Does Caulerpa prolifera with Its Bacterial Coating Represent a Promising Association for Seawater Phytoremediation of Diesel Hydrocarbons?
Abstract
1. Introduction
2. Results
2.1. Diesel Exposition of C. prolifera in Controlled Conditions
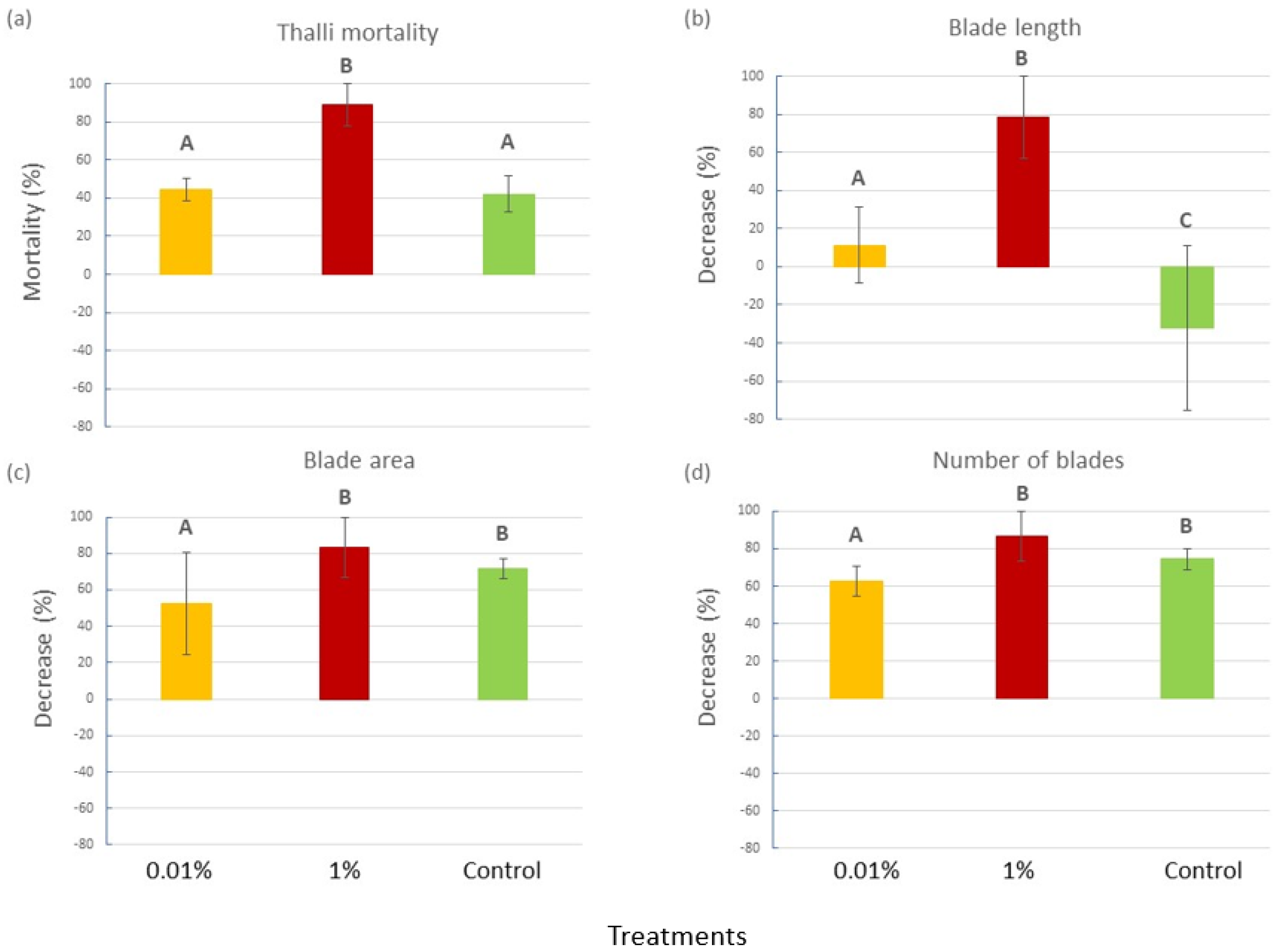
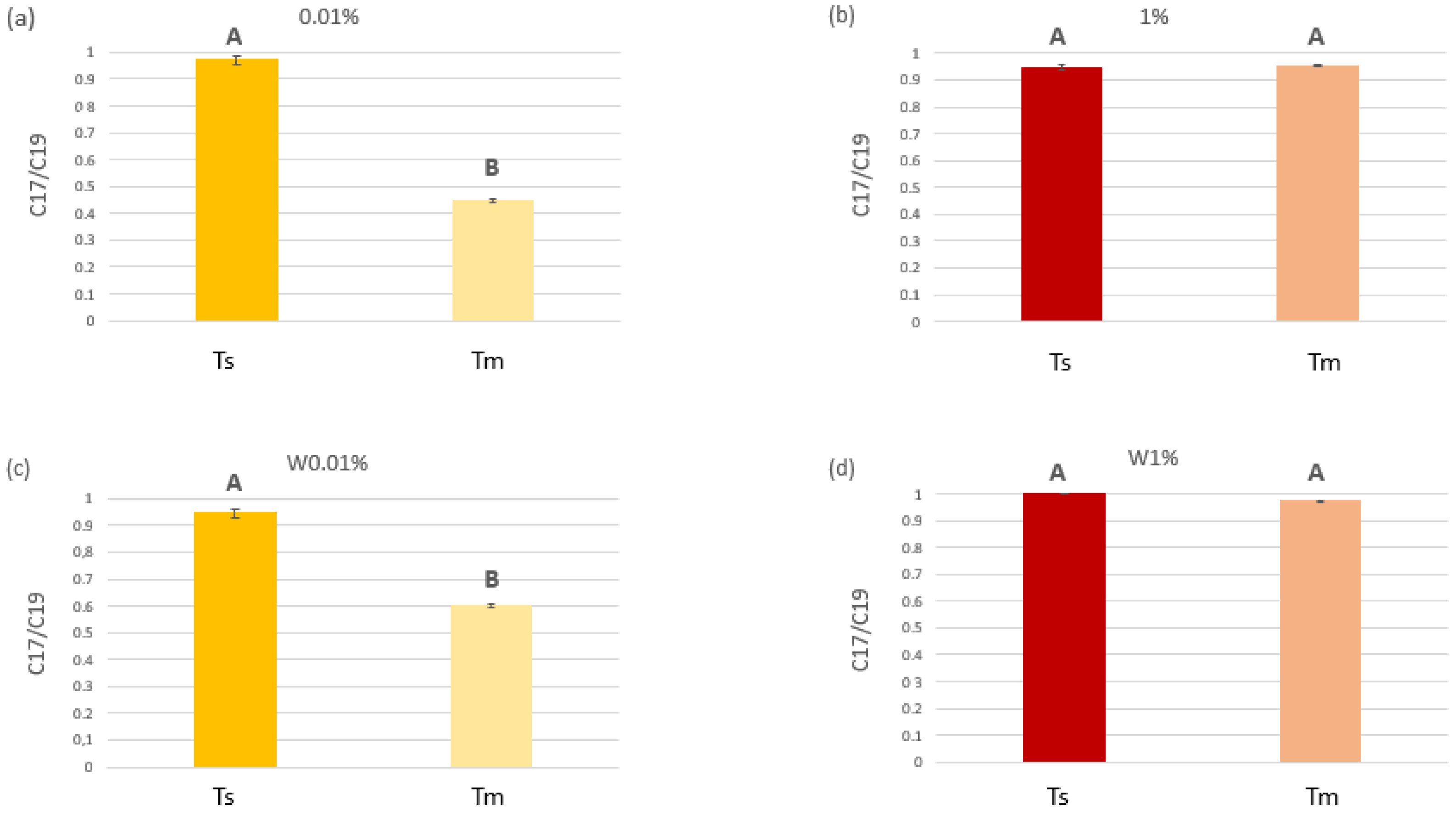
2.1.1. C. prolifera Functional Traits
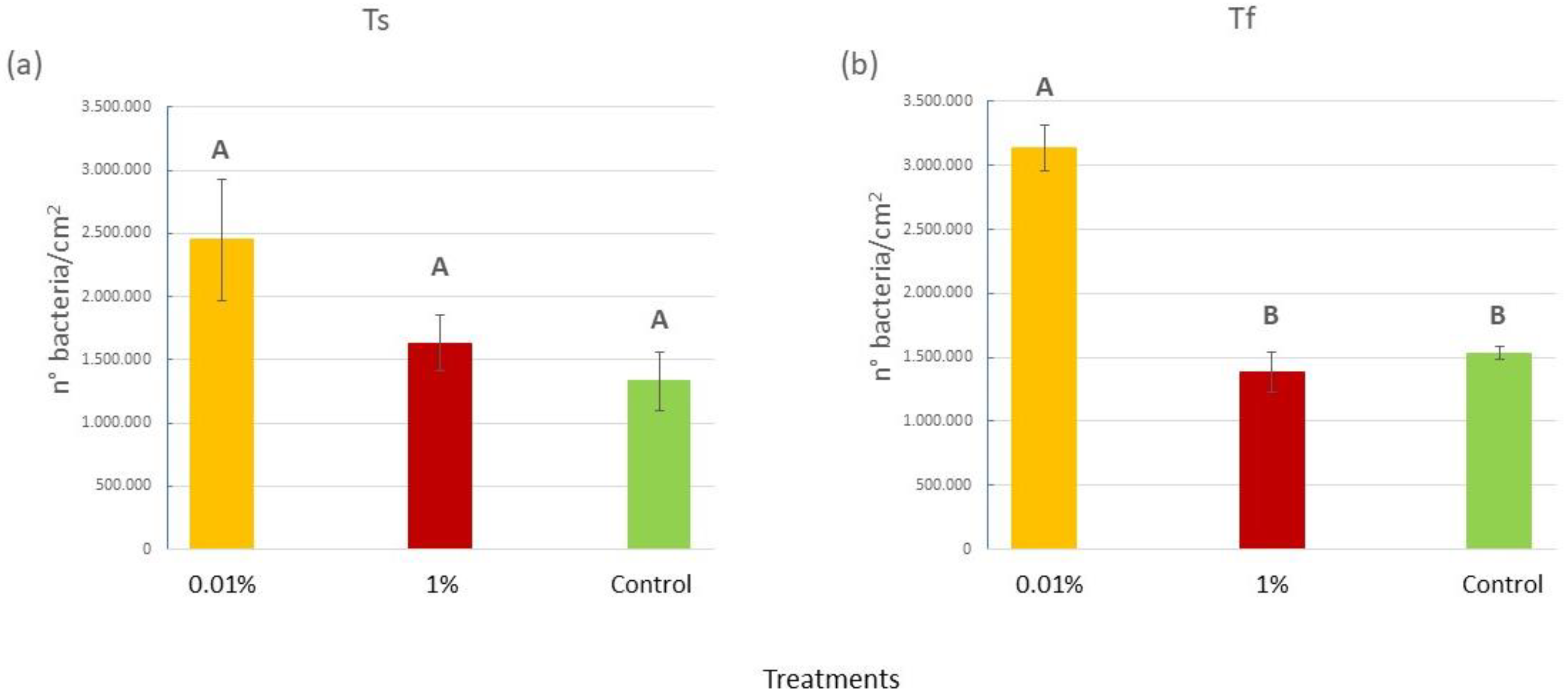
2.1.2. Bacterial Abundance on C. prolifera Blades
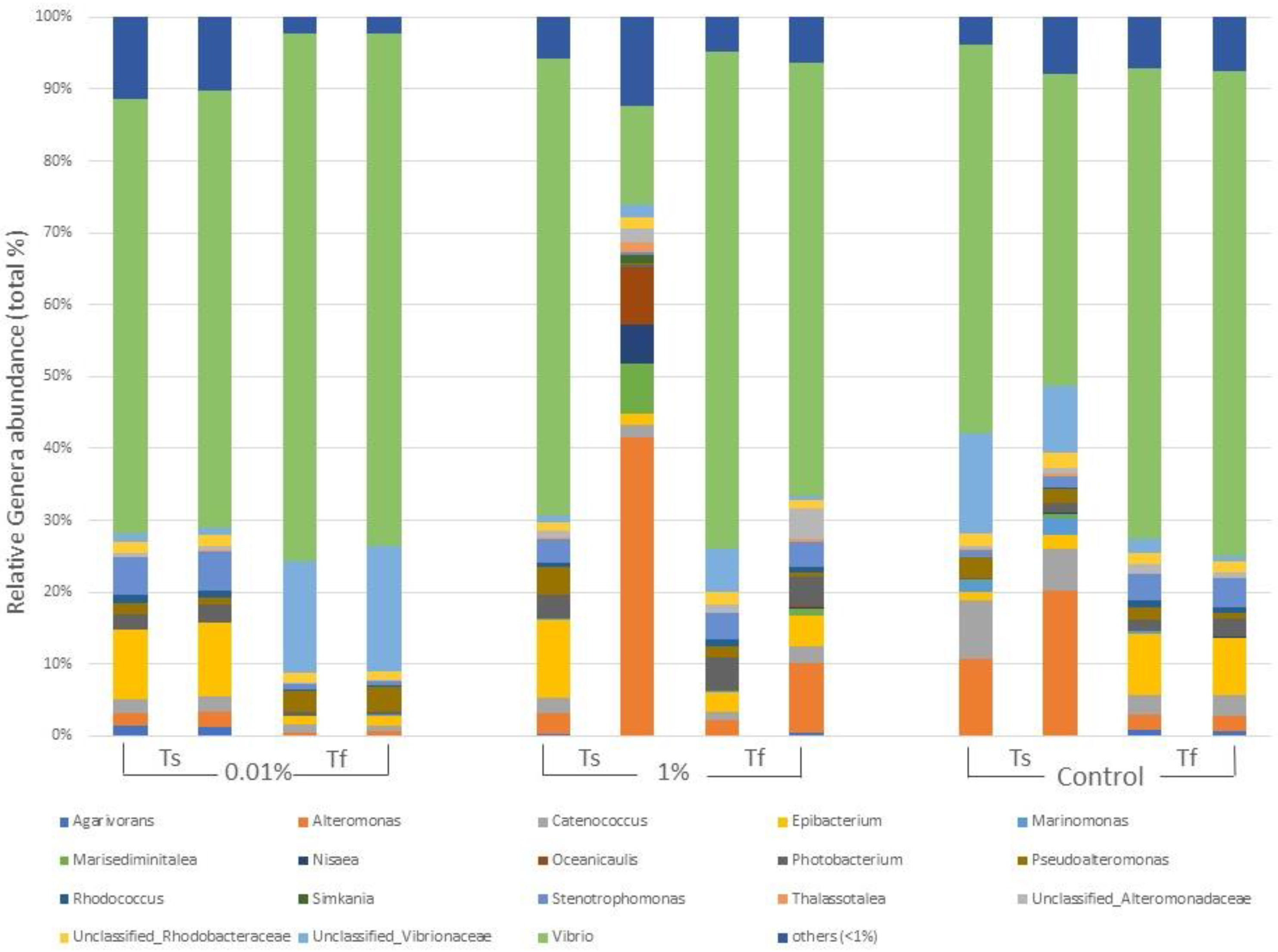
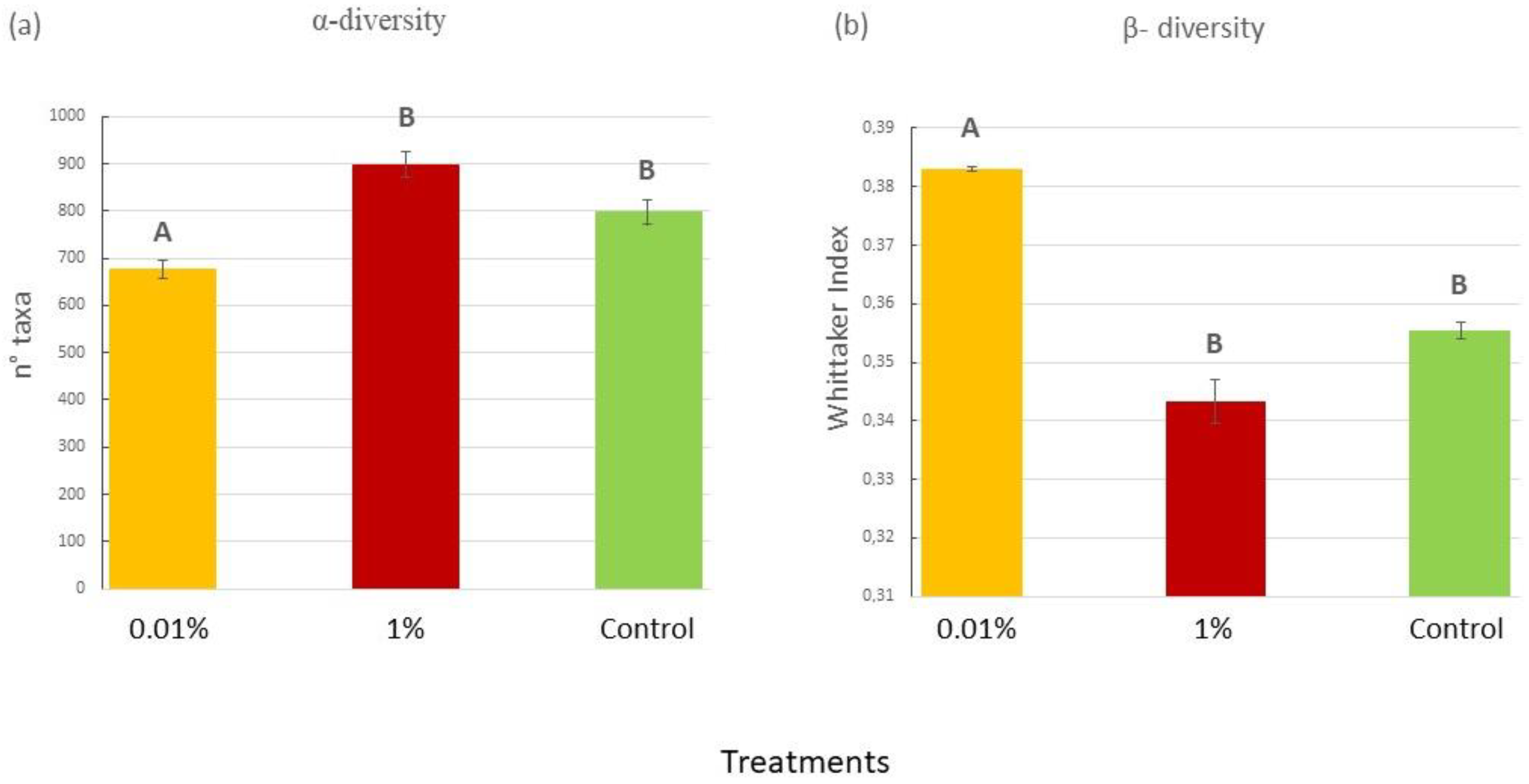
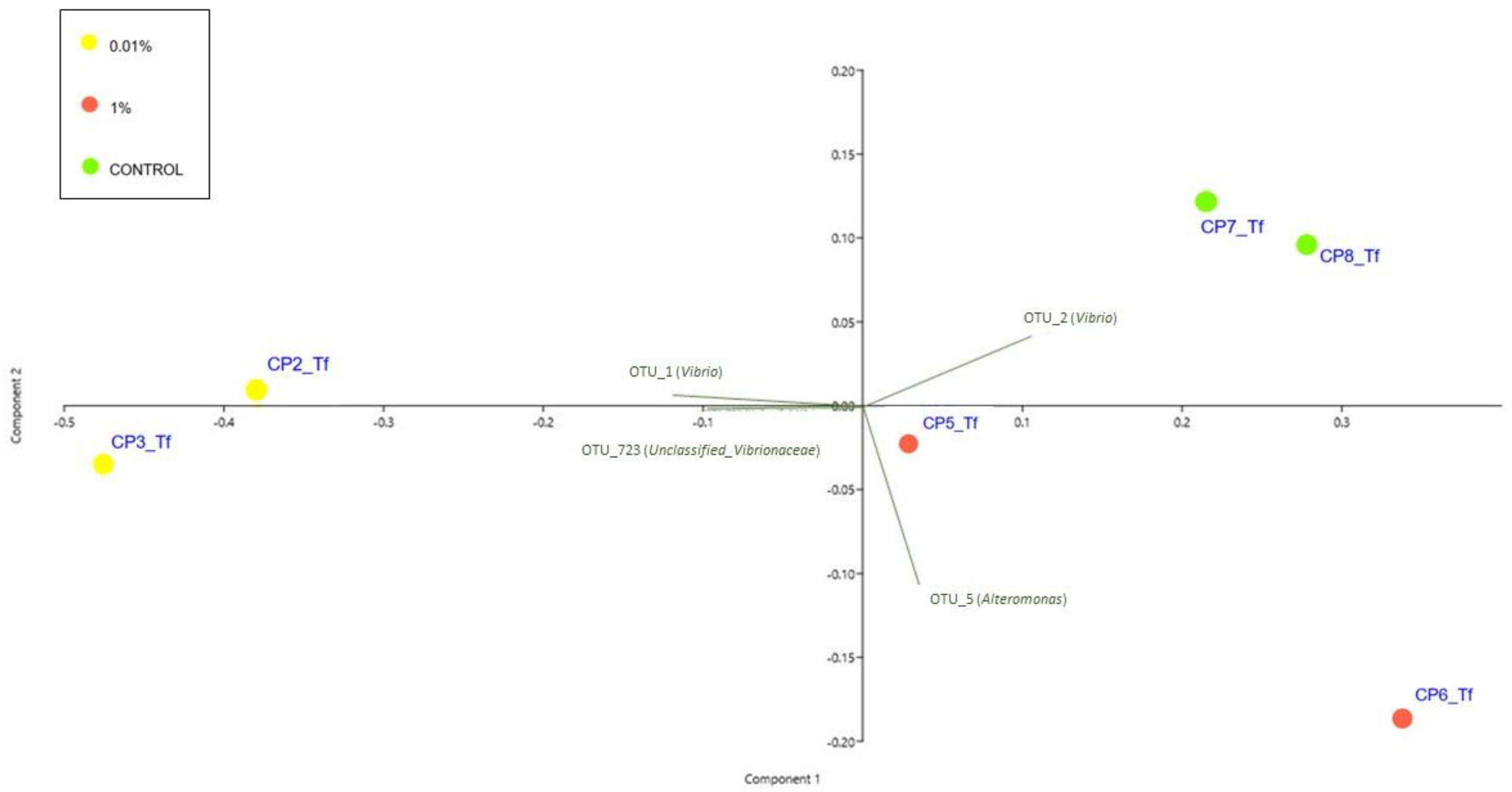
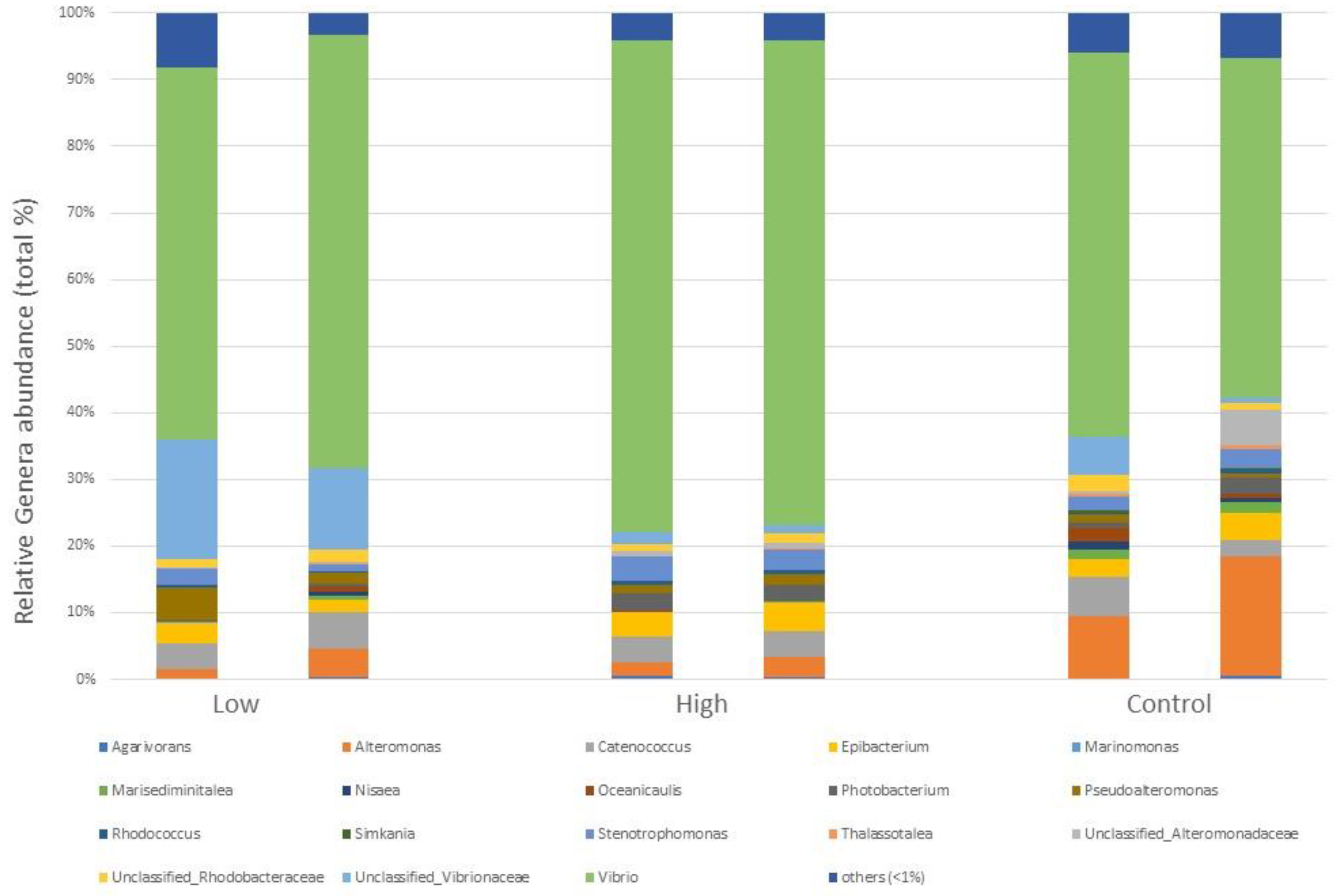
2.1.3. Characterization of Bacterial Communities
2.1.4. Water Diesel-Derived Hydrocarbon Degradation
2.2. Natural Exposure of C. prolifera to Diesel Fuel in Marine Environments
2.2.1. Bacterial Abundance
2.2.2. Characterization of Bacterial Communities
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. C. prolifera Functional Traits
4.3. Algae-Associated Bacteria Counts
4.4. Bacterial Analysis Using High-Throughput Sequencing
4.5. Gas-Chromatography Analysis of Water Diesel-Derived Hydrocarbon Concentration and Biodegradation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stepanyan, O.V.; Voskoboinikov, G.M. Effect of oil and oil products on morphofunctional parameters of marine macrophytes. Russ. J. Mar. Biol. 2006, 32, S32–S39. [Google Scholar] [CrossRef]
- Mearns, A.J.; Reish, D.J.; Oshida, P.S.; Ginn, T.; Rempel-Hester, M.A.; Arthur, C.; Rutherford, N. Effects of pollution on marine organisms. Water Environ. Res. 2013, 85, 1206–1300. [Google Scholar] [CrossRef]
- Shakhmatova, O.A.; Milchakova, N.A. Effect of environmental conditions on Black Sea macroalgae catalase activity. Int. J. Algae 2014, 16, 377–391. [Google Scholar] [CrossRef]
- Pilatti, F.K.; Ramlov, F.; Schmidt, E.C.; Kreusch, M.; Pereira, D.T.; Costa, C.; Oliveira, E.R.D.; Bauer, C.M.; Rocha, M.; Bouzon, Z.L.; et al. In vitro exposure of Ulva lactuca Linnaeus (Chlorophyta) to gasoline—Biochemical and morphological alterations. Chemosphere 2016, 156, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Ryzhik, I.; Pugovkin, D.; Makarov, M.; Roleda, M.Y.; Basova, L.; Voskoboynikov, G. Tolerance of Fucus vesiculosus exposed to diesel water-accommodated fraction (WAF) and degradation of hydrocarbons by the associated bacteria. Environ. Pollut. 2019, 254, 113072. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Yu, H. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef]
- Nikolopoulou, M.; Kalogerakis, N. Enhanced bioremediation of crude oil utilizing lipophilic fertilizers combined with biosurfactants and molasses. Mar. Pollut. Bull. 2008, 56, 1855–1861. [Google Scholar] [CrossRef]
- Turner, N.R.; Renegar, D.A. Petroleum hydrocarbon toxicity to corals: A review. Mar. Pollut. Bull. 2017, 119, 1–16. [Google Scholar] [CrossRef]
