Cinnamon Oil Alleviates Acetaminophen-Induced Uterine Toxicity in Rats by Abrogation of Oxidative Stress, Apoptosis, and Inflammation
Abstract
1. Introduction
2. Results
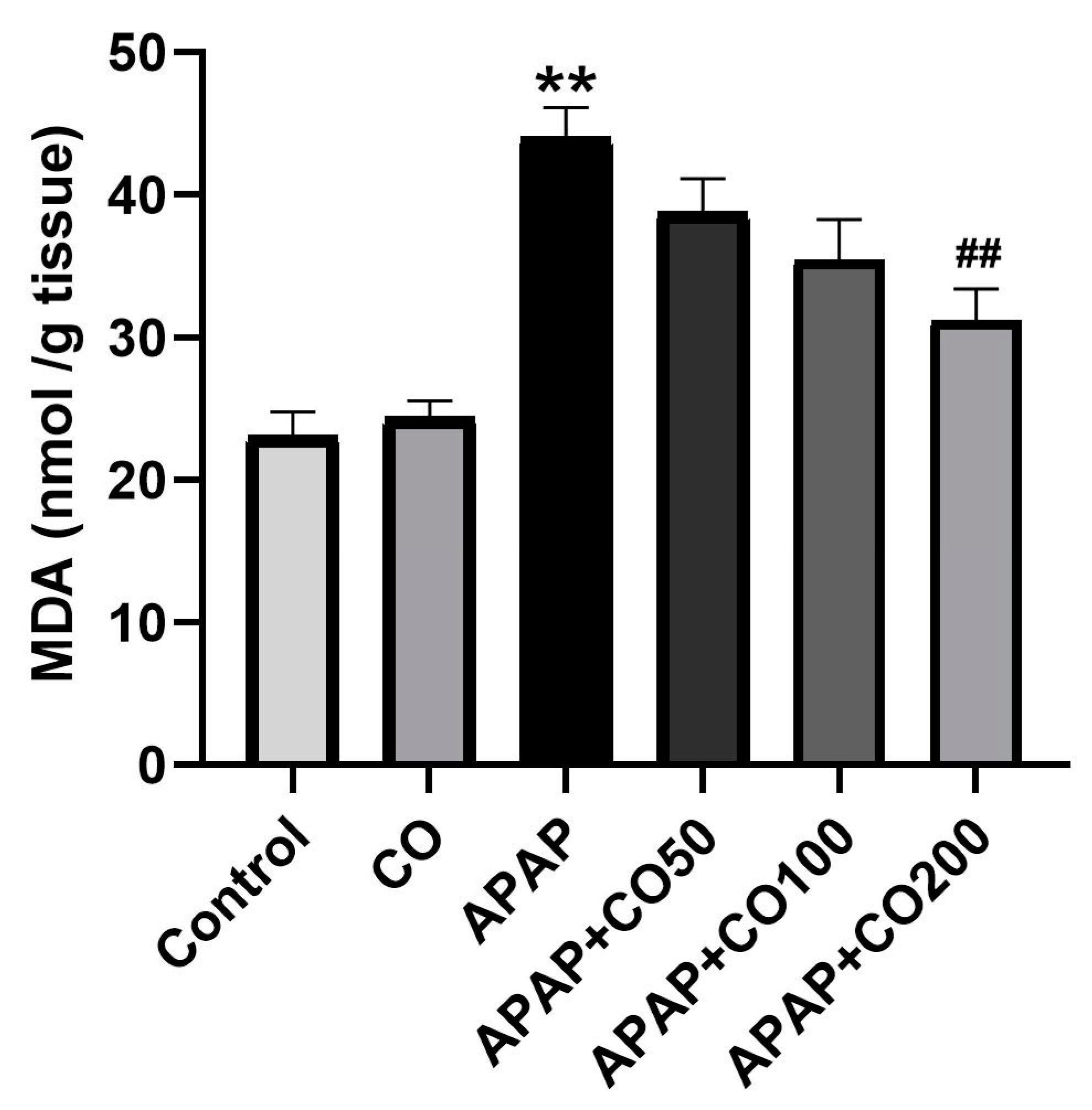
2.1. Effect of CO on LPO
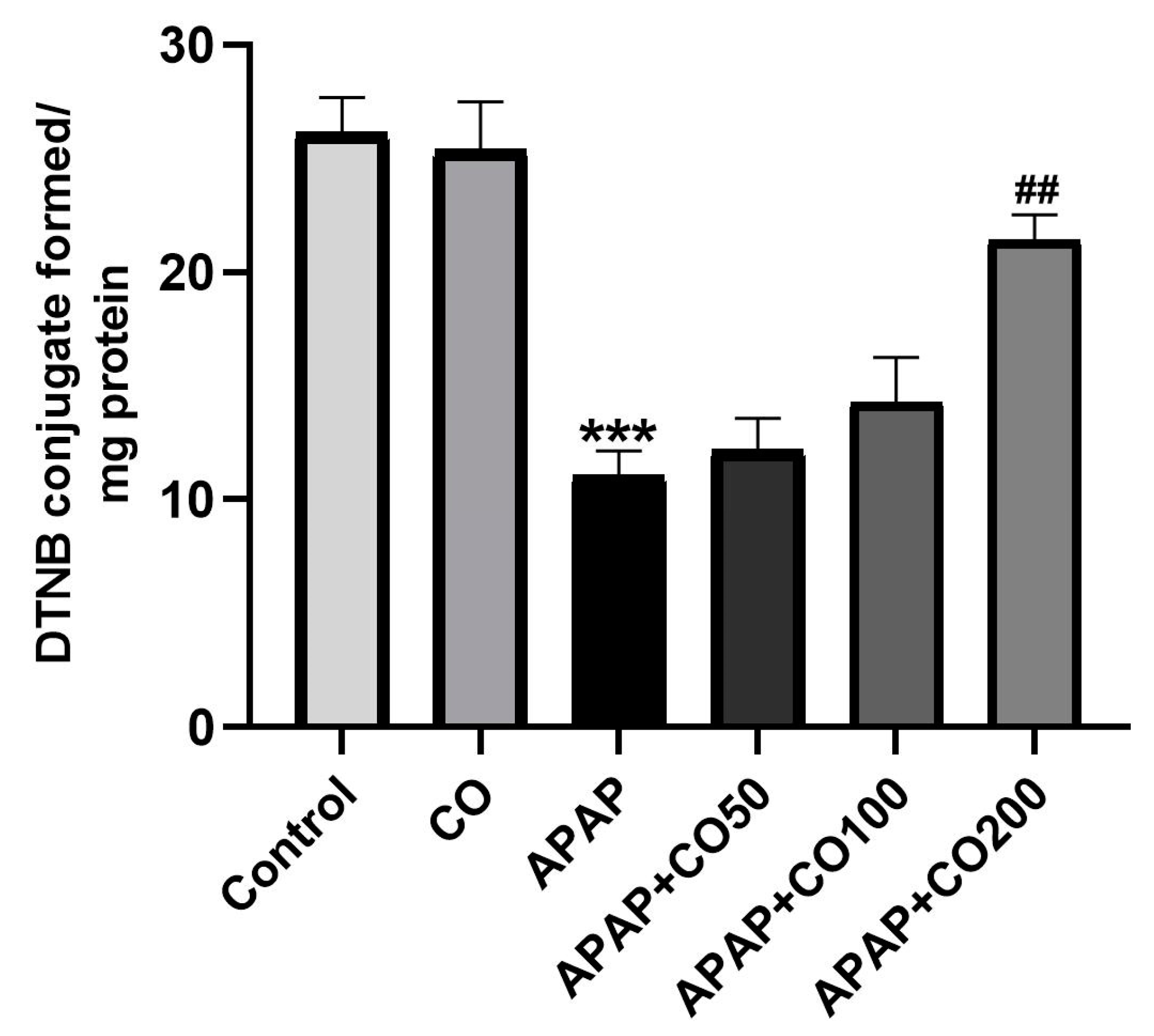
2.2. Effect of CO on GSH Level
2.3. CO Treatment Protected the Activities of Superoxide Dismutase (SOD) and Catalase Enzymes in the Uterus
2.4. CO Treatment Protected the Activities of Glutathione Peroxidase (GPx) and Glutathione Reductase (GR) Enzymes in the Uterus
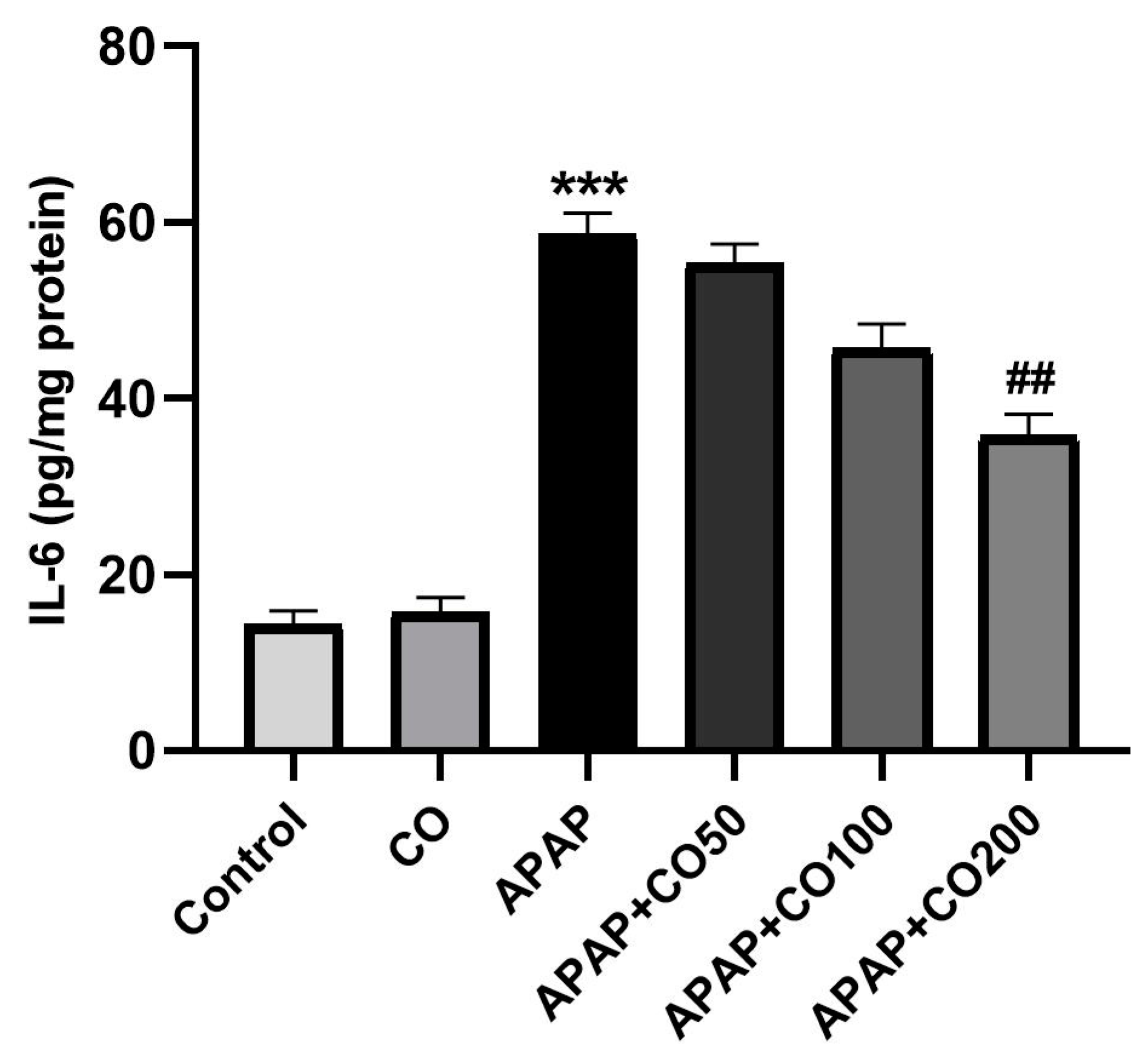
2.5. Role of CO on APAP-Induced Toxicity in Proinflammatory Cytokines
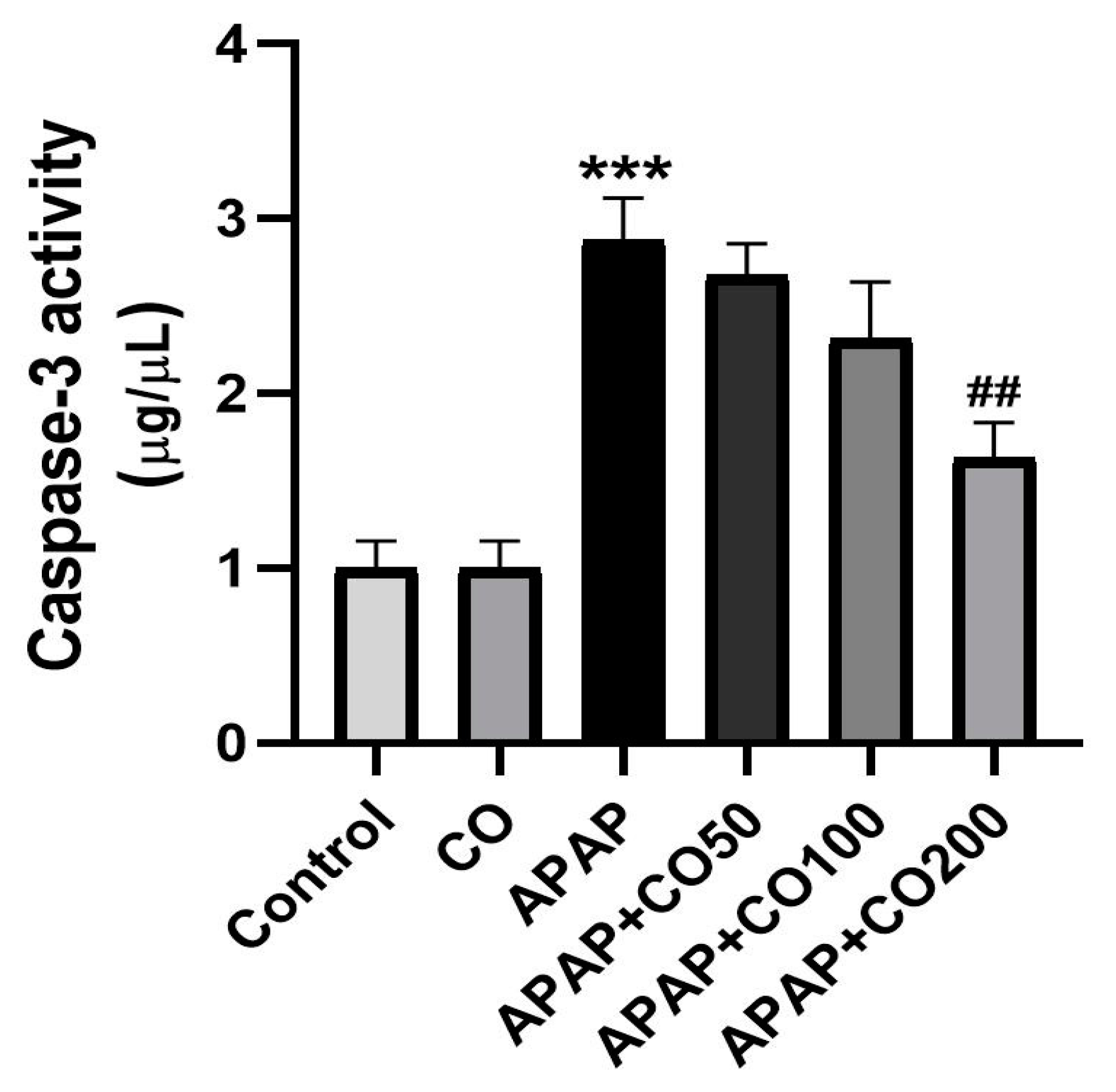
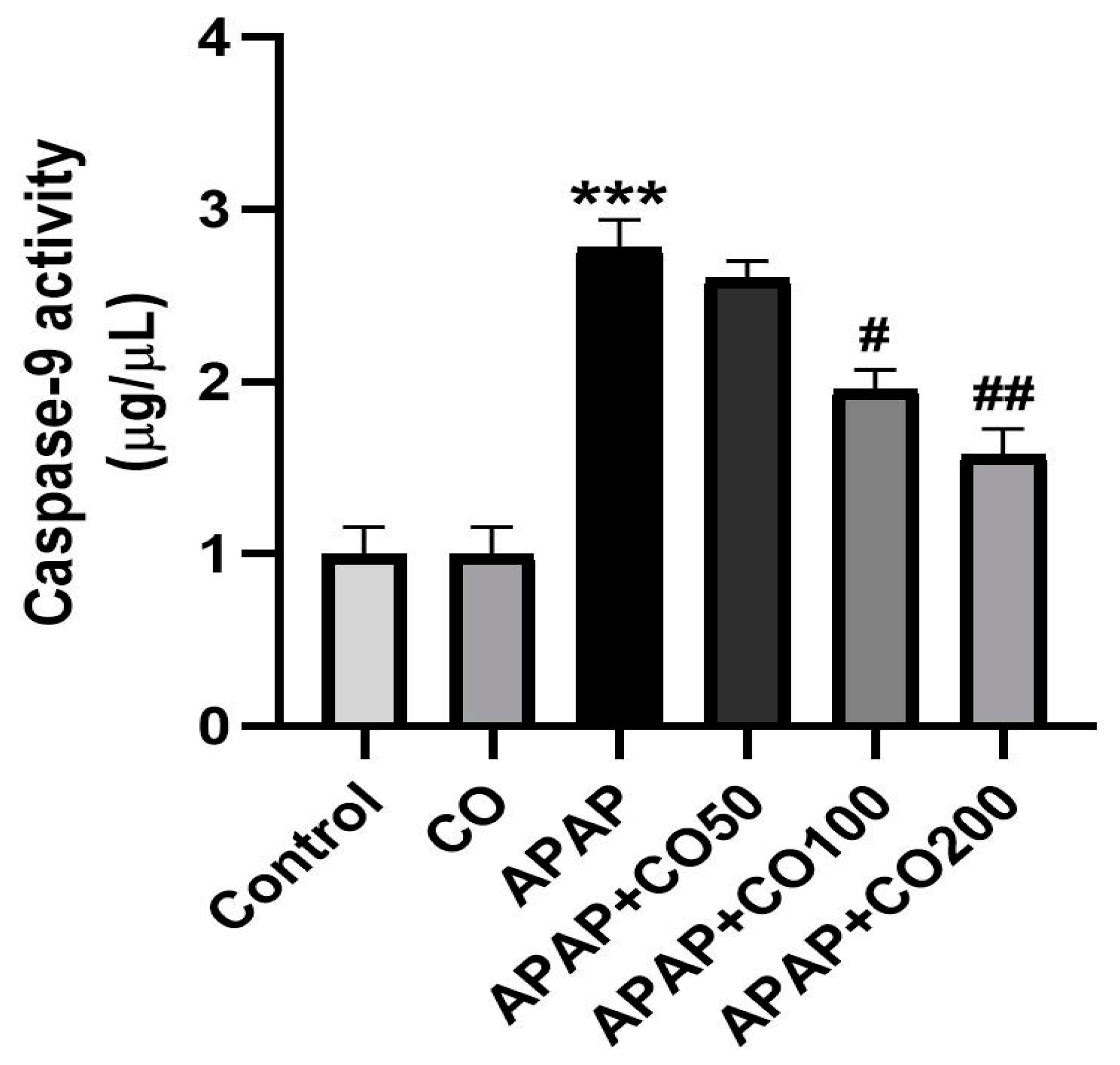
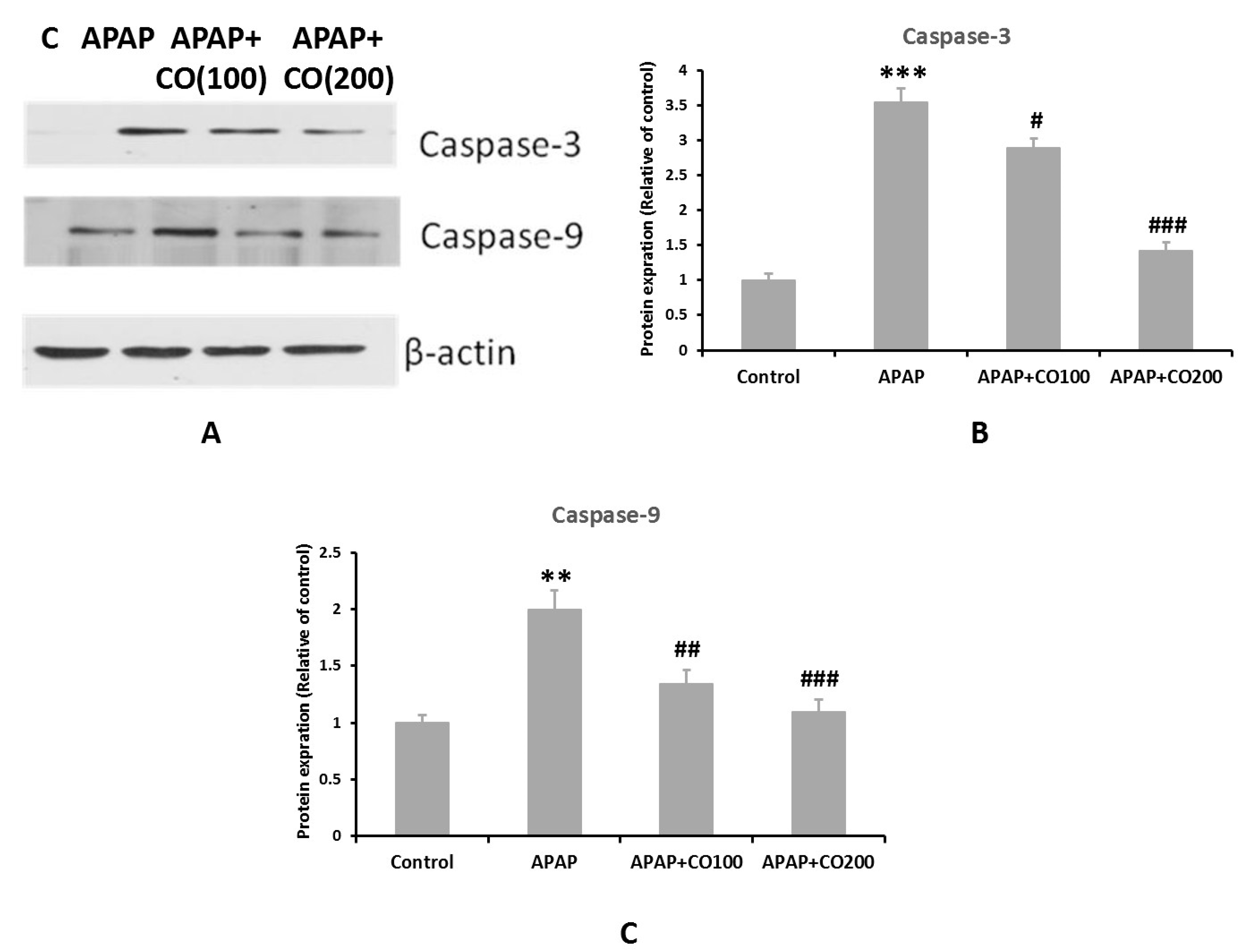
2.6. CO Treatment Suppresses Expression of Activated Caspases 3 and 9
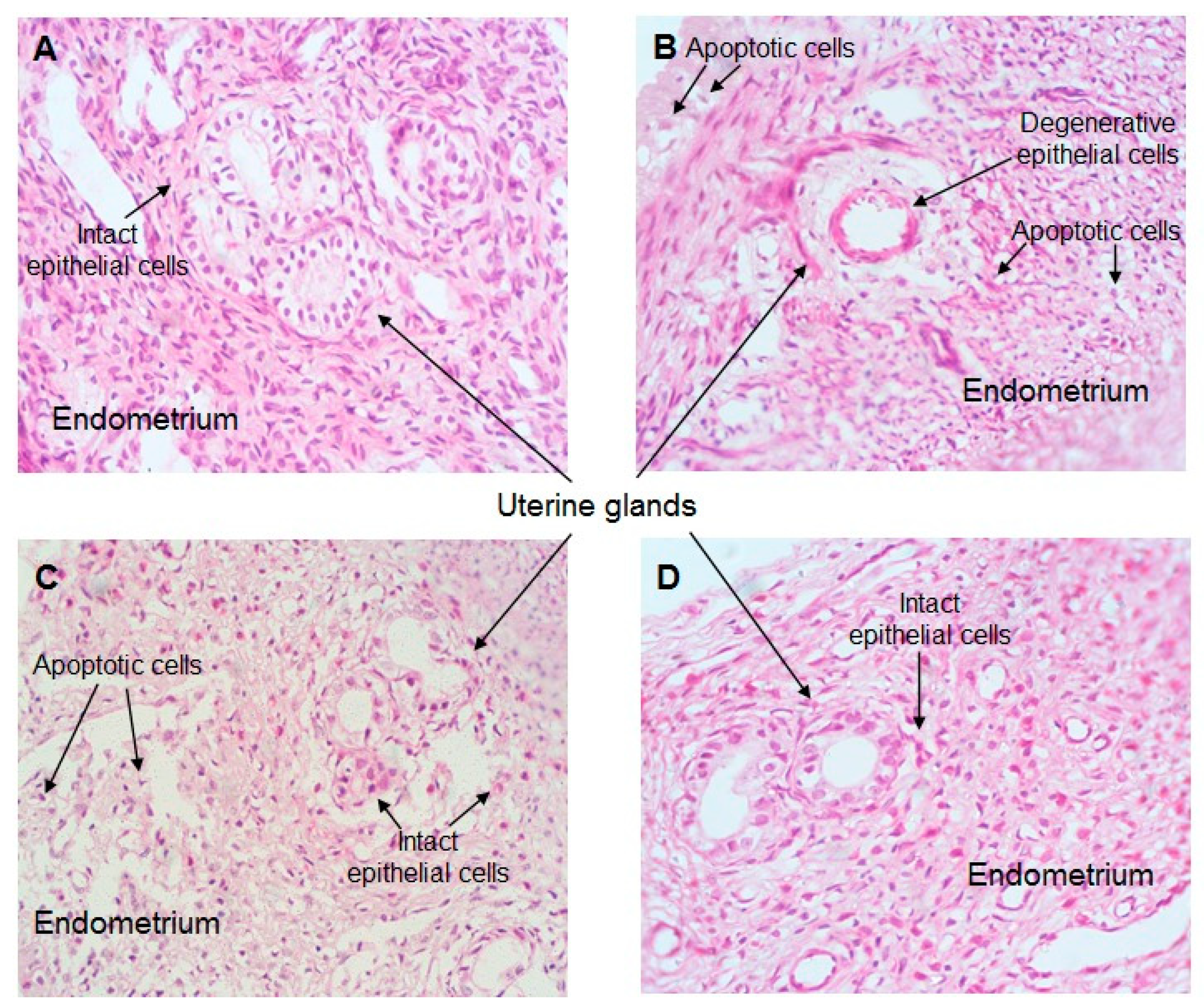
2.7. Effect of CO Treatment on Histopathology of the Uterus
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Animals
3.3. Experimental Design
3.4. Uterus Tissue Sample Preparation
3.5. Estimation of Lipid Peroxidation
3.6. Estimation of Reduced Glutathione (GSH)
3.7. Estimation of the Activity of the Superoxide Dismutase (SOD)
3.8. Estimation of Catalase Activity
3.9. Assay of Glutathione Reductase (GR) and Glutathione Peroxidase (GPx) Activity
3.10. Assay of Inflammatory Cytokine (IL-6 and IL-1 Beta) and Apoptosis Markers (Caspases 3 and 9)
3.11. Western Blot Analysis of Caspases 3 and 9
3.12. Uterus Histopathological Studies
3.13. Estimation of Protein
