Inhibition of the CYP Enzymatic System Responsible of Heterocyclic Amines Bioactivation by an Asclepias subulata Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Ethanolic Extract of Asclepias subulata
2.3. Animals
2.4. Preparation of Rat Liver Microsomes
2.5. CYP1A1 and CYP1A2 Activities
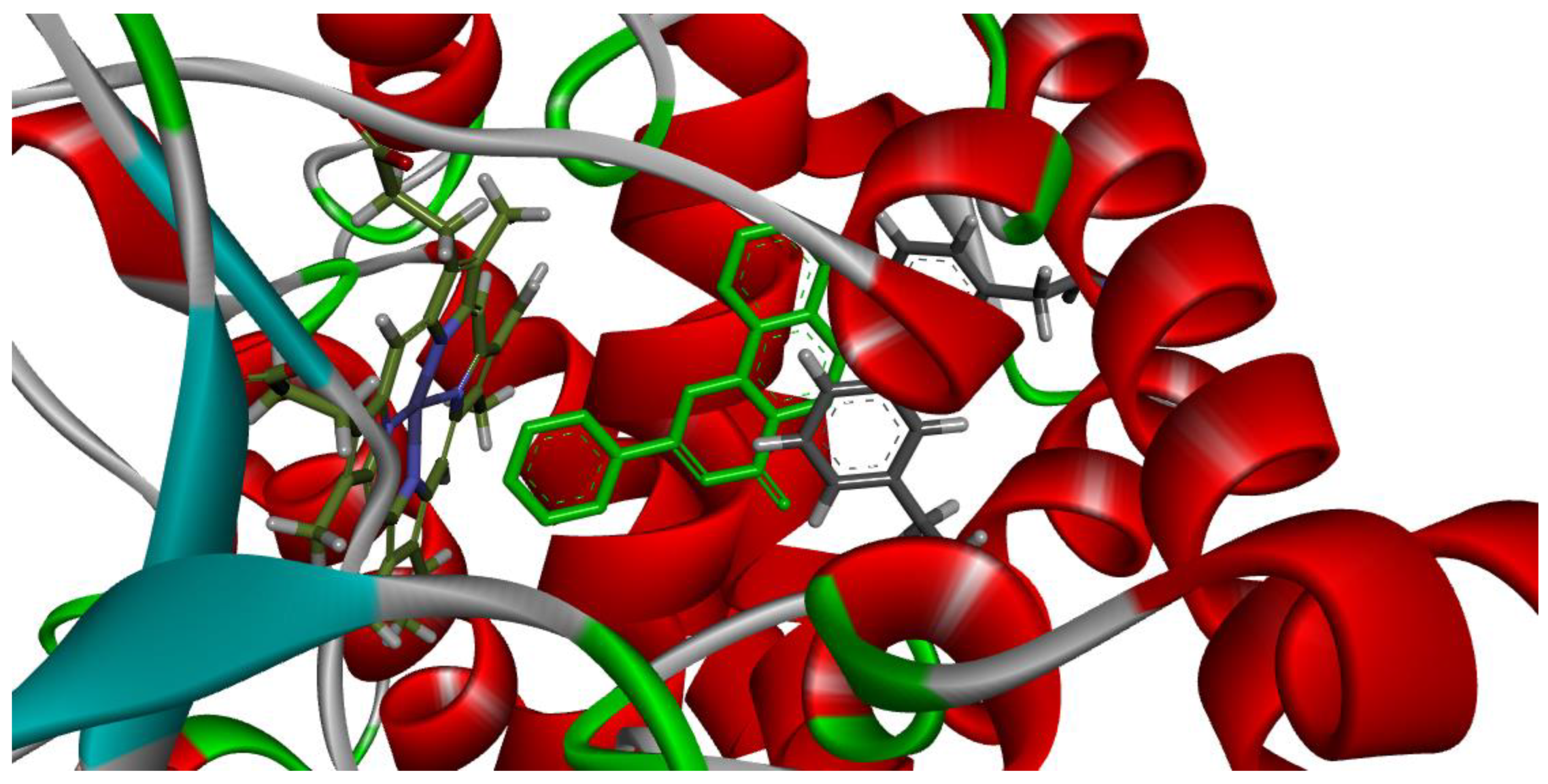
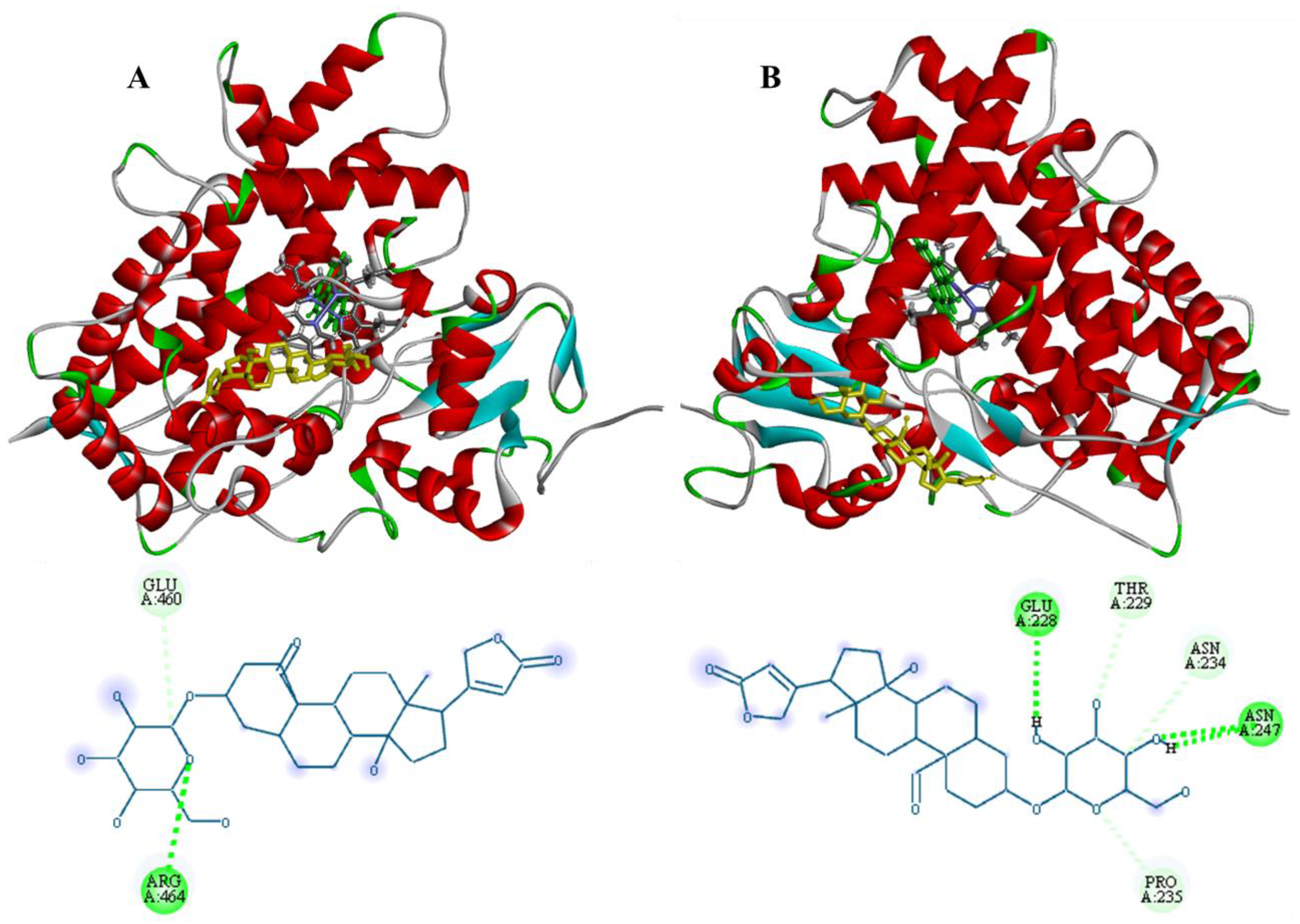
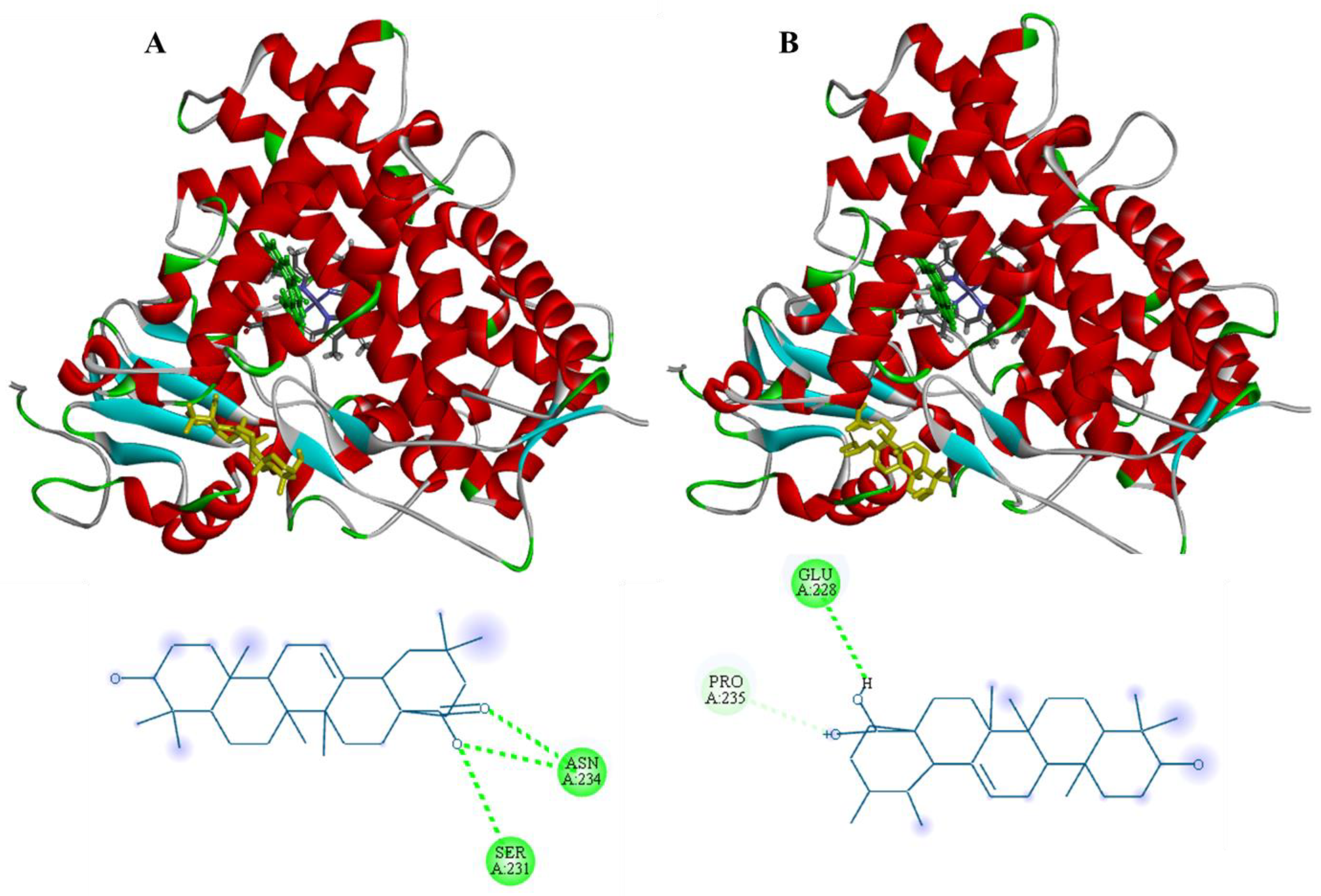
2.6. Molecular Docking
2.7. Statistical Analysis
3. Results and Discussion
