Temporal Changes of Leaf Spectral Properties and Rapid Chlorophyll—A Fluorescence under Natural Cold Stress in Rice Seedlings
Abstract
1. Introduction
2. Results
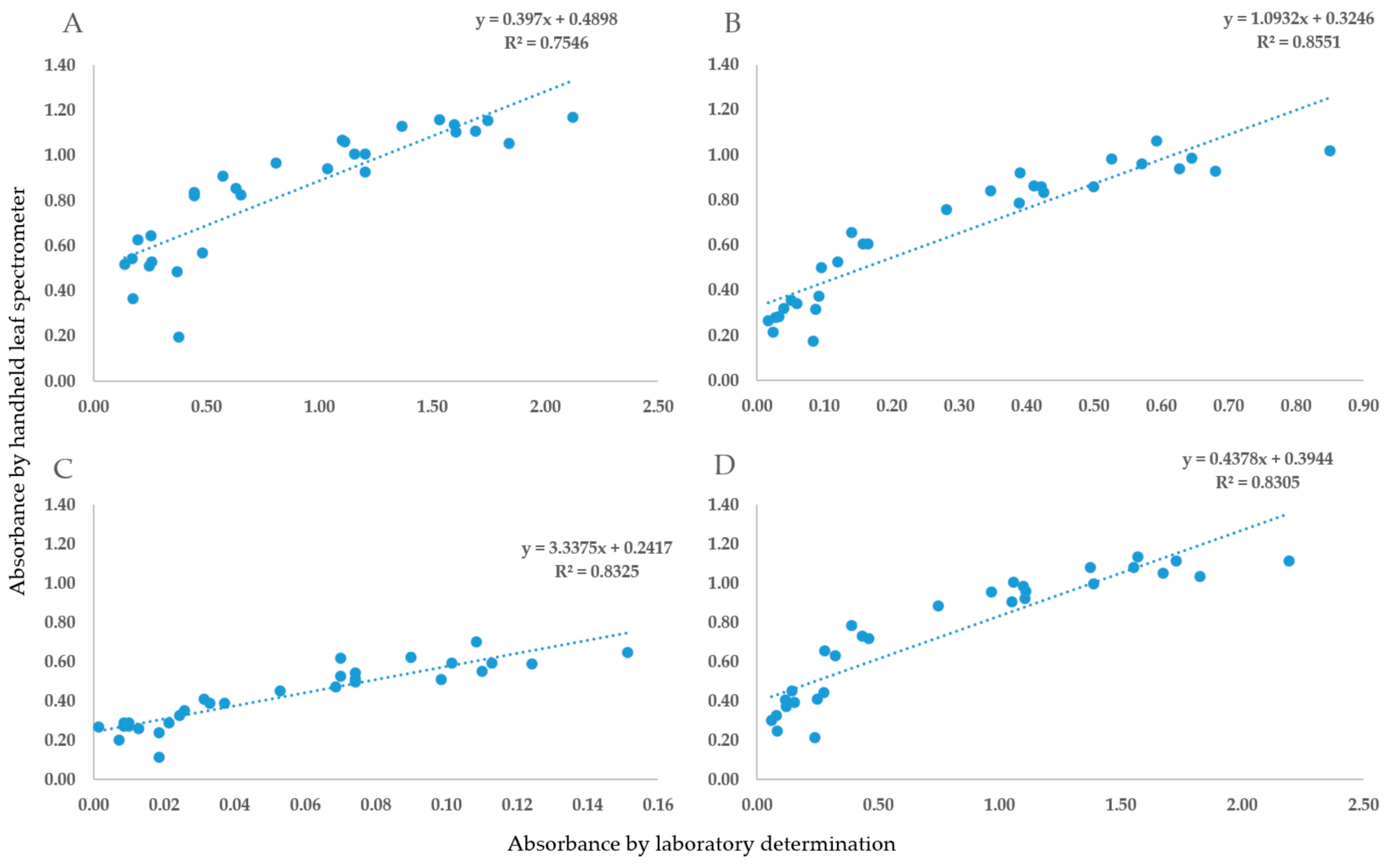
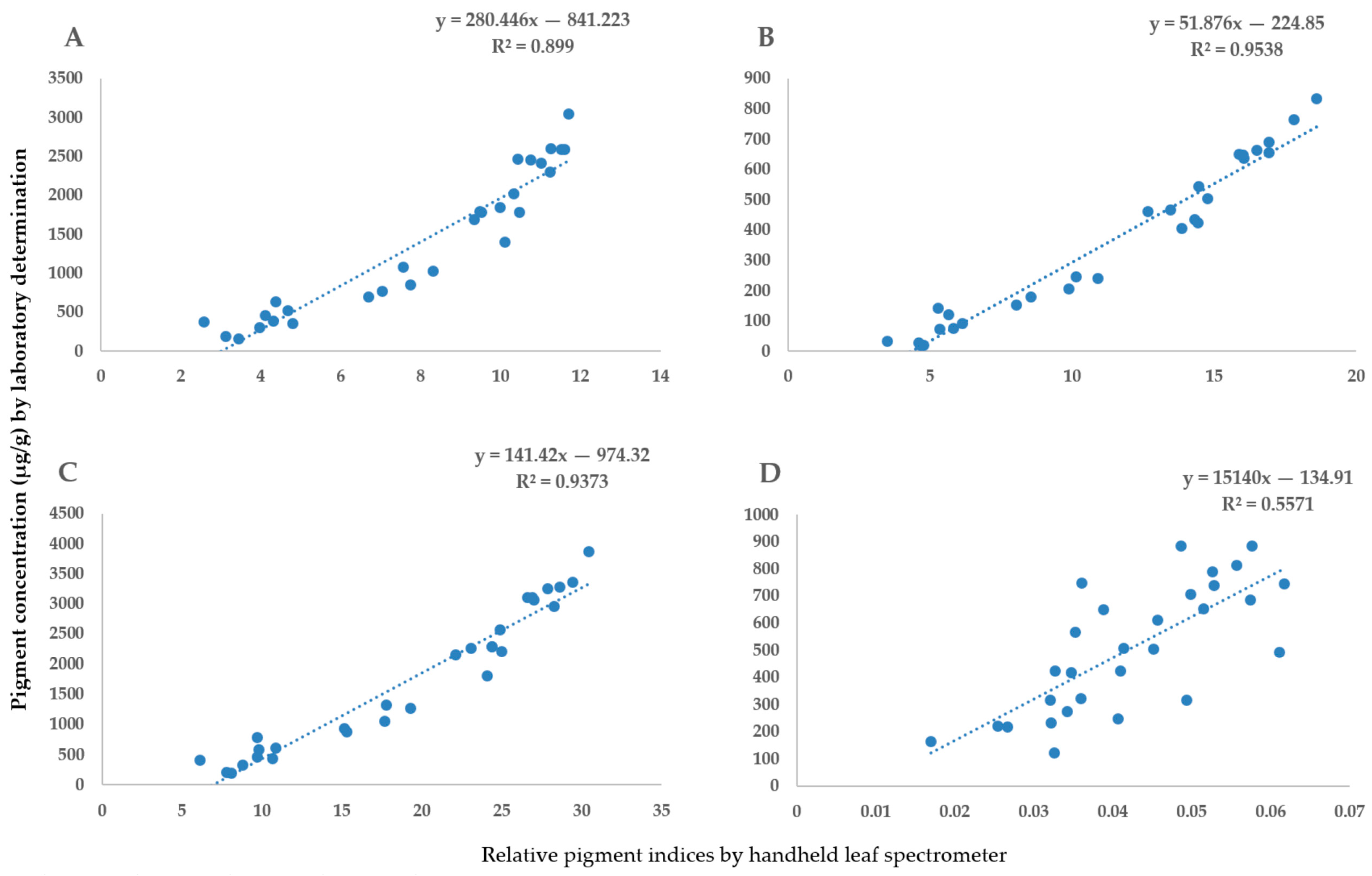
2.1. The Validation of Handheld Spectrometer Data
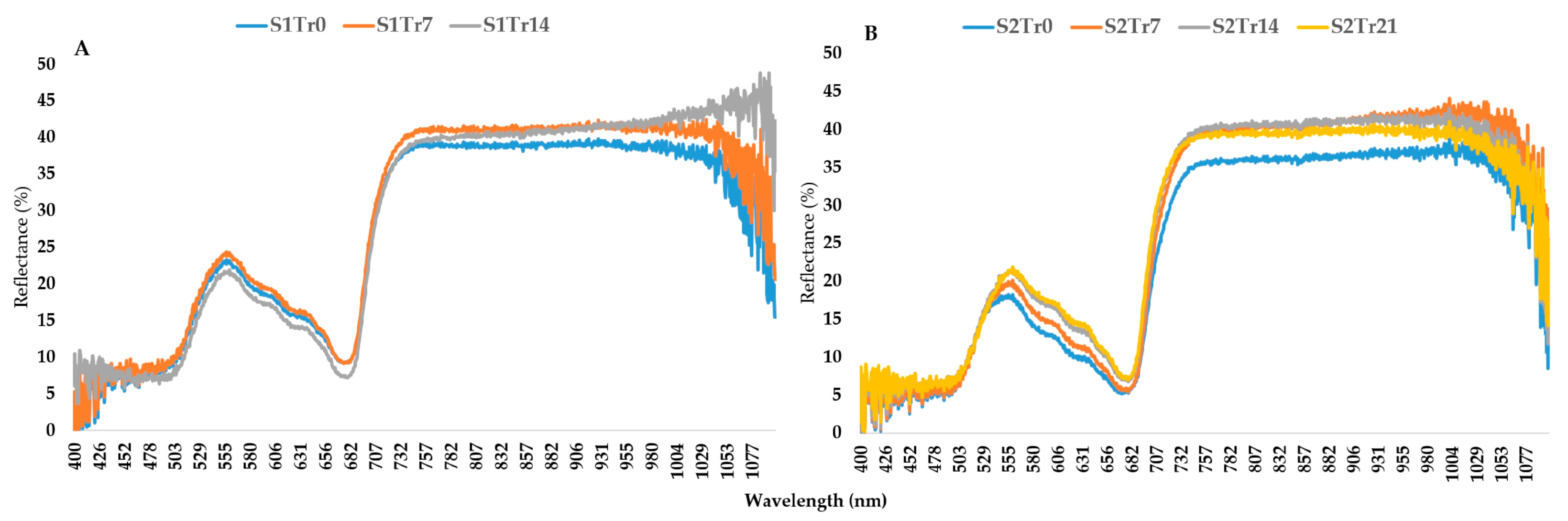
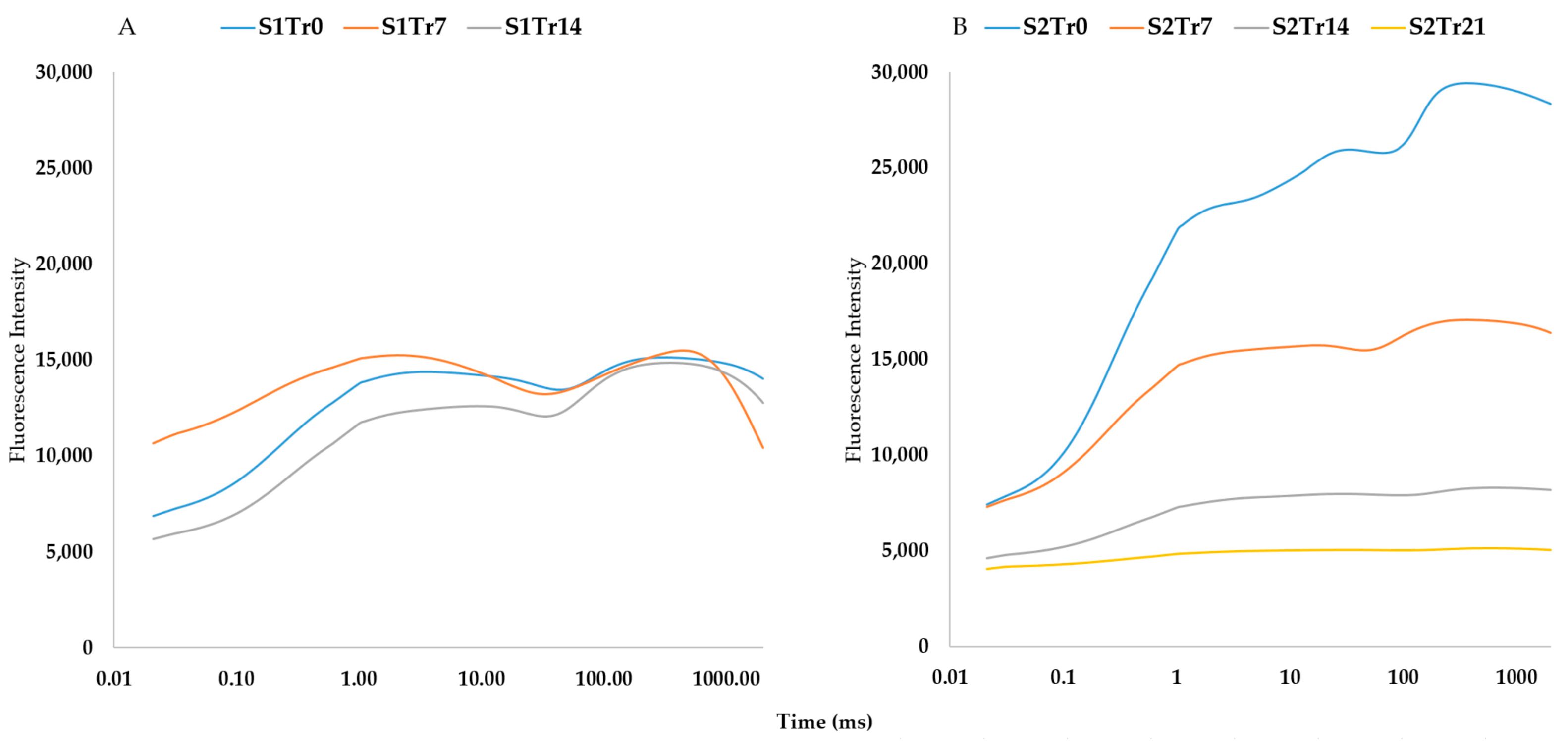
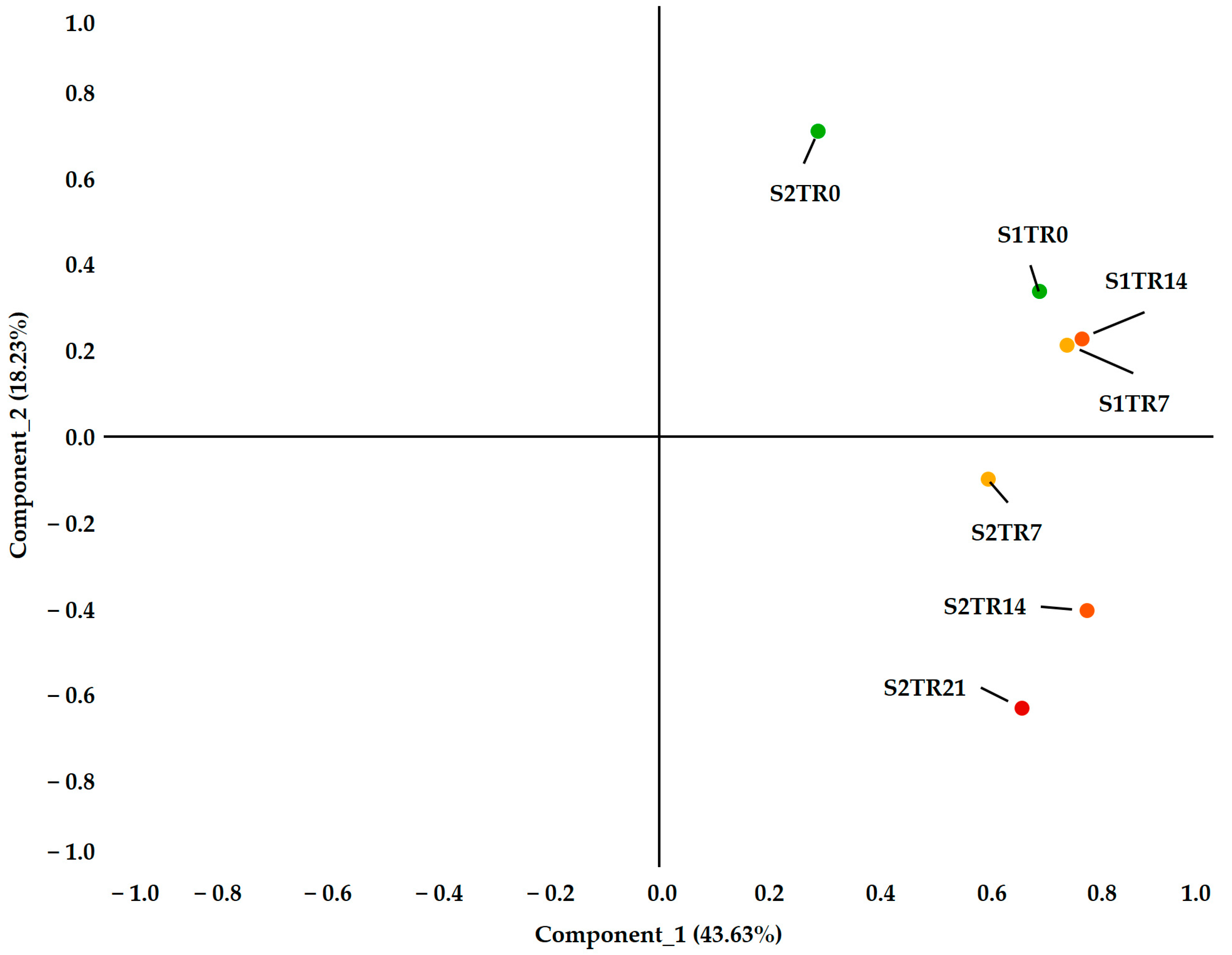
2.2. The Characteristics of the Seasonal Variation and Treatments
2.3. The Wavelengths That Are Affected by Low Temperatures
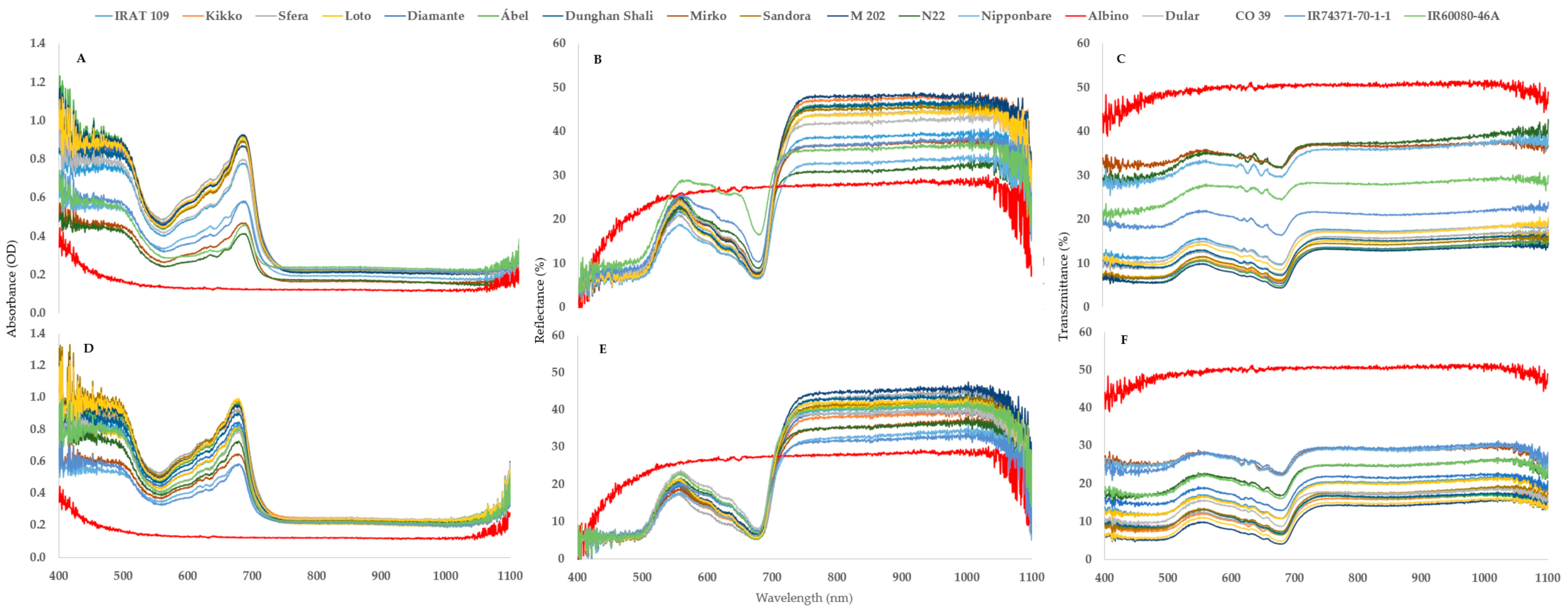
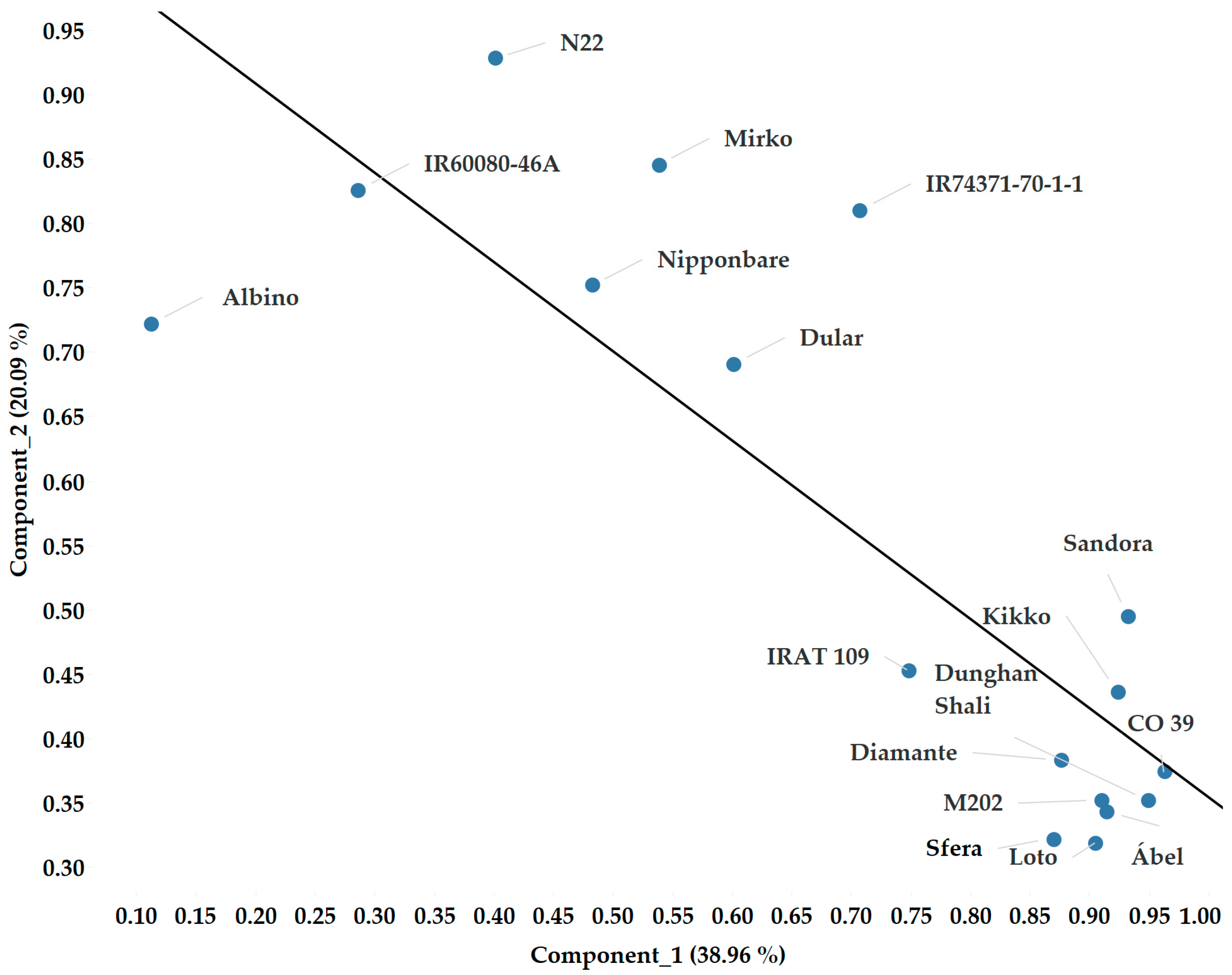
2.4. The Effects of the Genotypes
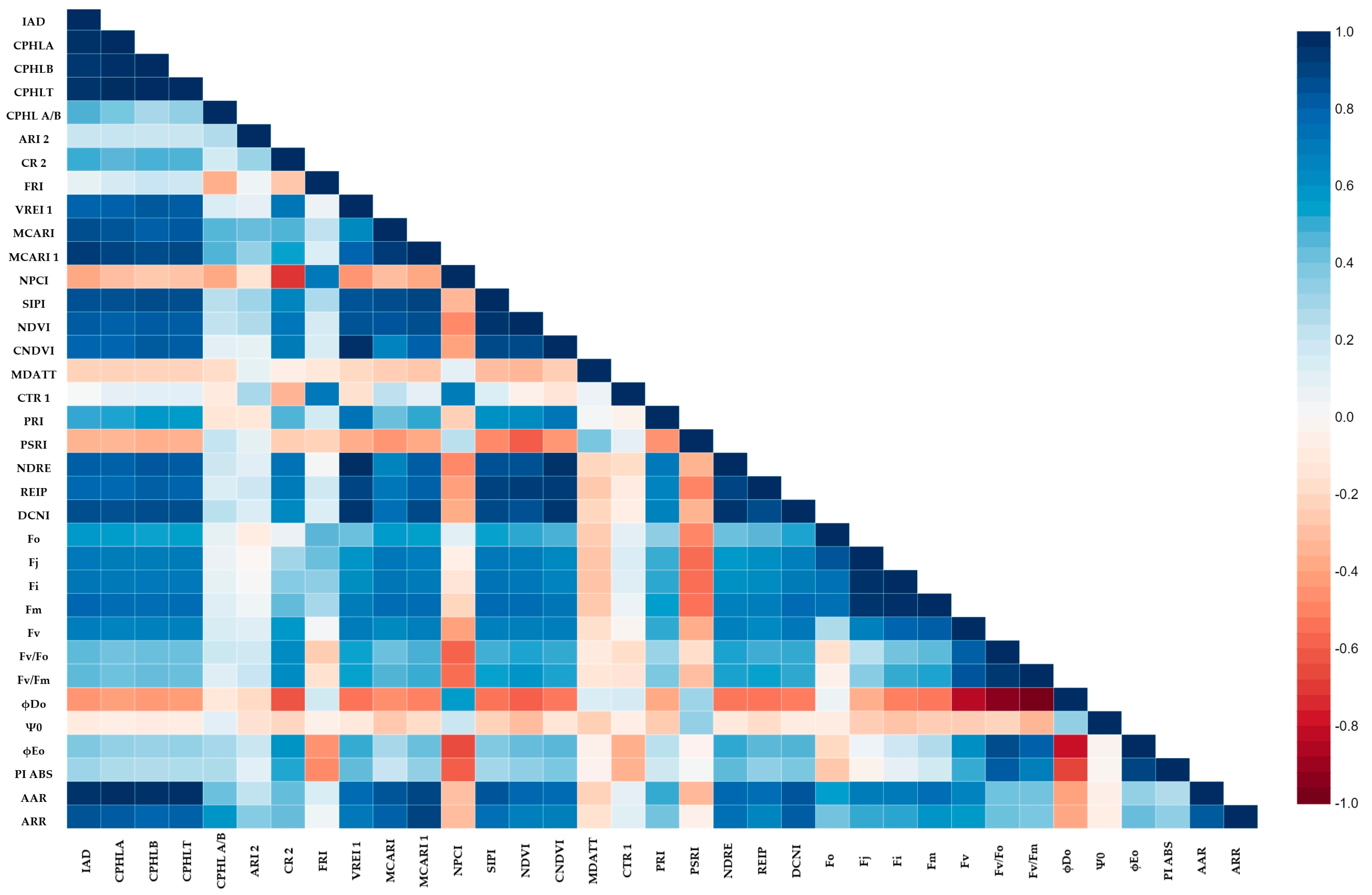
2.5. The Relationship among Spectral Indices and Photosyntetic Activity
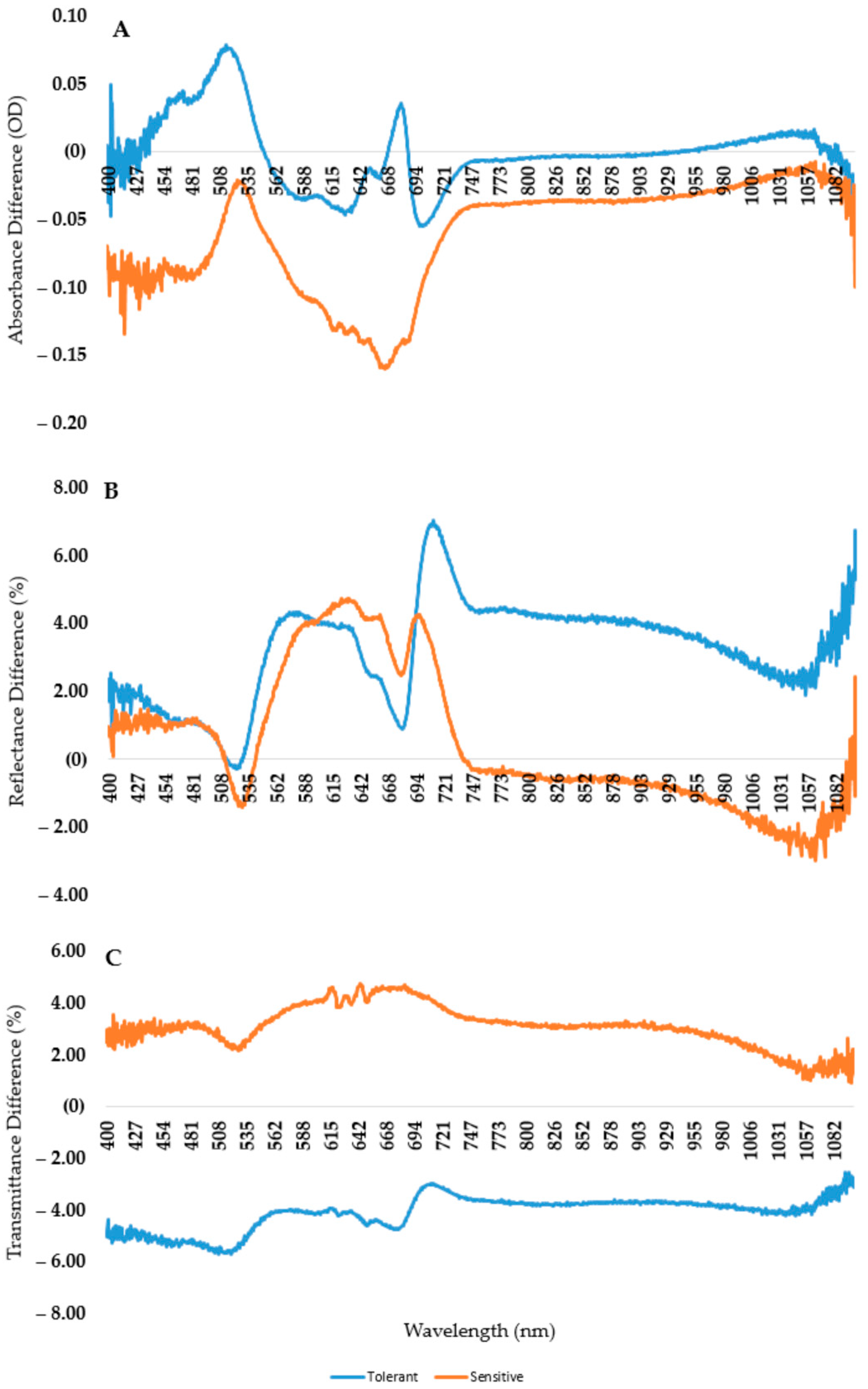
2.6. The Differences between Tolerant and Sensitive Genotypes
3. Discussion
4. Materials and Methods
5. Conclusions
- (1)
- The spring testing environment is more suitable for marking differences among genotypes based on their long-term stress responses. On the other hand, in autumn, the susceptibility of each variety to chlorophyll degradation is higher. Thus, the extent of the decline can be determined better;
- (2)
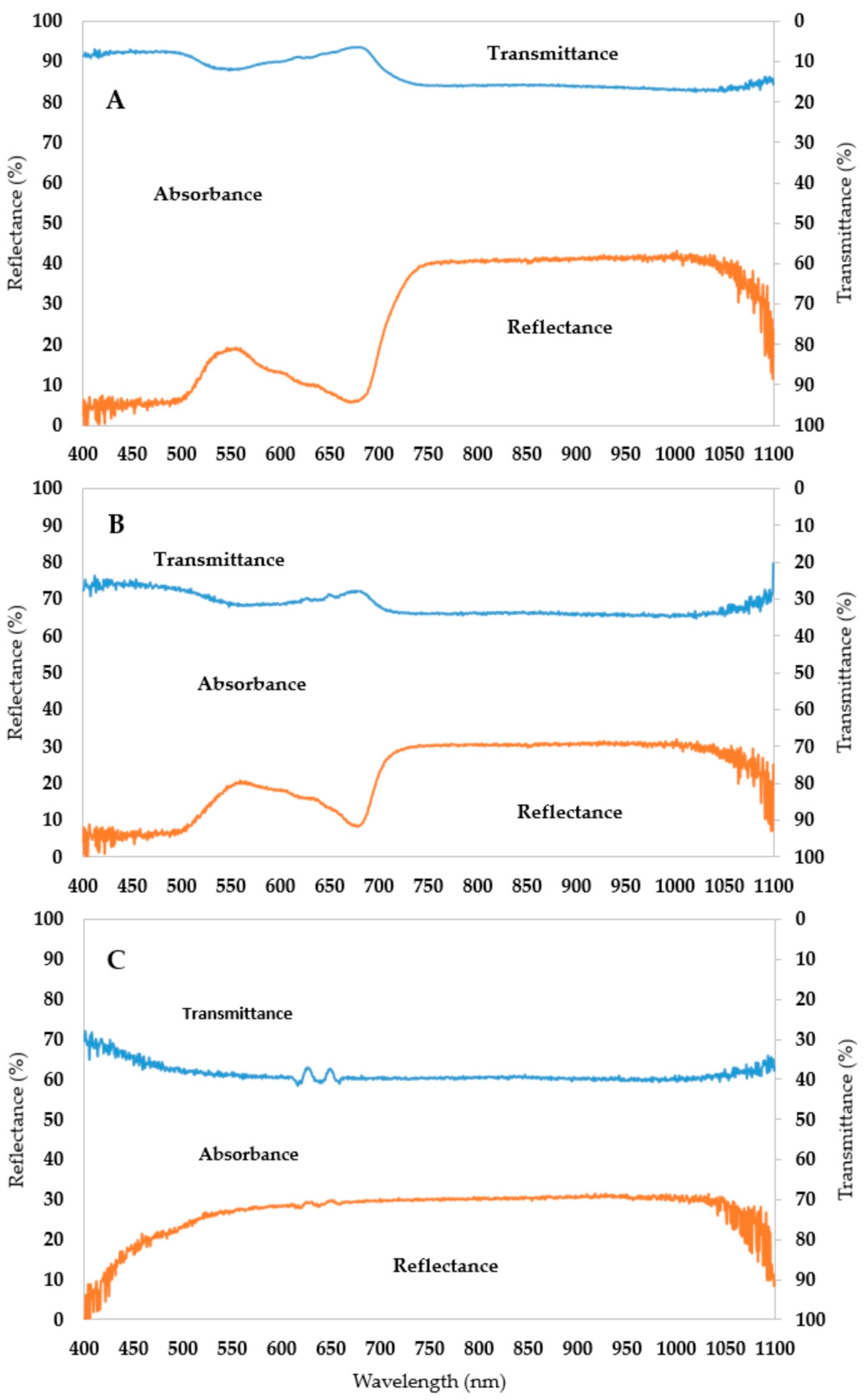
- The spectral pattern of an albino leaf can be characterized with a narrow absorbance range. The mean amount of the reflected light is around 30% and the transmitted amount is 40% throughout the whole spectrum;
- (3)
- Albino plants are the theoretical endpoint of chlorophyll degradation, so the new difference indices (AAR and ARR) are suitable for better describing the shape of the curve and the extent of chlorophyll degradation;
- (4)
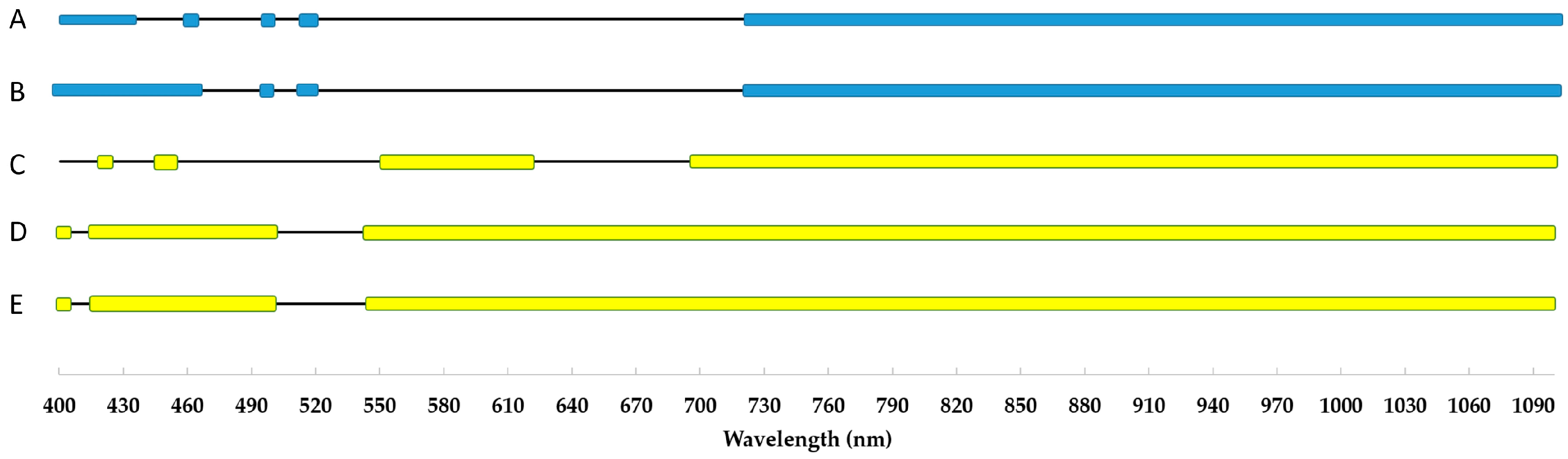
- The most stable wavelength range to cold stress was 525–535 nm, while the most sensitive was above 700 nm in the reflectance curve;
- (5)
- Almost all wavelengths outside the 525–535 nm range are suitable for differentiating between tolerant and sensitive varieties based on the control and cold-treated spectrograph difference.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- San Bautista, A.; Fita, D.; Franch, B.; Castiñeira-Ibáñez, S.; Arizo, P.; Sánchez-Torres, M.J.; Becker-Reshef, I.; Uris, A.; Rubio, C. Crop Monitoring Strategy Based on Remote Sensing Data (Sentinel-2 and Planet), Study Case in a Rice Field after Applying Glycinebetaine. Agronomy 2022, 12, 708. [Google Scholar] [CrossRef]
- Brinkhoff, J.; Dunn, B.W.; Robson, A.J.; Dunn, T.S.; Dehaan, R.L. Modeling Mid-Season Rice Nitrogen Uptake Using Multispectral Satellite Data. Remote Sens. 2019, 11, 1837. [Google Scholar] [CrossRef]
