Abstract
Commiphora gileadensis (L.) C. Chr is a perennial plant existing mainly in the southern and western mountains of the Arabian Peninsula. In the Makkah province, the remaining populations are threatened by many factors such as overcutting, overgrazing, and urban developments. These dangers are expected to be aggravated by the progression of aridification factors arising from climate change. To overcome the decline in remaining populations of this valuable species, a timely evaluation of the population’s genetic variables and genetic structure is vital for the conservation of existing C. gileadensis populations. In this study, we used 61 SSR primers to achieve this objective. Only 50 loci showed polymorphisms, which led to further analysis of the population genetics for 600 genotypes that were collected from 50 populations of C. gileadensis found in 10 different sites in the Makkah region: Gebel Al Muliesaa, Wadi Albathna, Wadi Houra, Wadi Albaidaa, Wadi Elebiedia, Gebel Kniethl, Wadi Sayaa, Wadi Elbarasa, Wadi Alfawara, and Wadi Alkharar. The results showed an obvious decrease in genetic diversity variables in all studied populations. The range of PPL was between 8 and 40; additionally, the low HT value of 0.804 and the high value of inbreeding, Fis = 0.238, reflected a severe lack of heterozygotes. High levels of FST and GST and low gene flow indicate considerable segregation among the C. gileadensis populations, which creates a barrier to gene migration. Our data suggest the need for conservation planning for C. gileadensis in order to avoid the species’ forthcoming extinction. Efforts should be largely oriented around managing water consumption, prohibiting overcutting and overgrazing, and establishing appropriate seed banks.
1. Introduction
Many members of the genus Commiphora are listed as endangered due to the overcollection of their populations for utilisation in medicinal and economical purposes (Burseraceae [Myrrh family]) [1]. Commiphora gileadensis (L.) C. Chr is considered one of the most economically and medicinally valuable trees grown in the Northern Hemisphere. Its distribution is mainly centred in the Red Sea region in the southern and western mountains of Saudi Arabia and other mountainous habitats in neighbouring countries within the Southern Arabian Peninsula and East Africa [2,3]. The well-known name of this tree in Saudi Arabia is “Besham” or Balsam, and its economic value is largely due to its use in the perfume industry [4,5] as well as its many medicinal applications, e.g., as a remedy for respiratory system diseases [6].
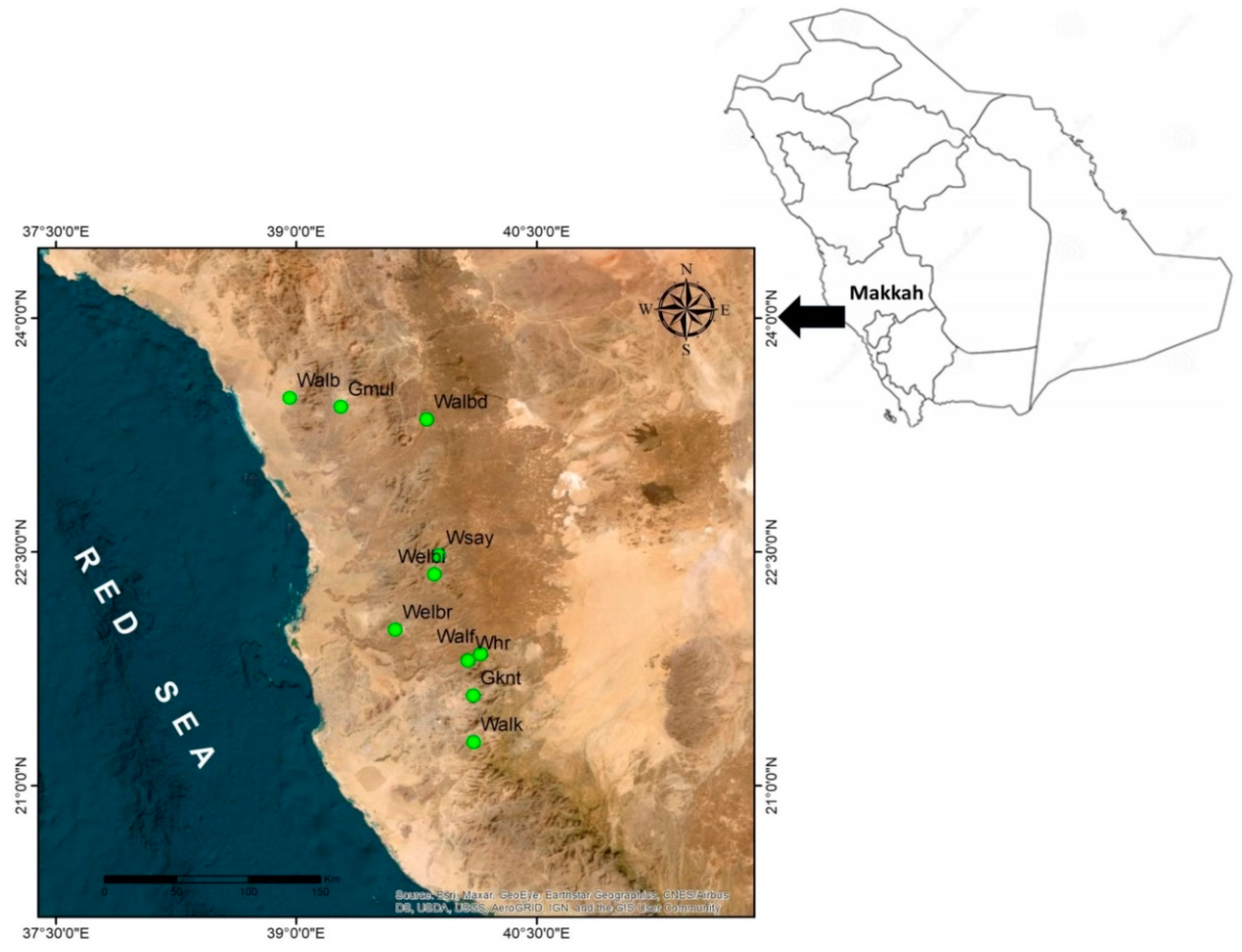
Commiphora gileadensis is found in few remaining populations, and it is mainly associated with the bottom of rocky mountains in the south and western regions of Saudi Arabia where its distribution is centred in Makkah province (Figure 3). The species was originally estimated to have between 1800 and 3000 populations that declined to the current estimate of only 60 populations (personal observation). The apparent reduction in the population numbers and population size of C. gileadensis could be attributed to progressive climate change conditions in the region [7] which, exacerbated by anthropogenic impacts [8,9], are anticipated to lead to a greater decline in the sizes of existing C. gileadensis populations and other associated plant taxa in arid habitats such as the Makkah region.
