Chemopreventive Effect of Cooked Chickpea on Colon Carcinogenesis Evolution in AOM/DSS-Induced Balb/c Mice
Abstract
:1. Introduction
2. Results
2.1. Body Weight
2.2. Macro- and Microscopic Evaluation
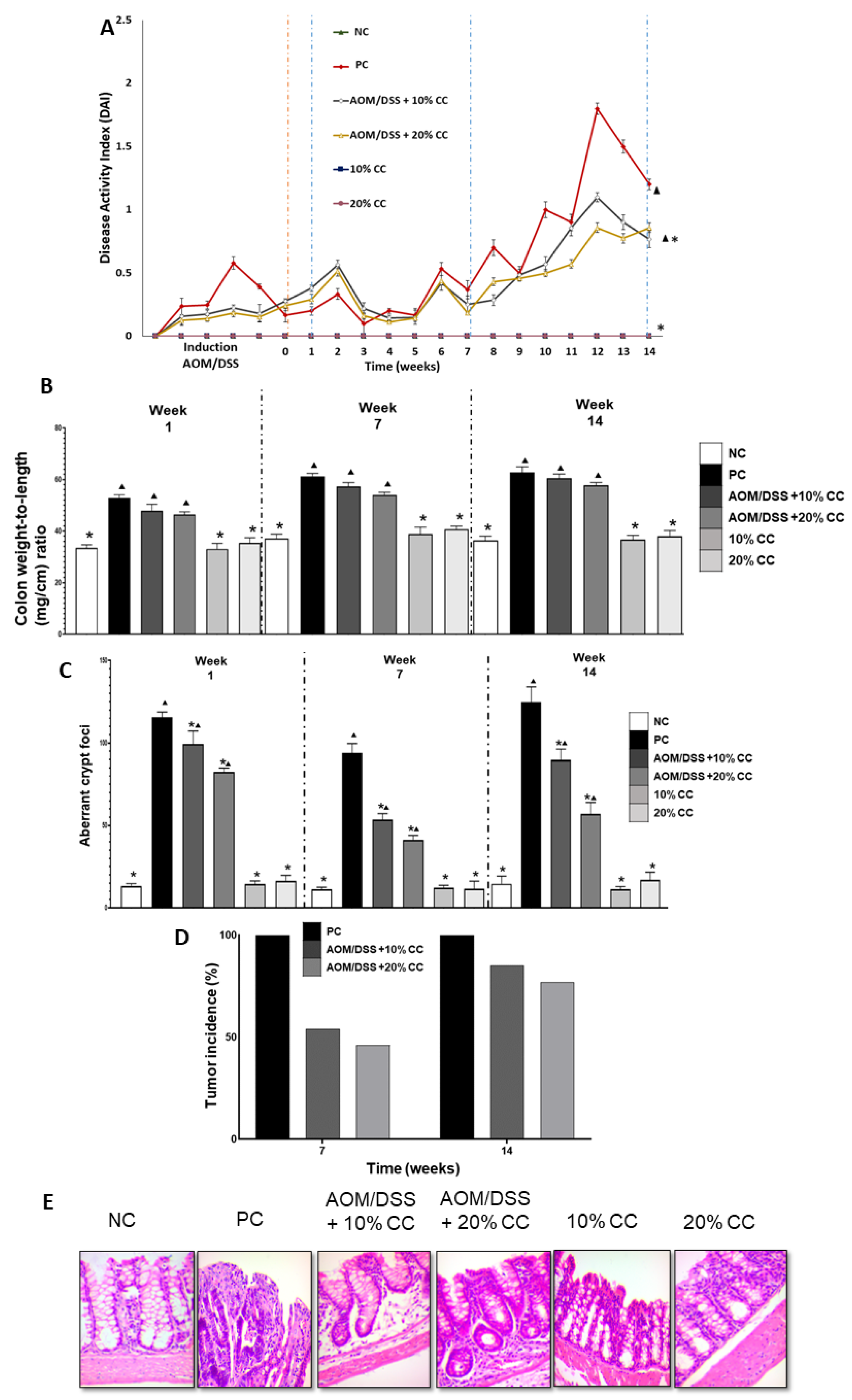
2.2.1. Disease Activity Index (DAI)
2.2.2. Colon Weight to Length Ratio (Colon Wt/L Ratio)
2.2.3. Number of Aberrant Crypt Foci (ACF)
2.2.4. Tumor Incidence
2.2.5. Histological Examination
2.3. Quantification of AgNOR, PCNA, β-Catenin, iNOS, and COX-2
2.3.1. Proliferation Markers: AgNOR, PCNA, and β-Catenin
2.3.2. Inflammation Markers: iNOS and COX-2
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Chickpea (Cicer arietinum) Seed
4.3. Animals, Induction of Colon Cancer, and Diets
4.4. Toxicity Signs
4.5. Colon Weight/Length (mg/cm) Ratio
4.6. Number of Aberrant Crypt Foci (ACF)
4.7. Tumor Incidence
4.8. Histological Examination
4.9. Quantification of AgNOR, PCNA, β-Catenin, iNOS, and COX-2
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GCO. Cancer Today: Fact Sheets. Available online: https://gco.iarc.fr/today/ (accessed on 2 November 2022).