- Franch, B.; Bautista, A.S.; Fita, D.; Rubio, C.; Tarrazó-Serrano, D.; Sánchez, A.; Skakun, S.; Vermote, E.; Becker-Reshef, I.; Uris, A. Within-Field Rice Yield Estimation Based on Sentinel-2 Satellite Data. Remote Sens. 2021, 13, 4095. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Li, D.; Wang, C.; Jiang, H.; Zheng, Q.; Peng, Z. Estimation of Paddy Rice Nitrogen Content and Accumulation Both at Leaf and Plant Levels from UAV Hyperspectral Imagery. Remote Sens. 2021, 13, 2956. [Google Scholar] [CrossRef]
- Wang, F.; Yao, X.; Xie, L.; Zheng, J.; Xu, T. Rice Yield Estimation Based on Vegetation Index and Florescence Spectral Information from UAV Hyperspectral Remote Sensing. Remote Sens. 2021, 13, 3390. [Google Scholar] [CrossRef]
- Perros, N.; Kalivas, D.; Giovos, R. Spatial Analysis of Agronomic Data and UAV Imagery for Rice Yield Estimation. Agriculture 2021, 11, 809. [Google Scholar] [CrossRef]
- Sun, J.; Yang, J.; Shi, S.; Chen, B.; Du, L.; Gong, W.; Song, S. Estimating Rice Leaf Nitrogen Concentration: Influence of Regression Algorithms Based on Passive and Active Leaf Reflectance. Remote Sens. 2017, 9, 951. [Google Scholar] [CrossRef]
- Arias, F.; Zambrano, M.; Broce, K.; Medina, C.; Pacheco, H.; Nunez, Y.; Arias, F.; Zambrano, M.; Broce, K.; Medina, C.; et al. Hyperspectral Imaging for Rice Cultivation: Applications, Methods and Challenges. AIMS Agric. Food 2021, 6, 273–307. [Google Scholar] [CrossRef]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An Investigation into Robust Spectral Indices for Leaf Chlorophyll Estimation. ISPRS J. Photogramm. Remote Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of Leaf Reflectances in Narrow Wavebands as Indicators of Plant Stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Sishodia, R.; Ray, R.; Singh, S. Applications of Remote Sensing in Precision Agriculture: A Review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Merzlyak, M.N.; Solovchenko, A.E.; Smagin, A.I.; Gitelson, A.A. Apple Flavonols during Fruit Adaptation to Solar Radiation: Spectral Features and Technique for Non-Destructive Assessment. J. Plant Physiol. 2005, 162, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, W.; Avila, L.; Earl, H.; Lukens, L. Leaf Spectral Reflectance of Maize Seedlings and Its Relationship to Cold Tolerance. Crop Sci. 2018, 58, 2569–2580. [Google Scholar] [CrossRef]
- Ishikawa, D.; Hoogenboom, G.; Hakoyama, S.; Ishiguro, E. A Potential of the Growth Stage Estimation for Paddy Rice by Using Chlorophyll Absorption Bands in the 400–1100 Nm Region. J. Agric. Meteorol. 2015, 71, 24–31. [Google Scholar] [CrossRef]