Commiphora gileadensis plants could be affected by a considerable decline in genetic diversity as a consequence of genetic drift, which is a key reason for the apparent low fitness and thus severe inability of many populations to adapt to ambient environmental confrontations [10,11,12]. Therefore, elucidating the population genetic variables and genetic structures of the remaining plant populations of C. gileadensis is crucial to conserve and restore this valuable plant species [13] and may support conservation plans for other plant taxa [14].
One of the main limiting factors for plant species with low population sizes is their potential for outcrossing and performing successful seed setting, especially under the stress of arid conditions. For C. gileadensis, self-incompatibility represents an extra stress that endangers its existence. This species has a floral structure like other members of Burseraceae and is recognizable by small, actinomorphic, and slightly odoriferous flowers; these features are considered to promote obligate outcrossing [15]. This reproductive system can severely impact population genetics corresponding to the survival of plant populations in arid habitats and can cause the loss of polymorphic genes, genetic drift, as well as progressive inbreeding [16,17].
As a result of the ambient climate change conditions connected with human over-utilisation in the Makkah province, the existing plant taxa—including remaining populations of C. gileadensis—are prone to the risk of extinction because of the ongoing decline in population size and potential loss of genetic diversity. Genetic analysis is pivotal for detecting genetic variation [18] using SSR (simple sequence repeats). Loci are considered to be of great value for measuring the gene diversity and genetic structures in collapsing plant populations of C. gileadensis due to their high potential to detect repeat regions with variable sequences in the target genome. These loci are well characterized as co-dominant molecular markers [19,20,21,22,23]. SSR markers were successfully applied to assess population genetics and genetic structure in other rare plant species [24,25,26].
Our research aims to elucidate the genetic diversity and genetic structure patterns of C. gileadensis populations under the xeric conditions of the Makkah province; by applying microsatellite loci, we can extract the required data for proposing mandatory conservation plans that are crucial to prevent the imminent threat of extinction for C. gileadensis and other associated plant species grown in this region.
2. Results
A total of 50 loci showed polymorphisms. The percentage of polymorphic loci (Table 1) was at its maximum value (40) in the Walb 5 and Walbd5 populations in Wadi Albathna and Wadi Albaidaa, respectively, whereas the minimum percentage of polymorphic loci (8) was detected in the Welbi1 population in Wadi Elebiedia. High selfing was indicated by our results for C. gileadensis, as the average inbreeding coefficient (Fis) was 0.238, verifying an obvious deficit of heterozygotes (Table 1).

Table 1.
The measurements of population genetic variables of C. gileadensis populations across studied sites in Makkah province.
The mean number of alleles per locus (Na) varied between 1.48 (Walb 5 population) and 1.08 (Welbi1 population), resulting in the mean number of effective alleles per locus (Ne) and the Shannon index (I). The highest number of private alleles was 0.087 and was calculated in the Gmul 3 population, while no private alleles were detected in Walb 1, Walb 4, Walbd5, Welbi2, Welbi3, Welbi5, Welbr2, Welbr3, Welbr5, Walk 2, and Walk 3 populations. Expected heterozygosity (He) ranged from 1.281, 0.217, and 0.138, respectively, in the Gmul 3 and Walb 5 populations to 1.037, 0.039, and 0.025, respectively, in the Welbi1 and Walbd2 populations (Table 1). The average total heterozygosity (HT) for all loci and populations was equal to 0.804.
