Identification and Expression of the CorA/MRS2/ALR Type Magnesium Transporters in Tomato
Abstract
1. Introduction
2. Results
2.1. Identifications of Mg2+ Transporters
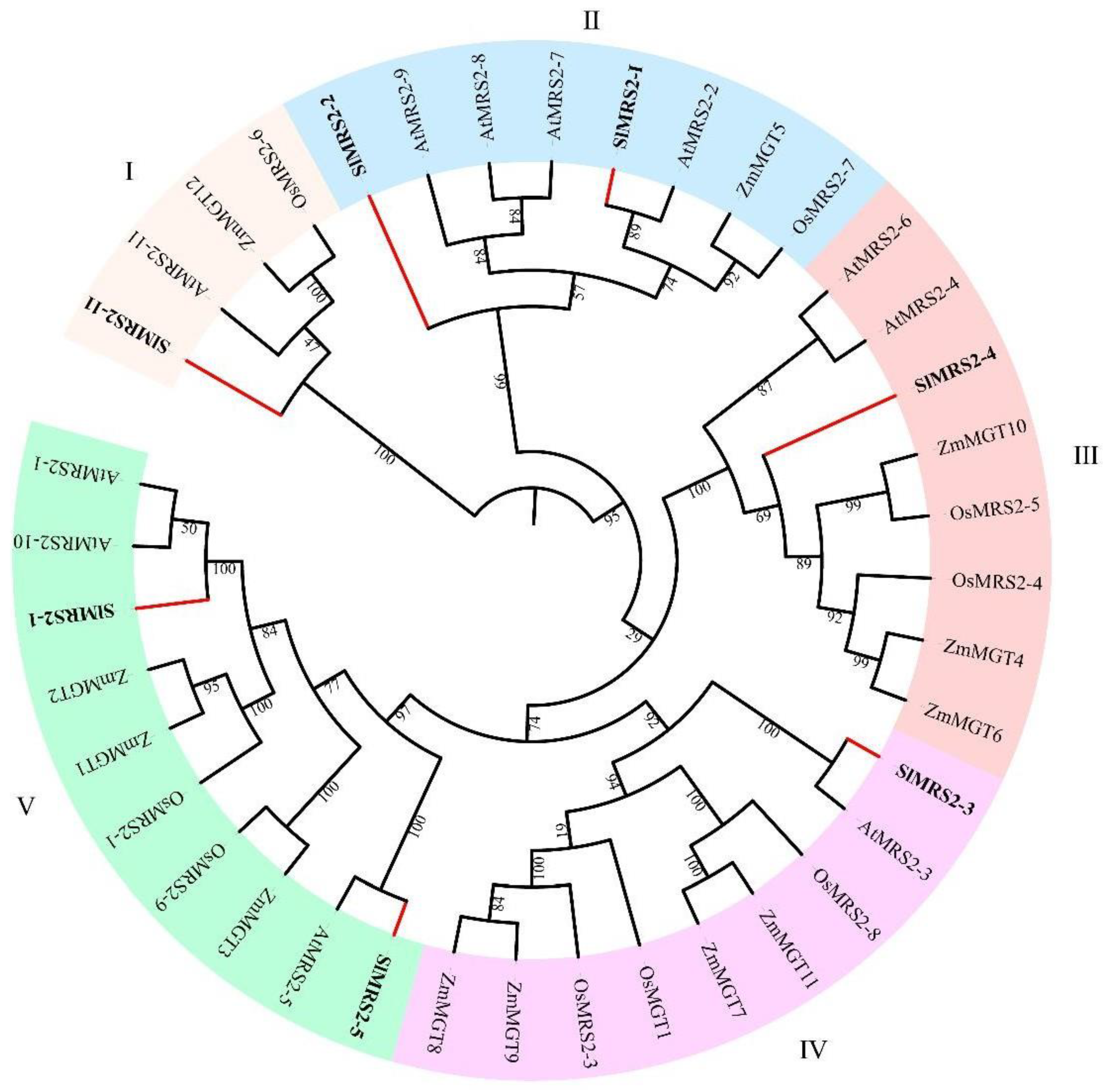
2.2. The phylogenetic Tree Analysis
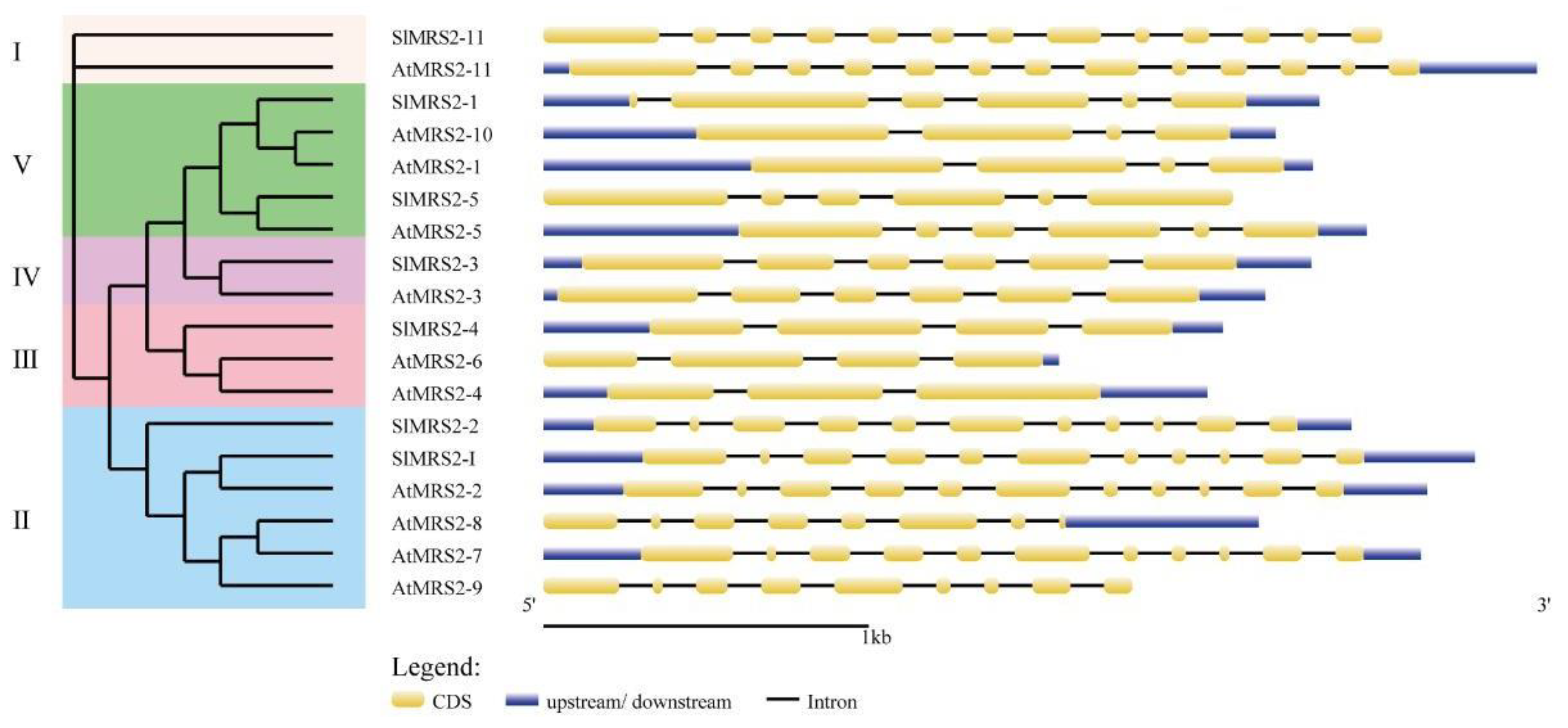
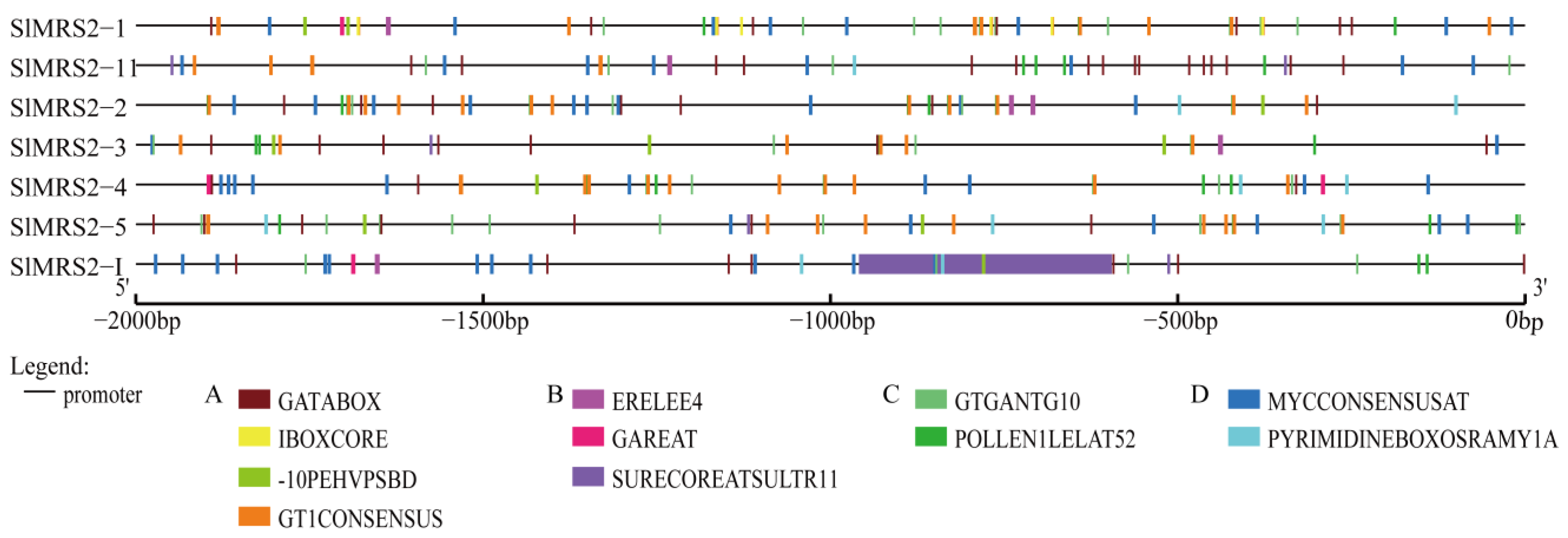
2.3. The Gene Structure, Conserved Motifs, and Cis-Acting Elements
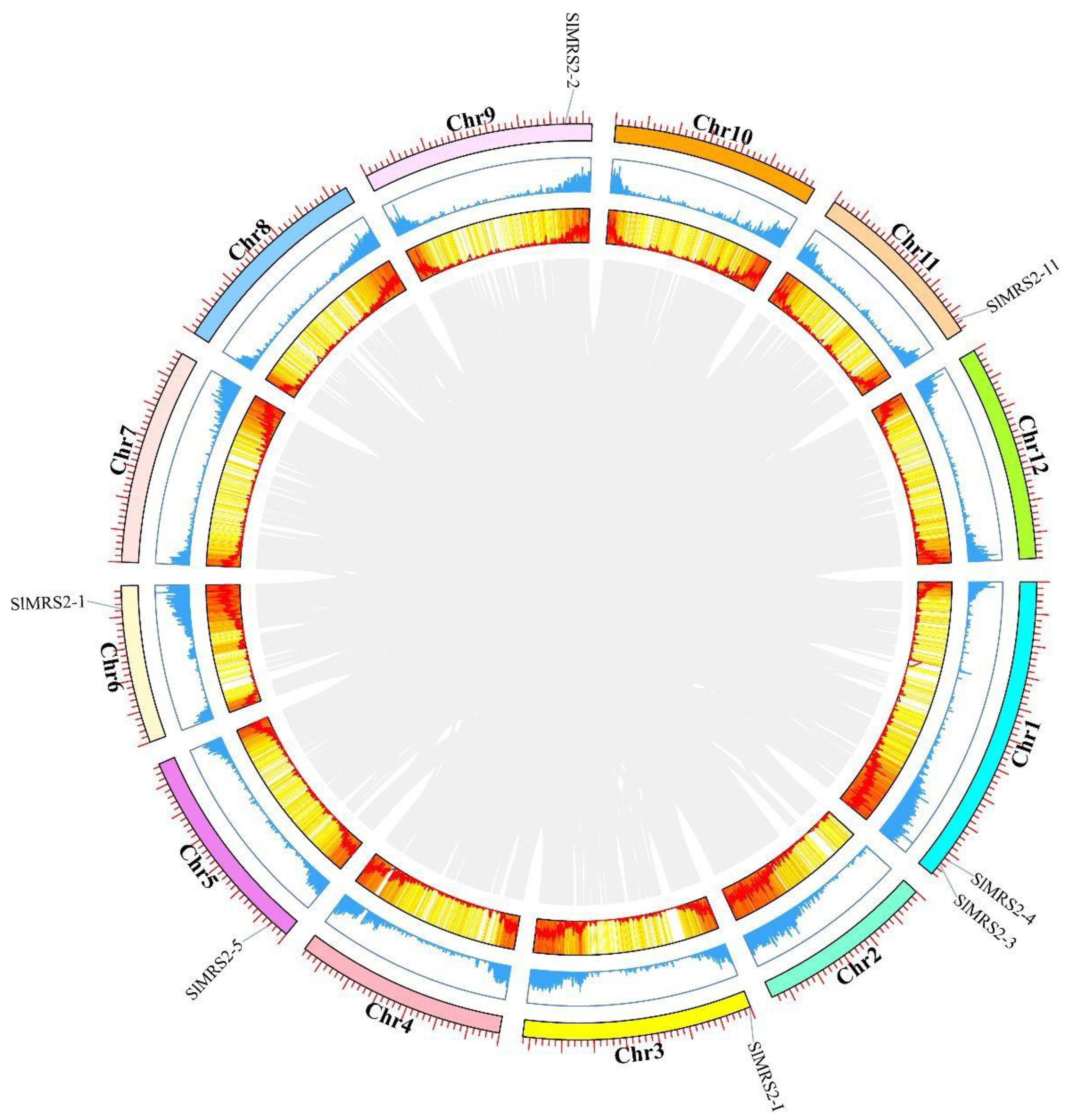
2.4. Chromosomal Location and Gene Distribution
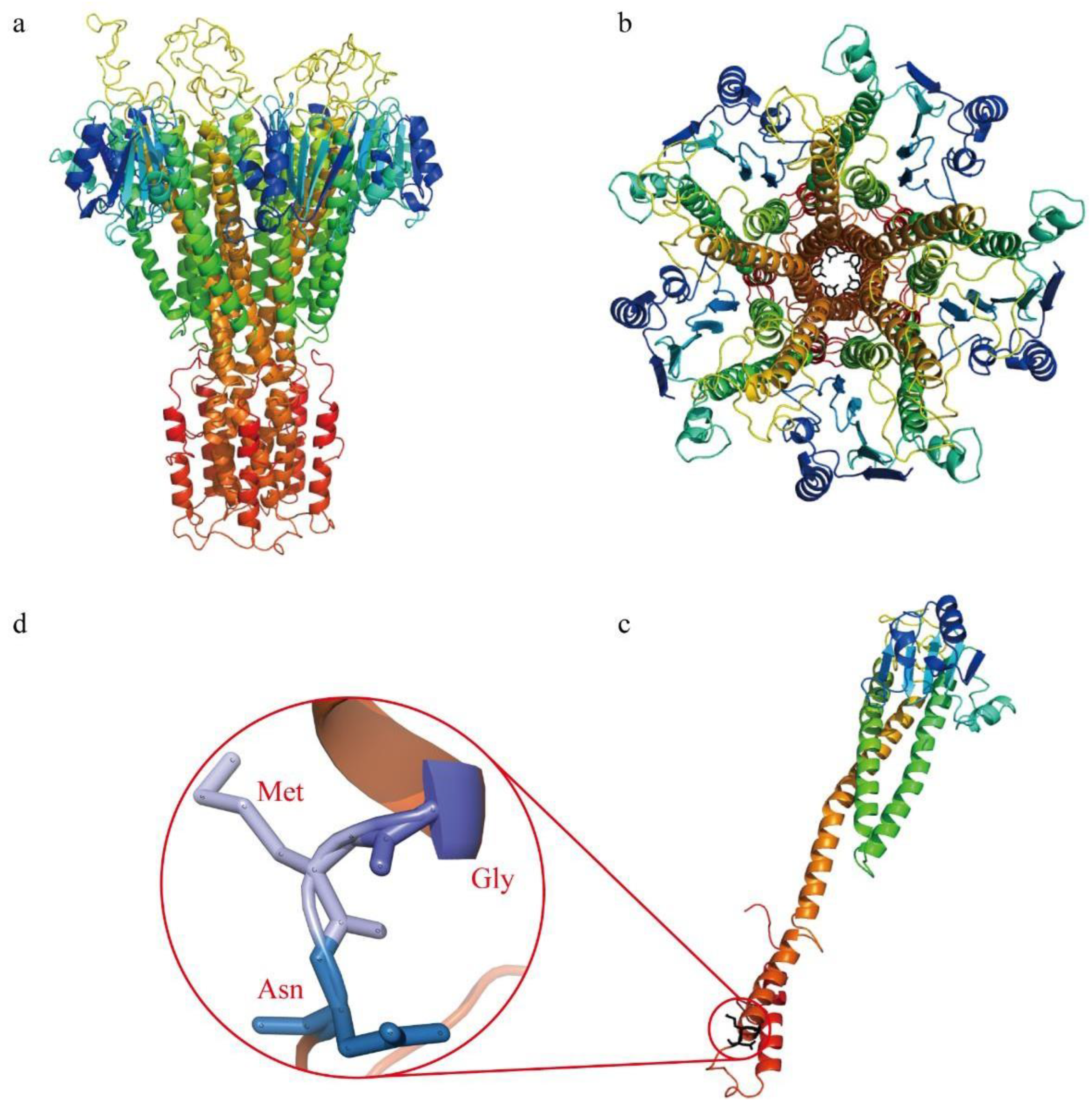
2.5. Three-Dimensional Structure Prediction of Magnesium Transporter
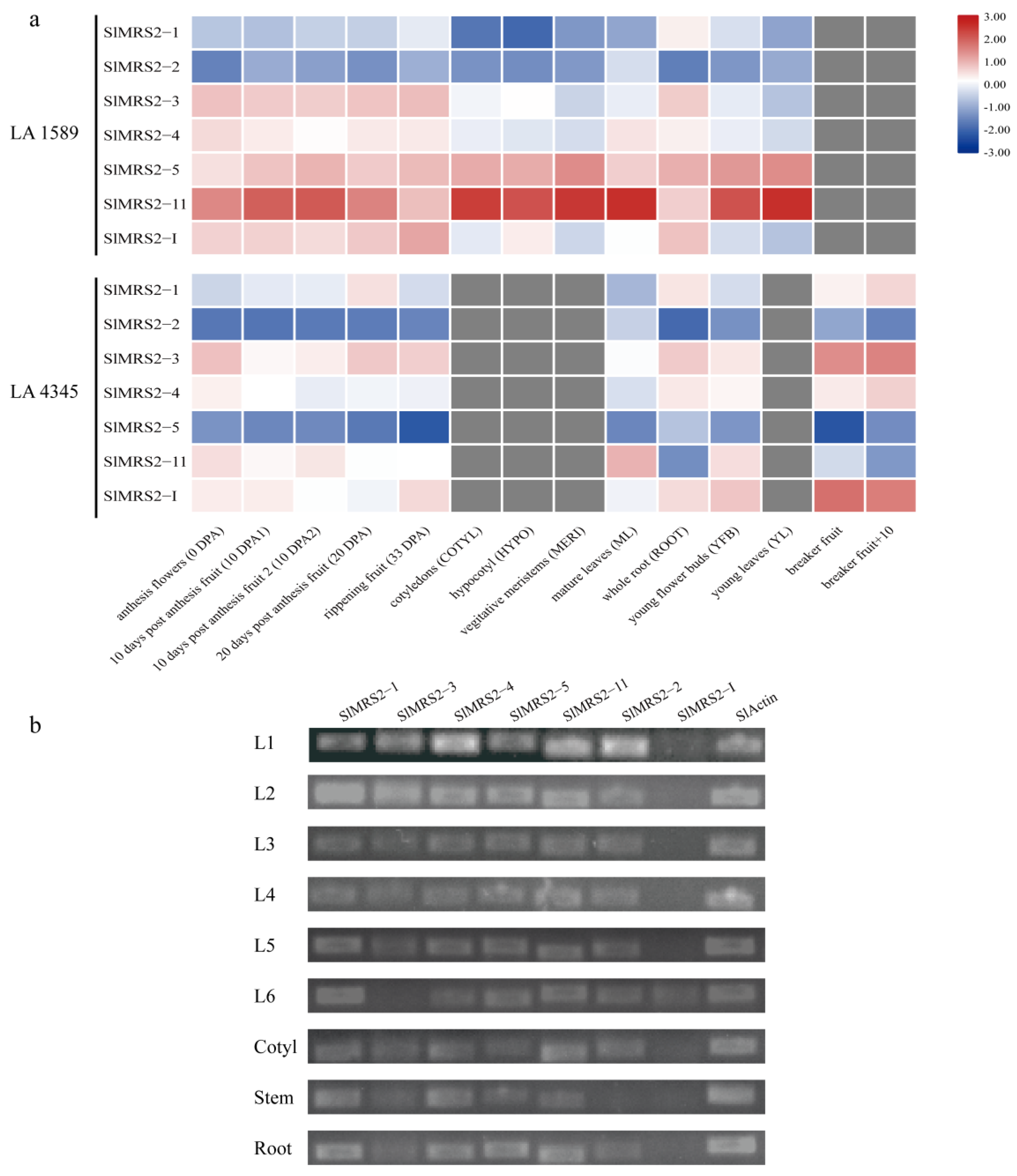
2.6. Expression Analysis of SlMRS2 Genes in Tomato
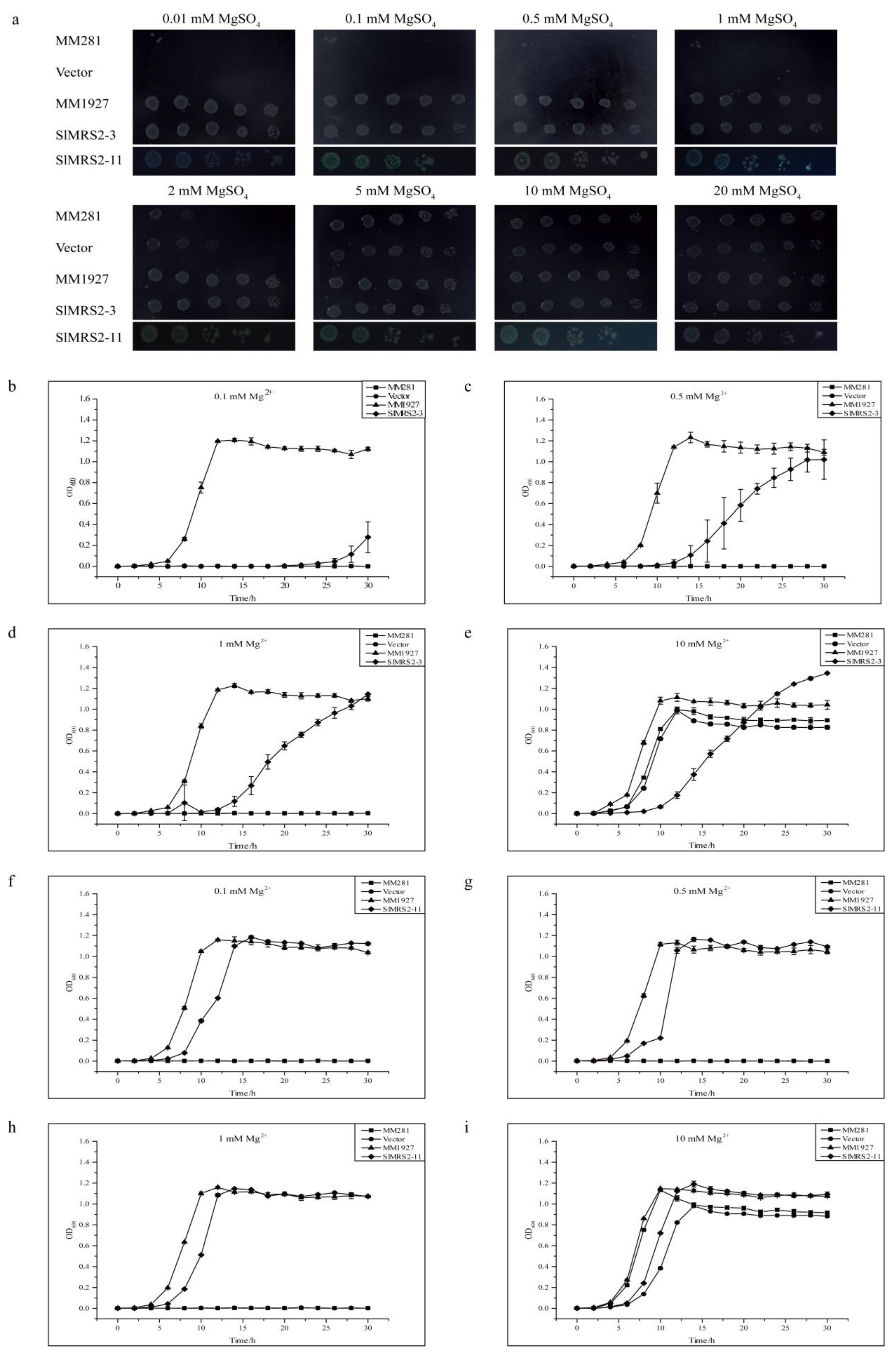
2.7. Function Complementation Analysis of SlMRS2 Genes
3. Discussion
4. Materials and Methods
4.1. Identification of Magnesium Transporters
4.2. Phylogenetic Analysis and Gene Distribution
4.3. Sequence Analysis and Protein Quaternary Structure
4.4. Plant Materials, Growth Conditions, and Treatments
4.5. Gene Cloning
4.6. Salmonella typhimurium Mutant Complementation Assay
4.7. The Expression Analysis and Quantitative Real-Time PCR
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2017, 74, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2012; p. 672. [Google Scholar] [CrossRef]
- Tunc-Ozdemir, M.; Tang, C.; Ishka, M.R.; Brown, E.; Groves, N.R.; Myers, C.T.; Rato, C.; Poulsen, L.R.; McDowell, S.; Miller, G.; et al. A Cyclic Nucleotide-Gated Channel (CNGC16) in Pollen Is Critical for Stress Tolerance in Pollen Reproductive Development. Plant Physiol. 2013, 161, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Nazim, H.; Liang, Z.S.; Yang, D.F. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Snacely, M.D.; Florer, J.B.; Miller, C.G.; Maguire, M.E. Magnesium transport in Salmonella typhimurium 28Mg2+ transport by the CorA, MgtA, and MgtB system. Bacterial 1989, 171, 4761–4766. [Google Scholar] [CrossRef] [PubMed]