3.1. Inhibition of CYP1A1 by ASE (Non-Heated and Heated)
3.2. Inhibition of CYP1A2 by ASE (Non-Heated and Heated)
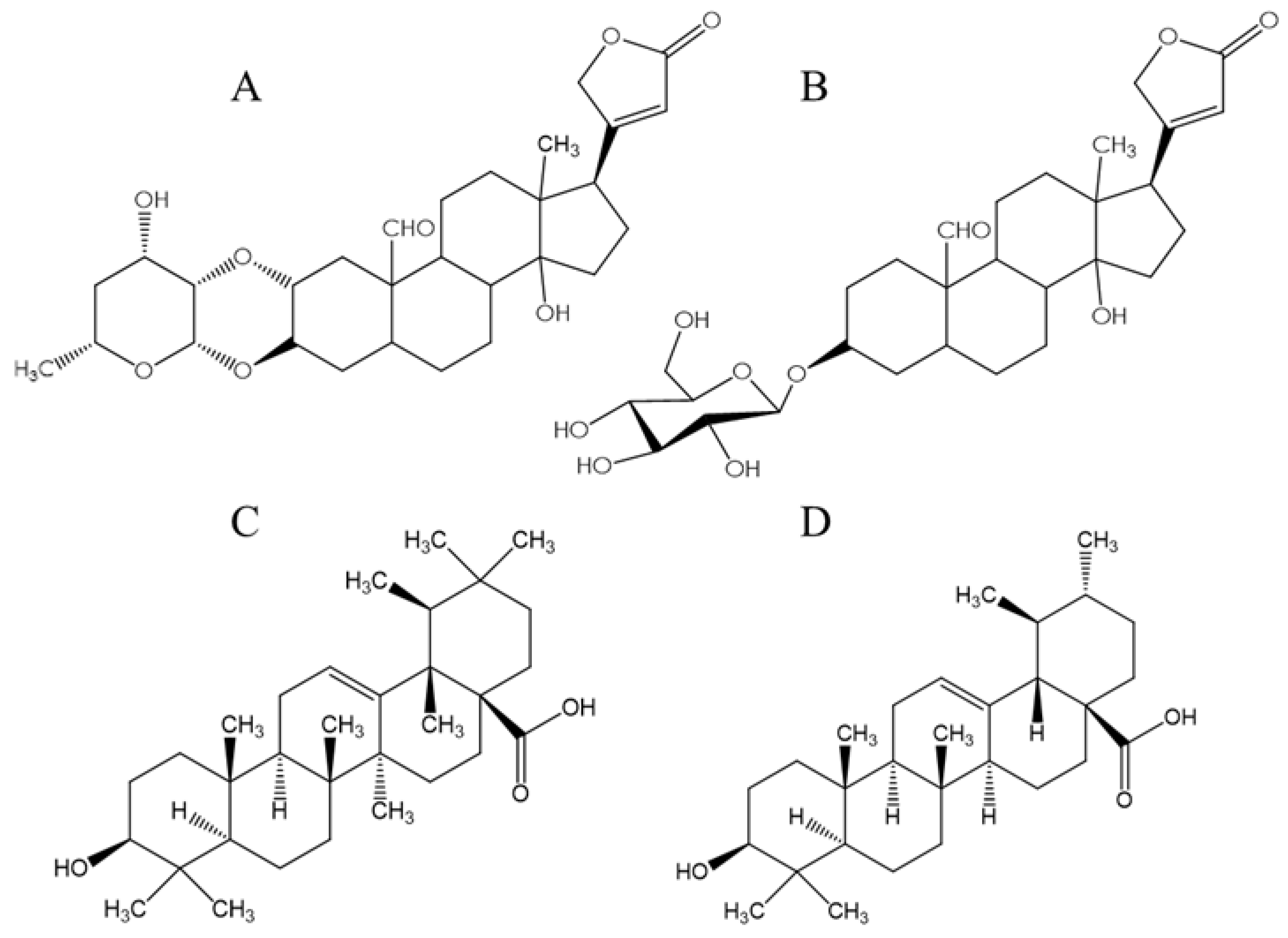
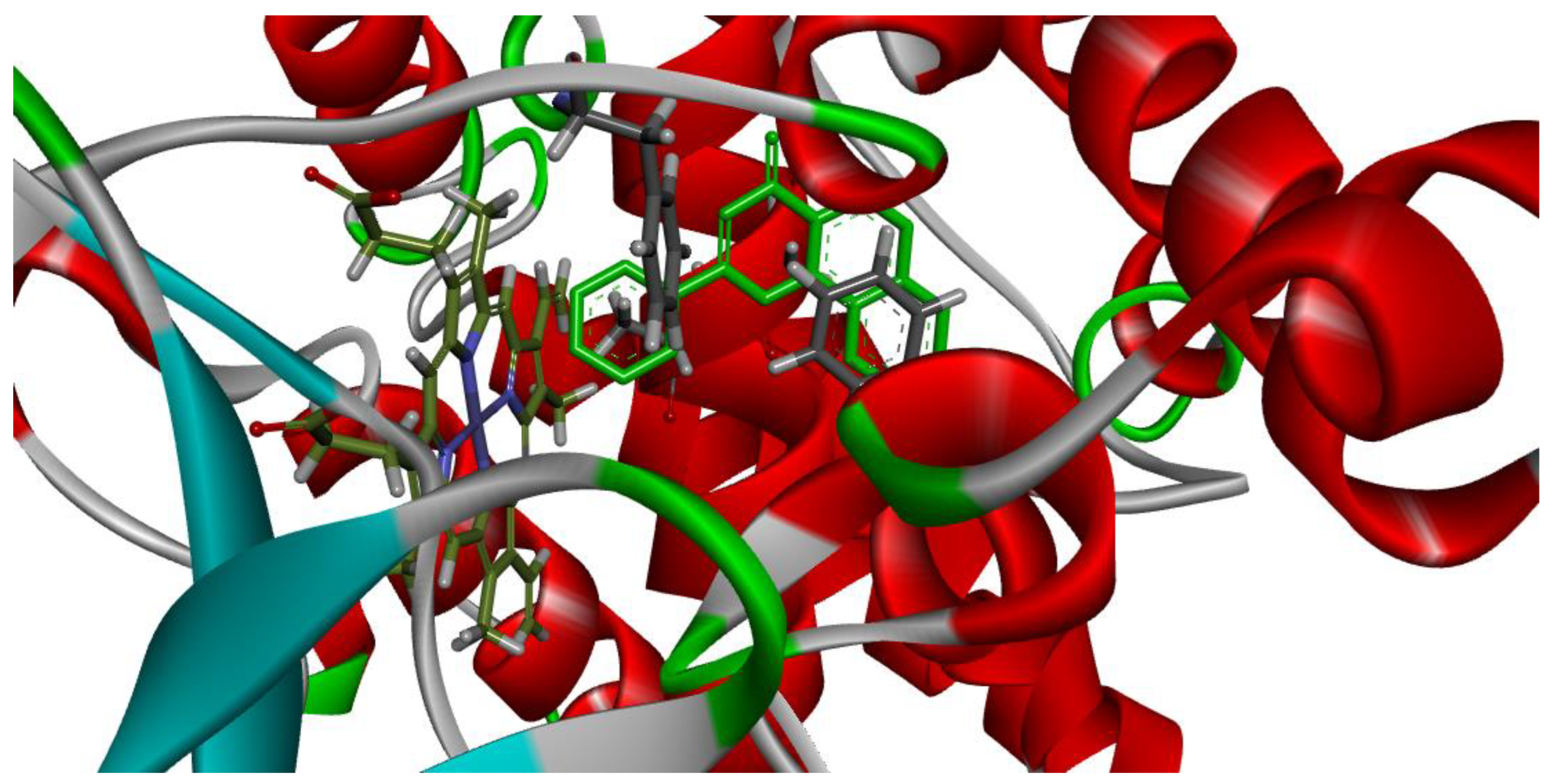
3.3. Molecular Docking
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Available online: https://www.iarc.who.int/featured-news/latest-global-cancer-data-cancer-burden-rises-to-18-1-million-new-cases-and-9-6-million-cancer-deaths-in-2018/ (accessed on 15 June 2023).