- Carter, G.A. Responses of Leaf Spectral Reflectance to Plant Stress. Am. J. Bot. 1993, 80, 239–243. [Google Scholar] [CrossRef]
- Ziosi, V.; Noferini, M.; Fiori, G.; Tadiello, A.; Trainotti, L.; Casadoro, G.; Costa, G. A New Index Based on Vis Spectroscopy to Characterize the Progression of Ripening in Peach Fruit. Postharvest Biol. Technol. 2008, 49, 319–329. [Google Scholar] [CrossRef]
- Tan, K.; Wang, S.; Song, Y.; Liu, Y.; Gong, Z. Estimating Nitrogen Status of Rice Canopy Using Hyperspectral Reflectance Combined with BPSO-SVR in Cold Region. Chemom. Intell. Lab. Syst. 2018, 172, 68–79. [Google Scholar] [CrossRef]
- Bandaru, V.; Daughtry, C.S.; Codling, E.E.; Hansen, D.J.; White-Hansen, S.; Green, C.E. Evaluating Leaf and Canopy Reflectance of Stressed Rice Plants to Monitor Arsenic Contamination. Int. J. Environ. Res. Public Health 2016, 13, 606. [Google Scholar] [CrossRef]
- Zhang, C.; Filella, I.; Garbulsky, M.F.; Peñuelas, J. Affecting Factors and Recent Improvements of the Photochemical Reflectance Index (PRI) for Remotely Sensing Foliar, Canopy and Ecosystemic Radiation-Use Efficiencies. Remote Sens. 2016, 8, 677. [Google Scholar] [CrossRef]
- Sukhova, E.; Sukhov, V. Analysis of Light-Induced Changes in the Photochemical Reflectance Index (PRI) in Leaves of Pea, Wheat, and Pumpkin Using Pulses of Green-Yellow Measuring Light. Remote Sens. 2019, 11, 810. [Google Scholar] [CrossRef]
- Cao, Z.; Yao, X.; Liu, H.; Liu, B.; Cheng, T.; Tian, Y.; Cao, W.; Zhu, Y. Comparison of the Abilities of Vegetation Indices and Photosynthetic Parameters to Detect Heat Stress in Wheat. Agric. For. Meteorol. 2019, 265, 121–136. [Google Scholar] [CrossRef]
- Roy Choudhury, M.; Christopher, J.; Das, S.; Apan, A.; Menzies, N.W.; Chapman, S.; Mellor, V.; Dang, Y.P. Detection of Calcium, Magnesium, and Chlorophyll Variations of Wheat Genotypes on Sodic Soils Using Hyperspectral Red Edge Parameters. Environ. Technol. Innov. 2022, 27, 102469. [Google Scholar] [CrossRef]
- Raper, T.B.; Varco, J.J. Canopy-Scale Wavelength and Vegetative Index Sensitivities to Cotton Growth Parameters and Nitrogen Status. Precis. Agric 2015, 16, 62–76. [Google Scholar] [CrossRef]
- Xing, N.; Huang, W.; Xie, Q.; Shi, Y.; Ye, H.; Dong, Y.; Wu, M.; Sun, G.; Jiao, Q. A Transformed Triangular Vegetation Index for Estimating Winter Wheat Leaf Area Index. Remote Sens. 2020, 12, 16. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Ding, W.; Wu, L. Monitoring Stress Levels on Rice with Heavy Metal Pollution from Hyperspectral Reflectance Data Using Wavelet-Fractal Analysis. Int. J. Appl. Earth Obs. Geoinf. 2011, 13, 246–255. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef]