- Graschopf, A.; Stadler, J.A.; Hoellerer, M.K.; Eder, S.; Sieghardt, M.; Kohlwein, S.D.; Schweyen, R.J. The yeast plasma membrane protein Alr1 controls Mg2+ homeostasis and is subject to Mg2+-dependent control of its synthesis and degradation. J. Biol. Chem. 2001, 276, 16216–16222. [Google Scholar] [CrossRef]
- Lee, J.M.; Gardner, R.C. Residues of the yeast ALR1 protein that are critical for magnesium uptake. Curr. Genet. 2006, 49, 7–20. [Google Scholar] [CrossRef]
- Bui, D.M.; Gregan, J.; Jarosch, E.; Ragnini, A.; Schweyen, R.J. The Bacterial Magnesium Transporter CorA Can Functionally Substitute for Its Putative Homologue Mrs2p in the Yeast Inner Mitochondrial Membrane. J. Biol. Chem. 1999, 274, 20438–20443. [Google Scholar] [CrossRef]
- Gregan, J.; Bui, D.M.; Pillich, R.; Fink, M.; Zsurka, G.; Schweyen, R.J. The mitochondrial inner membrane protein Lpe10p, a homologue of Mrs2p, is essential for magnesium homeostasis and group II intron splicing in yeast. Mol. Gen. Genet. 2001, 264, 773–781. [Google Scholar] [CrossRef]
- Pisat, N.P.; Pandey, A.; Macdiarmid, C.W. MNR2 regulates intracellular magnesium storage in Saccharomyces cerevisiae. Genetics 2009, 183, 873–884. [Google Scholar] [CrossRef]
- Knoop, V.; Groth-Malonek, M.; Gebert, M.; Eifler, K.; Weyand, K. Transport of magnesium and other divalent cations: Evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol. Genet. Genom. 2005, 274, 205–216. [Google Scholar] [CrossRef]
- Li, L.; Tutone, A.F.; Drummond, R.S.; Gardner, R.C.; Luan, S. A novel family of magnesium transport genes in Arabidopsis. Plant Cell 2001, 13, 2761–2775. [Google Scholar] [CrossRef]
- Gebert, M.; Meschenmoser, K.; Svidova, S.; Weghuber, J.; Schweyen, R.; Eifler, K.; Lenz, H.; Weyand, K.; Knoop, V. A Root-Expressed Magnesium Transporter of the MRS2/MGT Gene Family in Arabidopsis thaliana Allows for Growth in Low-Mg2+ Environments. Plant Cell 2009, 21, 4018–4030. [Google Scholar] [CrossRef]
