Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era
Abstract
1. Introduction
2. Methodology
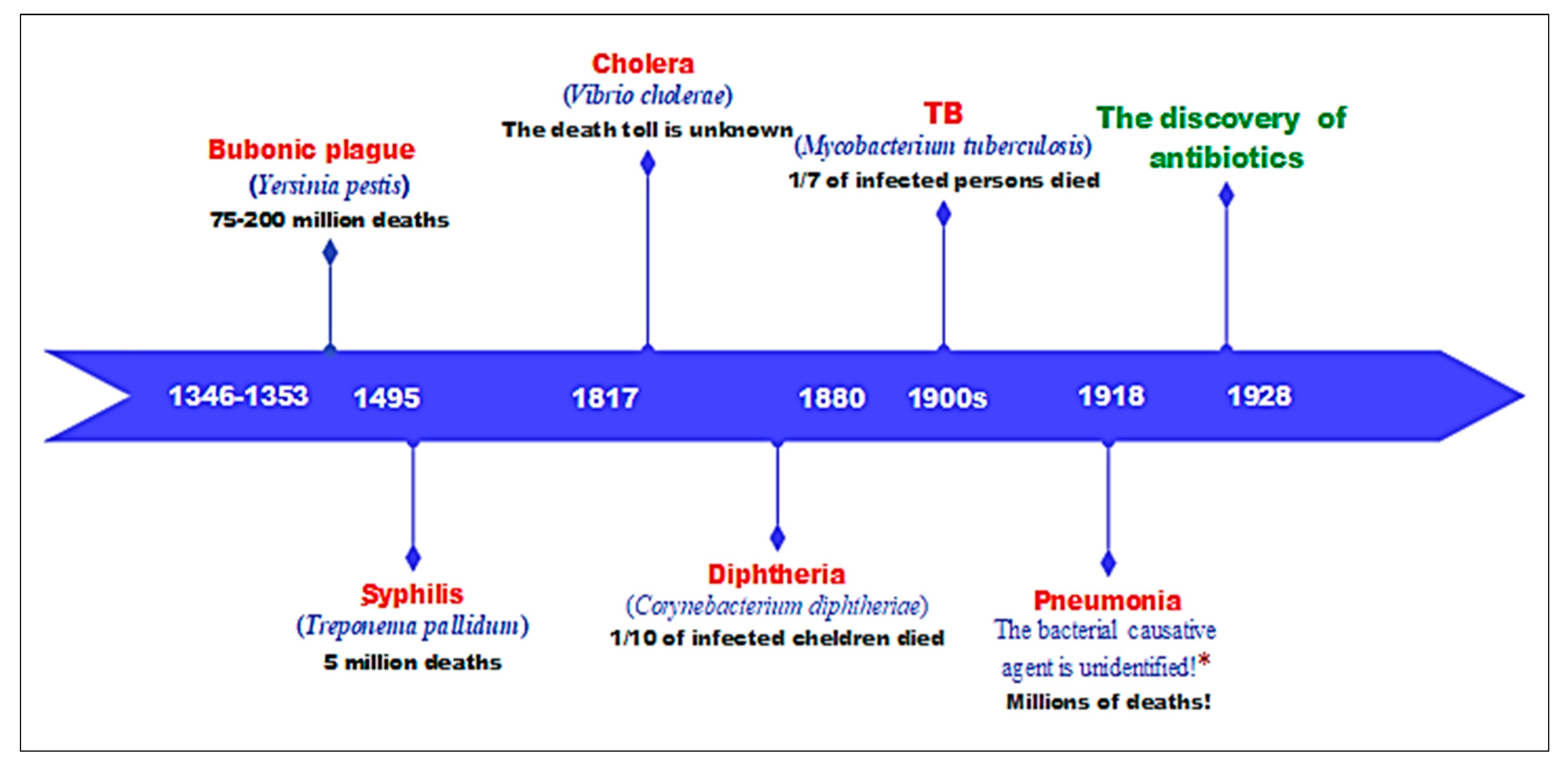
3. The Pre-Antibiotic Era
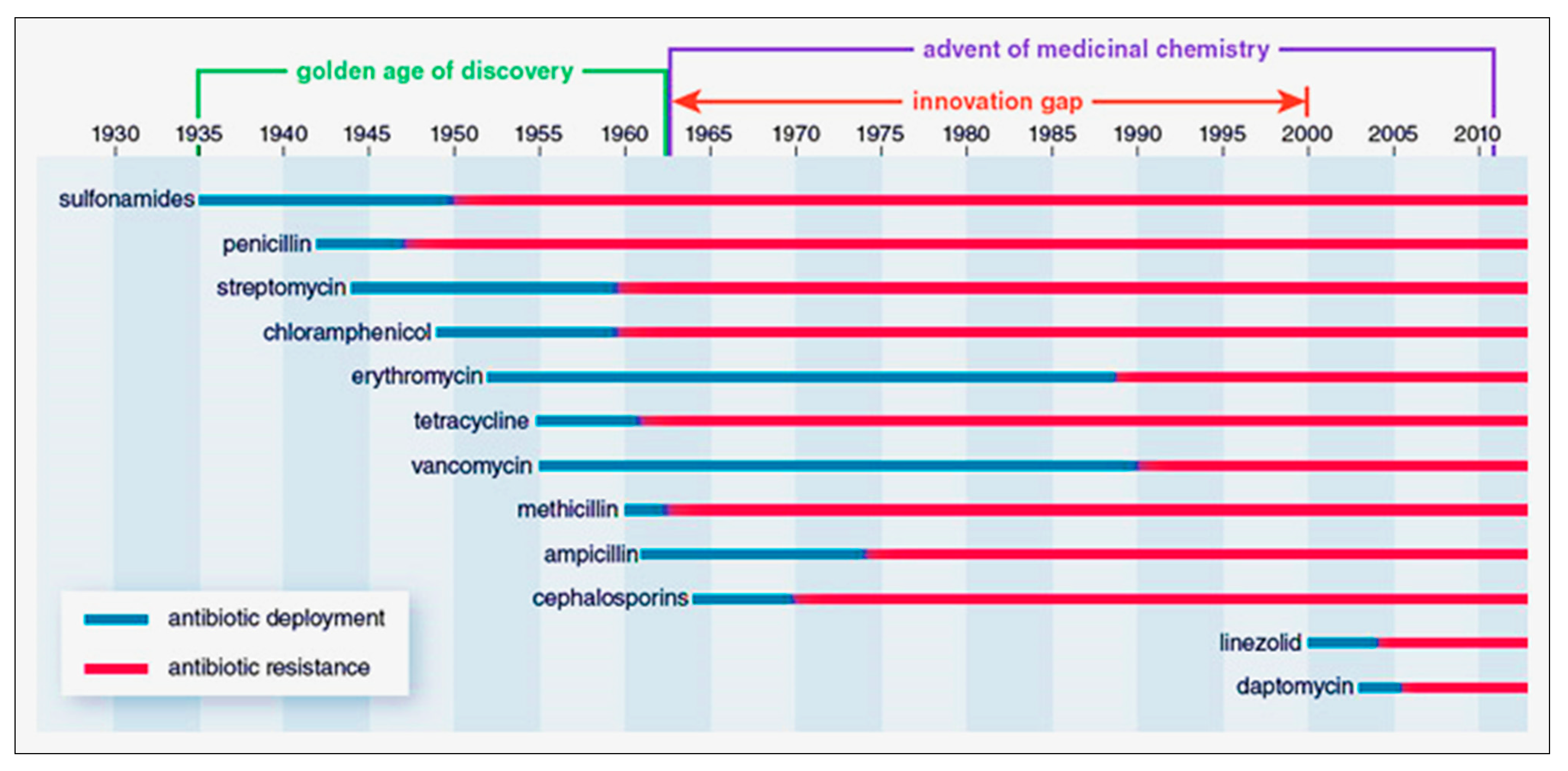
4. The Golden Era of Antibiotics
5. The Post-Antibiotic Era
6. Antibiotics at a Crossroads: Unraveling the Missteps
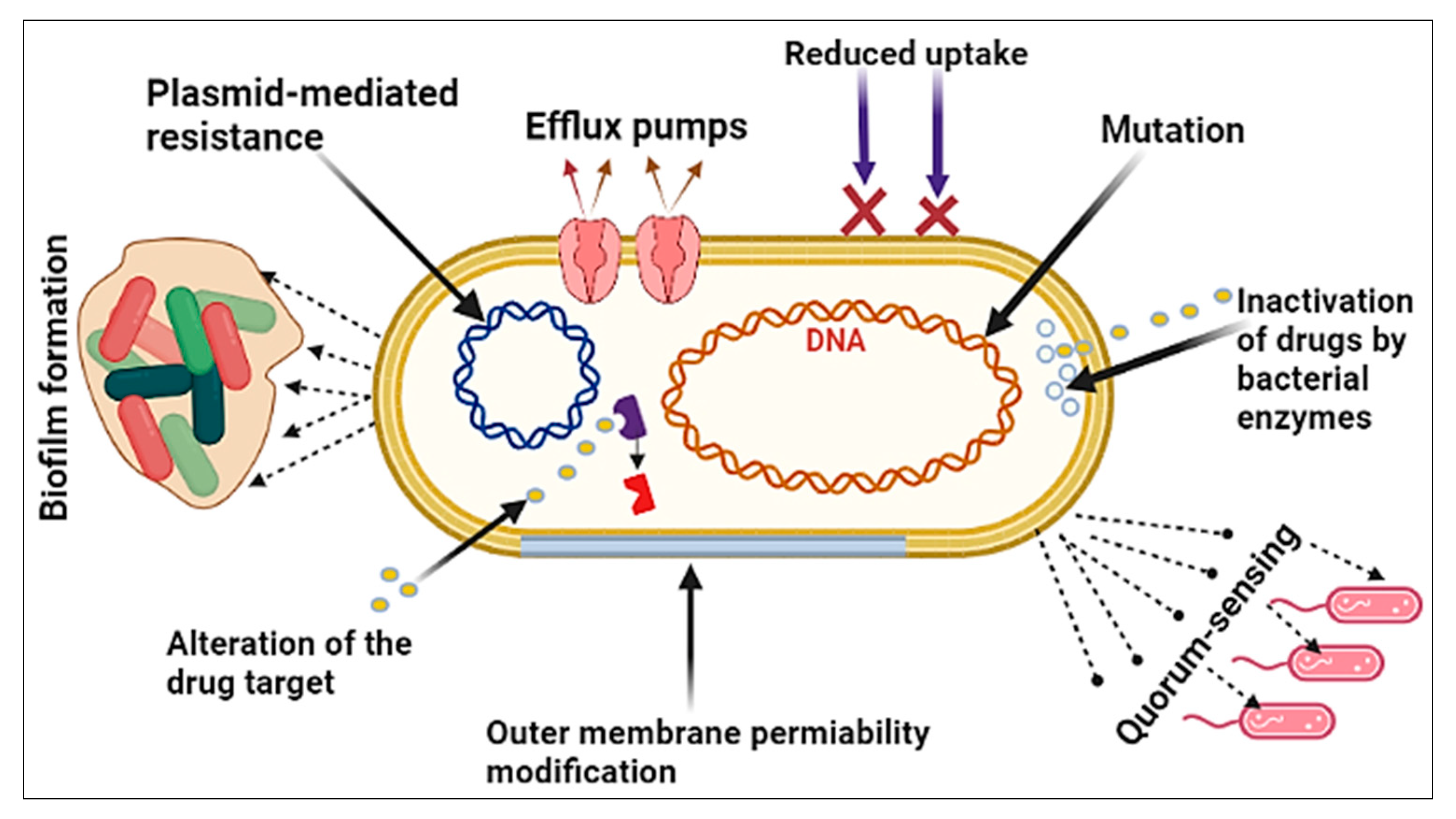
6.1. The Bacterial Factor
- (1)
- Mutation: A spontaneous alteration in the DNA sequence of the gene may affect the trait for which it codes. A single base-pair change may alter the expression of one or more amino acids, thereby changing the enzyme or cell structure and resulting in resistance to the targeted antibiotic [66].
