Identification of Potential Phytochemical/Antimicrobial Agents against Pseudoperonospora cubensis Causing Downy Mildew in Cucumber through In-Silico Docking
Abstract
1. Introduction
2. Material and Methods
2.1. Homology Modeling
| Sl. No. | Sequence Description | Length of Proteins | Sequence of Amino Acids |
|---|---|---|---|
| 1 | Cytochrome oxidase subunit 1 of P. cubensis (Accession No. AEA38564.1) | 412 | MNFQNIKNWSTRWLFSTNHKDIGTXYLIFSAFAGIVGTTLSILIRIELAQPGNQIFMGNHQLYNVVVTAHAFVMVFFLVMPALIGGFGNWFVPLMIGAPDMAFPRMNNISFWLLPPALLLLISSAIVESGAGTGWAVYPPLSSVQAHSGPSVDLAIFSLHLTGISSLLGAINFISTIYNMRAPGLSFHRLPLFVWSILITAFLLLLTLPVLAGAITMLLTDRNLNTSFYDPSGGGDPVLYQHLFWFFGHPEVYVLILPAFGIISQVSAYFAKKNVFGYLGMVYAMLSIGLLGSIVWAHHMFTVGLDVDTRAYFSAATMIIAVPTGIKIFSWLATLWGGSLKFETPLLFTLGFILLFVMGGVTGVVMSNSGLDIALHDTYYIVGHFHYVLSMGAIFGIFTGFYFWIGKISGRR |
| 2 | QNE 4 effector protein P. cubensis (Accession No. ADW27474.1) | 517 | MMPPAKLVAYIAVASSIVLARYEASTDITSTSDANKLSISAPSDPVQHDTKQLLRTSDTAVTKDNEERMFNAAGLKRASTMSHFADVHGLPHEPLAPHLHDTYDPAGASHPPVLPYTGEAKAHEDLQHAASTSNPLKKISPADTQLTEGENNEAEILKRIMTLMQPVAPRALKRKRKLPDGTETQLQWNESDILDIYEKHKDKFLNIMNEWWLNGLGPQAFERMILENQLPTSIYEDYVMFHAAKDEEMYEHFAKWQNEGILPKEIEEKINAVLPKARKAPLVVRLENKYEVFYKKKQPFEAYRTKLLDEDTEPEEAERLKSKKWDRLRVVLKVRSSQRKTKFTLQWFRKHPNEFLLKSIQEGTPPEDIRSVLGLARLEGLKLFKHPNYEYYLKYLKLWFQTHSTEHWQERVPKGMPPEDVRFILGLGQLKGSEFSQHPNFPEYIKFFELWHEAYTRKKMKEWMQLNTPLDEAFAKLAIRDHNDVEFIVDKSDLYMKQYENEWKKKHPTLRTPAVST |
2.2. Molecular Docking
2.2.1. Ligands’ Source and Fungal Receptor Proteins
2.2.2. Preparation of Ligands and Target Proteins
2.2.3. Active Site Prediction and Molecular Docking
2.3. In Vitro Evaluation of Botanicals
2.3.1. Aqueous Extraction
2.3.2. Methanolic Extraction
3. Results
3.1. Modeling and Physicochemical Properties of Proteins
3.1.1. Prediction of the 3D Structure of Proteins of P. cubensis
3.1.2. Template Selection
3.1.3. Ramachandran Plot Analysis
3.1.4. Physico-Chemical Properties of Two Proteins of P. cubensis

| Sl. No. | Ramachandran Plot Statistics | QNE4 | Cytochrome Oxidase Subunit 1 | ||
|---|---|---|---|---|---|