3.14. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, L.P.; Mayeux, P.R.; Hinson, J.A. “Acetaminophen- induced hepatotoxicity,” Drug Metabolism and Disposition: The Biological Fate of Chemicals. Drug Metab. Dispos. 2003, 31, 1499–1506. [Google Scholar] [CrossRef]
- Bagnall, W.E.; Kelleher, J.; Walker, B.E.; Losowsky, M.S. The gastrointestinal absorption of paracetamol in the rat. J. Pharm. Pharmacol. 1979, 31, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Ashafaq, M.; Alshahrani, S.; Siddiqui, R.; Ahmed, R.A.; Khuwaja, G.; Islam, F. Cinnamon oil against acetaminophen-induced acute liver toxicity by attenuating inflammation, oxidative stress and apoptosis. Toxicol. Rep. 2020, 7, 1296–1304. [Google Scholar] [CrossRef]
- Alshahrani, S.; Ashafaq, M.; Hussain, S.; Mohammed, M.; Sultan, M.; Jali, A.M.; Siddiqui, R.; Islam, F. Renoprotective effects of cinnamon oil against APAP-Induced nephrotoxicity by ameliorating oxidative stress, apoptosis and inflammation in rats. Saudi Pharm. J. 2021, 29, 194–200. [Google Scholar] [CrossRef]
- Ashafaq, M.; Hussain, S.; Alshahrani, S.; Madkhali, O.; Siddiqui, R.; Khuwaja, G.; Alam, M.I.; Islam, F. Role of cinnamon oil against acetaminophen overdose induced neurological aberrations through brain stress and cytokine upregulation in rat brain. Drug Chem. Toxicol. 2020, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Briant, R.H.; Dorrington, R.E.; Cleal, J.; Williams, F.M. The Rate of Acetaminophen Metabolism in the Elderly and the Young. J. Am. Geriatr. Soc. 1976, 24, 359–361. [Google Scholar] [CrossRef]
- Brodie, B.B.; Axelrod, J. The fate of acetophenetidin (phenacetin) in man and methods for the estimation of acetophenetidin and its metabolites in biological material. J. Pharmac. Exp. Therap. 1949, 97, 58–67. [Google Scholar]