- (2)
- Plasmid-mediated resistance: The spread of antibiotic resistance genes that are on plasmids. The plasmids can be moved between bacteria of the same species or between bacteria of different species by conjugation. Plasmids often have a lot of different antibiotic resistance genes on them, which help spread multidrug-resistant (MDR) bacteria. Antibiotic resistance caused by MDR plasmids severely limits the treatment options for infections caused by Gram-negative bacteria [67].
- (3)
- Biofilm formation: Bacteria that stick to damaged tissue or transplanted medical devices often enclose themselves in a wet matrix of polysaccharides and peptides, forming a slimy coating called a biofilm. The biofilm’s resistance to antibiotics is dependent on complex multicellular mechanisms [68].
- (4)
- Quorum-sensing: A bacterial communication strategy that is dependent on the bacterial population density. It entails the use of tiny dissolved signaling molecules to stimulate the expression of a large number of genes that regulate a wide variety of activities, including antibiotic resistance [69]. This process has been clearly shown in the development of resistance when Pseudomonas aeruginosa moves to a new niche and is exposed to antibiotics [70].
- (5)
- Outer membrane permeability modification: The outer membrane of Gram-negative bacteria, in particular, acts as a strong barrier to antibacterial treatments. Antibiotics may pass through the outer membrane in two general ways: through a lipid-mediated pathway for hydrophobic antibiotics or through general diffusion porins for hydrophilic antibiotics. Bacteria are extremely efficient at using both of these mechanisms to enhance their resistance to antibiotics through modifications to these macromolecules [71].
- (6)
- Efflux pumps: Bacteria use this process to expel harmful solutes (e.g., antibiotics) from the cell. Antibiotic efflux in bacteria was first reported in the 1970s for tetracyclines. Since then, multidrug efflux pumps have received a lot of attention, and this pathway has been found in a variety of MDR bacterial species, particularly in Gram-negative bacteria [72].
- (7)
- Reduced uptake: Antibiotic-resistant bacteria reduce membrane permeability in one of two ways: by increasing the efflux or by decreasing the uptake. The reduced uptake (decreasing uptake) was shown to be responsible for beta-lactam resistance in Gram-negative bacteria, as beta-lactams need to penetrate the periplasmic region to bind the penicillin-binding protein targets located in the cytoplasmic membrane [71].
- (8)
- (9)
- Alternation of the antibiotic target: Antibiotic target-site alteration is a frequent mechanism of resistance; it occurs to evade the antibiotic’s effect by interfering with its target site. To do this, bacteria have developed a variety of strategies, including target protection (preventing the antibiotic from reaching its receptor) and changes via mutation of the target site, resulting in a lower sensitivity to the drug [75].
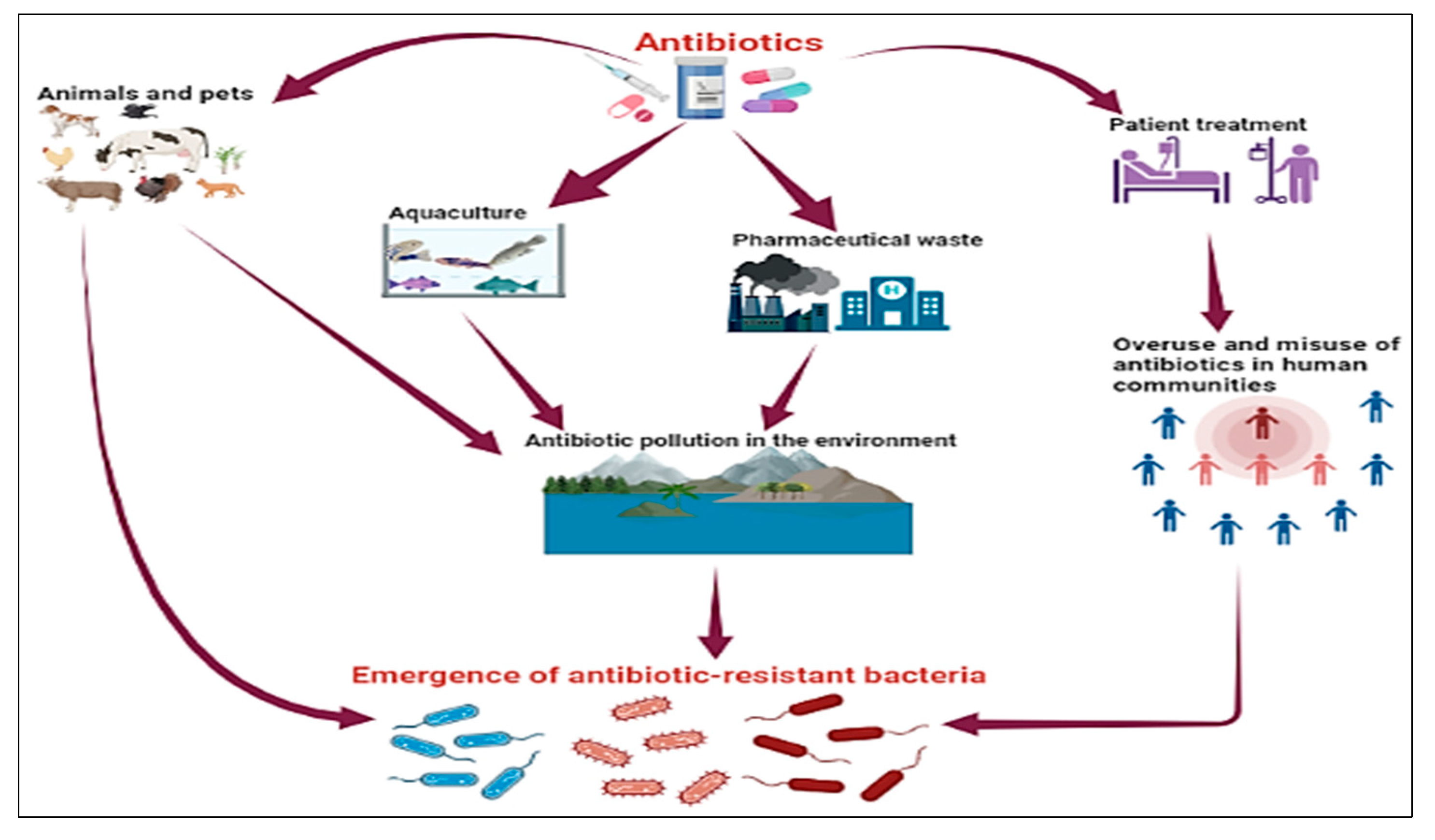
6.2. The Human Factor
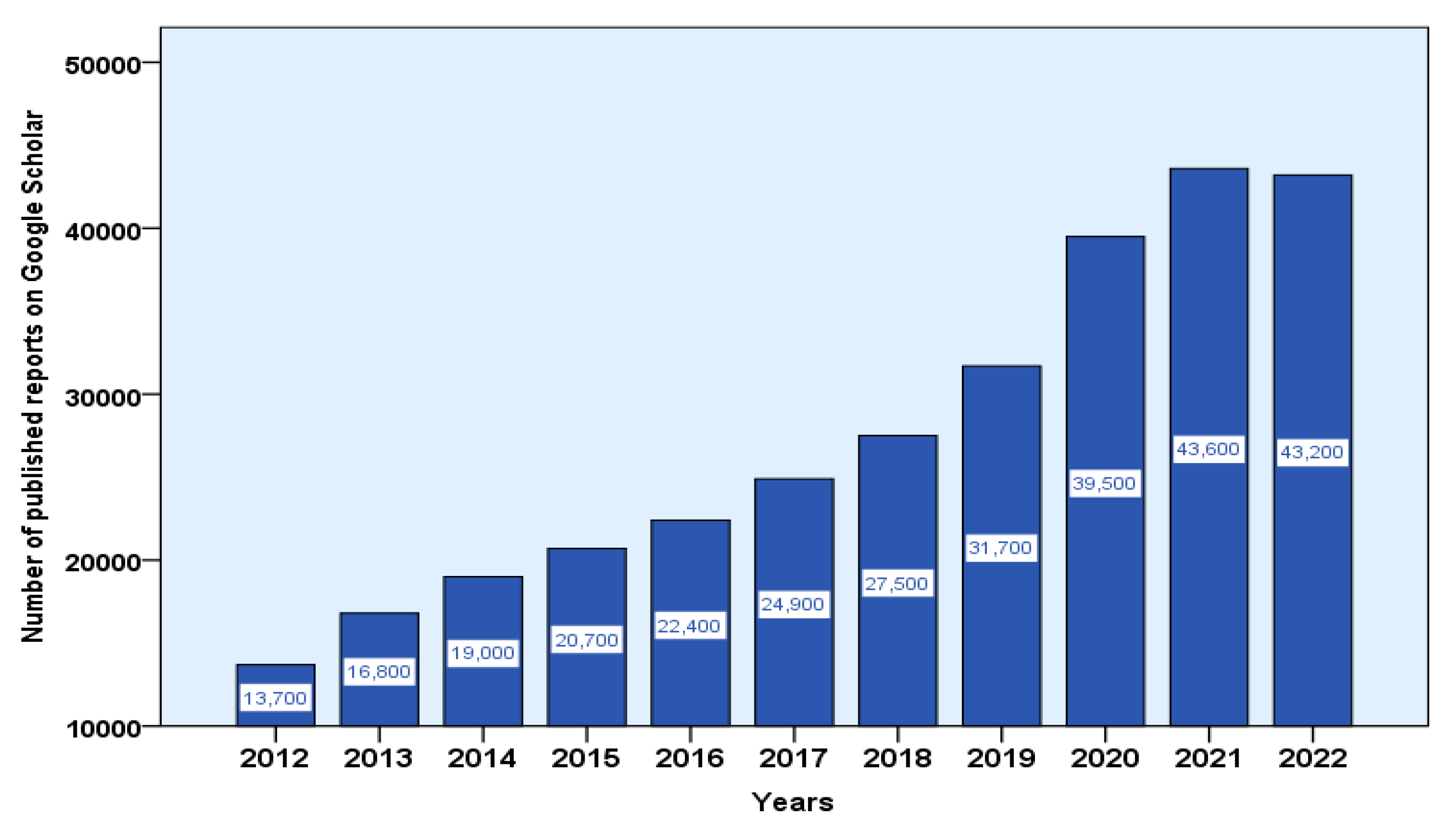
7. Emerging Global Focus on Therapeutic Applications of Medicinal Plants
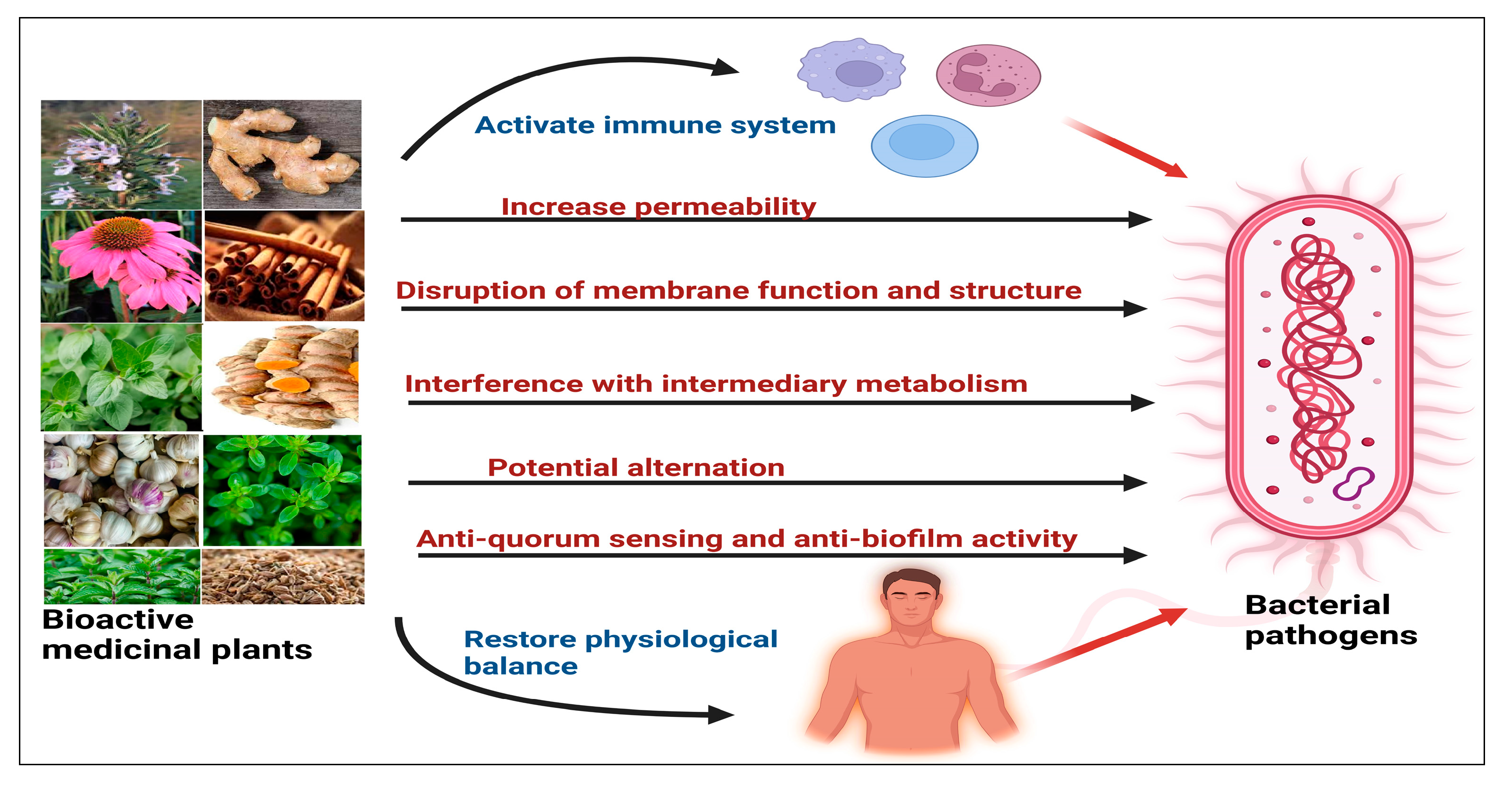
8. The Mechanisms of Plant-Derived Antibacterial Agents
9. Major Phytochemical Classes with Potent Antibacterial Activity
9.1. Phenolic Compounds
9.2. Alkaloids
9.3. Saponins
9.4. Terpenoids
9.5. Other Compounds
10. Medicinal Plants versus Antibiotics
- (i)
- Medicinal plants offer advantages over conventional antibiotics in terms of availability and cost-effectiveness. They are easily accessible and more economical compared to large-scale antibiotic production, as they do not require extensive and expensive chemical and pharmaceutical procedures. In contrast, synthesizing new antibiotics is a complex and time-consuming process that is prone to setbacks and high costs. Developing a novel antibiotic typically demands significant resources, taking 10 to 15 years and costing over USD one billion [126,127].
- (ii)