- Mao, D.; Chen, J.; Tian, L.; Liu, Z.; Yang, L.; Tang, R.; Li, J.; Lu, C.; Yang, Y.; Shi, J.; et al. Arabidopsis Transporter MGT6 Mediates Magnesium Uptake and Is Required for Growth under Magnesium Limitation. Plant Cell 2014, 26, 2234–2248. [Google Scholar] [CrossRef]
- Zhang, L.D.; Peng, Y.Y.; Li, J.; Tian, X.Y.; Chen, Z.C. OsMGT1 Confers Resistance to Magnesium Deficiency by Enhancing the Import of Mg in Rice. Int. J. Mol. Sci. 2019, 20, 207. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, N.; Ding, J.Z.; Liu, C.; Du, H.M.; Huang, K.F.; Cao, M.J.; Lu, Y.L.; Gao, S.B.; Zhang, S.Z. The maize CorA/MRS2/MGT-type Mg transporter, ZmMGT10, responses to magnesium deficiency and confers low magnesium tolerance in transgenic Arabidopsis. Plant Mol. Biol. 2017, 95, 269–278. [Google Scholar] [CrossRef]
- Oda, K.; Kamiya, T.; Shikanai, Y.; Shigenobu, S.; Yamaguchi, K.; Fujiwara, T. The Arabidopsis Mg Transporter, MRS2-4, is Essential for Mg Homeostasis under Both Low and High Mg Conditions. Plant Cell Physiol. 2016, 57, 754–763. [Google Scholar] [CrossRef]
- Yan, Y.W.; Mao, D.D.; Yang, L.; Qi, J.L.; Zhang, X.X.; Tang, Q.L.; Li, Y.P.; Tang, R.J.; Luan, S. Magnesium Transporter MGT6 Plays an Essential Role in Maintaining Magnesium Homeostasis and Regulating High Magnesium Tolerance in Arabidopsis. Front. Plant Sci. 2018, 9, 274. [Google Scholar] [CrossRef]
- Berezin, I.; Brook, E.; Mizrahi, K.; Mizrachy-Dagry, T.; Elazar, M.; Zhou, S.; Shaul, O. Overexpression of the vacuolar metal/proton exchanger AtMHX in tomato causes decreased cell expansion and modifications in the mineral content. Funct. Plant Biol. 2008, 35, 15. [Google Scholar] [CrossRef]
- Conn, S.J.; Conn, V.; Tyerman, S.D.; Kaiser, B.N.; Leigh, R.A.; Gilliham, M. Magnesium transporters, MGT2/MRS2-1 and MGT3/MRS2-5, are important for magnesium partitioning within Arabidopsis thaliana mesophyll vacuoles. New Phytol. 2011, 190, 583–594. [Google Scholar] [CrossRef]