- Heading, R.C.; Nimmo, J.; Prescott, L.F.; Tothill, P. The dependence of paracetamol absorption on the rate of gastric emptying. Br. J. Pharmacol. 1973, 47, 415–421. [Google Scholar] [CrossRef]
- Prescott, L. Gastrointestinal Absorption of Drugs. Med. Clin. N. Am. 1974, 58, 907–916. [Google Scholar] [CrossRef]
- Den Braver, M.W.; Vermeulen, N.P.E.; Commandeur, J.N.M. Generic method for the absolute quantification of glutathione S-conjugates: Application to the conjugates of acetaminophen, clozapine and diclofenac. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1046, 185–194. [Google Scholar] [CrossRef]
- Mugford, C.A.; Tarloff, J.B. The contribution of oxidation and deacetylation to acetaminophen nephrotoxicity in female Sprague-Dawley rats. Toxicol. Lett. 1997, 93, 15–22. [Google Scholar] [CrossRef]
- Lalert, L.; Ji-Au, W.; Srikam, S.; Chotipinit, T.; Sanguanrungsirikul, S.; Srikiatkhachorn, A.; Grand, S.M.-L. Alterations in Synaptic Plasticity and Oxidative Stress Following Long-Term Paracetamol Treatment in Rat Brain. Neurotox. Res. 2019, 37, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Huo, Y.; Yin, S.; Hu, H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018, 17, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-T.; Deng, J.-S.; Huang, W.-C.; Shieh, P.-C.; Chung, M.-I.; Huang, G.-J. Salvianolic Acid C against Acetaminophen-Induced Acute Liver Injury by Attenuating Inflammation, Oxidative Stress, and Apoptosis through Inhibition of the Keap1/Nrf2/HO-1 Signaling. Oxidative Med. Cell. Longev. 2019, 2019, 9056845. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, A.C.; Sun, R.; Mishin, V.; Hall, L.B.; Laskin, J.D.; Laskin, D.L. Role of galectin-3 in acetaminophen-induced hepatotoxicity and inflammatory mediator production. Toxicol. Sci. 2012, 127, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.T.; Arjumand, W.; Nafees, S.; Seth, A.; Ali, N.; Rashid, S.; Sultana, S. Hesperidin alleviates acetaminophen induced toxicity in wistar rats by abrogation of oxidative stress, apoptosis and inflammation. Toxicol. Lett. 2012, 208, 149–161. [Google Scholar] [CrossRef]
- Trease, G.E.; Evans, W.C. Trease & Evans. Pharmacognosy, 13th ed.; Bailliére Tindall: London, UK, 1989. [Google Scholar]
- De Smet, P.A.G.M. The Role of Plant-Derived Drugs and Herbal Medicines in Healthcare. Drugs 1997, 54, 801–840. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Manna, P.; Sil, P.C. Taurine protects acetaminophen-induced oxidative damage in mice kidney through APAP urinary excretion and CYP2E1 inactivation. Toxicology 2010, 269, 24–34. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, G.; Huang, L.; Pang, H.; Zhang, N.; Chen, Y.; Wang, G. Hepatoprotective Effect of Polyphenol-Enriched Fraction from Folium Microcos on Oxidative Stress and Apoptosis in Acetaminophen-Induced Liver Injury in Mice. Oxidative Med. Cell. Longev. 2017, 2017, 3631565. [Google Scholar] [CrossRef]
- Murcia, M.A.; Egea, I.; Romojaro, F.; Parras, P.; Jiménez, A.M.; Martínez-Tomé, M. Antioxidant Evaluation in Dessert Spices Compared with Common Food Additives. Influence of Irradiation Procedure. J. Agric. Food Chem. 2004, 52, 1872–1881. [Google Scholar] [CrossRef]
- Tuzcu, Z.; Orhan, C.; Sahin, N.; Juturu, V.; Sahin, K. Cinnamon Polyphenol Extract Inhibits Hyper-lipidemia and Inflammation by Modulation of Transcription Factors in High-Fat Diet-Fed Rats. Oxidative Med. Cell. Longev. 2017, 2017, 1583098. [Google Scholar] [CrossRef]