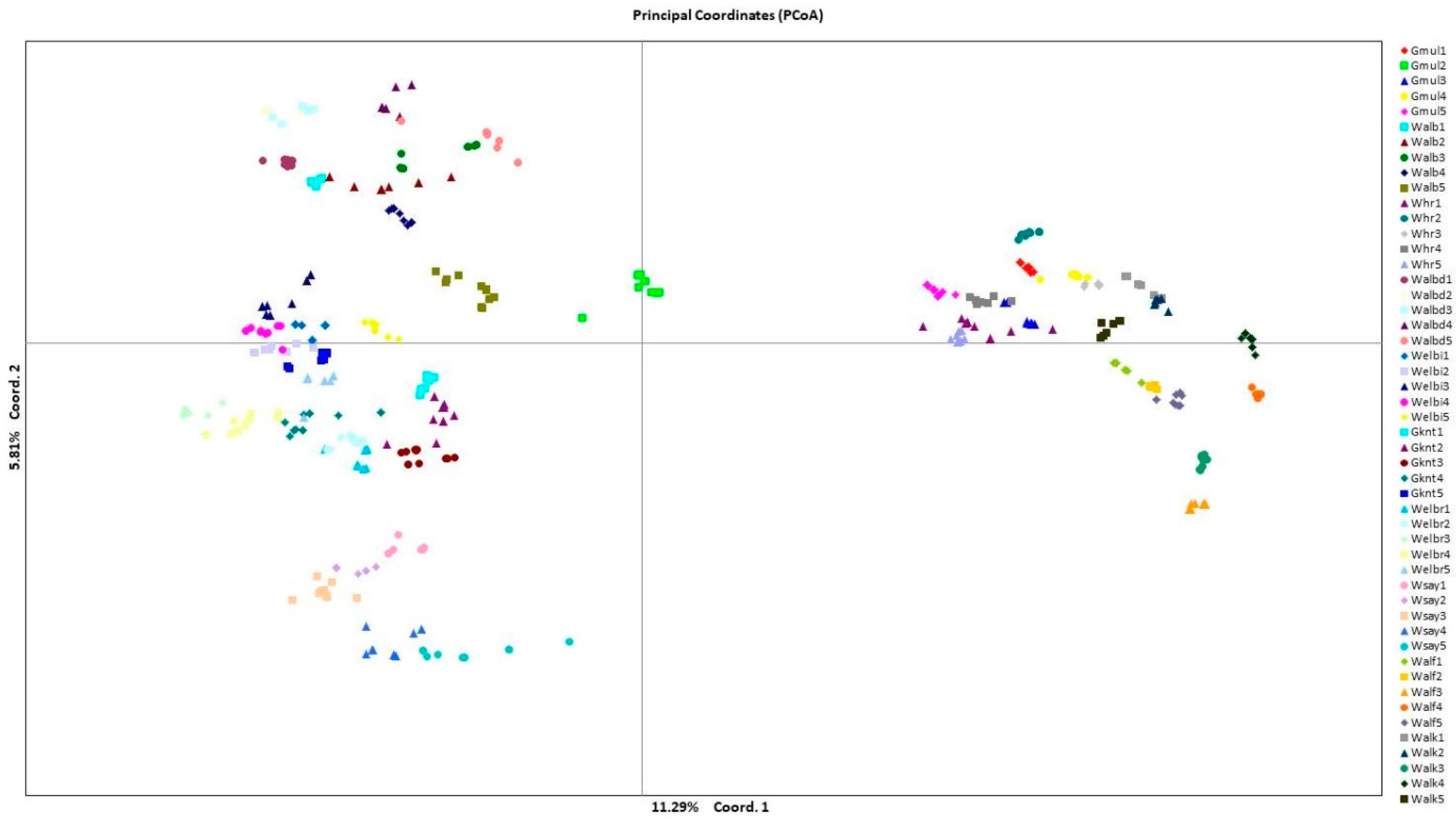
The PCoA results (Figure 1) indicated that five out of seven principal components were significant (eigenvalue > 1) and considered as 99.9 of the sum variation. The main five significant components were Na, Ne, I, the number of private alleles, and Ho.
Figure 1.
Principal coordinate analysis to categorize C. gileadensis populations based on pairwise genetic distance among 600 individual genotypes belonging to 50 studied populations with coloured and polygon codes for each population acronym.
The analysis categorized the studied populations of C. gileadensis into four groups. The upper-right group comprised individuals who belonged to populations of Gebel Al Muliesaa, Wadi Alkharar, and Wadi Houra; the upper-left group contained individuals in populations from Wadi Albathna, Wadi Elebiedia, and Wadi Albaidaa; the lower-left group contained populations from Gebel Kniethl, Wadi Elbarasa, and Wadi Sayaa; and the lower-right group contained Wadi Alfawara populations.
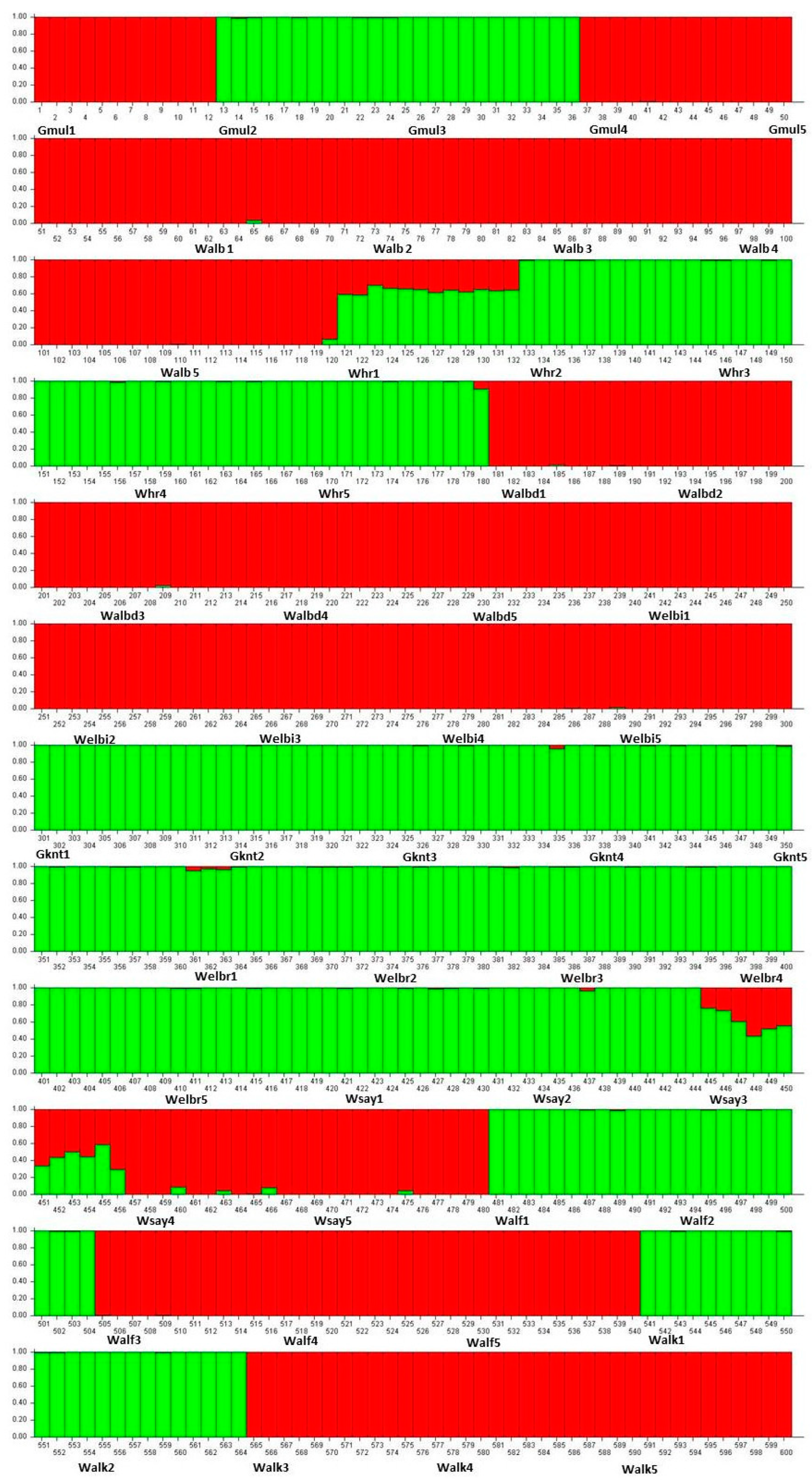
Evanno’s method [27] indicated that K = 2 was optimal among the 50 populations of C. gileadensis (Figure 2).