- Ishijima, S.; Uda, M.; Hirata, T.; Shibata, M.; Kitagawa, N.; Sagami, I. Magnesium uptake of Arabidopsis transporters, AtMRS2-10 and AtMRS2-11, expressed in Escherichia coli mutants: Complementation and growth inhibition by aluminum. Bba-Biomembranes 2015, 1848, 1376–1382. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, R.; Li, L.; Huang, J. The Magnesium Transporter MGT10 Is Essential for Chloroplast Development and Photosynthesis in Arabidopsis thaliana. Mol. Plant 2017, 10, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Wang, B.; Lou, Y.; Han, W.J.; Lu, J.Y.; Li, D.D.; Li, L.G.; Zhu, J.; Yang, Z.N. Magnesium Transporter 5 plays an important role in Mg transport for male gametophyte development in Arabidopsis. Plant J. 2015, 84, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Qi, Y.; Zhao, J.; Li, Y.; Wang, R.; Shao, J.; Liu, X.; An, L.; Yu, F. Mutations in the Arabidopsis AtMRS2-11/AtMGT10/VAR5 Gene Cause Leaf Reticulation. Front. Plant Sci. 2017, 8, 2007. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.S.M.; Tutone, A.; Li, Y.C.; Gardner, R.C. A putative magnesium transporter AtMRS2-11 is localized to the plant chloroplast envelope membrane system. Plant Sci. 2006, 170, 78–89. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, C.; Zhou, L.N.; Zhao, Z.; Li, Y.H.; Qu, M.; Huang, K.F.; Zhang, L.; Lu, Y.L.; Cao, M.J.; et al. Molecular and functional characterization of the magnesium transporter gene ZmMGT12 in maize. Gene 2018, 665, 167–173. [Google Scholar] [CrossRef]
- Wang, Y.J.; Hua, X.T.; Xu, J.S.; Chen, Z.C.; Fan, T.Q.; Zeng, Z.H.; Wang, H.B.; Hour, A.L.; Yu, Q.Y.; Ming, R.; et al. Comparative genomics revealed the gene evolution and functional divergence of magnesium transporter families in Saccharum. BMC Genom. 2019, 20, 83. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Tan, H.; Yang, X.; Tian, L.F.; Luan, S.; Chen, L.B.; Li, D.P. An endoplasmic reticulum magnesium transporter is essential for pollen development in Arabidopsis. Plant Sci. 2015, 231, 212–220. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.G.; Liu, Z.H.; Yuan, Y.J.; Guo, L.L.; Mao, D.D.; Tian, L.F.; Chen, L.B.; Luan, S.; Li, D.P. Magnesium transporter AtMGT9 is essential for pollen development in Arabidopsis. Cell Res. 2009, 19, 887–898. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Wang, P.; Jiao, H.J.; Tang, C.; Liu, X.; Jing, Y.H.; Zhang, S.L.; Wu, J.Y. Phylogenetic and expression analysis of the magnesium transporter family in pear, and functional verification of PbrMGT7 in pear pollen. J. Hortic. Sci. Biotech. 2018, 93, 51–63. [Google Scholar] [CrossRef]
- Ishijima, S.; Manabe, Y.; Shinkawa, Y.; Hotta, A.; Tokumasu, A.; Ida, M.; Sagami, I. The homologous Arabidopsis MRS2/MGT/CorA-type Mg2+ channels, AtMRS2-10 and AtMRS2-1 exhibit different aluminum transport activity. Bba-Biomembranes 2018, 1860, 2184–2191. [Google Scholar] [CrossRef]
- Chen, Z.C.; Yamaji, N.; Motoyama, R.; Nagamura, Y.; Ma, J.F. Up-Regulation of a Magnesium Transporter Gene OsMGT1 Is Required for Conferring Aluminum Tolerance in Rice. Plant Physiol. 2012, 159, 1624–1633. [Google Scholar] [CrossRef]
- Zhao, C.B.; Sun, K.; Chen, S.S.; Liang, C.; Meng, J.J.; Tang, Y.F.; Song, S.Y. Characterization the complete chloroplast genome of the tomato (Solanum lycopersicum L.) from China. Mitochondrial DNA B 2019, 4, 1374–1376. [Google Scholar] [CrossRef]
- Kobayashi, N.I.; Tanoi, K. Critical Issues in the Study of Magnesium Transport Systems and Magnesium Deficiency Symptoms in Plants. Int. J. Mol. Sci. 2015, 16, 23076–23093. [Google Scholar] [CrossRef]
- Saito, T.; Kobayashi, N.I.; Tanoi, K.; Iwata, N.; Suzuki, H.; Iwata, R.; Nakanishi, T.M. Expression and Functional Analysis of the CorA-MRS2-ALR-Type Magnesium Transporter Family in Rice. Plant Cell Physiol. 2013, 54, 1673–1683. [Google Scholar] [CrossRef]