- Rivero, S.M.; Elías, R.; Vallariona, E.A. First survey of macroinfauna in the Mar del Plata Harbour (Argentina), and the use of polychaetes as pollution indicators. Rev. De. Biol. Mar. Y Oceanogr. 2005, 40, 101–108. [Google Scholar]
- Ingole, B.; Sivadas, S.; Nanajkar, M.; Sautya, S.; Nag, A. A comparative study of macrobenthic community from harbours along the central west coast of India. Environ. Monit. Assess. 2009, 154, 135–146. [Google Scholar] [CrossRef]
- Loya, Y.; Rinkevich, B. Effects of oil pollution on coral reef communities. Mar. Ecol. Prog. Ser. 1980, 3, 180. [Google Scholar] [CrossRef]
- Sarıkoç, S. Fuels of the diesel-gasoline engines and their properties. Diesel Gasol. Engines 2020, 31, 89904. [Google Scholar]
- Lominchar, M.A.; Santos, A.; De Miguel, E.; Romero, A. Remediation of aged diesel contaminated soil by alkaline activated persulfate. Sci. Total Environ. 2018, 622, 41–48. [Google Scholar] [CrossRef]
- Monteiro, L.; Traunspurger, W.; Roeleveld, K.; Lynen, F.; Moens, T. Direct toxicity of the water-soluble fractions of a crude and a diesel-motor oil on the survival of free-living nematodes. Ecol. Indic. 2018, 93, 13–23. [Google Scholar] [CrossRef]
- Khalid, F.E.; Lim, Z.S.; Sabri, S.; Gomez-Fuentes, C.; Zulkharnain, A.; Ahmad, S.A. Bioremediation of diesel contaminated marine water by bacteria: A review and bibliometric analysis. J. Mar. Sci. Eng. 2021, 9, 155. [Google Scholar] [CrossRef]
- Ramadass, K.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Toxicity of diesel water accommodated fraction toward microalgae, Pseudokirchneriella subcapitata and Chlorella sp. MM3. Ecotoxicol. Environ. Saf. 2017, 142, 538–543. [Google Scholar] [CrossRef]
- Rice, S.D.; Short, J.W.; Karinen, J.F. Comparative oil toxicity and comparative animal sensitivity. In Proceedings of the Fate and Effects of Petroleum Hydrocarbons in Marine Ecosystems and Organisms: Proceedings of a Symposium, Olympic Hotel, Seattle, WA, USA, 10–12 November 1976. [Google Scholar]
- Coull, B.C.; Chandler, G.T. Pollution and meiofauna: Field, laboratory and mesocosm studies. Oceanogr. Mar. Biol. Annu. Rev. 1992, 30, 191–271. [Google Scholar]
- Pezeshki, S.R.; Hester, M.W.; Lin, Q.; Nyman, J.A. The effects of oil spill and clean-up on dominant US Gulf coast marsh macrophytes: A review. Environ. Pollut. 2000, 108, 129–139. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Li, N.; Lou, Y.; Zhao, X. Effect of oil spill stress on fatty acid stable carbon isotope composition of Ulva pertusa. Sci. Total Environ. 2019, 649, 1443–1451. [Google Scholar] [CrossRef]
- Endeshaw, A.; Birhanu, G.; Zerihun, T.; Misganaw, W. Application of microorganisms in bioremediation-review. J. Environ. Microb. 2017, 1, 2–9. [Google Scholar]
- Prince, R.C. Petroleum spill bioremediation in marine environments. Crit. Rev. Microbiol. 1993, 19, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M. Microbial hydrocarbon degradation—Bioremediation of oil spills. J. Chem. Technol. Biotechnol. 1991, 52, 149–156. [Google Scholar] [CrossRef]