- Góngora, M.; Matthes, K.L.; Castaño, P.R.; Linseisen, J.; Rohrmann, S. Dietary Heterocyclic Amine Intake and Colorectal Adenoma Risk: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2019, 28, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimura, T.; Wakabayashi, K.; Nakagama, H.; Nagao, M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004, 95, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Mosley, D.; Su, T.; Murff, H.J.; Smalley, W.E.; Ness, R.M.; Zheng, W.; Shrubsole, M.J. Meat intake, meat cooking methods, and meat-derived mutagen exposure and risk of sessile serrated lesions. Am. J. Clin. Nutr. 2020, 111, 1244–1251. [Google Scholar] [CrossRef]
- Chevereau, M.; Glatt, H.; Zalko, D.; Cravedi, J.-P.; Audebert, M. Role of human sulfotransferase 1A1 and N-acetyltransferase 2 in the metabolic activation of 16 heterocyclic amines and related heterocyclics to genotoxicants in recombinant V79 cells. Arch. Toxicol. 2017, 91, 3175–3184. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, W.; Zhu, L.; Mao, L.; Zhang, Y. Recent advances in heterocyclic aromatic amines: An update on food safety and hazardous control from food processing to dietary intake. Compr. Rev. Food Sci. Food Saf. 2020, 19, 124–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waladkhani, A.R.; Clemens, M.R. Dietary phytochemicals in prevention and therapy of cancer. In Botanical Medicine in Clinical Practice; CAB International: Wallingford, UK, 2008; pp. 377–387. [Google Scholar]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of phytochemicals in cancer chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-S.; Tsai, M.-L.; Ho, C.-T.; Wang, Y.-J.; Pan, M.-H. Chemopreventive effect of natural dietary compounds on xenobiotic-induced toxicity. J. Food Drug. Anal. 2017, 25, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [Green Version]
- Picking, D.; Chambers, B.; Barker, J.; Shah, I.; Porter, R.; Naughton, D.P.; Delgoda, R. Inhibition of cytochrome P450 activities by extracts of Hyptis verticillata Jacq.: Assessment for potential HERB-drug interactions. Molecules 2018, 23, 430. [Google Scholar] [CrossRef] [Green Version]
- Rodeiro, I.; Donato, M.T.; Jimenez, N.; Garrido, G.; Molina-Torres, J.; Menendez, R.; Castell, J.V.; Gomez-Lechon, M.J. Inhibition of human P450 enzymes by natural extracts used in traditional medicine. Phytother. Res. 2009, 23, 279–282. [Google Scholar] [CrossRef]