- Li, H.; Du, H.; Huang, K.; Chen, X.; Liu, T.; Gao, S.; Liu, H.; Tang, Q.; Rong, T.; Zhang, S. Identification, and Functional and Expression Analyses of the CorA/MRS2/MGT-Type Magnesium Transporter Family in Maize. Plant Cell Physiol. 2016, 57, 1153–1168. [Google Scholar] [CrossRef]
- Payandeh, J.; Pfoh, R.; Pai, E.F. The structure and regulation of magnesium selective ion channels. Bba-Biomembranes 2013, 1828, 2778–2792. [Google Scholar] [CrossRef]
- Lunin, V.V.; Dobrovetsky, E.; Khutoreskaya, G.; Zhang, R.; Joachimiak, A.; Doyle, D.A.; Bochkarev, A.; Maguire, M.E.; Edwards, A.M.; Koth, C.M. Crystal structure of the CorA Mg2+ transporter. Nature 2006, 440, 833–837. [Google Scholar] [CrossRef]
- Schmitz, J.; Tierbach, A.; Lenz, H.; Meschenmoser, K.; Knoop, V. Membrane protein interactions between different Arabidopsis thaliana MRS2-type magnesium transporters are highly permissive. Bba-Biomembranes 2013, 1828, 2032–2040. [Google Scholar] [CrossRef]
- Song, C.; Guo, J.; Sun, W.; Wang, Y. Whole Genome Duplication of Intra- and Inter-chromosomes in the Tomato Genome. J. Genet. Genom. 2012, 39, 361–368. [Google Scholar] [CrossRef]
- Consortium, T.T.G.; Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D.; et al. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635. [Google Scholar] [CrossRef]
- Goytain, A.; Hines, R.M.; El-Husseini, A.; Quamme, G.A. NIPA1(SPG6), the basis for autosomal dominant form of hereditary spastic paraplegia, encodes a functional Mg2+ transporter. J. Biol. Chem. 2007, 282, 8060–8068. [Google Scholar] [CrossRef] [PubMed]
- Goytain, A.; Hines, R.M.; Quamme, G.A. Functional characterization of NIPA2, a selective Mg2+ transporter. Am. J. Physiol. Cell Physiol. 2008, 295, C944–C953. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Vuylsteke, M.; Coppens, F.; Cristescu, S.M.; Harren, F.J.M.; Inze, D.; Verbruggen, N. Systems analysis of the responses to long-term magnesium deficiency and restoration in Arabidopsis thaliana. New Phytol. 