- Ripoll, J.; Bertin, N.; Bidel, L.P.R.; Urban, L. A User’s View of the Parameters Derived from the Induction Curves of Maximal Chlorophyll a Fluorescence: Perspectives for Analyzing Stress. Front. Plant Sci. 2016, 7, 1679. [Google Scholar] [CrossRef]
- Kalaji, H.; Govindjee, G.; Bosa, K.; Janusz, K.; Zuk-Golaszewska, K. Effects of Salt Stress on Photosystem II Efficiency and CO2 Assimilation in Two Syrian Barley Landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Peng, Y.; Zeng, A.; Zhu, T.; Fang, S.; Gong, Y.; Tao, Y.; Zhou, Y.; Liu, K. Using Remotely Sensed Spectral Reflectance to Indicate Leaf Photosynthetic Efficiency Derived from Active Fluorescence Measurements. J. Appl. Remote Sens. 2017, 11, 026034. [Google Scholar] [CrossRef]
- Gamon, J.; Serrano, L.; Surfus, J.S. The Photochemical Reflectance Index: An Optical Indicator of Photosynthetic Radiation Use Efficiency across Species, Functional Types, and Nutrient Levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, L.; Hu, H.; Zheng, K.; Jin, Q. Monitoring Leaf Chlorophyll Fluorescence with Spectral Reflectance in Rice (Oryza sativa L.). Procedia Eng. 2011, 15, 4403–4408. [Google Scholar] [CrossRef]
- Andaya, V.; Mackill, D. QTLs Conferring Cold Tolerance at the Booting Stage of Rice Using Recombinant Inbred Lines from a Japonica × Indica Cross. Appl. Genet. 2003, 106, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Andaya, V.C.; Mackill, D.J. Mapping of QTLs Associated with Cold Tolerance during the Vegetative Stage in Rice. J. Exp. Bot. 2003, 54, 2579–2585. [Google Scholar] [CrossRef]
- Freitas, G.M.d.; Thomas, J.; Liyanage, R.; Lay, J.O.; Basu, S.; Ramegowda, V.; Amaral, M.N.d.; Benitez, L.C.; Braga, E.J.B.; Pereira, A. Cold Tolerance Response Mechanisms Revealed through Comparative Analysis of Gene and Protein Expression in Multiple Rice Genotypes. PLoS ONE 2019, 14, e0218019. [Google Scholar] [CrossRef]
- Kaimori, N.; Takahashi, N. Genecological studies on chlorotic behaviors of rice plants at low temperature. Bull. Inst. Agri. Res. Tohoku Univ. 1981, 32, 73–79. [Google Scholar]
- Basuchaudhuri, P. Cold Tolerance in Rice Cultivation; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-306-86664-4. [Google Scholar]
- Yoshida, T.; Ono, T. Environmental Differences in Leaf Stomatal Frequency of Rice. Jpn. J. Crop Sci. 1978, 47, 506–514. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with Reduced Stomatal Density Conserves Water and Has Improved Drought Tolerance under Future Climate Conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef]