- Rendic, S.; Guengerich, F.P. Contributions of human enzymes in carcinogen metabolism. Chem. Res. Toxicol. 2012, 25, 1316–1383. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Estrada, M.J.; Contreras, C.A.V.; Escobar, A.G.; Juarez, L.A.M.; Meza, N.G.; Zepeda, R.E.R. Antiproliferative and apoptotic activities of extracts of Asclepias subulata. Pharm. Biol. 2015, 53, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Brewer, F.; Jaimes, V.J.; Zárraga, L.C. Usos de las especies del género Asclepias, L. (Apocynaceae, Asclepiadoideae), información del Herbario Nacional de México, MEXU. Polibotánica 2008, 25, 155–171. [Google Scholar]
- Gutiérrez-Pacheco, S.L.; Valenzuela-Melendres, M.; Hernández-Mendoza, A.; Burgos-Hernández, A.; Robles-Zepeda, R.E.; Peña-Ramos, E.A. Antimutagenic effect of an Asclepias subulata extract against heterocyclic aromatic amines commonly found in cooked meat and its heat stability. Food Chem. 2020, 322, 126725. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.; Jayaprakasha, G.; Jena, B. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Rascón-Valenzuela, L.; Velázquez, C.; Garibay-Escobar, A.; Vilegas, W.; Medina-Juárez, L.; Gámez-Meza, N.; Robles-Zepeda, R. Apoptotic activities of cardenolide glycosides from Asclepias subulata. J. Ethnopharmacol. 2016, 193, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Reed, L.; Arlt, V.M.; Phillips, D.H. The role of cytochrome P450 enzymes in carcinogen activation and detoxication: An in vivo–in vitro paradox. Carcinogenesis 2018, 39, 851–859. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Estrada, M.; Velázquez-Contreras, C.; Garibay-Escobar, A.; Sierras-Canchola, D.; Lapizco-Vázquez, R.; Ortiz-Sandoval, C.; Burgos-Hernández, A.; Robles-Zepeda, R.E. In vitro antioxidant and antiproliferative activities of plants of the ethnopharmacopeia from northwest of Mexico. BMC Complement. Altern. Med. 2013, 13, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Matsushima; Sawamura, M.; Hara, K.; Sugimura, T. A safe substitute for polychlorinated biphenyls as an inducer of metabolic activation system. In In Vitro Metabolic Activation in Mutagenesis Testing; Elsevier/North-Holland: Amsterdam, The Netherlands, 1976; pp. 85–88. [Google Scholar]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. Genet. Toxicol. Environ. Mutagen. Subj. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Burke, M.; Thompson, S.; Weaver, R.J.; Wolf, C.R.; Mayers, R.T. Cytochrome P450 specificities of alkoxyresorufin O-dealkylation in human and rat liver. Biochem. Pharmacol. 1994, 48, 923–936. [Google Scholar] [CrossRef]