2010, 187, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, H.H.; Fang, X.Z.; Zhang, Y.S.; Jin, C.W. Auxin Acts Downstream of Ethylene and Nitric Oxide to Regulate Magnesium Deficiency-Induced Root Hair Development in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 1452–1465. [Google Scholar] [CrossRef]
- Guo, W.; Cong, Y.; Hussain, N.; Wang, Y.; Liu, Z.; Jiang, L.; Liang, Z.; Chen, K. The remodeling of seedling development in response to long-term magnesium toxicity and regulation by ABA-DELLA signaling in Arabidopsis. Plant Cell Physiol. 2014, 55, 1713–1726. [Google Scholar] [CrossRef]
- Guo, W.; Chen, S.; Hussain, N.; Cong, Y.; Liang, Z.; Chen, K. Magnesium stress signaling in plant: Just a beginning. Plant Signal. Behav. 2015, 10, e992287. [Google Scholar] [CrossRef]
- Sheng, Y.J.; Ding, Y.W.; Fu, Y.Y.; Yang, D.F.; Liang, Z.; Guo, W.L. The Research Development of the Response Mechanisms to Magnesium Stresses in Plants. Bot. Res. 2015, 04, 97–106. [Google Scholar] [CrossRef]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant 2008, 133, 692–704. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Liao, L.L.; Liu, S.; Nie, M.M.; Li, J.; Zhang, L.D.; Ma, J.F.; Chen, Z.C. Magnesium Deficiency Triggers SGR-Mediated Chlorophyll Degradation for Magnesium Remobilization. Plant Physiol. 2019, 181, 262–275. [Google Scholar] [CrossRef]
- Coordinators, N.R. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Schrodinger, LLC. The PyMOL Molecular Graphics System; Version 1.8.; Schrodinger, LLC.: New York, NY, USA, 2015. [Google Scholar]
- Carvajal, M.; Martínez, V.; Cerdá, A. Influence of magnesium and salinity on tomato plants grown in hydroponic culture. J. Plant Nutr. 1999, 22, 177–190. [Google Scholar] [CrossRef]