- PAHO/WHO. Colorectal Cancer. Available online: https://www3.paho.org/hq/index.php?option=com_content&view=article&id=11761:colorectal-cancer&Itemid=41765&lang=en#gsc.tab=0 (accessed on 10 September 2022).
- Zhou, H.; Huang, T.; Xiong, Y.; Peng, L.; Wang, R.; Zhang, G.J. The prognostic value of proliferating cell nuclear antigen expression in colorectal cancer: A meta-analysis. Medicine 2018, 97, e13752. [Google Scholar] [CrossRef] [PubMed]
- Duchartre, Y.; Kim, Y.-M.; Kahn, M. The Wnt signaling pathway in cancer. Crit. Rev. Oncol. Hematol. 2016, 99, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Savithri, V.; Sudha, S.; Shameena, P.M.; Varghese, I. A clinicopathologic study of odontogenic Keratocyst (okc) and the role of AgNORs in cell proliferation. Oral. Maxillofac. Pathol. J. 2010, 1, 976–1225. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.-J.; Yeom, Y.; Kim, Y.-S.; Kim, E.; Shin, J.-H.; Seok, P.R.; Woo, M.J.; Kim, Y. Effect of vitamin C on azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced colitis-associated early colon cancer in mice. Nutr. Res. Pract. 2018, 12, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Kohno, H.; Suzuki, R.; Yamada, Y.; Sugie, S.; Mori, H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003, 94, 965–973. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, S.; Xu, Y.; Sheng, S.; Qian, S.Y.; Huo, X. Nitric oxide synthase inhibitors 1400 W and L-NIO inhibit angiogenesis pathway of colorectal cancer. Nitric Oxide 2019, 83, 33–39. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western diet and the immune system: An inflammatory connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, P.; Langman, J.S. ABC of colorectal cancer: Epidemiology. BMJ 2000, 321, 805–808. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Oomah, B.D.; Loarca-Piña, G.; Vergara-Castañeda, H.A. Common Beans and Their Non-Digestible Fraction: Cancer Inhibitory Activity—An Overview. Foods 2013, 2, 374–392. [Google Scholar] [CrossRef] [Green Version]
- León-Espinosa, E.B.; Sánchez-Chino, X.; Garduño-Siciliano, L.; Álvarez-González, R.I.; Dávila-Ortiz, G.; Madrigal-Bujaidar, E.; Téllez-Medina, D.I.; Jiménez-Martínez, C. Hypocholesterolemic and Anticarcinogenic Effect of Vicia faba Protein Hydrolyzates. Nutr. Cancer 2016, 68, 856–864. [Google Scholar] [CrossRef]
- Sánchez-Chino, X.M.; Jiménez Martínez, C.; León-Espinosa, E.B.; Garduño-Siciliano, L.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Vásquez-Garzón, V.R.; Baltiérrez-Hoyos, R.; Dávila-Ortiz, G. Protective effect of chickpea protein hydrolysates on colon carcinogenesis associated with a hypercaloric diet. J. Am. Coll. Nutr. 2018, 38, 162–170. [Google Scholar] [CrossRef]
- Borzì, A.M.; Biondi, A.; Basile, F.; Luca, S.; Vicari, E.S.D.; Vacante, M. Olive Oil Effects on Colorectal Cancer. Nutrients 2018, 11, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storniolo, C.E.; Martínez-Hovelman, N.; Martínez-Huélamo, M.; Lamuela-Raventos, R.M.; Moreno, J.J. Extra Virgin Olive Oil Minor Compounds Modulate Mitogenic Action of Oleic Acid on Colon Cancer Cell Line. J. Agric. Food Chem. 2019, 67, 11420–11427. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Overy, A.J.; Büsselberg, D. Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression. Biomolecules 2020, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Wu, R.; Gaspar, J.M.; Sargsyan, D.; Su, Z.Y.; Zhang, C.; Gao, L.; Cheng, D.; Li, W.; Wang, C.; et al. DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis-accelerated colon cancer in mice. Carcinogenesis 2018, 39, 669–680. [Google Scholar] [CrossRef] [Green Version]