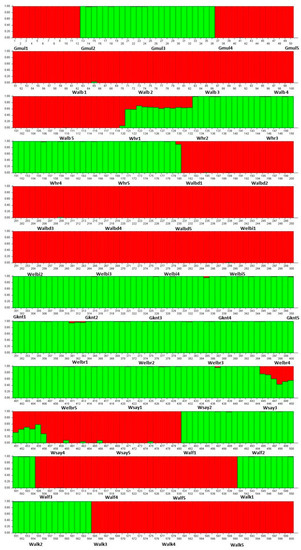
Figure 2.
Population structure of 600 genotypes of C. gileadensis belonging to 50 populations that were grouped into two subgroups based on 50 SSR markers (K = 2); the acronyms of each population were written next to the genotypes where they belonged. The green colour referred for the first subgroup and the red colour referred to the second subgroup.
The AMOVA showed substantial genetic differentiation among the studied C. gileadensis populations where FST = 0.896 and was higher with RST = 0.980. The maximum genetic differentiation was observed between different populations (98, p = 0.001), while the minimum value (1, p = 0.010) was measured among individuals in the same population. The gene flow of Nm = 0.024 is a low value for the gene migration among population per generation. G-statistic measurements showed a higher value of FST = 0.913 and Gst value = 0.908 compared to the FST resulting from AMOVA, which confirmed high genetic differentiation among different populations.
3. Discussion
In this study, all the genetic diversity variables of C. gileadensis showed a modest to extreme decline in measurements among all the enduring populations analysed and was in agreement with other studies on closely related plant species of Burseraceae. This indicates an extensive decline in gene diversity and meets the corresponding severe environmental circumstances [28,29,30,31,32,33,34] confirmed by the comparison of the high genetic differentiation FST values, which measured for C. gileadensis (FST = 0.896) with other rare plant species in similar plant habitats in South Sinai, and they revealed considerable values of genetic differentiation, e.g., Primula boveana (FST = 0.737) [35] and Cotoneaster orbicularis (FST = 0.634) [32].
The main reason behind the lack of genetic diversity in the existing populations may be their small population size. A leading factor underlying this decline could be that C. gileadensis populations are subjected to high genetic drift and inbreeding, which exacerbate the problem of decline in polymorphic alleles as an imminent result of encountering environmental conditions [36,37,38].
The distribution outline of genetic diversity locations among the studied populations revealed a considerable range of variability, with relatively modest polymorphisms measured in the populations of Gebel Al Muliesaa, Wadi Alkharar, and Wadi Houra, which could be attributed to the relative abundance of water reserves in these regions. Wadi Albathna and Wadi Albaidaa are located at the foot of the Bany Ayoub Mountain region, where water reserves are more abundant due to frequent floods caused by rain in this location. Wadi Elbarasa has low-altitude valleys characterized by water aggregations that allow for the growth of a few plant populations. The slight gorges in Gebel Kniethl (750–1000 m a.s.l) facilitate the growth of plant populations [39], revealing the same connection between genetic diversity maintenance in rare plant populations and water availability in desert habitats.
The PCoA determined the association between the genetic diversity in C. gileadensis and the abundance of water reserves. The PCoA subdivided populations with relatively high polymorphisms, showing sites with water abundance on the right and left upper parts of the PCoA axis: Gebel Al Muliesaa, Wadi Alkharar, and Wadi Houra were in the upper-right group and populations from Wadi Albathna, Wadi Elebiedia, and Wadi Albaidaa were in the upper-left group. The STRUCTURE analysis results for the existence of only two subpopulations for the studied 500 genotypes of C. gileadensis and the notable values of genetic differentiation among populations, calculated using AMOVA and confirmed with G-statistics, indicated considerable isolation among the population sites. The main reason for this high isolation could be the increasing activities of human inhabitants, which include overcutting for cosmetic and medicinal purposes. This can also include excessive overgrazing by camels and sheep herds owned by local tribes in the area or other tribes inhabiting the southern region of Saudi Arabia with drier climatic conditions [40] that become extremely hazardous during spring. Moreover, water reserves are at risk of high depletion due to the recent increasing human populations associated with growing industries and petroleum refinery companies in the region [7,41].