- Kasajima, I.; Ebana, K.; Yamamoto, T.; Takahara, K.; Yano, M.; Kawai-Yamada, M.; Uchimiya, H. Molecular Distinction in Genetic Regulation of Nonphotochemical Quenching in Rice. Proc. Natl. Acad. Sci. USA 2011, 108, 13835–13840. [Google Scholar] [CrossRef]
- Kasajima, I. Difference in Oxidative Stress Tolerance between Rice Cultivars Estimated with Chlorophyll Fluorescence Analysis. BMC Res. Notes 2017, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Lee, S.-C.; Hong, S.K.; An, K.; An, G.; Kim, S.-R. Ectopic Expression of a Cold-Responsive OsAsr1 CDNA Gives Enhanced Cold Tolerance in Transgenic Rice Plants. Mol. Cells 2009, 27, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Zhang, G. The Next Generation of Rice: Inter-Subspecific Indica-Japonica Hybrid Rice. Front. Plant Sci. 2022, 13, 857896. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.A.; Khan, M.N.; Bhat, M.A.; Shikari, A.B.; Wani, S.H.; Sofi, N.R.; Khan, M.I.; Lone, R.A. Cold Tolerance at Germination and Seedling Stages of Rice: Methods of Evaluation and Characterization of Thirty Rice Genotypes under Stress Conditions. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 1103–1109. [Google Scholar] [CrossRef]
- Cruz, R.P.d.; Milach, S.C.K. Cold Tolerance at the Germination Stage of Rice: Methods of Evaluation and Characterization of Genotypes. Sci. Agric. 2004, 61, 1–8. [Google Scholar] [CrossRef]
- Hyun, U.-J.; Yeo, S.-M.; Lee, S.-B.; Lee, J.-H.; Jeong, J.-M.; Seong, Y.-K.; Seo, D.-H.; Won, Y.-J.; Ahn, E.-K.; Lee, J.H.; et al. Optimization of Temperature Regime to Screen Cold Tolerant Rice Seedlings. Plant Breed. Biotechnol. 2016, 4, 176–187. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance Spectral Features and Non-Destructive Estimation of Chlorophyll, Carotenoid and Anthocyanin Content in Apple Fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical Properties and Nondestructive Estimation of Anthocyanin Content in Plant Leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Tao, R. Characterizing and Estimating Fungal Disease Severity of Rice Brown Spot with Hyperspectral Reflectance Data. Rice Sci. 2008, 15, 232–242. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, X.; Jiang, L.; Li, X.; Yang, S.; Li, Y. Hyperspectral Reflectance Characteristics of Rice Canopies under Changes in Diffuse Radiation Fraction. Remote Sens. 2022, 14, 285. [Google Scholar] [CrossRef]