- Herbal medicines interact safely with the body’s vital systems, exhibiting minimal side effects. They are efficiently eliminated through the excretory system and often have synergistic effects that promote physiological balance. In contrast, many antibiotics are either semisynthetic derivatives/chemically synthesized, with potential negative side effects and a risk of contributing to antibiotic resistance with frequent use [128,129].
- (iii)
- (iv)
- Medicinal plant products pose minimal pollution risks and can be extracted using eco-friendly methods. In contrast, antibiotics necessitate reduced usage due to their negative effects on soil and water pollution. The annual manufacturing and residue discharge of antibiotics contribute significantly to a pollution load estimated between 100,000 and 200,000 tons [131,132].
- (v)
- Medicinal plants exhibit multiple complementary and synergistic mechanisms of action, rendering them highly promising for addressing antibiotic-resistant bacteria. In contrast, pathogenic bacteria have developed diverse mechanisms and strategies to significantly evade the effectiveness of antibiotics that rely on a single molecule [133,134].
10.1. Safety of Antibacterial Phytochemicals
10.2. Effectiveness of Plant-Based Compounds
10.3. Pharmaceutical Company Contentment
- (i)
- The high cost of synthesizing novel antibacterial chemical compounds compared to the low cost of manufacturing antibacterial agents from natural products [138].
- (ii)
- Large pharmaceutical corporations have departed the market of synthetic antibiotics due to a lack of financial incentives and profits [139].
- (iii)
- Synthetic antibiotics have not been able to stop the spread of bacterial pathogens that have become highly resistant [140].
- (iv)
- The abundance of phytochemical molecules isolated from medicinal plants that are powerful against bacterial infections and have been scientifically confirmed [87].
- (v)
- New advances in biotechnology make it possible to manufacture novel antibacterial drugs from plants with great efficiency [1].
11. Plants Exhibiting Antibacterial Potential against WHO-Designated High-Priority Pathogens
12. Synergistic Interactions of Phytochemicals against Bacterial Pathogens
12.1. The Synergistic Activity of Plant Molecules with Antibiotics
12.2. Synergistic Combinations of Bioactive Plant Molecules
12.3. The Synergistic Activity of Plant Molecules with Nanomaterials
13. Challenges in the Field of Plant-Based Drug Discovery
14. New Perspectives on Antibacterial Agents
15. Future Directions
- (i)
- AI-driven precision medicine augments health-related tasks, providing highly personalized diagnostic and therapeutic information. Tailoring antibacterial treatments using medicinal plants to an individual’s genetics, bacterial profile, and health conditions maximizes efficacy and minimizes antibiotic resistance. This approach aims to prevent infections, reduce the disease burden, and lower healthcare costs for all [187].
- (ii)
- Modernization and Integration of Traditional and Modern Medicine: In global healthcare and infection control, more recognition and respect will be gained by traditional medicine and indigenous knowledge. The integration of traditional herbal remedies into evidence-based medical practices will be facilitated by collaboration between medicinal plants and modern pharmacoepidemiology. Such integration is already being applied in some countries, such as China and India [188].
- (iii)
- Rise of Plant Biotechnology: The enhancement of the antibacterial properties of medicinal plants will be crucially influenced by biotechnology and bioinformatics. Metabolic Engineering Strategies will be utilized to enhance the production of bioactive compounds, making them more potent and effective against drug-resistant bacteria [189].
- (iv)
- Combination Therapies: A more prevalent approach will involve the use of medicinal plant combinations with synergistic antibacterial effects or with conventional antibiotics. Specific herbal mixtures that work together to combat bacterial infections more effectively than single compounds will be the focus of researchers [190].