- Yulug, B.; Kilic, E.; Altunay, S.; Ersavas, C.; Orhan, C.; Dalay, A.; Tuzcu, M.; Sahin, N.; Juturu, V.; Sahin, K. Cinnamon Polyphenol Extract Exerts Neuroprotective Activity in Traumatic Brain In-jury in Male Mice. CNS Neurol. Disord. Drug Targets 2018, 17, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Thiele, K.; Solano, M.E.; Huber, S.; Flavell, R.A.; Kessler, T.; Barikbin, R.; Jung, R.; Karimi, K.; Tiegs, G.; Arck, P.C. Prenatal Acetaminophen Affects Maternal Immune and Endocrine Adaptation to Pregnancy, Induces Placental Damage, and Impairs Fetal Development in Mice. Am. J. Pathol. 2015, 185, 2805–2818. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; Van den Anker, J.N. Perinatal and neonatal use of paracetamol for pain relief. In Seminars in Fetal & Neonatal Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–6. [Google Scholar]
- Horowitz, R.S.; Dart, R.C.; Jarvie, D.R.; Bearer, C.F.; Gupta, U. Placental Transfer of N-Acetylcysteine Following Human Maternal Acetaminophen Toxicity. J. Toxicol. Clin. Toxicol. 1997, 35, 447–451. [Google Scholar] [CrossRef]
- Hussain, S. Comparative efficacy of the curcumin against H2O2 induced ROS in cervical cancer biopsies and Hela cell line. Int. J. Adv. Pharm. Med. Bioallied Sci. 2017, 123, 209–212. [Google Scholar]
- Ashafaq, M.; Tabassum, H.; Parvez, S. Modulation of Behavioral Deficits and Neurodegeneration by Tannic Acid in Experimental Stroke Challenged Wistar Rats. Mol. Neurobiol. 2016, 54, 5941–5951. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Mitka, M. FDA Asks Physicians to Stop Prescribing High-Dose Acetaminophen Products. JAMA 2014, 311, 563. [Google Scholar] [CrossRef]