| Residues | Percentage (%) | Residues | Percentage (%) | ||
| 1 | Residues in most favored regions [A, B, L] | 92 | 86.8 | 140 | 82.8 |
| 2 | Residues in additional allowed regions [a, b, l, p] | 13 | 12.3 | 27 | 16.0 |
| 3 | Residues in generously allowed regions [~a,~b,~l,~p] | 0 | 0.0 | 2 | 1.2 |
| 4 | Residues in disallowed regions | 1 | 0.9 | 0 | 0.0 |
| 5 | Number of non-glycine and non-proline residues | 106 | 100.0 | 169 | 100.0 |
| 6 | Number of end-residues (except Gly and Pro) | 2 | 2 | ||
| 7 | Number of glycine residues (shown in triangles) | 4 | 7 | ||
| 8 | Number of proline residues | 5 | 10 | ||
| 9 | Total number of residues | 117 | 188 | ||
| Sl. No | Description | QNE4 | Cytochrome Oxidase Subunit 1 |
|---|---|---|---|
| 1 | Number of amino acids | 517 | 412 |
| 2 | Molecular weight (Daltons) | 60,203.75 | 45,283.65 |
| 3 | Theoretical pI | 7.08 | 8.70 |
| 4 | Negatively charged residues | 76 | 14 |
| 5 | Positively charged residues | 75 | 16 |
| 6 | Ext. coefficient M−1 cm−1 | 88,810 | 82,850 |
| 7 | Instability index | 40.53 | 25.94 |
| 8 | Aliphatic index | 75.71 | 114.78 |
| 9 | Grand average of hydropathicity (GRAVY) | −0.726 | −0.796 |
3.2. Molecular Docking Studies
| Group | Sl. No. | Compound | Dock Score for Binding Affinity (kcal/mol) | |
|---|---|---|---|---|
| Cytochrome Oxidase Subunit 1 | QNE Effector Protein P. cubensis | |||
| Terpenoids | 1 | Cucurbitacin-A | −7.9 | −8.1 |
| 2 | Cucurbitacin-B | −7.8 | −8.3 | |
| 3 | Cucurbitacin-C | −7.4 | −7.4 | |
| 4 | Cucurbitacin-D | −8.0 | −8.2 | |
| 5 | Cucurbitacin-E | −8.0 | −8.1 | |
| 6 | Cucurbitacin-I | −8.3 | −8.0 | |
| Glucosyl flavones | 7 | Cucumerin-A | −7.8 | −9.1 |
| 8 | Cucumerin-B | −7.7 | −8.5 | |
| Flavonoids | 9 | Vitexin | −7.0 | −7.5 |
| 10 | Isovitexin | −7.6 | −8.0 | |
| 11 | Orientin | −7.1 | −7.4 | |
| 12 | Isoorientin | −7.4 | −7.9 | |
| Megastigmane derivatives | 13 | Cucumegastigmane-I | −5.3 | −5.4 |
| 14 | Cucumegastigmane-II | −6.2 | −7.8 | |
| 15 | (+)-Dehydrovomifoliol | −5.0 | −6.3 | |
| Indolic secondary metabolites | 16 | Indole-3-aldehyde | −4.4 | −5.0 |