- Katsoulas, N.; Elvanidi, A.; Ferentinos, K.P.; Kacira, M.; Bartzanas, T.; Kittas, C. Crop Reflectance Monitoring as a Tool for Water Stress Detection in Greenhouses: A Review. Biosyst. Eng. 2016, 151, 374–398. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance Indices Associated with Physiological Changes in Nitrogen- and Water-Limited Sunflower Leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Gates, D.M.; Keegan, H.J.; Schleter, J.C.; Weidner, V.R. Spectral Properties of Plants. Appl. Opt. 1965, 4, 11–20. [Google Scholar] [CrossRef]
- Viljevac Vuletić, M.; Spanic, V. Characterization of Photosynthetic Performance during Natural Leaf Senescence in Winter Wheat: Multivariate Analysis as a Tool for Phenotypic Characterization. Photosynthetica 2020, 58, 301–313. [Google Scholar] [CrossRef]
- Pérez-Patricio, M.; Camas-Anzueto, J.L.; Sanchez-Alegría, A.; Aguilar-González, A.; Gutiérrez-Miceli, F.; Escobar-Gómez, E.; Voisin, Y.; Rios-Rojas, C.; Grajales-Coutiño, R. Optical Method for Estimating the Chlorophyll Contents in Plant Leaves. Sensors 2018, 18, 650. [Google Scholar] [CrossRef]
- Vogelmann, J.E.; Rock, B.N.; Moss, D.M. Red Edge Spectral Measurements from Sugar Maple Leaves. Int. J. Remote Sens. 1993, 14, 1563–1575. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral Vegetation Indices and Novel Algorithms for Predicting Green LAI of Crop Canopies: Modeling and Validation in the Context of Precision Agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Penuelas, J.; Frederic, B.; Filella, I. Semi-Empirical Indices to Assess Carotenoids/Chlorophyll-a Ratio from Leaf Spectral Reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral Image Analysis Techniques for the Detection and Classification of the Early Onset of Plant Disease and Stress. Plant Methods 2017, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Lu, X.; Zhao, W.; Liu, Y.; Wang, Z.; Omasa, K. Comparing Vegetation Indices for Remote Chlorophyll Measurement of White Poplar and Chinese Elm Leaves with Different Adaxial and Abaxial Surfaces. J. Exp. Bot. 2015, 66, 5625–5637. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Fitzgerald, G.; Belford, R.; Christensen, L. Detection of Nitrogen Deficiency in Wheat from Spectral Reflectance Indices and Basic Crop Eco-Physiological Concepts. Aust. J. Agric. Res. 2006, 57, 781–789. [Google Scholar] [CrossRef]
- Mistele, B.; Schmidhalter, U. Estimating the Nitrogen Nutrition Index Using Spectral Canopy Reflectance Measurements. Eur. J. Agron. 2008, 29, 184–190. [Google Scholar] [CrossRef]
- Chen, P.; Haboudane, D.; Tremblay, N.; Wang, J.; Vigneault, P.; Li, B. New Spectral Indicator Assessing the Efficiency of Crop Nitrogen Treatment in Corn and Wheat. Remote Sens. Environ. 2010, 114, 1987–1997. [Google Scholar] [CrossRef]
- Strasser, R.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]