- Papp, K.M.; Maguire, M.E. The CorA Mg2+ transporter does not transport Fe2+. J. Bacteriol. 2004, 186, 7653–7658. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| Code | Gene Symbol | Gene Locus | Gene Name | Gene Length (bp) | Protein Length (aa) | pI | MW (kDa) | Chromosome | Chromosome Location | Subcellular Localization | TM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LOC101257182 | Solyc06g068490 | SlMRS2-1 | 5655 | 461 | 5.01 | 52.4 | 6 | 42564308~42570140 | plas | 2 |
| 2 | LOC101245882 | Solyc09g065920 | SlMRS2-2 | 4759 | 377 | 4.79 | 42.53 | 9 | 64439955~64444758 | chlo | 2 |
| 3 | LOC101261964 | Solyc01g106900 | SlMRS2-3 | 8358 | 498 | 4.56 | 54.98 | 1 | 94503194~94511499 | chlo | 2 |
| 4 | LOC101247864 | Solyc01g103890 | SlMRS2-4 | 6520 | 432 | 5.53 | 49.12 | 1 | 92285408~92291894 | nucl/plas | 2 |
| 5 | LOC101267022 | Solyc05g012220 | SlMRS2-5 | 5349 | 415 | 4.8 | 46.84 | 5 | 5498595~5506278 | plas | 2 |
| 6 | LOC101267965 | Solyc11g066660 | SlMRS2-11 | 8959 | 447 | 6.09 | 50.37 | 11 | 52722376~52732626 | plas | 2 |
| 7 | LOC101259511 | Solyc03g005390 | SlMRS2-I | 10485 | 395 | 4.72 | 44.2 | 3 | 224633~235094 | vacu | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Khan, S.; Tong, M.; Hu, H.; Yin, L.; Huang, J. Identification and Expression of the CorA/MRS2/ALR Type Magnesium Transporters in Tomato. Plants 2023, 12, 2512. https://doi.org/10.3390/plants12132512
Liu W, Khan S, Tong M, Hu H, Yin L, Huang J. Identification and Expression of the CorA/MRS2/ALR Type Magnesium Transporters in Tomato. Plants. 2023; 12(13):2512. https://doi.org/10.3390/plants12132512
Chicago/Turabian StyleLiu, Wen, Shahbaz Khan, Mengying Tong, Haiyan Hu, Liyan Yin, and Jiaquan Huang. 2023. "Identification and Expression of the CorA/MRS2/ALR Type Magnesium Transporters in Tomato" Plants 12, no. 13: 2512. https://doi.org/10.3390/plants12132512
APA StyleLiu, W., Khan, S., Tong, M., Hu, H., Yin, L., & Huang, J. (2023). Identification and Expression of the CorA/MRS2/ALR Type Magnesium Transporters in Tomato. Plants, 12(13), 2512. https://doi.org/10.3390/plants12132512