- Banat, I.M. Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: A review. Bioresour. Technol. 1995, 51, 1–12. [Google Scholar] [CrossRef]
- Prince, R.C.; Lessard, R.R.; Clark, J.R. Bioremediation of marine oil spills. Oil Sci. Technol. 2003, 58, 463–468. [Google Scholar] [CrossRef]
- Adebusoye, S.A.; Ilori, M.O.; Amund, O.O.; Teniola, O.D.; Olatope, S.O. Microbial degradation of petroleum hydrocarbons in a polluted tropical stream. World J. Microbiol. Biotechnol. 2007, 23, 1149–1159. [Google Scholar] [CrossRef]
- Liu, W.; Luo, Y.; Teng, Y.; Li, Z.; Ma, L.Q. Bioremediation of oily sludge contaminated soil by stimulating indigenous microbes. Environ. Geochem. Health 2010, 32, 23–29. [Google Scholar] [CrossRef]
- Dave, D.A.E.G.; Ghaly, A.E. Remediation technologies for marine oil spills: A critical review and comparative analysis. Am. J. Environ. Sci. 2011, 7, 423–440. [Google Scholar] [CrossRef]
- Vergeynst, L.; Wegeberg, S.; Aamand, J.; Lassen, P.; Gosewinkel, U.; Fritt-Rasmussen, J.; Gustavson, K.; Mosbech, A. Biodegradation of marine oil spills in the Arctic with a Greenland perspective. Sci. Total Environ. 2018, 626, 1243–1258. [Google Scholar] [CrossRef]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Korda, A.P.A.R.; Santas, P.; Tenente, A.; Santas, R. Petroleum hydrocarbon bioremediation: Sampling and analytical techniques, in situ treatments and commercial microorganisms currently used. Appl. Microbiol. Biotechnol. 1997, 48, 677–686. [Google Scholar] [CrossRef]
- Pandey, B.; Fulekar, M.H. Bioremediation technology: A new horizon for environmental clean-up. Biol. Med. 2012, 4, 51. [Google Scholar]
- Dindar, E.; Şağban, F.O.T.; Başkaya, H.S. Bioremediation of petroleum-contaminated soil. J. Biol. Env. Sci. 2013, 7, 39–47. [Google Scholar]
- Smitha, M.S.; Singh, S.; Singh, R. Microbial biotransformation: A process for chemical alterations. J. Bacteriol. Mycol. Open Access. 2017, 4, 85. [Google Scholar]
- McGuinness, M.; Dowling, D. Plant-associated bacterial degradation of toxic organic compounds in soil. Int. J. Environ. Res. Publ. Health 2009, 6, 2226–2247. [Google Scholar] [CrossRef]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Kuffner, M.; Sessitsch, A. Soil type affects plant colonization, activity and catabolic gene expression of inoculated bacterial strains during phytoremediation of diesel. J. Hazard. Mater. 2011, 186, 1568–1575. [Google Scholar] [CrossRef]
- Teng, Y.; Shen, Y.; Luo, Y.; Sun, X.; Sun, M.; Fu, D.; Li, Z.; Christie, P. Influence of Rhizobium meliloti on phytoremediation of polycyclic aromatic hydrocarbons by alfalfa in an aged contaminated soil. J. Hazard. Mater. 2011, 186, 1271–1276. [Google Scholar] [CrossRef]