| Value | F | Hypothesis df | Error df | Sig. | |
|---|---|---|---|---|---|
| Genotype | 0.000 | 11.733 | 512.000 | 6921.549 | 0.000 |
| Seasonal variation | 0.094 | 144.406 | 32.000 | 478.000 | 0.000 |
| Tmax | 0.000 | 64.409 | 192.000 | 2987.519 | 0.000 |
| Tmean | 0.000 | 64.409 | 192.000 | 2987.519 | 0.000 |
| Tmin | 0.000 | 64.409 | 192.000 | 2987.519 | 0.000 |
| Air Temperature | ||||||
|---|---|---|---|---|---|---|
| Date | Day | Code | Tmax | Tmean | Tmin | |
| Spring | 19 May 2021 | 0 | S1Tr0 | 19.27 | 13.99 | 9.17 |
| 26 May 2021 | 7 | S1Tr7 | 21.27 | 15.20 | 9.59 | |
| 2 June 2021 | 14 | S1Tr14 | 21.24 | 15.13 | 9.20 | |
| Autumn | 27 September 2021 | 0 | S2Tr0 | 24.16 | 18.19 | 9.83 |
| 4 October 2021 | 7 | S2Tr7 | 20.16 | 15.85 | 9.81 | |
| 11 October 2021 | 14 | S2Tr14 | 16.60 | 12.53 | 7.18 | |
| 18 October 2021 | 21 | S2Tr21 | 13.93 | 8.76 | 1.48 | |
| Name | Country of Origin | Varietal Group |
|---|---|---|
| Ábel | Hungary | temperate japonica |
| Dunghan Shali | Hungary | temperate japonica |
| Sandora (HSC 55) | Hungary | temperate japonica |
| Kikko | Italy | temperate japonica |
| Sfera | Italy | temperate japonica |
| Loto | Italy | temperate japonica |
| Diamante | Chile | temperate japonica |
| M 202 | U.S.A. | temperate japonica |
| Nipponbare | Japan | temperate japonica |
| Mirko | Italy | tropical japonica |
| IRAT 109 | Ivory Coast | tropical japonica |
| IR60080-46A | Philippines | tropical japonica |
| N22 | India | aus |
| Dular | India | aus |
| CO 39 | Philippines | indica |
| IR74371-70-1-1 | Philippines | indica |
| Index | Formulation | Reference |
|---|---|---|
| Absorbance Difference Index (IAD) | A670 − A720 | [17] |
| Chlorophyll A (CPHLA) | (12.7 × A663) − (2.59 × A645) | [57] |
| Chlorophyll B (CPHLB) | (22.9 × A645) − (4.7 × A663) | [57] |
| Chlorophyll TOTAL (CPHLT) | (8.2 × A663) + (20.2 × A645) | [57] |
| Chlorophyll a/b Ratio (CPHLA/CPHLB) | CPHLA/CPHLB | [42] |
| Anthocyanin Reflectance Index 2 (ARI 2) | R800 × (1/R550) − (1/R700) | [50] |
| Carotenoid Reflectance Index 2 (CRI 2) | (1/R510) − (1/R700) | [12] |
| Flavonols Reflectance Index (FRI) | (1/R410 − 1/R460) × R800 | [13] |
| Vogelmann Index (VREI 1) | R740/R720 | [58] |
| Modified Chlorophyll Absorption Ratio Index (MCARI) | ((R700 − R670) − 0.2 × (R700 − R550)) × (R700/R670) | [59] |
| Modified Chlorophyll Absorption Ratio Index 1 (MCARI1) | 1.2 × (2.5 × (R800 − R670) − 1.3 × (R800 − R550)) | [60] |
| Normalized Pigment Chlorophyll Index (NPCI) | (R680 − R430)/(R680 + R430) | [54] |
| Structure Intensive Pigment Index (SIPI) | (R800 − R445)/(R800 + R680) | [61] |