- (v)
- Standardization and Quality Control: Significant efforts will be made to standardize the production and quality control of herbal drugs to ensure their safety and efficacy as antibacterial agents. Regulations and guidelines will be put in place to maintain consistency across different formulations [191].
- (vi)
- Alternative Delivery Systems: Innovative delivery systems, such as nanoencapsulation and targeted drug delivery will be employed to enhance the bioavailability and targeted action of medicinal plant compounds against bacterial infections [192].
- (vii)
- Regulatory Support and Incentives for Global Collaboration and Research Sharing: International collaboration among researchers, governments, and pharmaceutical companies will be considered essential for advancing medicinal plant research. Open-access databases will be instrumental in facilitating the sharing of knowledge and data to accelerate drug discovery [193].
16. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| AMR | Antimicrobial resistance |
| CNS | Central nervous system |
| CDC | Centers for Disease Control and Prevention |
| DNA | Deoxyribonucleic acid |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| G(–) | Gram-negative bacteria |
| G(+) | Gram-positive bacteria |
| MDR | Multidrug-resistant bacteria |
| RNA | Ribonucleic acid |
| TB | Tuberculosis |
| WHO | World Health Organization |
References
- Abdallah, E.M. Plants: An alternative source for antimicrobials. J. Appl. Pharm. Sci. 2011, 1, 16–20. [Google Scholar]
- Thomson, W.A.R.; Schultes, R.E. Medicines from the Earth; McGraw-Hill: New York, NY, USA, 1978. [Google Scholar]
- Galway-Witham, J.; Stringer, C. How did Homo sapiens evolve? Science 2018, 360, 1296–1298. [Google Scholar] [CrossRef]
- Klein, R.G. Population structure and the evolution of Homo sapiens in Africa. Evol. Anthropol. Issues News Rev. 2019, 28, 179–188. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1. [Google Scholar] [CrossRef]
- Dervendzi, V. Contemporary Treatment with Medicinal Plants; Tabernakul: Skopje, Republic of Macedonia, 1992; pp. 5–43. [Google Scholar]
- Cook, H.J. Physicians and natural history. Cult. Nat. Hist. 1996, 2, 91–105. [Google Scholar]
- Goodman, L.S.; Gilman, A. The Pharmacological Basis of Therapeutics: A Textbook of Pharmacology, Toxicology and Therapeutics for Physicians and Medical Students; Macmillan: New York, NY, USA, 1943. [Google Scholar]
- Conly, J.; Johnston, B. Where are all the new antibiotics? The new antibiotic paradox. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 159–160. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Schcolnik-Cabrera, A. Current Approaches to Overcome Antimicrobial Resistance. Curr. Med. Chem. 2023, 30, 3–4. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Smith, H.; Sweet, C. Cooperation between viral and bacterial pathogens in causing human respiratory disease. In Polymicrobial Diseases; Wiley: Hoboken, NJ, USA, 2002; pp. 199–212. [Google Scholar]
- Taubenberger, J.K.; Reid, A.H.; Fanning, T.G. The 1918 influenza virus: A killer comes into view. Virology 2000, 274, 241–245. [Google Scholar] [CrossRef]
- Huremović, D. Brief history of pandemics (pandemics throughout history). In Psychiatry of Pandemics; Springer: Berlin/Heidelberg, Germany, 2019; pp. 7–35. [Google Scholar]
- Vuorinen, H. Diseases in the Ancient World; Hippokrates: Helsinki, Finland, 1997; pp. 74–97. [Google Scholar]
- Dols, M.W. Plague in early Islamic history. J. Am. Orient. Soc. 1974, 94, 371–383. [Google Scholar] [CrossRef]
- Jaladat, A.; Mostafavi, E. Diagnosis, treatment and reports of plague outbreaks during Islamic civilization. J. Islam. Iran. Tradit. Med. 2017, 8, 223–230. [Google Scholar]
- Brundage, J.F.; Shanks, G.D. Deaths from bacterial pneumonia during 1918–19 influenza pandemic. Emerg. Infect. Dis. 2008, 14, 1193. [Google Scholar] [CrossRef]
- McEvedy, C. The bubonic plague. Sci. Am. 1988, 258, 118–123. [Google Scholar] [CrossRef]
- Akiva Lehrer, K. Towards a General Pedagogic Model of Plagues and Pandemics. Rev. Bus. Financ. Stud. 2020, 11, 41–76. [Google Scholar]
- Felman, Y.M. Syphilis: From 1495 Naples to 1989 AIDS. Arch. Dermatol. 1989, 125, 1698–1700. [Google Scholar] [CrossRef]
- Sarbu, I.; Matei, C.; Benea, V.; Georgescu, S. Brief history of syphilis. J. Med. Life 2014, 7, 4. [Google Scholar]
- McGrew, R.E. The first cholera epidemic and social history. Bull. Hist. Med. 1960, 34, 61–73. [Google Scholar]
- Barua, D. History of cholera. In Cholera; Springer: Berlin/Heidelberg, Germany, 1992; pp. 1–36. [Google Scholar]
- Davis, L.G. A diphtheria epidemic in the early eighties. Minn. Hist. 1934, 15, 434–438. [Google Scholar]
- Truelove, S.A.; Keegan, L.T.; Moss, W.J.; Chaisson, L.H.; Macher, E.; Azman, A.S.; Lessler, J. Clinical and epidemiological aspects of diphtheria: A systematic review and pooled analysis. Clin. Infect. Dis. 2020, 71, 89–97. [Google Scholar] [CrossRef]
- Daniel, T.M.; Bates, J.H.; Downes, K.A. History of tuberculosis. Tuberc. Pathog. Prot. Control 1994, 16, 13–24. [Google Scholar]
- Barberis, I.; Bragazzi, N.L.; Galluzzo, L.; Martini, M. The history of tuberculosis: From the first historical records to the isolation of Koch’s bacillus. J. Prev. Med. Hyg. 2017, 58, E9. [Google Scholar] [PubMed]
- Prabhu, R.; Singh, V. The history of tuberculosis: Past, present, and future. Adv. Microbiol. 2019, 9, 931–942. [Google Scholar] [CrossRef]
- Klugman, K.P.; Astley, C.M.; Lipsitch, M. Time from Illness Onset to Death, 1918 Influenza and Pneumococcal Pneumonia; National Institute of Health: Bethesda, MD, USA, 2009. [Google Scholar]
- McAuley, J.L.; Hornung, F.; Boyd, K.L.; Smith, A.M.; McKeon, R.; Bennink, J.; Yewdell, J.W.; McCullers, J.A. Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host Microbe 2007, 2, 240–249. [Google Scholar] [CrossRef]
- Walker, W. The Aberdeen typhoid outbreak of 1964. Scott. Med. J. 1965, 10, 466–479. [Google Scholar] [CrossRef]
- Marshall, E. Sverdlovsk: Anthrax Capital? Soviet doctors answer questions about an unusual anthrax epidemic once thought to have been triggered by a leak from a weapons lab. Science 1988, 240, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Khwaif, J.; Hayyawi, A.; Yousif, T. Cholera outbreak in Baghdad in 2007: An epidemiological study. EMHJ-East. Mediterr. Health J. 2010, 16, 584–589. [Google Scholar] [CrossRef]
- Crawford, D.H. Deadly Companions: How Microbes Shaped Our History; OUP Oxford: New York, NY, USA, 2007. [Google Scholar]
- Karlen, A. Man and Microbes: Disease and Plagues in History and Modern Times; Simon and Schuster: New York, NY, USA, 1996. [Google Scholar]
- Kawano, M. Novel antimicrobial method to tackle drug-resistant bacteria. Impact 2021, 2021, 48–50. [Google Scholar] [CrossRef]
- Young, M.; Craig, J. Urgent global action is needed on multi drug-resistant tuberculosis (MDR-TB)–can small cone moxa contribute to a global response? Eur. J. Integr. Med. 2020, 37, 101072. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 27 February 2017. 2020. Available online: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 20 February 2022).