Larger decreases in the population size of C. gileadensis and further isolation are anticipated with increasing temperatures and water deficiency conditions, as indicated by the fluctuations of rain frequency in these sites [8,9]. Moreover, the constant influence of increasing temperatures could pose a greater risk to the reproductive capabilities of C. gileadensis flowers—as well as have a negative impact on pollination potential—and is thus expected to increase selfing [33,42], as revealed by the excessive low values of the measured inbreeding coefficient (Fis).
C. gileadensis is characterized by drupe-type fruit with one seed that is considered relatively heavy for wind dispersal (sizes range from 3.5 to 4.8 cm), which supports our computed low level of gene flow among C. gileadensis populations. For this reason, the extensive anthropogenic and climatic causes of isolation have promoted the elevation of the genetic differentiation value among the remaining populations of C. gileadensis. This phenomenon was clearly outlined in the PCoA. The values of the calculated gene flow decreased considerably from the values required for preventing an increase in genetic drift [43]. The concurrent influences of genetic drift and gene flow could aggravate the future drop in gene diversity among the remaining populations of C. gileadensis.
4. Materials and Methods
4.1. Plant Materials
The sampling of C. gileadensis in the mountains and plains of the Makkah province included fifty populations from ten different sites (Table 2, Figure 3). From every site, five populations were selected for sampling. All studied populations were located at the bottom of the rocky mountains between the Alabwaa village and the Makkah metropolitan area. The highest number of individuals was found in a population located in Wadi Albaidaa at the bottom of the Ayoub mountains east of Abwaa (Figure 2). The lowest number of individuals (16) was the Whr1 population in the Wadi Houra site, which was the result of extensive human activities in the area—such as overcutting—and overgrazing by sheep and cattle herds over the whole site.

Table 2.
Information of the studied sites and populations of Commiphora gileadensis in Makkah province (acronym of 3–4 letters refers to population name, and the number refers to different populations within same site).
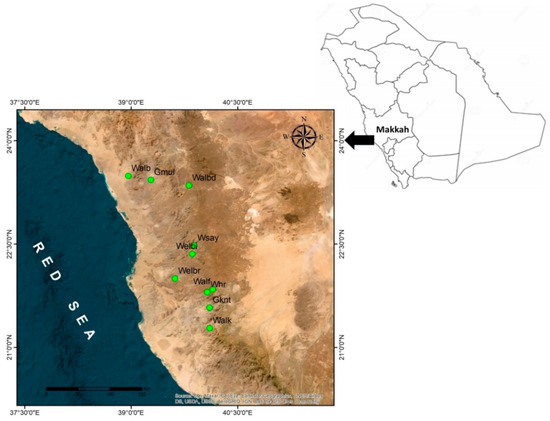
Figure 3.
The studied sites of Commiphora gileadensis in the Makkah province, Kingdom of Saudi Arabia (Gmul: Gebel Al Muliesaa; Walb: Wadi Albathna; Whr: Wadi Houra; Walbd: Wadi Albaidaa; Welbi: Wadi Elebiedia; Gknt: Gebel Kniethl; Welbr: Wadi Elbarasa; Wsay: Wadi Sayaa; Walf: Wadi Alfawara; Walk: Wadi Alkharar).
Twelve individuals were sampled from each of the studied populations. Two to three leaflets were preserved directly in liquid nitrogen and then placed at −20 °C in a freezer for further DNA isolation.
4.2. Genomic DNA Extraction and PCR Tests
Isolation of DNA from the preserved leaflet samples for 600 plant individuals was performed using a DNeasy Plant Mini Kit (Qiagen, Germantown, MD, USA). Fifty loci revealing polymorphisms were recognized using sixty-one formerly published primers for other species belonging to the Burseraceae family [28,29,30,31]. The polymorphic primer tests were performed for all sampled individuals (Table S1). A master mix for PCR trials was prepared according to procedures outlined in [34]. PCR tests were carried out using a C1000 Thermal Cycler (BioRad, Hercules, CA, USA). The PCR reaction conditions were as follows: initial denaturation at 95 °C for 5 min; followed by 45 cycles at 94 °C for 35 s for denaturation; 55 °C for 40 s for annealing; and 72 °C for 5 min for final extension.
The products of the PCR reactions were sequenced using a 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) with LIZ500 as a size standard. The sequences of amplified fragments were determined using GeneMapper 4.0 (Applied Biosystems, Foster City, CA, USA), and the lengths of the amplified fragments ranged from 112 to 300 bp in accordance with [44].
4.3. Population Genetic Analysis
The variables of genetic diversity, genetic structure, inbreeding, and G-statistics were calculated using GenAlEx 6.5 [45], measuring the genetic differentiation among the populations with RST for microsatellite loci [46].
The genetic structure for 50 populations of C. gileadensis was conducted using the Bayesian clustering method in STRUCTURE version 2.3.4 [47], with the admixture model implemented and K values (number of potential clusters) ranging from one to ten. The burn-in period and Markov Chain Monte Carlo (MCMC) conducted 100,000 iterations [27]. The optimal K value was determined using the method of Evanno et al. [27] as implemented in STRUCTURE Harvester [48].
AMOVA was applied with 999 permutations to assess genetic differentiation among populations [49,50]. Gene flow (Nm) was calculated via the private allele method [51]. The analysis of heterozygosity (Ho), the expected heterozygosity (He) under Hardy–Weinberg equilibrium, and Wright’s fixation index (F = 1 − Ho/He) were tested for each locus in each population to test deviations from the Hardy–Weinberg equilibrium and thereby determine inbreeding in existing populations of C. gileadensis. Principal coordinate analysis (PCoA) was performed using GenAlEx 6.5 [45] based on pairwise genetic distance data among 600 individual genotypes belonging to 50 studied populations.
