.00 ± 0.58 ab | 2.06 ± 0.22 ab | 0.13 ± 0.01 b | 1.93 ± 0.03 ab | 27.67 ± 0.33 a | 4.39 ± 0.61 a | 0.32 ± 0.02 a | 4.07 ± 0.19 a | 7 ± 0.00 a | 0.31 ± 0.01 a |
| Treatments | Chl a | Chl b | Car | Chl a + b | Total Pigments | Total Soluble Sugars | Starch | Total Carbohydrates |
|---|---|---|---|---|---|---|---|---|
| Control | 0.93 ± 0.07 c | 0.13 ± 0.08 e | 0.34 ± 0.06 c | 1.06 ± 0.05 d | 1.40 ± 0.04 c | 15.85 ± 0.28 a | 260.98 ± 1.83 a | 276.83 ± 2.11 a |
| 10 ppm AgNO3 | 0.94 ± 0.06 c | 0.13 ± 0.02 e | 0.28 ± 0.07 e | 1.07 ± 0.04 cd | 1.35 ± 0.03 c | 8.90 ± 0.21 d | 187.56 ± 1.83 d | 196.46 ± 2.11 d |
| 50 ppm AgNO3 | 0.84 ± 0.08 d | 0.13 ± 0.06 e | 0.21 ± 0.07 f | 0.97 ± 0.06 e | 1.18 ± 0.08 d | 6.59 ± 0.49 e | 167.93 ± 3.31 f | 174.52 ± 3.52 f |
| 10 ppm cAgNPs | 1.33 ± 0.16 b | 0.29 ± 0.03 b | 0.37 ± 0.08 b | 1.62 ± 0.09 b | 1.99 ± 0.07 b | 11.10 ± 0.49 c | 198.46 ± 1.83 c | 209.56 ± 2.11 c |
| 50 ppm cAgNPs | 0.95 ± 0.08 c | 0.16 ± 0.09 d | 0.28 ± 0.05 e | 1.11 ± 0.03 c | 1.39 ± 0.06 c | 6.61 ± 0.35 e | 169.73 ± 0.21 f | 176.34 ± 0.70 f |
| 10 ppm bAgNPs | 1.56 ± 0.04 a | 0.31 ± 0.07 a | 0.39 ± 0.07 a | 1.87 ± 0.08 a | 2.26 ± 0.15 a | 13.66 ± 0.28 b | 209.88 ± 3.03 b | 223.54 ± 3.52 b |
| 50 ppm bAgNPs | 1.34 ± 0.17 b | 0.28 ± 0.06 c | 0.29 ± 0.08 d | 1.62 ± 0.04 b | 1.91 ± 0.13 b | 6.83 ± 0.28 e | 173.17 ± 1.76 e | 180.00 ± 2.11 e |
| Treatments | H2O2 (µmol/g FW) | CAT (mmol H2O2/min/g FW) | POX (U/min/g FW) | PPO (U/min/g FW) |
|---|---|---|---|---|
| Control | 18.75 ± 0.12 g | 5.78 ± 0.02 e | 52.92 ± 0.03 e | 65.28 ± 0.03 c |
| 10 ppm AgNO3 | 35.35 ± 0.32 b | 7.44 ± 0.03 d | 76.54 ± 0.05 d | 45.83 ± 0.04 f |
| 50 ppm AgNO3 | 58.85 ± 0.45 a | 2.51 ± 0.05 f | 42.36 ± 0.04 f | 29.17 ± 0.02 g |
| 10 ppm cAgNPs | 28.35 ± 0.22 e | 9.04 ± 0.01 b | 85.28 ± 0.06 c | 69.17 ± 0.02 b |
| 50 ppm cAgNPs | 33.16 ± 0.25 c | 7.61 ± 0.03 d | 75.14 ± 0.07 d | 51.11 ± 0.03 e |
| 10 ppm bAgNPs | 21.35 ± 0.12 f | 10.09 ± 0.01 a | 101.64 ± 0.03 a | 77.22 ± 0.04 a |
| 50 ppm bAgNPs | 32.45 ± 0.12 d | 8.35 ± 0.02 c | 88.67 ± 0.11 b | 57.50 ± 0.05 d |
| pH | EC (dSm−1) | K+ (meq/100 g) | Na+ (meq/100 g) | Ca++ (meq/100 g) | Mg++ (meq/100 g) | CO3-- (meq/100 g) | HCO3− (meq/100 g) | Cl− (meq/100 g) |
|---|---|---|---|---|---|---|---|---|
| 7.97 | 0.88 | 0.54 | 4.26 | 2.60 | 0.72 | - | 2.08 | 3.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhammad, B.A.; Abdel-Aziz, H.M.M.; Seleiman, M.F.; Tourky, S.M.N. How Can Biological and Chemical Silver Nanoparticles Positively Impact Physio-Chemical and Chloroplast Ultrastructural Characteristics of Vicia faba Seedlings? Plants 2023, 12, 2509. https://doi.org/10.3390/plants12132509
Alhammad BA, Abdel-Aziz HMM, Seleiman MF, Tourky SMN. How Can Biological and Chemical Silver Nanoparticles Positively Impact Physio-Chemical and Chloroplast Ultrastructural Characteristics of Vicia faba Seedlings? Plants. 2023; 12(13):2509. https://doi.org/10.3390/plants12132509
Chicago/Turabian StyleAlhammad, Bushra Ahmed, Heba M. M. Abdel-Aziz, Mahmoud F. Seleiman, and Shaimaa M. N. Tourky. 2023. "How Can Biological and Chemical Silver Nanoparticles Positively Impact Physio-Chemical and Chloroplast Ultrastructural Characteristics of Vicia faba Seedlings?" Plants 12, no. 13: 2509. https://doi.org/10.3390/plants12132509
APA StyleAlhammad, B. A., Abdel-Aziz, H. M. M., Seleiman, M. F., & Tourky, S. M. N. (2023). How Can Biological and Chemical Silver Nanoparticles Positively Impact Physio-Chemical and Chloroplast Ultrastructural Characteristics of Vicia faba Seedlings? Plants, 12(13), 2509. https://doi.org/10.3390/plants12132509