- de Camargo, A.C.; Favero, B.T.; Morzelle, M.C.; Franchin, M.; Alvarez-Parrilla, E.; de la Rosa, L.A.; Geraldi, M.V.; Maróstica Júnior, M.R.; Shahidi, F.; Schwember, A.R. Is Chickpea a Potential Substitute for Soybean? Phenolic Bioactives and Potential Health Benefits. Int. J. Mol. Sci. 2019, 20, 2644. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Liu, C.; Sathe, S.K. Quality of a chickpea-based high protein snack. J. Food Sci. 2019, 84, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Megías, C.; Cortés-Giraldo, I.; Alaiz, M.; Vioque, J.; Girón-Calle, J. Isoflavones in chickpea (Cicer arietinum) protein concentrates. J. Funct. Foods 2016, 21, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, H.; Vasconcelos, M.; Gil, A.M.; Pinto, E. Benefits of pulse consumption on metabolism and health: A systematic review of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; De Souza, R.J.; Choo, V.L.; Ha, V.; Cozma, A.I.; Chiavaroli, L.; Mirrahimi, A.; Blanco Mejia, S.; Di Buono, M.; Bernstein, A.M. Effects of dietary pulse consumption on body weight: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 1213–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGinley, J.N.; Fitzgerald, V.K.; Neil, E.S.; Omerigic, H.M.; Heuberger, A.L.; Weir, T.L.; McGee, R.; Vandemark, G.; Thompson, H.J. Pulse Crop Effects on Gut Microbial Populations, Intestinal Function, and Adiposity in a Mouse Model of Diet-Induced Obesity. Nutrients 2020, 12, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Chino, X.M.; Jiménez-Martínez, C.; Vásquez-Garzón, V.R.; Álvarez-González, I.; Villa-Treviño, S.; Madrigal-Bujaidar, E.; Dávila-Ortiz, G.; Baltiérrez-Hoyos, R. Cooked chickpea consumption inhibits colon carcinogenesis in mice induced with azoxymethane and dextran sulfate sodium. J. Am. Coll. Nutr. 2017, 36, 391–398. [Google Scholar] [CrossRef]
- Tanaka, T. Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int. J. Inflam 2012, 2012, 658786. [Google Scholar] [CrossRef] [Green Version]
- Parang, B.; Barrett, C.W.; Williams, C.S. AOM/DSS Model of Colitis-Associated Cancer. Methods Mol. Biol. 2016, 1422, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Murillo, G.; Choi, U.K.; Pan, O.; Constantinou, A.I.; Mehta, R.G. Efficacy of garbanzo and soybean flour in suppression of aberrant crypt foci in the colons of CF-1 mice. Anticancer. Res. 2004, 24, 3049–3056. [Google Scholar]
- Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Campos-Vega, R.; Gallegos-Corona, M.A.; González de Mejía, E.; Loarca-Piña, G. Physicochemical and nutraceutical properties of moringa (Moringa oleifera) leaves and their effects in an in vivo AOM/DSS-induced colorectal carcinogenesis model. Food Res. Int. 2018, 105, 159–168. [Google Scholar] [CrossRef]
- Elimrani, I.; Koenekoop, J.; Dionne, S.; Marcil, V.; Delvin, E.; Levy, E.; Seidman, E.G. Vitamin D reduces colitis-and inflammation-associated colorectal cancer in mice independent of NOD2. Nutr. Cancer 2017, 69, 276–288. [Google Scholar] [CrossRef]
- Silva, K.A.; Dong, J.; Dong, Y.; Dong, Y.; Schor, N.; Tweardy, D.J.; Zhang, L.; Mitch, W.E. Inhibition of Stat3 activation suppresses caspase-3 and the ubiquitin–proteasome system, leading to preservation of muscle mass in cancer cachexia. J. Biol. Chem. 2015, 290, 11177–11187. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.H.; Kim, N.; Shim, Y.K.; Choi, Y.J.; Nam, R.H.; Choi, Y.J.; Ham, M.H.; Suh, J.H.; Lee, S.M.; Lee, C.M.; et al. Adequate Dextran Sodium Sulfate-induced Colitis Model in Mice and Effective Outcome Measurement Method. J. Cancer Prev. 2015, 20, 260–267. [Google Scholar] [CrossRef]