5. Conclusions and Recommendations
Our current research represents the first assessment of distribution patterns of population genetic variables and genetic structures among and within the remaining populations of C. gileadensis in habitats of the Makkah region; it was carried out in order to contribute to the conservation management and protection for C. gileadensis as an economically and medicinally valuable plant species. This species is confronted by the danger of forthcoming extinction due to an extreme loss of gene polymorphism associated with excessive interpopulation genetic differentiation and severe inbreeding.
The conservation plan of C. gileadensis should be based on long-term and progressive actions and should be oriented mainly to prevent the continuous degradation of its populations and its habitats. Many efforts could be considered in this area. Firstly, the results of the present study suggest that we should establish enclosures of a wire-fence type to protect populations that are subjected to severe low genetic polymorphisms [52], as indicated in our study for Wadi Alkharar, Wadi Albaidaa, Wadi Alfawara, and Gebel Al Muliesaa. Such enclosures are crucial to prevent the dangers of overgrazing from camels and sheep in these locations; they should be monitored regularly to observe vegetation parameters and detect any further alterations in the protected populations. Secondly, management plans for water resources should be proposed to promote water utilisation, enhancing the reuse of wastewater and the effective usage and storage of rainwater and underground water. These plans should be incorporated into undergraduate learning programs in educational institutes, in addition to increasing public awareness for managing water consumption among inhabitants of the Makkah region.
Thirdly, the apparent decline in genetic diversity and the considerable genetic differentiation in these populations could be reclaimed via performing a test of interpopulation crosses among the populations with the highest genetic differentiation values (FST) and higher selfing, potentially increasing the population fitness [53]. Many factors should be considered during the design of the interpopulation crosses to alleviate the consequences of outbreeding depression, such as the genetic distance between the concerned populations, their latitude, and the genetic diversity magnitude, e.g., crosses between extremely low genetic diversity populations resulted in the outbreeding value in F1 [54]. Moreover, a test bank for the collected seeds from the studied remaining populations can be established [53]. The collected seeds should be planted in greenhouses as nurseries, and seedlings with strong vegetative characteristics could be planted into populations with the lowest genetic diversity resembling that of the populations from which the parent seeds were collected; this would reduce potential consequences, including further inbreeding and an extreme decline of gene flow. Parts of the collected viable seeds should be preserved using suitable procedures for seed maintenance in well-equipped test banks; these test banks would be of high value for future C. gileadensis conservation efforts in its habitats. The recommended measures for the conservation of C. gileadensis could be applicable to the other rare species in the genus Commiphora which grow in similar arid habitats.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12132506/s1, Table S1: The tested primers status for Commiphora gileadensis.
Author Contributions
Conceptualization, H.M.; methodology, H.M. and Z.M.A.-H.; software, H.M.; formal analysis, K.H.A.; investigation, H.M. and Z.M.A.-H.; resources, H.M., K.H.A. and Z.M.A.-H.; data curation, K.H.A.; writing—original draft, H.M., K.H.A. and Z.M.A.-H.; writing—review and editing, H.M.; visualization, H.M. and K.H.A.; project administration, H.M., K.H.A. and Z.M.A.-H.; funding acquisition, H.M., K.H.A. and Z.M.A.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education and Deanship of Scientific Research (DSR), at King Abdulaziz University, Jeddah, Saudi Arabia: under grant no. IFPIP: 728-662-1443.
Data Availability Statement
Not applicable.
Acknowledgments
This present research work was funded by Institutional Fund Projects under grant no. (IFPIP: 728-662-1443). The authors gratefully acknowledge technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mathur, M.; Mathur, P.; Purohit, H. Ecological niche modelling of a critically endangered species Commiphora wightii (Arn.) Bhandari using bioclimatic and non-bioclimatic variables. Ecol. Process 2023, 12, 8. [Google Scholar] [CrossRef]
- Miller, A.G.; Morris, M. Plants of Dhofar, the Southern Region of Oman: Traditional, Economic and Medicinal Uses; The Office of the Advisor for Conservation of the Environment, Diwan of Royal Court Sultanate of Oman: Muscat, Oman, 1988. [Google Scholar]
- Wood, J.R.I. A Handbook of the Yemen Flora. With Color Illustrations by Hugo Haig-Thomas; Royal Botanic Gardens: Kew, UK, 1997. [Google Scholar]
- Shen, T.; Li, G.H.; Wang, X.N.; Lou, H.X. The genus Commiphora: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2012, 142, 319–330. [Google Scholar] [CrossRef]
- Mahr, D. Commiphora: An Introduction to the Genus. Cactus Succul. J. 2012, 84, 140–154. [Google Scholar] [CrossRef]