- Yousaf, S.; Afzal, M.; Reichenauer, T.G.; Brady, C.L.; Sessitsch, A. Hydrocarbon degradation, plant colonization and gene expression of alkane degradation genes by endophytic Enterobacter ludwigii strains. Environ. Pollut. 2011, 159, 2675–2683. [Google Scholar] [CrossRef]
- Khan, S.; Afzal, M.; Iqbal, S.; Khan, Q.M. Plant–bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 2013, 90, 1317–1332. [Google Scholar] [CrossRef]
- Dhote, M.; Kumar, A.; Jajoo, A.; Juwarkar, A. Assessment of hydrocarbon degradation potentials in a plant–microbe interaction system with oil sludge contamination: A sustainable solution. Int. J. Phytoremediation 2017, 19, 1085–1092. [Google Scholar] [CrossRef]
- Escalante-Espinosa, E.; Gallegos-Martínez, M.E.; Favela-Torres, E.; Gutiérrez-Rojas, M. Improvement of the hydrocarbon phytoremediation rate by Cyperus laxus Lam. inoculated with a microbial consortium in a model system. Chemosphere 2005, 59, 405–413. [Google Scholar] [CrossRef]
- Alarcón, A.; Davies, F.T., Jr.; Autenrieth, R.L., Jr.; Zuberer, D.A. Arbuscular mycorrhiza and petroleum-degrading microorganisms enhance phytoremediation of petroleum-contaminated soil. Int. J. Phytorem. 2008, 10, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Robert, F.M.; Sun, W.H.; Toma, M.; Jones, R.K.; Tang, C.-S. Interactions among buffelgrass, phenanthrene and phenanthrene-degrading bacteria in gnotobiotic microcosms. J. Environ. Sci. Health 2008, 43, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Golubev, S.N.; Muratova, A.Y.; Wittenmayer, L.; Bondarenkova, A.D.; Hirche, F.; Matora, L.Y.; Merbach, W.; Turkovskaya, O.V. Rhizosphere indole-3-acetic acid as a mediator in the Sorghum bicolor–phenanthrene–Sinorhizobium meliloti interactions. Plant Physiol. Biochem. 2011, 49, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Sessitsch, A. The inoculation method affects colonization and performance of bacterial inoculant strains in the phytoremediation of soil contaminated with diesel oil. Int. J. Phytorem. 2012, 14, 35–47. [Google Scholar] [CrossRef]
- Phang, S.M.; Chu, W.L.; Rabiei, R. Phycoremediation. Algae World 2015, 26, 357–389. [Google Scholar]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef]
- Voskoboinikov, G.M.; Matishov, G.G.; Metelkova, L.O.; Zhakovskaya, Z.A.; Lopushanskaya, E.M. Participation of the green algae Ulvaria obscura in bioremediation of sea water from oil products. Dokl. Biol. Sci. 2018, 481, 139–141. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y.; Liu, H.; Liu, S.; Qin, Y.; Li, P. Advances in cultivation, wastewater treatment application, bioactive components of Caulerpa lentillifera and their biotechnological applications. PeerJ 2019, 7, e6118. [Google Scholar] [CrossRef]
- Arimoto, A.; Nishitsuji, K.; Higa, Y.; Arakaki, N.; Hisata, K.; Shinzato, C.; Shoguchi, E. A siphonous macroalgal genome suggests convergent functions of homeobox genes in algae and land plants. Dna Res. 2019, 26, 183–192. [Google Scholar] [CrossRef]