| 17 | Indole-3-carboxylic acid | −4.8 | −5.5 | |
| Flavone glucosides | 18 | Isoscoparin | −7.5 | −8.5 |
| 19 | Saponarin | −8.1 | −7.3 | |
| 20 | Vicenin-2 | −7.1 | −8.0 | |
| 21 | Apigenin-7-O-glucoside | −7.5 | −8.5 | |
| 22 | Quercetin-3-O-glucoside | −6.8 | −7.6 | |
| 23 | Isorhamnetin-3-O-glucoside | −6.5 | −6.9 | |
| 24 | Kaemferol-3-O-rhamnoside | −7.2 | −7.4 | |
| Polyphenol | 25 | 4-hydroxycinnamic acid | −4.8 | −5.8 |
| Antimicrobial compounds | 26 | Carrageenan | −7.0 | −8.0 |
| 27 | Acyclovir | −5.0 | −5.7 | |
| 28 | 5-Azacytidine | −5.4 | −5.8 | |
| 29 | Cytarbine | −5.4 | −5.9 | |
| 30 | Ribavirin | −5.2 | −5.8 | |
| 31 | Ridovudine | −6.0 | −7.0 | |
| 32 | Ningnanmycin | −6.0 | −7.8 | |
| 33 | Vidarabine | −5.7 | −6.6 | |
| 34 | Acycloguanosine | −5.0 | −5.5 | |
| 35 | 2-Thiouracil | −3.3 | −4.6 | |
| 36 | Moroxydine hydrochloride | −4.7 | −5.2 | |
| 37 | Luotonin A | −7.8 | −6.9 | |
| 38 | Tylophorinine | −7.1 | −8.1 | |
| 39 | Antofine | −7.2 | −6.6 | |
| 40 | Deoxytylophorinine | −7.2 | −6.7 | |
| 41 | Pyrroloisoquinoline | −5.0 | −7.4 | |
| 42 | Pulmonarin-A | −4.8 | −5.5 | |
| 43 | Pulmonarin-B | −5.0 | −5.9 | |
| 44 | Streptindole | −6.1 | −7.6 | |
| 45 | Tryptanthrin | −6.8 | −7.7 | |
| 46 | Essramycin | −6.6 | −7.9 | |
| 47 | Chlorogenic acid | −6.7 | −7.3 | |
| 48 | Peonidin | −6.3 | −7.2 | |
| 49 | Swertianolin | −8.0 | −7.8 | |
| 50 | Zidovudine | −5.8 | −6.6 | |
| Clove | 51 | Eugenol | −4.7 | −5.3 |
| 52 | Eugenol acetate | −4.7 | −5.4 | |
| 53 | (E)-β-caryophyllene | −5.6 | −6.8 | |
| Garlic | 54 | Allyl acetate | −6.6 | −7.2 |
| 55 | Allicin | −3.4 | −3.7 | |
| 56 | Allixin | −4.9 | − 5.8 | |
| 57 | Alliin | −3.9 | −4.5 | |
| Neemm | 58 | Azadiractin a | −3.7 | 3.5 |
| 59 | Nibolin b | −3.4 | −3.4 | |
| 60 | Azadiractin b | −3.2 | −3.6 | |
| 61 | Nimbin | −5.0 | −5.5 | |
| Tulsi | 62 | Gallic acid | −4.5 | −5.1 |
| 63 | Catechol | −4.1 | −5.0 | |
| 64 | Cinnamic acid | −3.7 | −4.4 | |
| Pudina | 65 | Menthol | −3.3 | −4.8 |
| Fungicides | 66 | Azoxystrobin | −7.2 | −8.1 |
| 67 | Ridomil | −5.3 | −5.3 | |