- Rosenblatt-Farrell, N. The Landscape of Antibiotic Resistance; National Institute of Environmental Health Sciences: Durham, NC, USA, 2009. [Google Scholar]
- Walsh, C. Where will new antibiotics come from? Nat. Rev. Microbiol. 2003, 1, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Hesse, L.; O’Neill, A.J. Exploiting current understanding of antibiotic action for discovery of new drugs. J. Appl. Microbiol. 2002, 92, 4S–15S. [Google Scholar] [CrossRef]
- Mohr, K.I. History of antibiotics research. In How to Overcome the Antibiotic Crisis; Springer: Berlin/Heidelberg, Germany, 2016; pp. 237–272. [Google Scholar]
- Dantas, G.; Sommer, M.O. How to fight back against antibiotic resistance. Am. Sci. 2014, 102, 42–51. [Google Scholar] [CrossRef]
- Mousavifar, L.; Roy, R. Alternative therapeutic strategies to fight bacterial infections. Innate Immun. 2018, 13, 25. [Google Scholar] [CrossRef]
- Lerner, P.I. Producing penicillin. New Engl. J. Med. 2004, 351, 524. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.L.; Levy, S.B. The history of the tetracyclines. Ann. N. Y. Acad. Sci. 2011, 1241, 17–32. [Google Scholar] [CrossRef]
- Golkar, T.; Zieliński, M.; Berghuis, A.M. Look and outlook on enzyme-mediated macrolide resistance. Front. Microbiol. 2018, 9, 1942. [Google Scholar] [CrossRef]
- Kumagai, T.; Tamai, S.; Abe, T.; Hikda, M. Current status of oral carbapenem development. Curr. Med. Chem. Anti-Infect. Agents 2002, 1, 1–14. [Google Scholar] [CrossRef]
- Pallo-Zimmerman, L.M.; Byron, J.K.; Graves, T.K. Fluoroquinolones: Then and now. Compend. Contin. Educ. Vet. 2010, 32, E1–E9. [Google Scholar]
- Jad, Y.E.; Acosta, G.A.; Naicker, T.; Ramtahal, M.; El-Faham, A.; Govender, T.; Kruger, H.G.; de la Torre, B.G.; Albericio, F. Synthesis and biological evaluation of a teixobactin analogue. Org. Lett. 2015, 17, 6182–6185. [Google Scholar] [CrossRef]
- Moore, S.A.; Tyring, S.K.; Moore, A.Y. New Classes of Broad-Spectrum Antibiotics and New Mechanisms of Delivery. In Overcoming Antimicrobial Resistance of the Skin; Springer: Berlin/Heidelberg, Germany, 2021; pp. 215–223. [Google Scholar]
- Klug, D.M.; Idiris, F.I.; Blaskovich, M.A.; von Delft, F.; Dowson, C.G.; Kirchhelle, C.; Roberts, A.P.; Singer, A.C.; Todd, M.H. There is no market for new antibiotics: This allows an ope n approach to research and development. Wellcome Open Res. 2021, 6, 146. [Google Scholar] [CrossRef]
- Boyd, N.K.; Teng, C.; Frei, C.R. Brief overview of approaches and challenges in new antibiotic development: A focus on drug repurposing. Front. Cell. Infect. Microbiol. 2021, 11, 684515. [Google Scholar] [CrossRef] [PubMed]
- Zetts, R.M.; Garcia, A.M.; Doctor, J.N.; Gerber, J.S.; Linder, J.A.; Hyun, D.Y. Primary Care Physicians’ Attitudes and Perceptions towards Antibiotic Resistance and Antibiotic Stewardship: A National Survey; Open forum infectious diseases, 2020; Oxford University Press US: Seattle, WA, USA, 2020; p. ofaa244. [Google Scholar]
- Kzhyshkowska, J.; De Berardinis, P.; Bettencourt, P. Research and Development: The Past, Present and Future, Including Novel Therapeutic Strategies. In Fighting an Elusive Enemy: Staphylococcus aureus and Its Antibiotic Resistance, Immune-Evasion and Toxic Mechanisms; Frontiers: Lausanne, Switzerland, 2022. [Google Scholar]
- WHO. 2020 antibacterial agents in clinical and preclinical development: An overview and analysis. In WHO 2020 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Overbye, K.M.; Barrett, J.F. Antibiotics: Where did we go wrong? Drug Discov. Today 2005, 10, 45–52. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling drug-resistant infections globally: Final report and recommendations; APO: Melbourne, Australia, 2016. [Google Scholar]
- Bennett, J.E.; Dolin, R.; Blaser, M.J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases E-book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Tortora, G.J.; Case, C.L.; Funke, B.R. Microbiologia, 12th ed.; Artmed Editora: Floresta, Brazil, 2016. [Google Scholar]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W.; Barghoorn, E.S.; Maser, M.D.; Gordon, R.O. Electron microscopy of fossil bacteria two billion years old. Science 1965, 149, 1365–1367. [Google Scholar] [CrossRef] [PubMed]
- Admassie, M. Current review on molecular and phenotypic mechanism of bacterial resistance to antibiotic. Sci. J. Clin. Med. 2018, 7, 13. [Google Scholar] [CrossRef]
- Schultsz, C.; Geerlings, S. Plasmid-mediated resistance in Enterobacteriaceae. Drugs 2012, 72, 1–16. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Sanz-García, F.; Martínez, J.L. Antibiotic resistance evolution is contingent on the quorum-sensing response in Pseudomonas aeruginosa. Mol. Biol. Evol. 2019, 36, 2238–2251. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.L.; Islam, M.R.; Paul, B.K.; Ahmed, K.; Bhuyian, T. Computational modeling and analysis of gene regulatory interaction network for metabolic disorder: A bioinformatics approach. Biointerface Res. Appl. Chem. 2020, 10, 5910–5917. [Google Scholar]
- De Pascale, G.; Wright, G.D. Antibiotic resistance by enzyme inactivation: From mechanisms to solutions. Chembiochem 2010, 11, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Labricciosa, F.M.; Barbadoro, P.; Pagani, L.; Ansaloni, L.; Brink, A.J.; Carlet, J.; Khanna, A.; Chichom-Mefire, A.; Coccolini, F. The Global Alliance for Infections in Surgery: Defining a model for antimicrobial stewardship—Results from an international cross-sectional survey. World J. Emerg. Surg. 2017, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.K.; Miellet, S.; McGlinn, A.; Fish, J.; Meedya, S.; Reynolds, N.; Van Oijen, A.M. The drivers of antibiotic use and misuse: The development and investigation of a theory driven community measure. BMC Public Health 2019, 19, 1425. [Google Scholar] [CrossRef]
- Wise, R.; Hart, T.; Cars, O.; Streulens, M.; Helmuth, R.; Huovinen, P.; Sprenger, M. Antimicrobial Resistance. Br. Med. J. Publ. Group 1998, 317, 609–610. [Google Scholar] [CrossRef]
- Rather, I.A.; Kim, B.-C.; Bajpai, V.K.; Park, Y.-H. Self-medication and antibiotic resistance: Crisis, current challenges, and prevention. Saudi J. Biol. Sci. 2017, 24, 808–812. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Gedebou, M.; Bilal, N. Nosocomial infections and misuse of antibiotics in a provincial community hospital, Saudi Arabia. J. Hosp. Infect. 2002, 50, 115–121. [Google Scholar] [CrossRef]
- Wolff, M.J. Use and misuse of antibiotics in Latin America. Clin. Infect. Dis. 1993, 17 (Suppl. S2), S346–S351. [Google Scholar] [CrossRef]
- Phillips, I.; Casewell, M.; Cox, T.; De Groot, B.; Friis, C.; Jones, R.; Nightingale, C.; Preston, R.; Waddell, J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2004, 53, 28–52. [Google Scholar] [CrossRef] [PubMed]
- Karp, B.E.; Engberg, J. Comment on: Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2004, 54, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Larsson, D.J. Antibiotics in the environment. Upsala J. Med. Sci. 2014, 119, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Significance of antibiotics in the environment. J. Antimicrob. Chemother. 2003, 52, 5–7. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2021, 20, 193–205. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Emeka, P.; Badger-Emeka, L.; Fateru, F. In vitro antimicrobial activities of Acalypha ornate leaf extracts on bacterial and fungal clinical isolates. J. Herb. Med. 2012, 2, 136–142. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems; World Health Organization: Geneva, Switzerland, 2004.
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Moloney, M.G. Natural products as a source for novel antibiotics. Trends Pharmacol. Sci. 2016, 37, 689–701. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Sultan, M.T.; Buttxs, M.S.; Qayyum, M.M.N.; Suleria, H.A.R. Immunity: Plants as effective mediators. Crit. Rev. Food Sci. Nutr. 2014, 54, 1298–1308. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Camele, I.; Elshafie, H.S.; Caputo, L.; De Feo, V. Anti-quorum sensing and antimicrobial effect of Mediterranean plant essential oils against phytopathogenic bacteria. Front. Microbiol. 2019, 10, 2619. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Patra, A.K. An overview of antimicrobial properties of different classes of phytochemicals. In Dietary Phytochemicals and Microbes; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. [Google Scholar]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Vermerris, W.; Nicholson, R. Phenolic Compound Biochemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Khameneh, B.; Iranshahy, M.; Ghandadi, M.; Ghoochi Atashbeyk, D.; Fazly Bazzaz, B.S.; Iranshahi, M. Investigation of the antibacterial activity and efflux pump inhibitory effect of co-loaded piperine and gentamicin nanoliposomes in methicillin-resistant Staphylococcus aureus. Drug Dev. Ind. Pharm. 2015, 41, 989–994. [Google Scholar] [CrossRef]
- Gradišar, H.; Pristovšek, P.; Plaper, A.; Jerala, R. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J. Med. Chem. 2007, 50, 264–271. [Google Scholar] [CrossRef]