- Pietroletti, M.; Capobianchi, A.; Ragosta, E.; Mecozzi, M. Preliminary evaluation of hydrocarbon removal power of Caulerpa racemosa in seawater by means of infrared and visible spectroscopic measurements. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 673–679. [Google Scholar] [CrossRef]
- Bambaranda, B.M.; Tsusaka, T.W.; Chirapart, A.; Salin, K.R.; Sasaki, N. Capacity of Caulerpa lentillifera in the removal of fish culture effluent in a recirculating aquaculture system. Processes 2019, 7, 440. [Google Scholar] [CrossRef]
- Meinesz, A. Répartition de Caulerpa prolifera (Forskal) Lamouroux sur les côtes continentales françaises de la Méditerranée. Tethys 1973, 4, 843–858. [Google Scholar]
- Ganteaume, A.; Bonhomme, P.; Emery, E.; Hervé, G.; Boudouresque, C.F. Impact sur la prairie à Posidonia oceanica de l’amarrage des bateaux de croisière, au large du port de Porquerolles (Provence, France, Méditerranée). Sci. Rep. Port-Cros Natl. Park. 2005, 21, 163–173. [Google Scholar]
- Antrim, L.D.; Thom, R.M.; Gardiner, W.W.; Cullinan, V.I.; Shreffler, D.K.; Bienert, R.W. Effects of petroleum products on bull kelp (Nereocystis luetkeana). Mar. Biol. 1995, 122, 23–31. [Google Scholar] [CrossRef]
- Spivak, A.C.; Vanni, M.J.; Mette, E.M. Moving on up: Can results from simple aquatic mesocosm experiments be applied across broad spatial scales? Freshw. Biol. 2011, 56, 279–291. [Google Scholar] [CrossRef]
- Ryzhik, I.V.; Makarov, M.V. Effect of diesel fuel film on green algae Ulva lactuca L. and Ulvaria obscura (Kützing) Gayral ex Bliding of the Barents Sea. Proc. IOP Conf. Ser. Earth Environ. Sci. 2019, 302, 012029. [Google Scholar]
- Ryzhik, I.V.; Kosobryukhov, A.A.; Markovskaya, E.F.; Makarov, M.V. Physiological Parameters of Fucus vesiculosus and Fucus serratus in the Barents Sea during a Tidal Cycle. Complex. Biol. Syst. Adapt. Toler. Extrem. Environ. 2018, 48, 48–56. [Google Scholar]
- Beer, S.; Israel, A. Photosynthesis of Ulva sp: III. O2 effects, carboxylase activities, and the CO2 incorporation pattern. Plant Physiol. 1986, 81, 937–938. [Google Scholar] [CrossRef]
- Kottuparambil, S.; Park, J. Anthracene phytotoxicity in the freshwater flagellate alga Euglena agilis Carter. Sci. Rep. 2019, 9, 15323. [Google Scholar] [CrossRef]
- Schramm, W. Effects of oil pollution on gas exchange in Prophyra umbilicalis when exposed to air. In Proceedings of the 7th International Symposium on Seaweed Research, Sapporo, Japan, 8–12 August 1971. [Google Scholar]
- Geiselbrecht, A.D.; Herwig, R.P.; Deming, J.W.; Staley, J.T. Enumeration and phylogenetic analysis of polycyclic aromatic hydrocarbon-degrading marine bacteria from Puget Sound sediments. Appl. Environ. Microbiol. 1996, 62, 3344–3349. [Google Scholar] [CrossRef]
- Haines, J.R.; Wrenn, B.A.; Holder, E.L.; Strohmeier, K.L.; Herrington, R.T.; Venosa, A.D. Measurement of hydrocarbon-degrading microbial populations by a 96-well plate most-probable-number procedure. J. Ind. Microbiol. 1996, 16, 36–41. [Google Scholar] [CrossRef]