- Shi, N.; Clinton, S.K.; Liu, Z.; Wang, Y.; Riedl, K.M.; Schwartz, S.J.; Zhang, X.; Pan, Z.; Chen, T. Strawberry Phytochemicals Inhibit Azoxymethane/Dextran Sodium Sulfate-Induced Colorectal Carcinogenesis in Crj: CD-1 Mice. Nutrients 2015, 7, 1696–1715. [Google Scholar] [CrossRef] [Green Version]
- Monk, J.M.; Lepp, D.; Wu, W.; Graf, D.; McGillis, L.H.; Hussain, A.; Carey, C.; Robinson, L.E.; Liu, R.; Tsao, R.; et al. Chickpea-supplemented diet alters the gut microbiome and enhances gut barrier integrity in C57Bl/6 male mice. J. Funct. Foods 2017, 38, 663–674. [Google Scholar] [CrossRef]
- Ju, J.; Lee, G.-Y.; Kim, Y.-S.; Chang, H.K.; Do, M.-S.; Park, K.-Y. Bamboo salt suppresses colon carcinogenesis in C57BL/6 mice with chemically induced colitis. J. Med. Food 2016, 19, 1015–1022. [Google Scholar] [CrossRef]
- Suzuki, R.; Kohno, H.; Sugie, S.; Nakagama, H.; Tanaka, T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis 2006, 27, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Bobe, G.; Barrett, K.G.; Mentor-Marcel, R.A.; Saffiotti, U.; Young, M.R.; Colburn, N.H.; Albert, P.S.; Bennink, M.R.; Lanza, E. Dietary cooked navy beans and their fractions attenuate colon carcinogenesis in azoxymethane-induced ob/ob mice. Nutr. Cancer 2008, 60, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Luna-Vital, D.A.; González-de Mejía, E.; Loarca-Piña, G. Dietary peptides from Phaseolus vulgaris L. reduced AOM/DSS-induced colitis-associated colon carcinogenesis in Balb/c mice. Plant Food Hum. Nutr. 2017, 72, 445–447. [Google Scholar] [CrossRef]
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal carcinoma: Pathologic aspects. J. Gastrointest. Oncol. 2012, 3, 153. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, L.; Danilewicz, M.; Fendler, W.; Suzin, J.; Spych, M.; Piekarski, J.; Tylinski, W.; Chalubinska, J.; Topczewska-Tylinska, K.; Cialkowska-Rysz, A. The AgNORs count in predicting long-term survival in serous ovarian cancer. Arch. Med. Sci. 2014, 10, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, P.; Sudhandiran, G. Luteolin inhibits cell proliferation during Azoxymethane-induced experimental colon carcinogenesis via Wnt/β-catenin pathway. Invest. New Drugs 2011, 29, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Girón-Calle, J.; Vioque, J.; del Mar Yust, M.; Pedroche, J.; Alaiz, M.; Millán, F. Effect of chickpea aqueous extracts, organic extracts, and protein concentrates on cell proliferation. J. Med. Food 2004, 7, 122–129. [Google Scholar] [CrossRef]
- Qasim, B.J.; Ali, H.H.; Hussein, A.G. Immunohistochemical expression of PCNA and CD34 in colorectal adenomas and carcinomas using specified automated cellular image analysis system: A clinicopathologic study. Saudi J. Gastroentero. 2012, 18, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Guajardo-Flores, D.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Evaluation of the antioxidant and antiproliferative activities of extracted saponins and flavonols from germinated black beans (Phaseolus vulgaris L.). Food Chem. 2013, 141, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Girón-Calle, J.; Alaiz, M.; Vioque, J. Effect of chickpea protein hydrolysates on cell proliferation and in vitro bioavailability. Food Res. Int. 2010, 43, 1365–1370. [Google Scholar] [CrossRef]