- Duan, F.; Li, X.; Cai, S.; Xin, G.; Wang, Y.; Du, D.; He, S.; Huang, B.; Guo, X.; Zhao, H. Haloemodin as novel antibacterial agent inhibiting DNA gyrase and bacterial topoisomerase I. J. Med. Chem. 2014, 57, 3707–3714. [Google Scholar] [CrossRef]
- Poomanee, W.; Chaiyana, W.; Mueller, M.; Viernstein, H.; Khunkitti, W.; Leelapornpisid, P. In-vitro investigation of anti-acne properties of Mangifera indica L. kernel extract and its mechanism of action against Propionibacterium acnes. Anaerobe 2018, 52, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.I.; Ryu, J.; Jin, C.H.; Kim, D.-G.; Kim, J.M.; Seo, K.-S.; Kim, J.-B.; Kim, S.H.; Ahn, J.-W.; Kang, S.-Y. Phenolic compounds in extracts of Hibiscus acetosella (Cranberry Hibiscus) and their antioxidant and antibacterial properties. Molecules 2020, 25, 4190. [Google Scholar] [CrossRef]
- Lu, Y.; Joerger, R.; Wu, C. Study of the chemical composition and antimicrobial activities of ethanolic extracts from roots of Scutellaria baicalensis Georgi. J. Agric. Food Chem. 2011, 59, 10934–10942. [Google Scholar] [CrossRef]
- Fujita, M.; Shiota, S.; Kuroda, T.; Hatano, T.; Yoshida, T.; Mizushima, T.; Tsuchiya, T. Remarkable Synergies between Baicalein and Tetracycline, and Baicalein and β-Lactams against Methicillin-Resistant Staphylococcus aureus. Microbiol. Immunol. 2005, 49, 391–396. [Google Scholar] [CrossRef]
- Holler, J.G.; Slotved, H.-C.; Mølgaard, P.; Olsen, C.E.; Christensen, S.B. Chalcone inhibitors of the NorA efflux pump in Staphylococcus aureus whole cells and enriched everted membrane vesicles. Bioorganic Med. Chem. 2012, 20, 4514–4521. [Google Scholar] [CrossRef]
- Bribi, N. Pharmacological activity of alkaloids: A review. Asian J. Bot. 2018, 1, 1–6. [Google Scholar]
- Hegnauer, R. Biochemistry, distribution and taxonomic relevance of higher plant alkaloids. Phytochemistry 1988, 27, 2423–2427. [Google Scholar] [CrossRef]
- Mabhiza, D.; Chitemerere, T.; Mukanganyama, S. Antibacterial properties of alkaloid extracts from Callistemon citrinus and Vernonia adoensis against Staphylococcus aureus and Pseudomonas aeruginosa. Int. J. Med. Chem. 2016, 2016, 6304163. [Google Scholar] [CrossRef]
- Liu, M.; Han, J.; Feng, Y.; Guymer, G.; Forster, P.I.; Quinn, R.J. Antimicrobial Benzyltetrahydroisoquinoline-Derived Alkaloids from the Leaves of Doryphora aromatica. J. Nat. Prod. 2021, 84, 676–682. [Google Scholar] [CrossRef]
- Pech-Puch, D.; Forero, A.M.; Fuentes-Monteverde, J.C.; Lasarte-Monterrubio, C.; Martinez-Guitian, M.; González-Salas, C.; Guillén-Hernández, S.; Villegas-Hernández, H.; Beceiro, A.; Griesinger, C. Antimicrobial Diterpene Alkaloids from an Agelas citrina Sponge Collected in the Yucatán Peninsula. Mar. Drugs 2022, 20, 298. [Google Scholar] [CrossRef]
- Jia, W.; Wang, J.; Xu, H.; Li, G. Resistance of Stenotrophomonas maltophilia to fluoroquinolones: Prevalence in a university hospital and possible mechanisms. Int. J. Environ. Res. Public Health 2015, 12, 5177–5195. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Li, C.; Zhang, H.; Li, G.; Liu, X.; Wei, J. Prevalence of genes of OXA-23 carbapenemase and AdeABC efflux pump associated with multidrug resistance of Acinetobacter baumannii isolates in the ICU of a comprehensive hospital of Northwestern China. Int. J. Environ. Res. Public Health 2015, 12, 10079–10092. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, M.; Tamura, Y.; Masuda, H.; Mizutani, K.; Tanaka, O.; Ikeda, T.; Ohtani, K.; Kasai, R.; Yamasaki, K. Antiyeast steroidal saponins from Yucca schidigera (Mohave Yucca), a new anti-food-deteriorating agent. J. Nat. Prod. 2000, 63, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Lunga, P.K.; Qin, X.-J.; Yang, X.W.; Kuiate, J.-R.; Du, Z.Z.; Gatsing, D. Antimicrobial steroidal saponin and oleanane-type triterpenoid saponins from Paullinia pinnata. BMC Complement. Altern. Med. 2014, 14, 369. [Google Scholar] [CrossRef] [PubMed]
- Passi, S.; Aligiannis, N.; Pratsinis, H.; Skaltsounis, A.; Chinou, I. Biologically active Triterpenoids of Cephalaria ambrosioides roots. Planta Med. 2009, 75, 163–167. [Google Scholar] [CrossRef]
- Biva, I.J.; Ndi, C.P.; Semple, S.J.; Griesser, H.J. Antibacterial performance of terpenoids from the Australian plant Eremophila lucida. Antibiotics 2019, 8, 63. [Google Scholar] [CrossRef]
- Gartika, M.; Pramesti, H.T.; Kurnia, D.; Satari, M.H. A terpenoid isolated from sarang semut (Myrmecodia pendans) bulb and its potential for the inhibition and eradication of Streptococcus mutans biofilm. BMC Complement. Altern. Med. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Hu, B.-Y.; Liu, J.-W.; Cai, Y.; Chen, X.-C.; Qin, D.-P.; Cheng, Y.-X.; Zhang, Z.-D. Anti-mycobacterium tuberculosis terpenoids from Resina Commiphora. Molecules 2019, 24, 1475. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids1. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Anssary, A.A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. Herb. Med. 2019, 1, 13. [Google Scholar]
- Sørensen, J.L.; Giese, H. Influence of carbohydrates on secondary metabolism in Fusarium avenaceum. Toxins 2013, 5, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Kyung, K.H. Antimicrobial activity of volatile sulfur compounds in foods. In Volatile Sulfur Compounds in Food; ACS Publications: Washington, DC, USA, 2011; pp. 323–338. [Google Scholar]
- Pan, S.-Y.; Zhou, S.-F.; Gao, S.-H.; Yu, Z.-L.; Zhang, S.-F.; Tang, M.-K.; Sun, J.-N.; Ma, D.-L.; Han, Y.-F.; Fong, W.-F. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid. Based Complement. Altern. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef] [PubMed]
- Luepke, K.H.; Suda, K.J.; Boucher, H.; Russo, R.L.; Bonney, M.W.; Hunt, T.D.; Mohr III, J.F. Past, present, and future of antibacterial economics: Increasing bacterial resistance, limited antibiotic pipeline, and societal implications. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Chan, K. Progress in traditional Chinese medicine. Trends Pharmacol. Sci. 1995, 16, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Kirst, H.A. Developing new antibacterials through natural product research. Expert Opin. Drug Discov. 2013, 8, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Horne, M. The world is running out of antibiotics. Aust. Med. 2017, 29, 31. [Google Scholar]
- Howard, S.J.; Catchpole, M.; Watson, J.; Davies, S.C. Antibiotic resistance: Global response needed. Lancet Infect. Dis. 2013, 13, 1001–1003. [Google Scholar] [CrossRef]
- Alizadehdakhel, A.; Golzary, A. An environmental friendly process for extraction of active constituents from herbal plants. Environ. Energy Econ. Res. 2020, 4, 69–81. [Google Scholar]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Guilhelmelli, F.; Vilela, N.; Albuquerque, P.; Derengowski, L.d.S.; Silva-Pereira, I.; Kyaw, C.M. Antibiotic development challenges: The various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013, 4, 353. [Google Scholar] [CrossRef]
- Menezes, R.d.P.; Bessa, M.A.d.S.; Siqueira, C.d.P.; Teixeira, S.C.; Ferro, E.A.V.; Martins, M.M.; Cunha, L.C.S.; Martins, C.H.G. Antimicrobial, Antivirulence, and Antiparasitic Potential of Capsicum chinense Jacq. Extracts and Their Isolated Compound Capsaicin. Antibiotics 2022, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Salleh, W.M.N.H.W.; Hashim, N.A.; Fabarani, N.P.; Ahmad, F. Antibacterial Activity of Constituents from Piper retrofractum Vahl. and Piper arborescens Roxb. Agric. Conspec. Sci. 2020, 85, 269–280. [Google Scholar]
- Fu, Y.; Liu, W.; Liu, M.; Zhang, J.; Yang, M.; Wang, T.; Qian, W. In vitro anti-biofilm efficacy of sanguinarine against carbapenem-resistant Serratia marcescens. Biofouling 2021, 37, 341–351. [Google Scholar] [CrossRef]
- Gibbons, S. Phytochemicals for bacterial resistance-strengths, weaknesses and opportunities. Planta Medica 2008, 74, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Plackett, B. Why big pharma has abandoned antibiotics. Nature 2020, 586, S50. [Google Scholar] [CrossRef]
- Martens, E.; Demain, A.L. The antibiotic resistance crisis, with a focus on the United States. J. Antibiot. 2017, 70, 520–526. [Google Scholar] [CrossRef]
- Nocedo-Mena, D.; Rivas-Galindo, V.M.; Navarro, P.; Garza-González, E.; González-Maya, L.; Ríos, M.Y.; García, A.; Ávalos-Alanís, F.G.; Rodríguez-Rodríguez, J.; del Rayo Camacho-Corona, M. Antibacterial and cytotoxic activities of new sphingolipids and other constituents isolated from Cissus incisa leaves. Heliyon 2020, 6, e04671. [Google Scholar] [CrossRef]
- de Jesus Dzul-Beh, A.; Uc-Cachón, A.H.; González-Sánchez, A.A.; Dzib-Baak, H.E.; Ortiz-Andrade, R.; Barrios-García, H.B.; Jimenez-Delgadillo, B.; Molina-Salinas, G.M. Antimicrobial potential of the Mayan medicine plant Matayba oppositifolia (A. Rich.) Britton against antibiotic-resistant priority pathogens. J. Ethnopharmacol. 2023, 300, 115738. [Google Scholar] [CrossRef]
- Etemadi, S.; Barhaghi, M.S.; Leylabadlo, H.; Memar, M.; Mohammadi, A.; Ghotaslou, R. The synergistic effect of turmeric aqueous extract and chitosan against multidrug-resistant bacteria. New Microbes New Infect. 2021, 41, 100861. [Google Scholar] [CrossRef]