- Michaud, L.; Di Marco, G.; Bruni, V.; Giudice, A.L. Biodegradative potential and characterization of psychrotolerant polychlorinated biphenyl-degrading marine bacteria isolated from a coastal station in the Terra Nova Bay (Ross Sea, Antarctica). Mar. Pollut. Bull. 2007, 54, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.L.; Pant, A. Crude oil degradation by a marine actinomycete Rhodococcus sp. Indian. J. Geo-Mar. Sci. 2001, 30, 146–150. [Google Scholar]
- Hedlund, B.P.; Staley, J.T. Vibrio cyclotrophicus sp. nov., a polycyclic aromatic hydrocarbon (PAH)-degrading marine bacterium. Int. J. Syst. Evol. Microbiol. 2001, 51, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Leahy, J.G.; Colwell, R.R. Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 1990, 54, 305–315. [Google Scholar] [CrossRef]
- Bicca, F.C.; Fleck, L.C.; Ayub, M.A.Z. Production of biosurfactant by hydrocarbon degrading Rhodococcus ruber and Rhodococcus erythropolis. Rev. De Microbiol. 1999, 30, 231–236. [Google Scholar] [CrossRef]
- Semenov, A.M.; Fedorenko, V.N.; Semenova, E.V. Microorganisms on the surfaces of marine macrophytes in the northern seas of Russia and prospects for their practical application. Biosphere 2014, 6, 60–76. [Google Scholar]
- Díaz, M.P.; Boyd, K.G.; Grigson, S.J.; Burgess, J.G. Biodegradation of crude oil across a wide range of salinities by an extremely halotolerant bacterial consortium MPD-M, immobilized onto polypropylene fibers. Biotechnol. Bioeng. 2002, 79, 145–153. [Google Scholar] [CrossRef]
- Rosenberg, E. Hydrocarbon-oxidizing bacteria. Prokaryotes A Handb. Biol. Bact. 2006, 2, 564–577. [Google Scholar]
- Ryzhik, I.V.; Pugovkin, D.V.; Salakhov, D.O.; Klindukh, M.P.; Voskoboynikov, G.M. Physiological changes and rate of resistance of Acrosiphonia arcta (Dillwyn) Gain upon exposure to diesel fuel. Heliyon 2022, 8, e10177. [Google Scholar] [CrossRef]
- Voskoboinikov, G.; Pugovkin, D.; Metelkova, L. The experimental research of sorption and destruction of diesel fuel by fucus algae of the Barents Sea. IOP Conf. Ser. Earth Environ. Sci. 2021, 937, 022058. [Google Scholar] [CrossRef]
- Fernandes, N.; Steinberg, P.; Rusch, D.; Kjelleberg, S.; Thomas, T. Community structure and functional gene profile of bacteria on healthy and diseased thalli of the red seaweed Delisea pulchra. PLoS ONE 2012, 7, e50854. [Google Scholar] [CrossRef]
- Campbell, B.J.; Yu, L.; Heidelberg, J.F.; Kirchman, D.L. Activity of abundant and rare bacteria in a coastal ocean. Proc. Natl. Acad. Sci. USA 2011, 108, 12776–12781. [Google Scholar] [CrossRef]
- Johnson, C.N. Fitness factors in vibrios: A mini-review. Microb. Ecol. 2013, 65, 826–851. [Google Scholar] [CrossRef]