- Ghanem, C.I.; Pérez, M.J.; Manautou, J.E.; Mottino, A.D. Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity. Pharmacol. Res. 2016, 109, 119–131. [Google Scholar] [CrossRef]
- Eidi, A.; Mortazavi, P.; Bazargan, M.; Zaringhalam, J. Hepatoprotective activity of cinnamon ethanolic extract against ccl4-induced liver injury in rats. EXCLI J. 2012, 11, 495–507. [Google Scholar]
- Hussain, Z.; Khan, J.A.; Arshad, A.; Asif, P.; Rashid, H.; Arshad, M. Protective effects of Cinnamomum zeylanicum L. (Darchini) in acetaminophen-induced oxidative stress, hepatotoxicity and nephrotoxicity in mouse model. Biomed. Pharmacother. 2018, 109, 2285–2292. [Google Scholar] [CrossRef] [PubMed]
- Dorri, M.; Hashemitabar, S.; Hosseinzadeh, H. Cinnamon (Cinnamomum zeylanicum) as an antidote or a protective agent against natural or chemical toxicities: A review. Drug Chem. Toxicol. 2018, 41, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Kokabiyan, Z.; Yaghmaei, P.; Jameie, S.B.; Hajebrahimi, Z. Therapeutic Effects of Eugenol in Polycystic Ovarian Rats Induced by Estradiol Valerate: A Histopathological and A Biochemical Study. Int. J. Fertil. Steril. 2022, 16, 184–191. [Google Scholar] [PubMed]
- Mollova, S.; Dzhurmanski, A.; Fidan, H.; Bojilov, D.; Manolov, S.; Dincheva, I.; Stankov, S.; Stoyanova, A.; Ercisli, S.; Assouguem, A.; et al. Chemical Composition of Essential Oils from Nepeta transcaucasica Grossh. and Nepeta cataria L. Cultivated in Bulgaria and Their Antimicrobial and Antioxidant Activity. ACS Omega 2023, 8, 15441–15449. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Chen, C. Inhibition of acetaminophen-induced hepatotoxicity in mice by exogenous thymosinbeta-4 treatment. Int. Immunopharmacol. 2018, 61, 20–28. [Google Scholar] [CrossRef]