- Sah, S.K.; Rasool, U.; Hemalatha, S. Andrographis paniculata extract inhibit growth, biofilm formation in multidrug resistant strains of Klebsiella pneumoniae. J. Tradit. Complement. Med. 2020, 10, 599–604. [Google Scholar] [CrossRef]
- Rasool, U.; Parveen, A.; Sah, S.K. Efficacy of Andrographis paniculata against extended spectrum β-lactamase (ESBL) producing E. coli. BMC Complement. Altern. Med. 2018, 18, 244. [Google Scholar] [CrossRef]
- Nogbou, N.-D.; Mabela, D.R.; Matseke, B.; Mapfumari, N.S.; Mothibe, M.E.; Obi, L.C.; Musyoki, A.M. Antibacterial Activities of Monsonia Angustifolia and Momordica Balsamina Linn Extracts against Carbapenem-Resistant Acinetobacter Baumannii. Plants 2022, 11, 2374. [Google Scholar] [CrossRef]
- Eve, A.; Aliero, A.A.; Nalubiri, D.; Adeyemo, R.O.; Akinola, S.A.; Pius, T.; Nabaasa, S.; Nabukeera, S.; Alkali, B.; Ntulume, I. In vitro antibacterial activity of crude extracts of Artocarpus heterophyllus seeds against selected diarrhoea-causing superbug bacteria. Sci. World J. 2020, 2020, 9813970. [Google Scholar] [CrossRef]
- Dettweiler, M.; Marquez, L.; Lin, M.; Sweeney-Jones, A.M.; Chhetri, B.K.; Zurawski, D.V.; Kubanek, J.; Quave, C.L. Pentagalloyl glucose from Schinus terebinthifolia inhibits growth of carbapenem-resistant Acinetobacter baumannii. Sci. Rep. 2020, 10, 15340. [Google Scholar] [CrossRef]
- Qian, W.; Zhang, J.; Wang, W.; Wang, T.; Liu, M.; Yang, M.; Sun, Z.; Li, X.; Li, Y. Antimicrobial and antibiofilm activities of paeoniflorin against carbapenem-resistant Klebsiella pneumoniae. J. Appl. Microbiol. 2020, 128, 401–413. [Google Scholar] [CrossRef]
- Stefani, T.; Garza-González, E.; Rivas-Galindo, V.M.; Rios, M.Y.; Alvarez, L.; Camacho-Corona, M.d.R. Hechtia glomerata Zucc: Phytochemistry and activity of its extracts and major constituents against resistant bacteria. Molecules 2019, 24, 3434. [Google Scholar] [CrossRef]
- Sadiku, N.; Gbala, I.D.; Olorunnishola, K.S.; Shittu, B.A. Bioactivity of Khaya senegalensis (Desr.) A. Juss. and Tamarindus indica L. extracts on selected pathogenic microbes. Arab. J. Med. Aromat. Plants 2020, 6, 29–41. [Google Scholar]
- Núñez-Mojica, G.; Rivas-Galindo, V.M.; Garza-González, E.; Miranda, L.D.; Romo-Pérez, A.; Pagniez, F.; Picot, C.; Le Pape, P.; Bazin, M.-A.; Marchand, P. Antimicrobial and antileishmanial activities of extracts and some constituents from the leaves of Solanum chrysotrichum Schldl. Med. Chem. Res. 2021, 30, 152–162. [Google Scholar] [CrossRef]
- Alsaadi, W.A.; Bamagoos, A.A.; Hakeem, K.R. Phytochemical analysis and antibacterial activities of Avicennia marina (Forssk.) Vierh. extracts against Vancomycin-resistant Enterococcus faecium. Adv. Environ. Biol. 2022, 16, 5–12. [Google Scholar] [CrossRef]
- Muhsinah, A.B.; Maqbul, M.S.; Mahnashi, M.H.; Jalal, M.M.; Altayar, M.A.; Saeedi, N.H.; Alshehri, O.M.; Shaikh, I.A.; Khan, A.A.L.; Iqubal, S.S. Antibacterial activity of Illicium verum essential oil against MRSA clinical isolates and determination of its phyto-chemical components. J. King Saud Univ. Sci. 2022, 34, 101800. [Google Scholar] [CrossRef]
- Bruna, F.; Fernandez, K.; Urrejola, F.; Touma, J.; Navarro, M.; Sepulveda, B.; Larrazabal-Fuentes, M.; Paredes, A.; Neira, I.; Ferrando, M. Chemical composition, antioxidant, antimicrobial and antiproliferative activity of Laureliopsis philippiana essential oil of Chile, study in vitro and in silico. Arab. J. Chem. 2022, 15, 104271. [Google Scholar] [CrossRef]
- El Baaboua, A.; El Maadoudi, M.; Bouyahya, A.; Belmehdi, O.; Kounnoun, A.; Cheyadmi, S.; Ouzakar, S.; Senhaji, N.S.; Abrini, J. Evaluation of the combined effect of antibiotics and essential oils against Campylobacter multidrug resistant strains and their biofilm formation. South Afr. J. Bot. 2022, 150, 451–465. [Google Scholar] [CrossRef]
- Valizadeh, M.; ur Rehman, F.; Hassanzadeh, M.A.; Beigomi, M.; Fazeli-Nasab, B. Investigating the Antimicrobial Effects of Glycyrrhiza glabra and Salvia officinalis Ethanolic Extract against Helicobacter pylori. Int. J. Infect. 2021, 8, e114563. [Google Scholar] [CrossRef]
- Bueno, P.I.; Machado, D.; Lancellotti, M.; Gonçalves, C.P.; Marcucci, M.C.; Sawaya, A.C.H.F.; de Melo, A. In silico studies, chemical composition, antibacterial activity and in vitro antigen-induced phagocytosis of Stryphnodendron adstringens (Mart.) Coville. Res. Soc. Dev. 2022, 11, e35911225748. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Mrabti, H.N.; El Hachlafi, N.; El Kamili, T.; Elbouzidi, A.; Abdallah, E.M.; Flouchi, R.; Assaggaf, H.; Qasem, A.; Zengin, G.; et al. Integrated analysis of antimicrobial, antioxidant, and phytochemical properties of Cinnamomum verum: A comprehensive In vitro and In silico study. Biochem. Syst. Ecol. 2023, 110, 104700. [Google Scholar] [CrossRef]
- Antih, J.; Houdkova, M.; Urbanova, K.; Kokoska, L. Antibacterial activity of Thymus vulgaris L. essential oil vapours and their GC/MS analysis using solid-phase microextraction and syringe headspace Sampling Techniques. Molecules 2021, 26, 6553. [Google Scholar] [CrossRef]
- Bai, X.; Chen, T.; Liu, X.; Liu, Z.; Ma, R.; Su, R.; Li, X.; Lü, X.; Xia, X.; Shi, C. Antibacterial Activity and Possible Mechanism of Litsea cubeba Essential Oil against Shigella sonnei and Its Application in Lettuce. Foodborne Pathog. Dis. 2023, 20, 138–148. [Google Scholar] [CrossRef]
- Magnini, R.D.; Hilou, A.; Millogo-Koné, H.; Pagès, J.-M.; Davin-Regli, A. Acacia senegal extract rejuvenates the activity of phenicols on selected enterobacteriaceae multi drug resistant strains. Antibiotics 2020, 9, 323. [Google Scholar] [CrossRef]
- Maqbul, M.S.; Alshabi, A.M.; Khan, A.A.; Iqubal, S.S.; Shaikh, I.A.; Mohammed, T.; Habeeb, M.S.; Bokhari, Y.A.; Khan, K.A.; Hussain, M.S. Comparative Study of Moringa oleifera with Moringa peregrine Seed oil using GC-MS and its Antimicrobial Activity against Helicobacter pylori. Orient. J. Chem. 2020, 36, 481–492. [Google Scholar] [CrossRef]
- Stefanović, O.D. Synergistic activity of antibiotics and bioactive plant extracts: A study against Gram-positive and Gram-negative bacteria. Bact. Pathog. Antibact. Control 2018, 23, 23–48. [Google Scholar]
- Silva, D.M.; COSTA, P.A.; Ribon, A.O.; Purgato, G.A.; Gaspar, D.-M.; Diaz, M.A. Plant extracts display synergism with different classes of antibiotics. An. Da Acad. Bras. De Ciências 2019, 91, e20180117. [Google Scholar] [CrossRef] [PubMed]
- Aiyegoro, O.; Afolayan, A.; Okoh, A. In vitro antibacterial activities of crude extracts of the leaves of Helichrysum longifolium in combination with selected antibiotics. Afr. J. Pharm. Pharmacol. 2009, 3, 293–300. [Google Scholar]
- Radulovic, N.; Blagojevic, P.; Stojanovic-Radic, Z.; Stojanovic, N. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar] [PubMed]
- ADWAN, G.M.; Abu-Shanab, B.; Adwan, K.; Abu-Shanab, F. Antibacterial effects of nutraceutical plants growing in Palestine on Pseudomonas aeruginosa. Turk. J. Biol. 2006, 30, 239–242. [Google Scholar]
- Al-Bayati, F.A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J. Ethnopharmacol. 2008, 116, 403–406. [Google Scholar] [CrossRef]
- Harris, R. Synergism in the essential oil world. Int. J. Aromather. 2002, 12, 179–186. [Google Scholar] [CrossRef]
- Bag, A.; Chattopadhyay, R.R. Evaluation of synergistic antibacterial and antioxidant efficacy of essential oils of spices and herbs in combination. PLoS ONE 2015, 10, e0131321. [Google Scholar] [CrossRef]
- Zhang, Q.; Ying, Y.; Ping, J. Recent advances in plant nanoscience. Adv. Sci. 2022, 9, 2103414. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Modwi, A.; Al-Mijalli, S.H.; Mohammed, A.E.; Idriss, H.; Omar, A.S.; Afifi, M.; Al-Farga, A.; Goh, K.W.; Ming, L.C. In Vitro Influence of ZnO, CrZnO, RuZnO, and BaZnO Nanomaterials on Bacterial Growth. Molecules 2022, 27, 8309. [Google Scholar] [CrossRef]
- Abdellatif, A.A.; Alhathloul, S.S.; Aljohani, A.S.; Maswadeh, H.; Abdallah, E.M.; Hamid Musa, K.; El Hamd, M.A. Green Synthesis of Silver Nanoparticles Incorporated Aromatherapies Utilized for Their Antioxidant and Antimicrobial Activities against Some Clinical Bacterial Isolates. Bioinorg. Chem. Appl. 2022, 2022, 2432758. [Google Scholar] [CrossRef]
- Malaikozhundan, B.; Vinodhini, J.; Kalanjiam, M.A.R.; Vinotha, V.; Palanisamy, S.; Vijayakumar, S.; Vaseeharan, B.; Mariyappan, A. High synergistic antibacterial, antibiofilm, antidiabetic and antimetabolic activity of Withania somnifera leaf extract-assisted zinc oxide nanoparticle. Bioprocess Biosyst. Eng. 2020, 43, 1533–1547. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Shobiya, M. Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomed. Pharmacother. 2016, 84, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Roque-Borda, C.A.; da Silva, P.B.; Rodrigues, M.C.; Azevedo, R.B.; Di Filippo, L.; Duarte, J.L.; Chorilli, M.; Festozo Vicente, E.; Pavan, F.R. Challenge in the discovery of new drugs: Antimicrobial peptides against WHO-list of critical and high-priority bacteria. Pharmaceutics 2021, 13, 773. [Google Scholar] [CrossRef]