- Sansen, S.; Yano, J.K.; Reynald, R.L.; Schoch, G.A.; Griffin, K.J.; Stout, C.D.; Johnson, E.F. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J. Biol. Chem. 2007, 282, 14348–14355. [Google Scholar] [CrossRef] [Green Version]
- Walsh, A.A.; Szklarz, G.D.; Scott, E.E. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J. Biol. Chem. 2013, 288, 12932–12943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, G.P. Saponins. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Trugo, L.C., Finglas, P.M., Eds.; Academic Press: London, UK, 2003; pp. 5095–5098. [Google Scholar]
- Morsy, N. Cardiac glycosides in medicinal plants. In Aromatic and Medicinal Plants–Back to Nature; Intechopen: London, UK, 2017; pp. 29–45. [Google Scholar]
- Jeng, S.-N.; Shih, M.K.; Kao, C.M.; Liu, T.; Chen, S.-C. Antimutagenicity of ethanol extracts of bee glue against environmental mutagens. Food Chem. Toxicol. 2000, 38, 893–897. [Google Scholar] [CrossRef]
- Alvarez-Gonzalez, I.; Mojica, R.; Madrigal-Bujaidar, E.; Camacho-Carranza, R.; Escobar-García, D.; Espinosa-Aguirre, J. The antigenotoxic effects of grapefruit juice on the damage induced by benzo (a) pyrene and evaluation of its interaction with hepatic and intestinal Cytochrome P450 (Cyp) 1a1. Food Chem. Toxicol. 2011, 49, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Shields, M.; Niazi, U.; Badal, S.; Yee, T.; Sutcliffe, M.J.; Delgoda, R. Inhibition of CYP1A1 by quassinoids found in Picrasma excelsa. Planta Med. 2009, 75, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.M.; Seiber, J.; Keeler, R.; Johnson, A. Studies on the toxic principle of Asclepias eriocarpa and Asclepias labriformis. In Effects of Poisonous Plants on Livestock; Elsevier: Amsterdam, The Netherlands, 1978; pp. 273–284. [Google Scholar]
- Chen, K.; Brown, E.; Bliss, C.I. The digitalis-like principles of Calotropis compared with other cardiac substances. J. Pharmacol. Exp. Ther. 1942, 74, 223–234. [Google Scholar]
- Chen, K.; Henderson, F.G.; Anderson, R.C. Comparison of forty-two cardiac glycosides and aglycones. J. Pharmacol. Exp. Ther. 1951, 103, 420–430. [Google Scholar]