- Cermik, H.; Taslipinar, M.Y.; Aydin, I.; Agilli, M.; Aydin, F.N.; Ucar, F.; Alp, B.F.; Toygar, M.; Ozkan, E.; Altayli, E.; et al. The Relationship Between N-acetylcysteine, Hyperbaric Oxygen, and Inflammation in a Rat Model of Acetaminophen-induced Nephrotoxicity. Inflammation 2013, 36, 1145–1152. [Google Scholar] [CrossRef]
- Abdel-Wahab, W.M.; Moussa, F.I. Neuroprotective effect of N-acetylcysteine against cisplatin-induced toxicity in rat brain by modulation of oxidative stress and inflammation. Drug Des. Dev. Ther. 2019, 13, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, F.; Wang, K.; Liu, G.; Yang, M.; Luan, Y.; Zhao, Z. Protective effect of allyl methyl disulfide on acetaminophen-induced hepatotoxicity in mice. Chem. Biol. Interact. 2016, 249, 71–77. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Control | CO Only | APAP Only | APAP + CO 50 | APAP + CO 100 | APAP + CO 200 |
|---|---|---|---|---|---|---|
| SOD (nmol of epinephrine protected from oxidation/min/mg protein) | 250.27 ± 24 | 249.93 ± 19 (−0.13%) | 108.62 ± 16 *** (−56.59%) a | 115.33 ± 17 (6.17%) b | 156.57 ± 19 (44.14%) b | 200.66 ± 22 ## (84.73%) b |
| CAT (nmol H2O2 consumed/min/mg protein) | 14.26 ± 1.23 | 15.02 ± 1.06 (5.32%) | 8.62 ± 1.06 ** (−39.55%) a | 10.37 ± 0.91 (25.54%) b | 12.85 ± 1.72 # (55.13%) b | 13.02 ± 1.87 ## (57.62%) b |
| GPx (nmol of NADPH oxidized/min/mg protein) | 159.03 ± 17 | 157.87 ± 19 (−0.72%) | 67.79 ± 15 *** (−57.37%) a | 92.73 ± 11 (36.79%) b | 98.66 ± 13 ## (45.53%) b | 122.41 ± 18 ### (80.57%) b |