- Hlashwayo, D.F.; Barbosa, F.; Langa, S.; Sigauque, B.; Bila, C.G. A systematic review of In Vitro activity of medicinal plants from Sub-Saharan Africa against Campylobacter spp. Evid.-Based Complement. Altern. Med. 2020, 2020, 9485364. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Mwamatope, B.; Tembo, D.; Kampira, E.; Maliwichi-Nyirenda, C.; Ndolo, V. Seasonal Variation of Phytochemicals in Four Selected Medicinal Plants. Pharmacogn. Res. 2021, 13, 218–226. [Google Scholar] [CrossRef]
- Habeeb Rahuman, H.B.; Dhandapani, R.; Narayanan, S.; Palanivel, V.; Paramasivam, R.; Subbarayalu, R.; Thangavelu, S.; Muthupandian, S. Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnology 2022, 16, 115–144. [Google Scholar] [CrossRef]
- Ghosh, S.; Nandi, S.; Basu, T. Nano-Antibacterials Using Medicinal Plant Components: An Overview. Front. Microbiol. 2022, 12, 768739. [Google Scholar] [CrossRef]
- Liu, G.; Catacutan, D.B.; Rathod, K.; Swanson, K.; Jin, W.; Mohammed, J.C.; Chiappino-Pepe, A.; Syed, S.A.; Fragis, M.; Rachwalski, K. Deep learning-guided discovery of an antibiotic targeting Acinetobacter baumannii. Nat. Chem. Biol. 2023, 1–9. [Google Scholar] [CrossRef]
- Ali, T.; Ahmed, S.; Aslam, M. Artificial Intelligence for Antimicrobial Resistance Prediction: Challenges and Opportunities towards Practical Implementation. Antibiotics 2023, 12, 523. [Google Scholar] [CrossRef]
- Gupta, A.; Meshram, V.; Gupta, M.; Goyal, S.; Qureshi, K.; Jaremko, M.; Shukla, K. Fungal Endophytes: Microfactories of Novel Bioactive Compounds with Therapeutic Interventions; A Comprehensive Review on the Biotechnological Developments in the Field of Fungal Endophytic Biology over the Last Decade. Biomolecules 2023, 13, 1038. [Google Scholar] [CrossRef] [PubMed]
- Terreni, M.; Taccani, M.; Pregnolato, M. New antibiotics for multidrug-resistant bacterial strains: Latest research developments and future perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision medicine, AI, and the future of personalized health care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: Importance, challenges and future. J. Tradit. Complement. Med. 2017, 7, 234–244. [Google Scholar] [CrossRef]
- Sharma, M.; Koul, A.; Sharma, D.; Kaul, S.; Swamy, M.K.; Dhar, M.K. Metabolic engineering strategies for enhancing the production of bio-active compounds from medicinal plants. Nat. Bio-Act. Compd. 2019, 3, 287–316. [Google Scholar]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57. [Google Scholar] [PubMed]
- Nikam, P.H.; Kareparamban, J.; Jadhav, A.; Kadam, V. Future trends in standardization of herbal drugs. J. Appl. Pharm. Sci. 2012, 2, 38–44. [Google Scholar]
- Landriscina, A.; Rosen, J.; Friedman, A.J. Biodegradable chitosan nanoparticles in drug delivery for infectious disease. Nanomedicine 2015, 10, 1609–1619. [Google Scholar] [CrossRef]
- Cragg, G.M.; Boyd, M.R.; Khanna, R.; Kneller, R.; Mays, T.D.; Mazan, K.D.; Newman, D.J.; Sausville, E.A. International collaboration in drug discovery and development: The NCI experience. Pure Appl. Chem. 1999, 71, 1619–1633. [Google Scholar] [CrossRef]
| Degree of Priority | Bacterial Pathogen | Gram-Stain | Type of Resistance |
|---|---|---|---|
| Critical priority | Acinetobacter baumannii | G(–) | Carbapenem-resistant. |
| Pseudomonas aeruginosa | G(–) | Carbapenem-resistant. | |
| Enterobacteriaceae ** | G(–) | Carbapenem-resistant and third-generation-cephalosporin-resistant. | |
| High priority | Enterococcus faecium | G(+) | Vancomycin-resistant. |
| Staphylococcus aureus | G(+) | Methicillin-resistant and vancomycin-resistant. | |
| Helicobacter pylori | G(–) | Clarithromycin-resistant. | |
| Campylobacter spp. | G(–) | Fluoroquinolone-resistant. | |
| Salmonella spp. | G(–) | Fluoroquinolone-resistant. | |
| Neisseria gonorrhoeae | G(–) | Third-generation-cephalosporin-resistant and fluoroquinolone-resistant. | |
| Medium priority | Streptococcus pneumoniae | G(+) | Penicillin-resistant. |
| Haemophilus influenzae | G(–) | Ampicillin-resistant. | |
| Shigella spp. | G(–) | Fluoroquinolone-resistant. |
| Plant to Obtain the Extract/Part of the Plant | Extract or Compound Tested with Effective Action | Bioactive Compounds | Mechanism of Action | Isolates Exhibiting Superior Outcomes | MIC | Reference |
|---|---|---|---|---|---|---|
| Matayba oppositifolia/bark | Aqueous extract Hexane extract Ethyl acetate Methyl extract | Palmitic acid, friedelan-3-one, 7-dehydrodiosgenin. | - | Carbapenem-resistant A. baumannii Carbapenem-resistant K. pneumoniae Carbapenem-resistant P. aeruginosa Carbapenem-resistant Enterobacter spp. | 250–1000 µg/mL | [142] |
| Curcuma longa/rhizome | Aqueous extract | Turmeric and chitosan | - | Carbapenem-resistant P. aeruginosa | 1024 µg/mL | [143] |
| Andrographis paniculate/leaves | Ethyl acetate extract | Terpenoids and saponins | - | Carbapenem-resistant A. baumannii, β-Lactamase producing E. coli | 250–500 µg/mL 25 μg/mL | [144] [145] |
| Momordica Balsamina/fruit | Methyl extract | - | - | Carbapenem-resistant A. baumannii | 0.5 mg/mL | [146] |
| Artocarpus heterophyllus/seed | Hexane extract | - | - | Multidrug-resistant P. aeruginosa | 125 mg/mL | [147] |
| Schinus terebinthifolia/leaves | Pentagalloyl glucose | - | - | Carbapenem-resistant A. baumannii Carbapenem-resistant P. aeruginosa | 16–256 µg/mL | [148] |
| Cissus incisa/leaves | α-Amyrin-3-Oβ-D- glucopyranoside Cerebrosides mixture | - | - | Carbapenem-resistant P. aeruginosa | 100 μg/mL | [141] |
| Paeonia lactiflora/roots | Paeoniflorin | C23H28O11 | Breach of membrane integrity | Carbapenem-resistant K. pneumoniae | 1200 µg/mL | [149] |
| Hechtia glomerata/leaves | Hexane Extract Aqueous extract β-sitosterol β-sitosteryl acetate daucosterol, daucosteryl acetate | - | - | Carbapenem-resistant K. pneumoniae Carbapenem-resistant P. aeruginosa Carbapenem-resistant A. baumannii E. coli ESBL | 100–500 µg/mL | [150] |
| Khaya senegalensis/bark Tamarindus indica/bark | Aqueous extract Ethyl extract Methyl extract | - | - | Carbapenem-resistant E. coli | 25–400 mg/mL | [151] |
| Solanum chrysotrichum/leaves | Hexane extract Dichloromethane fraction Steroidal saponins | - | - | Carbapenem-resistant P. aeruginosa Carbapenem-resistant A. baumannii | 125–250 µg/mL | [152] |
| Avicennia marina/leaves | Ethanolic extract | Flavonoids, phenolics, triterpenes, and glycosides | Vancomycin-resistant E. faecalis | 4.0 mg/mL | [153] | |
| Illicium verum/seeds | Essential oils | Phenolics and flavonoids | Produce permanent damage to the cell membrane and cell contents | Methicillin-resistant S. aureus (MRSA) | 0.25–1.0 µg/mL | [154] |
| Laureliopsis philippiana/leaves | Essential oils | Eucalyptol, linalool, isozaphrol, isohomogenol, α-terpineol, and eudesmol | - | Helicobacter pylori (clinical isolates) | 64.0 µg/mL | [155] |
| Origanum Compactum/areal parts Lavandula stoechas/areal parts | Essential oils | - | Bactericidal and anti-biofilm formation | Multidrug-resistant Campylobacter spp. | 0.063% (v/v) | [156] |
| Salvia officinalis/leaves | Ethanolic extract | - | - | Multidrug-resistant Helicobacter pylori | 3.1–50.0 mg/mL | [157] |
| Stryphnodendron adstringens/bark | Ethanolic extract | Polyphenols and tannins | - | N. gonorrhoeae ATCC 49226 K. pneumoniae ATCC13693 MRSA (clinical isolate) S. pneumoniae ATCC 6303 | 3.125 mg/mL 12.5 mg/mL 3.125 mg/mL 0.78 mg/mL | [158] |
| Cinnamomum verum/inner bark | Essential oils | Cinnamaldehyde dimethyl acetal, cinnamaldehyde, and α-copaene | Bactericidal and inhibit bacterial DNA gyrase and topoisomerase | S. enterica (clinical isolate) E. coli ATCC 25922 | 0.5% v/v 0.25% v/v | [159] |
| Thymus vulgaris/areal parts | Essential oils | Phenolic monoterpenes, sesquiterpenoids (β-caryophyllene), phenylpropanoids, aliphatics, furanoids, and diterpenes. | H. influenzae ATCC 49247 S. aureus ATCC 29213 | 512.0 µg/mL 512.0–1024.0 µg/mL | [160] | |
| Litsea cubeba/undefined | Essential oils | - | Production of reactive oxygen species and destruction of the cell membrane | Shigella sonnei ATCC 25931 Shigella sonnei CMCC 51592 | 4.0 μL/mL 6.0 μL/mL | [161] |
| Acacia Senegal/leaves | Hydroethanolic extract | Phenolic compounds, flavonoids, and tannins | - | Multidrug-resistant E. coli Multidrug-resistant K. pneumoniae | 256.0 μg/mL >512.0 μg/mL | [162] |
| Moringa oleifera/seeds | Essential oils | Phenolic compounds and flavonoids | Bactericidal | Helicobacter pylori (clinical isolates) | 0.5 μg/mL | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. https://doi.org/10.3390/plants12173077
Abdallah EM, Alhatlani BY, de Paula Menezes R, Martins CHG. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants. 2023; 12(17):3077. https://doi.org/10.3390/plants12173077
Chicago/Turabian StyleAbdallah, Emad M., Bader Y. Alhatlani, Ralciane de Paula Menezes, and Carlos Henrique Gomes Martins. 2023. "Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era" Plants 12, no. 17: 3077. https://doi.org/10.3390/plants12173077
APA StyleAbdallah, E. M., Alhatlani, B. Y., de Paula Menezes, R., & Martins, C. H. G. (2023). Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants, 12(17), 3077. https://doi.org/10.3390/plants12173077