- Kim, V.; Lee, J.S.; Park, H.J.; Kim, J.-W.; Kim, C.J.; Shim, I.S.; Kim, N.J.; Han, S.M.; Lim, S. Inhibition of cytochrome P450 activities by oleanolic acid and ursolic acid in human liver microsomes. Life Sci. 2004, 74, 2769–2779. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Roche, L.; Santes-Palacios, R.; Herrera, J.A.; Hernández, S.L.; Riera, M.; Fernández, M.D.; Mesta, F.; Garrido, G.; Rodeiro, I.; Espinosa-Aguirre, J.J. Interaction of Thalassia testudinum metabolites with cytochrome P450 enzymes and its effects on benzo (a) pyrene-induced mutagenicity. Mar. Drugs 2020, 18, 566. [Google Scholar] [CrossRef]
- Sridhar., J.; Goyal, N.; Liu, J.; Foroozesh, M. Review of ligand specificity factors for CYP1A subfamily enzymes from molecular modeling studies reported to-date. Molecules 2017, 22, 1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
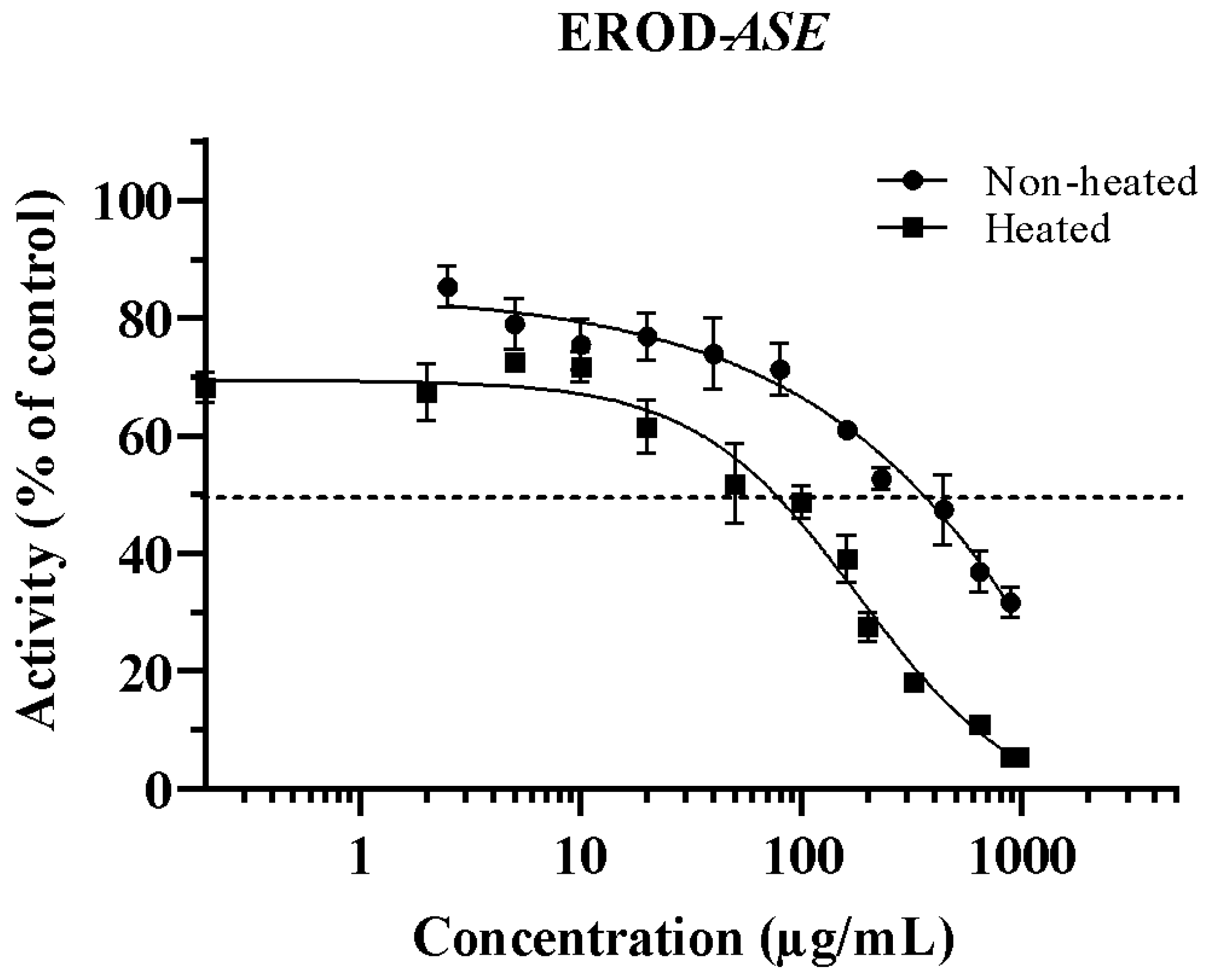
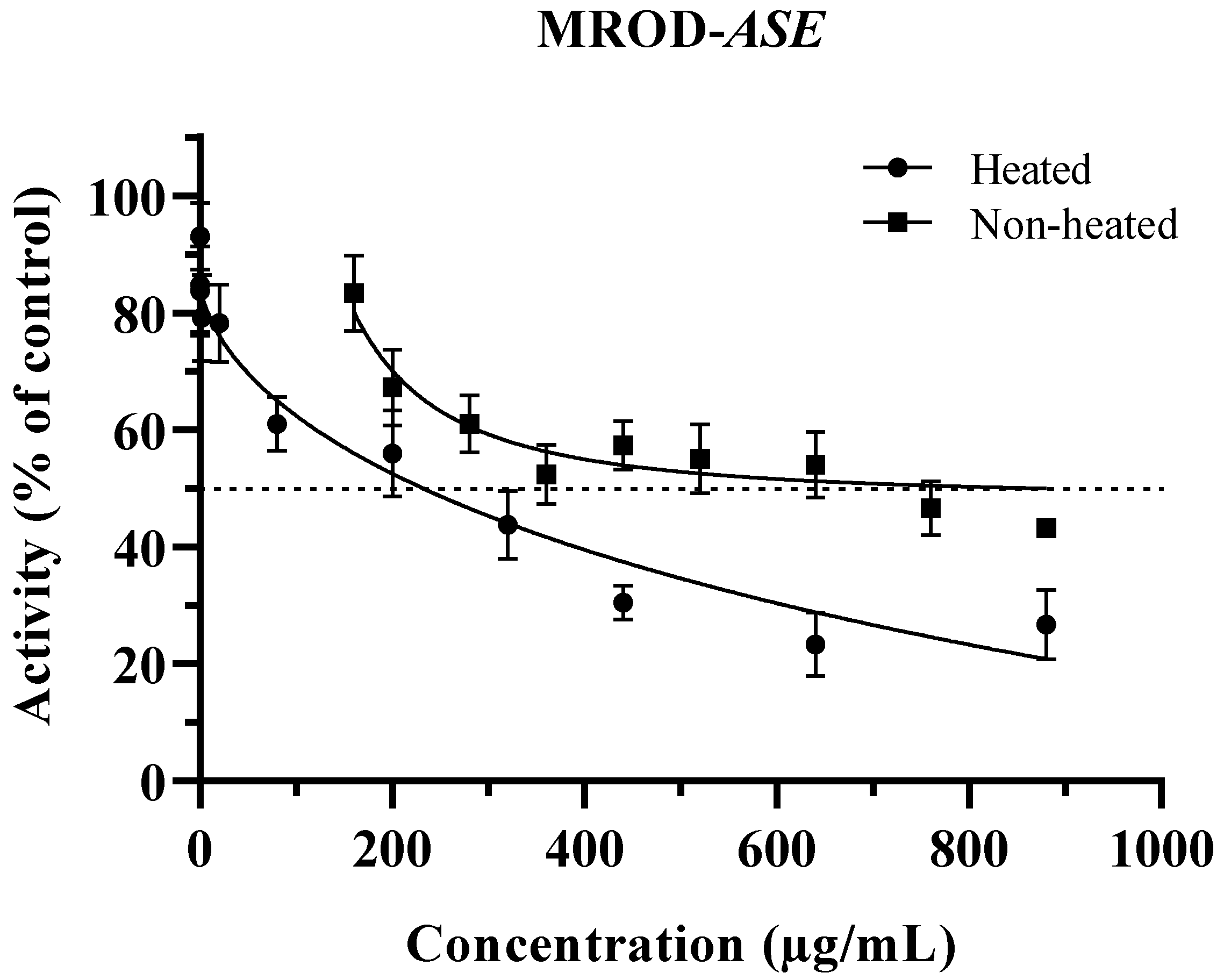

| IC50 (µg/mL) | ||
|---|---|---|
| Asclepias subulata Extract | EROD | MROD |
| Non-heated | 353.6 ± 4.51 a | 288.4 1 ± 5.81 |
| Heated 2 | 75.92 ± 3.97 b | 232.1 ± 7.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Pacheco, S.L.; Peña-Ramos, E.A.; Santes-Palacios, R.; Valenzuela-Melendres, M.; Hernández-Mendoza, A.; Burgos-Hernández, A.; Robles-Zepeda, R.E.; Espinosa-Aguirre, J.J. Inhibition of the CYP Enzymatic System Responsible of Heterocyclic Amines Bioactivation by an Asclepias subulata Extract. Plants 2023, 12, 2354. https://doi.org/10.3390/plants12122354
Gutiérrez-Pacheco SL, Peña-Ramos EA, Santes-Palacios R, Valenzuela-Melendres M, Hernández-Mendoza A, Burgos-Hernández A, Robles-Zepeda RE, Espinosa-Aguirre JJ. Inhibition of the CYP Enzymatic System Responsible of Heterocyclic Amines Bioactivation by an Asclepias subulata Extract. Plants. 2023; 12(12):2354. https://doi.org/10.3390/plants12122354
Chicago/Turabian StyleGutiérrez-Pacheco, Samaria Lisdeth, Etna Aida Peña-Ramos, Rebeca Santes-Palacios, Martin Valenzuela-Melendres, Adrián Hernández-Mendoza, Armando Burgos-Hernández, Ramón Enrique Robles-Zepeda, and Jesús Javier Espinosa-Aguirre. 2023. "Inhibition of the CYP Enzymatic System Responsible of Heterocyclic Amines Bioactivation by an Asclepias subulata Extract" Plants 12, no. 12: 2354. https://doi.org/10.3390/plants12122354
APA StyleGutiérrez-Pacheco, S. L., Peña-Ramos, E. A., Santes-Palacios, R., Valenzuela-Melendres, M., Hernández-Mendoza, A., Burgos-Hernández, A., Robles-Zepeda, R. E., & Espinosa-Aguirre, J. J. (2023). Inhibition of the CYP Enzymatic System Responsible of Heterocyclic Amines Bioactivation by an Asclepias subulata Extract. Plants, 12(12), 2354. https://doi.org/10.3390/plants12122354