- Graziano, M.; Rizzo, C.; Michaud, L.; Porporato, E.M.D.; De Domenico, E.; Spanò, N.; Lo Giudice, A. Biosurfactant production by hydrocarbon-degrading Brevibacterium and Vibrio isolates from the sea pen Pteroeides spinosum (Ellis, 1764). J. Basic. Microbiol. 2016, 56, 963–974. [Google Scholar] [CrossRef]
- Imron, M.F.; Kurniawan, S.B.; Titah, H.S. Potential of bacteria isolated from diesel-contaminated seawater in diesel biodegradation. Environ. Technol. Innov. 2019, 14, 100368. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, L.; Li, K.; Chen, C.; Lin, X.; Zhang, C.; Xie, Q. Enhanced bioremediation of diesel oil-contaminated seawater by a biochar-immobilized biosurfactant-producing bacteria Vibrio sp. LQ2 isolated from cold seep sediment. Sci. Total Environ. 2021, 793, 148529. [Google Scholar] [CrossRef]
- Rizzo, L.; Fraschetti, S.; Alifano, P.; Tredici, M.S.; Stabili, L. Association of Vibrio community with the Atlantic Mediterranean invasive alga Caulerpa cylindracea. J. Exp. Mar. Biol. Ecol. 2016, 475, 129–136. [Google Scholar] [CrossRef]
- Rizzo, L.; Pusceddu, A.; Stabili, L.; Alifano, P.; Fraschetti, S. Potential effects of an invasive seaweed (Caulerpa cylindracea, Sonder) on sedimentary organic matter and microbial metabolic activities. Sci. Rep. 2017, 7, 12113. [Google Scholar] [CrossRef]
- Stabili, L.; Rizzo, L.; Pizzolante, G.; Alifano, P.; Fraschetti, S. Spatial distribution of the culturable bacterial community associated with the invasive alga Caulerpa cylindracea in the Mediterranean Sea. Mar. Environ. Res. 2017, 125, 90–98. [Google Scholar] [CrossRef]
- Tahiluddin, A.; Daganio, J.; Lodovice, R.; Umpay, M.J. Abundance of heterotrophic marine bacteria, Vibrio, and marine fungi in green seaweed Caulerpa racemosa in Sibutu, Tawi-Tawi, Philippines. Sustain. Aquat. Res. 2022, 1, 56–62. [Google Scholar]
- Stabili, L.; Acquaviva, M.I.; Cavallo, R.A. Mytilus galloprovincialis filter feeding on the bacterial community in a Mediterranean coastal area (Northern Ionian Sea, Italy). Water Res. 2005, 39, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Caronni, S.; Delaria, M.A.; Gentili, R.; Montagnani, C.; Navone, A.; Panzalis, P.; Citterio, S. First Report of Gametogenesis and Spawning for the Invasive Alga Caulerpa cylindracea in the Tyrrhenian Sea: The Key Role of Water Motion and Temperature. Front. Mar. Sci. 2021, 8, 1724. [Google Scholar] [CrossRef]
- Bennett, S.; Wernberg, T.; Harvey, E.S.; Santana-Garcon, J.; Saunders, B.J. Tropical herbivores provide resilience to a climate-mediated phase shift on temperate reefs. Ecol. Lett. 2015, 18, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.M.; Aurand, D.; Bragin, G.E.; Clark, J.R.; Coelho, G.M.; Sowby, M.L.; Tjeerdema, R.S. Standardization of the preparation and quantitation of water-accommodated fractions of petroleum for toxicity testing. Mar. Pollut. Bull. 2000, 40, 1007–1016. [Google Scholar] [CrossRef]
- Jensen, P.R.; Kauffman, C.A.; Fenical, W. High recovery of culturable bacteria from the surfaces of marine algae. Mar. Biol. 1996, 126, 1–7. [Google Scholar] [CrossRef]