- González-Tejero, M.R.; Casares-Porcel, M.; Sánchez-Rojas, C.P.; Ramiro-Gutiérrez, J.M.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.E.; Censorii, E.; De Pasquale, C.; Della, A.; et al. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef]
- Tarawneh, Q.Y.; Chowdhury, S. Trends of Climate Change in Saudi Arabia: Implications on Water Resources. Climate 2018, 6, 8. [Google Scholar] [CrossRef]
- Issar, A.S. The impact of global warming on the water resources of the Middle East: Past, present and future. In Climate Changes and Water Resources in the Middle East and North Africa; Zereini, F., Hötzl, H., Eds.; Springer: Heidelberg, Germany, 2008. [Google Scholar]
- Soultan, A.; Wikelski, M.; Safi, K. Risk of biodiversity collapse under climate change in the Afro-Arabian region. Sci. Rep. 2019, 9, 955. [Google Scholar] [CrossRef]
- Luijten, S.H.; Dierick, A.; Gerard, J.; Oostermeijer, B.; Raijmann, L.E.J.; Den Nijs, H.C.M. Population size, genetic variation, and reproductive success in a rapidly declining, self-incompatible perennial (Arnica montana) in the Netherlands. Conserv. Biol. 2000, 14, 1776–1787. [Google Scholar]
- Hansson, B.; Westerberg, L. On the correlation between heterozygosity and fitness in natural populations. Mol. Ecol. 2002, 11, 2467–2474. [Google Scholar] [CrossRef]
- Bastiaan, S.; Hamish, G.S. Effects of genetic drift and gene flow on the selective maintenance of genetic variation. Genetics 2003, 194, 235–244. [Google Scholar]
- Shalabi, L.F.; Otaif, F.S. Commiphora Jacq (Burseraceae) in Saudi Arabia, Botanical, Phytochemical and Ethnobotanical Notes. Ecologies 2022, 3, 38–57. [Google Scholar] [CrossRef]
- Hatmaker, E.A.; Staton, M.E.; Dattilo, A.J.; Hadziabdic, D.; Rinehart, T.A.; Schilling, E.E.; Trigiano, R.N.; Wadl, P.A. Population Structure and Genetic Diversity within the endangered species Pityopsis ruthii (Asteraceae). Front. Plant Sci. 2018, 9, 943. [Google Scholar] [CrossRef]
- Raju, A.J.S.; Lakshmi, P.V.; Ramana, K.V.; Chandra, P.H. Entomophily, ornithophily and anemochory in the self-incompatible Boswellia ovalifoliolata Bal. & Henry (Burseraceae), an endemic and endangered medicinally important tree species. J. Threat. Taxa 2012, 4, 2673–2684. [Google Scholar]
- Keller, L.F.; Waller, D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002, 17, 230–241. [Google Scholar] [CrossRef]
- Vilas, C.; San Miguel, E.; Amaro, R.; García, C. Relative contribution of inbreeding depression and eroded adaptive diversity to extinction risk in small populations of shore campion. Conserv. Biol. 2005, 20, 229–238. [Google Scholar] [CrossRef]
- Kadam, U.S.; Lossie, A.C.; Schulz, B.; Irudayaraj, J. Gene expression analysis using conventional and imaging methods. In DNA and RNA Nanobiotechnologies in Medicine: Diagnosis and Treatment of Diseases; Springer: Berlin/Heidelberg, Germany, 2013; pp. 141–162. [Google Scholar]
- Kim, K.S.; Sappington, T.W. Microsatellite Data Analysis for Population Genetics. In Microsatellites. Methods in Molecular Biology (Methods and Protocols); Kantartzi, S., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 1006, pp. 271–295. [Google Scholar]
- Upadhyay, A.; Kadam, U.S.; Chacko, P.; Karibasappa, G.S. Microsatellite and RAPD analysis of grape (Vitis spp.) accessions and identification of duplicates/misnomers in germplasm collection. Indian J. Hortic. 2010, 67, 8–15. [Google Scholar]
- Upadhyay, A.; Kadam, U.S.; Chacko, P.M.; Aher, L.; Karibasappa, G.S. Microsatellite analysis to differentiate clones of Thompson seedless grapevine. Indian J. Hortic. 2010, 67, 260–263. [Google Scholar]
- Hinge, V.R.; Shaikh, I.M.; Chavhan, R.L.; Deshmukh, A.S.; Shelake, R.M.; Ghuge, S.A.; Dethe, A.M.; Suprasanna, P.; Kadam, U.S. Assessment of genetic diversity and volatile content of commercially grown banana (Musa spp.) cultivars. Sci. Rep. 2022, 12, 1–6. [Google Scholar] [CrossRef]
- Chavhan, R.L.; Sable, S.; Narwade, A.V.; Hinge, V.R.; Kalbande, B.B.; Mukherjee, A.K.; Chakrabarty, P.K.; Kadam, U.S. Multiplex molecular marker-assisted analysis of significant pathogens of cotton (Gossypium sp.). Biocatal. Agric. Biotechnol. 2023, 47, 102557. [Google Scholar] [CrossRef]
- Szczecińska, M.; Sramko, G.; Wołosz, K.; Sawicki, J. Genetic Diversity and Population Structure of the Rare and Endangered Plant Species Pulsatilla patens (L.) Mill in East Central Europe. PLoS ONE 2016, 11, e0151730. [Google Scholar] [CrossRef]
- Yu, Y.L.; Wang, H.C.; Yu, Z.X.; Schinnerl, J.; Tang, R.; Geng, Y.P.; Chen, G. Genetic diversity and structure of the endemic and endangered species Aristolochia delavayi growing along the Jinsha River. Plant Divers 2021, 43, 225–233. [Google Scholar] [CrossRef]
- Chen, L.; Pan, T.; Qian, H.; Zhang, M.; Yang, G.; Wang, X. Genetic Diversity and Population Structure Revealed by SSR Markers on Endemic Species Osmanthus serrulatus Rehder from Southwestern Sichuan Basin, China. Forests 2021, 12, 1365. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Misiewicz, T.M.; Barbosa, C.E.; Fine, P.V. Microsatellite primers for an Amazonian lowland tropical tree, Protium subserratum (Burseraceae). Am. J. Bot. 2012, 99, e465-7. [Google Scholar] [CrossRef]
- Maradani, B.S.; Gudasalamani, R.; Setty, S.; Chandrasekaran, R. Development of microsatellite markers for the resin-yielding, non-timber forest product species Boswellia serrata (Burseraceae). Appl. Plant Sci. 2018, 6, e01180. [Google Scholar] [CrossRef]