- Magee, P.J.; Owusu-Apenten, R.; McCann, M.J.; Gill, C.I.; Rowland, I.R. Chickpea (Cicer arietinum) and other plant-derived protease inhibitor concentrates inhibit breast and prostate cancer cell proliferation in vitro. Nutr. Cancer 2012, 64, 741–748. [Google Scholar] [CrossRef]
- Tanaka, H.; Kawaguchi, M.; Takano, H.; Shoda, S.; Miyoshi, T.; Iwasaki, R.; Hyodo, F.; Tomita, H.; Mori, T.; Hara, A. Activation of the Wnt/β-Catenin Signaling Pathway Causes Radioresistance in Colon Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, E682–E683. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Sharma, V.K.; Yadav, S.; Dey, S. Antiproliferative and apoptotic effects of black turtle bean extracts on human breast cancer cell line through extrinsic and intrinsic pathway. Chem. Cent. J. 2017, 11, 56. [Google Scholar] [CrossRef] [Green Version]
- Feregrino-Pérez, A.A.; Berumen, L.C.; García-Alcocer, G.; Guevara-Gonzalez, R.G.; Ramos-Gomez, M.; Reynoso-Camacho, R.; Acosta-Gallegos, J.A.; Loarca-Piña, G. Composition and chemopreventive effect of polysaccharides from common beans (Phaseolus vulgaris L.) on azoxymethane-induced colon cancer. J. Agric. Food Chem. 2008, 56, 8737–8744. [Google Scholar] [CrossRef]
- Canani, R.B.; Di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenet. 2012, 4, 4. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Comparative study on antiproliferation properties and cellular antioxidant activities of commonly consumed food legumes against nine human cancer cell lines. Food Chem. 2012, 134, 1287–1296. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Rao, C.V. Chemoprevention of Colon Cancer by iNOS-Selective Inhibitors. Immunopathol. Dis. Ther. 2012, 3, 155–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazewski, C.; Luna-Vital, D.; Berhow, M.; Gonzalez de Mejia, E. Reduction of colitis-associated colon carcinogenesis by a black lentil water extract through inhibition of inflammatory and immunomodulatory cytokines. Carcinogenesis 2020, 41, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Chung, K.-S.; Hwang, S.-J.; Yoon, Y.S.; Jang, Y.P.; Lee, J.K.; Lee, K.-T. Protective Effect of Cicer arietinum L. (Chickpea) Ethanol Extract in the Dextran Sulfate Sodium-Induced Mouse Model of Ulcerative Colitis. Nutrients 2020, 12, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Monk, J.M.; Lu, J.T.; Zarepoor, L.; Wu, W.; Liu, R.; Pauls, K.P.; Wood, G.A.; Robinson, L.; Tsao, R. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br. J. Nutr. 2014, 111, 1549–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpon, A.; Riezu-Boj, J.I.; Milagro, F.I.; Marti, A.; Razquin, C.; Martínez-González, M.A.; Corella, D.; Estruch, R.; Casas, R.; Fitó, M. Adherence to Mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. J. Physiol. Biochem. 2017, 73, 445–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef]
- Kim, B.H.; Chung, E.Y.; Min, B.-K.; Lee, S.H.; Kim, M.-K.; Min, K.R.; Kim, Y. Anti-inflammatory action of legume isoflavonoid sophoricoside through inhibition on cyclooxygenase-2 activity. Planta Med. 2003, 69, 474–476. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Paterson, J.; Bucknall, M.; Arcot, J. Interactions between phytochemicals from fruits and vegetables: Effects on bioactivities and bioavailability. Crit. Rev. Food Sci. Nutr. 2018, 58, 1310–1329. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, Y.; Shi, Z.; Everaert, N.; Ren, G. Synergistic Effect of Bioactive Anticarcinogens from Soybean on Anti-Proliferative Activity in MDA-MB-231 and MCF-7 Human Breast Cancer Cells In Vitro. Molecules 2018, 23, 1557. [Google Scholar] [CrossRef] [Green Version]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: A review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Cid-Gallegos, M.S.; Sánchez-Chino, X.M.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Vásquez-Garzón, V.R.; Baltiérrez-Hoyos, R.; Villa-Treviño, S.; Dávila-Ortíz, G.; Jiménez-Martínez, C. Modification of In Vitro and In Vivo Antioxidant Activity by Consumption of Cooked Chickpea in a Colon Cancer Model. Nutrients 2020, 12, 2572. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-González, I.; Garcia-Melo, F.; Vásquez-Garzón, V.R.; Villa-Treviño, S.; Madrigal-Santillán, E.; Morales-González, J.A.; Mendoza-Pérez, J.A.; Madrigal-Bujaidar, E. Evaluation of blueberry juice in mouse azoxymethane-induced aberrant crypts and oxidative damage. Evid. Based Complement. Altern. Med. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Trerè, D. AgNOR staining and quantification. Micron 2000, 31, 127–131. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Week 0 | Week 1 | Week 7 | Week 14 |
|---|---|---|---|---|
| Body weight (g) | ||||
| NC | 22.68 ± 0.31 | 25.40 ± 0.11 | 26.94 ± 0.25 | 28.9 ± 0.20 * |
| PC | 22.51 ± 0.26 | 24.01 ± 0.30 | 26.13 ± 0.13 | 20.47 ± 0.46 ▲ |
| AOM/DSS + 10% CC | 22.36 ± 0.46 | 25.46 ± 0.65 | 25.32 ± 0.38 | 25.91 ± 0.20 * |
| AOM/DSS + 20% CC | 22.55 ± 0.73 | 25.84 ± 0.61 | 25.70 ± 0.37 | 25.10 ± 0.48 * |
| 10% CC | 22.45 ± 0.23 | 26.55 ± 0.41 | 28.58 ± 0.18 | 30.10 ± 0.16 * |
| 20% CC | 22.61 ± 0.25 | 24.63 ± 0.44 | 26.71 ± 0.10 | 27.72 ± 0.25 * |
| % | |||||
|---|---|---|---|---|---|
| Group | Treatment | Normal | Dysplasia | Adenoma | Adenocarcinoma |
| 1 | NC | 100 | 0 | 0 | 0 |
| 2 | PC | 4.2 ± 3.7 a | 39.6 ± 3.2 a | 26.1 ± 8.1 c | 29.8 ± 12.1 c |
| 3 | AOM + 10% CC | 40.1 ± 4.0 b | 51 ± 5.0 c | 4.3 ± 5.0 b | 4.5 ± 4.7 b |
| 4 | AOM + 20% CC | 45.3 ± 4.0 c | 47.4 ± 1.3 b | 3.8 ± 2.8 a | 2.6 ± 1.8 a |
| 5 | 10% CC | 100 | 0 | 0 | 0 |
| 6 | 20% CC | 100 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cid-Gallegos, M.S.; Jiménez-Martínez, C.; Sánchez-Chino, X.M.; Madrigal-Bujaidar, E.; Vásquez-Garzón, V.R.; Baltiérrez-Hoyos, R.; Álvarez-González, I. Chemopreventive Effect of Cooked Chickpea on Colon Carcinogenesis Evolution in AOM/DSS-Induced Balb/c Mice. Plants 2023, 12, 2317. https://doi.org/10.3390/plants12122317
Cid-Gallegos MS, Jiménez-Martínez C, Sánchez-Chino XM, Madrigal-Bujaidar E, Vásquez-Garzón VR, Baltiérrez-Hoyos R, Álvarez-González I. Chemopreventive Effect of Cooked Chickpea on Colon Carcinogenesis Evolution in AOM/DSS-Induced Balb/c Mice. Plants. 2023; 12(12):2317. https://doi.org/10.3390/plants12122317
Chicago/Turabian StyleCid-Gallegos, María Stephanie, Cristian Jiménez-Martínez, Xariss M. Sánchez-Chino, Eduardo Madrigal-Bujaidar, Verónica R. Vásquez-Garzón, Rafael Baltiérrez-Hoyos, and Isela Álvarez-González. 2023. "Chemopreventive Effect of Cooked Chickpea on Colon Carcinogenesis Evolution in AOM/DSS-Induced Balb/c Mice" Plants 12, no. 12: 2317. https://doi.org/10.3390/plants12122317
APA StyleCid-Gallegos, M. S., Jiménez-Martínez, C., Sánchez-Chino, X. M., Madrigal-Bujaidar, E., Vásquez-Garzón, V. R., Baltiérrez-Hoyos, R., & Álvarez-González, I. (2023). Chemopreventive Effect of Cooked Chickpea on Colon Carcinogenesis Evolution in AOM/DSS-Induced Balb/c Mice. Plants, 12(12), 2317. https://doi.org/10.3390/plants12122317