| Normalized Difference Vegetation Index (NDVI) | (R800 − R680)/(R800 + R680) | [60] |
| Red Edge NDVI (RENDVI) | (R750 − R705)/(R750 + R705) | [62] |
| Modified DATT Index (MDATT) | (R719 − R726)/(R719 − R743) | [63] |
| Carter Index (CTR 1) | R695/R420 | [10] |
| Photochemical Reflectance Index (PRI) | (R531 − R570)/(R531 − R570) | [32] |
| Plant Senescence Reflectance Index (PSRI) | (R680 − R500)/R750 | [62] |
| Normalized Difference Red Edge (NDRE) | (R790 − R720)/(R790 + R720) | [64] |
| Red Edge Inflection Point (REIP) | 700 + 40 × (((R670 + R870)/2 − R700)/(R740 − R700)) | [65] |
| Double-peak Canopy Nitrogen Index (DCNI) | (R720 − R700)/((R700 − R670)/(R720 − R670 + 0.03)) | [66] |
| Albino Absorbance Ratio (AAR) | A672 of genotype/A672 of albino | This paper |
| Albino Reflectance Ratio (ARR) | Average of R800:R900/Average of R800:R900 of the albino | This paper |
| Fluorescence Intensity at 50 µs (Fo) | [67] | |
| Fluorescence Intensity at 2 ms (Fj) | [67] | |
| Fluorescence Intensity at 30 ms (Fi) | [67] | |
| Maximal Fluorescence Intensity (Fm) | [67] | |
| Variable Chlorophyll Fluorescence (Fv) | Fv = (Fm − Fo) | [67] |
| Maximum Quantum Yield of PSII (Fv/Fm) | Fv/Fm | [67] |
| Trapped Flux (Ψ0) | Psi_0 = 1 − Vj | [67] |
| (Fv/Fo) | (Fv/Fo) | [29] |
| Electron Transport Quantum Yield (ϕEo) | ϕEo = (1 − Fo/Fm)·Ψ0 | [29] |
| Thermal Dissipation Quantum Yield (ϕDo) | (Fo/Fm) | [29] |
| Performance Index (PIABS) | (RC/ABS) × (ϕPo/(1 − ϕPo)) × (ψ0/(1 − ψ0)) | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Székely, Á.; Szalóki, T.; Jancsó, M.; Pauk, J.; Lantos, C. Temporal Changes of Leaf Spectral Properties and Rapid Chlorophyll—A Fluorescence under Natural Cold Stress in Rice Seedlings. Plants 2023, 12, 2415. https://doi.org/10.3390/plants12132415
Székely Á, Szalóki T, Jancsó M, Pauk J, Lantos C. Temporal Changes of Leaf Spectral Properties and Rapid Chlorophyll—A Fluorescence under Natural Cold Stress in Rice Seedlings. Plants. 2023; 12(13):2415. https://doi.org/10.3390/plants12132415
Chicago/Turabian StyleSzékely, Árpád, Tímea Szalóki, Mihály Jancsó, János Pauk, and Csaba Lantos. 2023. "Temporal Changes of Leaf Spectral Properties and Rapid Chlorophyll—A Fluorescence under Natural Cold Stress in Rice Seedlings" Plants 12, no. 13: 2415. https://doi.org/10.3390/plants12132415
APA StyleSzékely, Á., Szalóki, T., Jancsó, M., Pauk, J., & Lantos, C. (2023). Temporal Changes of Leaf Spectral Properties and Rapid Chlorophyll—A Fluorescence under Natural Cold Stress in Rice Seedlings. Plants, 12(13), 2415. https://doi.org/10.3390/plants12132415