- Sgorbati, S.; Barbesti, S.; Citterio, S.; Bestetti, G.; DeVecchi, R. Characterization of number, DNA content, viability and cell size of bacteria from natural environments using DAPI/PI dual staining and flow cytometry. Minerva Biotecnol. 1996, 8, 9–15. [Google Scholar]
- Pittino, F.; Maglio, M.; Gandolfi, I.; Azzoni, R.S.; Diolaiuti, G.; Ambrosini, R.; Franzetti, A. Bacterial communities of cryoconite holes of a temperate alpine glacier show both seasonal trends and year-to-year variability. Ann. Glaciol. 2018, 59, 1–9. [Google Scholar] [CrossRef]
- Huber, J.A.; Mark Welch, D.B.; Morrison, H.G.; Huse, S.M.; Neal, P.R.; Butterfield, D.A.; Sogin, M.L. Microbial population structures in the deep marine biosphere. Science 2007, 318, 97–100. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, P.Y. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS ONE 2009, 4, e7401. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed; Elsevier Science B.V.: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Chebbi, A.; Hentati, D.; Zaghden, H.; Baccar, N.; Rezgui, F.; Chalbi, M.; Chamkha, M. Polycyclic aromatic hydrocarbon degradation and biosurfactant production by a newly isolated Pseudomonas sp. strain from used motor oil-contaminated soil. Int. Biodeterior. Biodegrad. 2017, 122, 128–140. [Google Scholar] [CrossRef]
- Underwood, A. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Gerald, B. A brief review of independent, dependent and one sample t-test. Int. J. Appl. Math. Theor. Phys. 2018, 4, 50–54. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 2001, 58, 626–639. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caronni, S.; Quaglini, L.A.; Franzetti, A.; Gentili, R.; Montagnani, C.; Citterio, S. Does Caulerpa prolifera with Its Bacterial Coating Represent a Promising Association for Seawater Phytoremediation of Diesel Hydrocarbons? Plants 2023, 12, 2507. https://doi.org/10.3390/plants12132507
Caronni S, Quaglini LA, Franzetti A, Gentili R, Montagnani C, Citterio S. Does Caulerpa prolifera with Its Bacterial Coating Represent a Promising Association for Seawater Phytoremediation of Diesel Hydrocarbons? Plants. 2023; 12(13):2507. https://doi.org/10.3390/plants12132507
Chicago/Turabian StyleCaronni, Sarah, Lara A. Quaglini, Andrea Franzetti, Rodolfo Gentili, Chiara Montagnani, and Sandra Citterio. 2023. "Does Caulerpa prolifera with Its Bacterial Coating Represent a Promising Association for Seawater Phytoremediation of Diesel Hydrocarbons?" Plants 12, no. 13: 2507. https://doi.org/10.3390/plants12132507
APA StyleCaronni, S., Quaglini, L. A., Franzetti, A., Gentili, R., Montagnani, C., & Citterio, S. (2023). Does Caulerpa prolifera with Its Bacterial Coating Represent a Promising Association for Seawater Phytoremediation of Diesel Hydrocarbons? Plants, 12(13), 2507. https://doi.org/10.3390/plants12132507