| GR (nmol of NADPH oxidized/min/mg protein) | 200.82 ± 24 | 203.11 ± 17 (1.14%) | 109.39 ± 16 *** (−45.52%) a | 118.09 ± 19 (7.95%) b | 125.28 ± 15 (14.65%) b | 171.79 ± 21 ### (57.04%) b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Alshahrani, S.; Siddiqui, R.; Khan, A.; Elhassan Taha, M.M.; Ahmed, R.A.; Jali, A.M.; Qadri, M.; Khairat, K.H.M.; Ashafaq, M. Cinnamon Oil Alleviates Acetaminophen-Induced Uterine Toxicity in Rats by Abrogation of Oxidative Stress, Apoptosis, and Inflammation. Plants 2023, 12, 2290. https://doi.org/10.3390/plants12122290
Hussain S, Alshahrani S, Siddiqui R, Khan A, Elhassan Taha MM, Ahmed RA, Jali AM, Qadri M, Khairat KHM, Ashafaq M. Cinnamon Oil Alleviates Acetaminophen-Induced Uterine Toxicity in Rats by Abrogation of Oxidative Stress, Apoptosis, and Inflammation. Plants. 2023; 12(12):2290. https://doi.org/10.3390/plants12122290
Chicago/Turabian StyleHussain, Sohail, Saeed Alshahrani, Rahimullah Siddiqui, Andleeb Khan, Manal Mohammed Elhassan Taha, Rayan A. Ahmed, Abdulmajeed M. Jali, Marwa Qadri, Khairat H. M. Khairat, and Mohammad Ashafaq. 2023. "Cinnamon Oil Alleviates Acetaminophen-Induced Uterine Toxicity in Rats by Abrogation of Oxidative Stress, Apoptosis, and Inflammation" Plants 12, no. 12: 2290. https://doi.org/10.3390/plants12122290
APA StyleHussain, S., Alshahrani, S., Siddiqui, R., Khan, A., Elhassan Taha, M. M., Ahmed, R. A., Jali, A. M., Qadri, M., Khairat, K. H. M., & Ashafaq, M. (2023). Cinnamon Oil Alleviates Acetaminophen-Induced Uterine Toxicity in Rats by Abrogation of Oxidative Stress, Apoptosis, and Inflammation. Plants, 12(12), 2290. https://doi.org/10.3390/plants12122290