- Koffi, K.G.; Heuertz, M.; Jans, R.; Hardy, O.J.; Vendramin, G.G.; Duminil, J. Characterization of new microsatellite loci isolated from Santiria trimera (Burseraceae). Am. J. Bot. 2012, 99, e334-6. [Google Scholar] [CrossRef]
- Rimlinger, A.; Marie, L.; Avana, M.L.; Bouka, G.U.; Zekraoui, L.; Mariac, C.; Carrière, S.M.; Duminil, J. New microsatellite markers for Dacryodes edulis (Burseraceae), an indigenous fruit tree species from Central Africa. Mol. Biol. Rep. 2020, 47, 2391–2396. [Google Scholar] [CrossRef]
- Mansour, H.; Sliwinska, E. Genetic Diversity and Inbreeding Level of Cotoneaster orbicularis Schltdl. in The Sinai Mountains Revealed by Microsatellite Markers and Flow Cytometry. Egypt. J. Bot. 2017, 2, 351–361. [Google Scholar] [CrossRef]
- Mansour, H.; Alsamadany, H.; Al-Hasawi, Z.M. Genetic diversity and genetic structure of Salvadora persica L., rare plant species in Rabigh province, Saudi Arabia: Implications for conservation. J. Taibah Univ. Sci. 2020, 14, 881–888. [Google Scholar] [CrossRef]
- Mansour, H.; Alsamadany, H.; Al-Hasawi, Z.M. Molecular Assessment of Genetic Diversity and Genetic Structure of Rhanterium epapposum Oliv. in Scarce Populations in Some Regions of Western Saudi Arabia. Plants 2022, 11, 1560. [Google Scholar] [CrossRef]
- Jimenez, A.; Mansour, H.; Keller, B.; Conti, E. Low genetic diversity and a high level of inbreeding in the Sinai primrose (Primula boveana), a species on the brink of extinction. Plant Syst. Evol. 2014, 300, 1199–1208. [Google Scholar] [CrossRef]
- Blomqvist, D.; Pauliny, A.; Larsson, M.; Flodin, L.A. Trapped in the extinction vortex? Strong genetic effects in a declining vertebrate population. BMC Evol. Biol. 2010, 10, 33. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Roldán-Ruiz, I.; Honnay, O. Evidence for demographic bottlenecks and limited gene flow leading to low genetic diversity in a rare thistle. Conserv. Genet. 2010, 11, 1979–1987. [Google Scholar] [CrossRef]
- Smyser, T.J.; Duchamp, J.E.; Johnson, S.A.; Larkin, J.L.; Rhodes, O.E., Jr. Consequences of metapopulation collapse: Comparison of genetic attributes between two Allegheny woodrat metapopulations. Conserv. Genet. 2012, 13, 849–858. [Google Scholar] [CrossRef]
- Al-Gharaibeh, M.M.; Hamasha, H.R.; Rosche, C.; Lachmuth, S.; Wesche, K.; Hensen, I. Environmental gradients shape the genetic structure of two medicinal Salvia species in Jordan. Plant Biol. 2017, 19, 227–238. [Google Scholar]
- Al-Rowaily, S.L.; El-Bana, M.I.; Al-Bakre, D.A.; Assaeed, A.M.; Hegazy, A.K.; Ali, M.B. Effects of open grazing and livestock exclusion on floristic composition and diversity in natural ecosystem of Western Saudi Arabia. Saudi. J. Biol. Sci. 2015, 22, 430–437. [Google Scholar] [CrossRef]
- Harter, T.; Davis, H.; Mathews, M.; Meyer, R. Shallow ground water quality on dairy farms with irrigated forage crops. J. Contam. Hydrol. 2022, 55, 287–315. [Google Scholar] [CrossRef] [PubMed]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Spieth, P.T. Gene flow and genetic differentiation. Genetics 1974, 78, 961–965. [Google Scholar] [CrossRef]
- Arif, I.A.; Khan, H.A.; Shobrak, M.; Al Homaidan, A.A.; Al Sadoon, M.; Al Farhan, A.H.; Bahkali, A.H. Interpretation of electrophoretograms of seven microsatellite loci to determine the genetic diversity of the Arabian Oryx. Genet. Mol. Res. 2010, 9, 259–265. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx v.6.5: Genetic analysis in Excel. Population genetic software for teaching and research. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Slatkin, M. A measure of population subdivision based on microsatellite allele frequencies. Genetics 1995, 139, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.; Stephens, M.; Rosenberg, N.; Donnelly, P. Association mapping in structured populations. Am. J. Hum. Genet. 2000, 67, 170–180. [Google Scholar] [CrossRef]
- Earl, E.A.; Von Holdt, B.M. Structure Harvester: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Res. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, Y.; Excoffier, L. A generic estimation of population subdivision using distances between alleles with special reference for microsatellite loci. Genetics 1996, 142, 1061–1064. [Google Scholar] [CrossRef]
- Barton, N.H.; Slatkin, M. A Quasi-equilibrium theory of the distribution of rare alleles in a subdivided population. Heredity 1986, 56, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Koyama, A.; Uchida, K.; Ozeki, M.; Iwasaki, T.; Nakahama, N.; Suka, T. Conservation of endangered and rare plants requires strategies additional to deer-proof fencing for conservation of sub-alpine plant diversity. Appl. Veg. Sci. 2021, 24, e12553. [Google Scholar] [CrossRef]
- Oakley, C.G.; Lundemo, S.; Ågren, J.; Schemske, D.W. Heterosis is common and inbreeding depression absent in natural populations of Arabidopsis thaliana. J. Evol. Biol. 2019, 32, 592–603. [Google Scholar] [CrossRef]
- Holsinger, K.E.; Gottlieb, L.D. Conservation of rare and endangered plants: Principles and prospects. In Genetics and Conservation of Rare Plants; Falk, D.A., Holsinger, K.E., Eds.; Oxford University Press: New York, NY, USA, 1991; pp. 195–208. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).