| 68 | Kresoxim methyl | −6.3 | −4.4 | |
| 69 | Curzate | −5.3 | −6.0 | |
| 70 | Oxalic acid | −3.3 | −5.3 | |
| 71 | Salicylic acid | −4.5 | −6.5 | |
| Sl. No. | Compound with PubChem ID | Structural and Chemical Formula | No. of H Bonds | Amino Acid Residue of QNE 4 Effector Protein Involved in Hydrogen Bonding with Ligand |
|---|---|---|---|---|
| 1 | Cucumerin-A 44257649 | 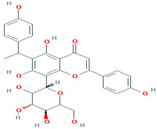 C29H28O11 | 4 | ARG339, TVR290, LEU 126, ASN134 |
| 2. | Cucumerin-B 44257648 |  C29H28O11 C29H28O11 | 1 | HIS110 |
| 3 | Isoscoparin 442611 | 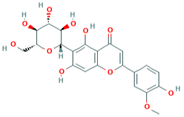 C22H22O11 C22H22O11 | 3 | SER109, HS110, GLY217 |
| 4 | Apigenin-7-O-glucoside 5280746 | 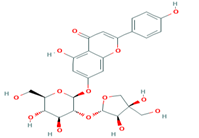 C26H28O14 | 4 | ASN214, SER109, MET224, GLY107 |
| 5 | Cucurbitacin-B 5281316 | 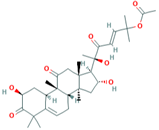 C32H46O8 | 1 | HIS110 |
| 6 | Cucurbitacin-D 5281318 |  C32H44O7 C32H44O7 | 3 | SER 125, TYR108,ARG146 |
| 7 | Cucurbitacin-A 5281315 | 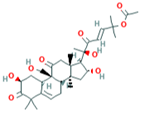 C32H46O9 | 2 | SER140, SER82 |
| 8 | Cucurbitacin-E 5281319 | 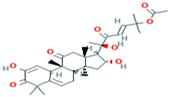 C32H44O8 C32H44O8 | 2 | SER 82, SER 109 |
| 9 | Cucurbitacin-I 5281321 | 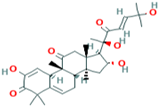 C30H42O7 C30H42O7 | 3 | LYS121,GLS127,ARG339 |
| 10 | Vicenin-2 442664 | 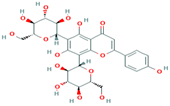 C27H30O15 C27H30O15 | 4 | SER109, HIS83, GLY107, SER82 |
| Sl. No. | Compound with PubChem ID | Structural and Chemical Formula | No. of H Bonds | Amino Acid Residue of QNE4 Effector Protein Involved in Hydrogen Bonding with Ligand |
|---|---|---|---|---|
| 1 | Azoxystrobin 3034285 | 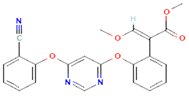 C22H17N3O5 | 1 | SER109 |
| 2 | Allyl acetate 11584 | 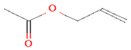 C5H8O2 | 2 | ASP86, HIS83 |
| 3 | Salicylic acid 338 |  C7H6O3 | 3 | ALA376,ARG377,LEU381 |
| 4 | Curzate 5364079 |  C7H10N4O3 | 4 | THR456,ALA376 |
| 5 | Allixin 86374 | 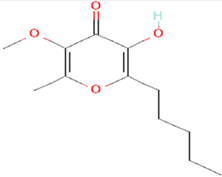 C12H18O4 | 2 | ARG285,GLN165 |

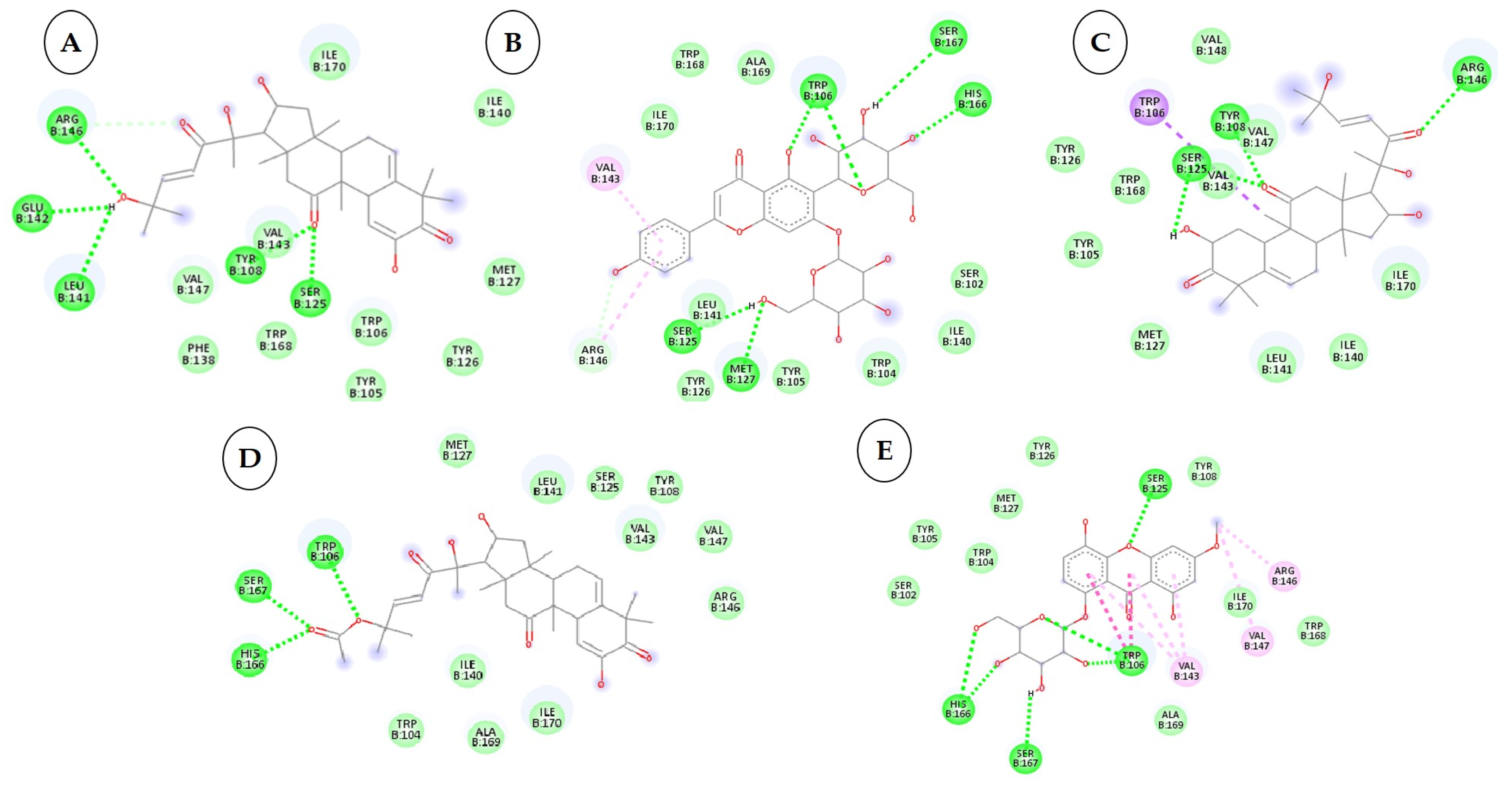
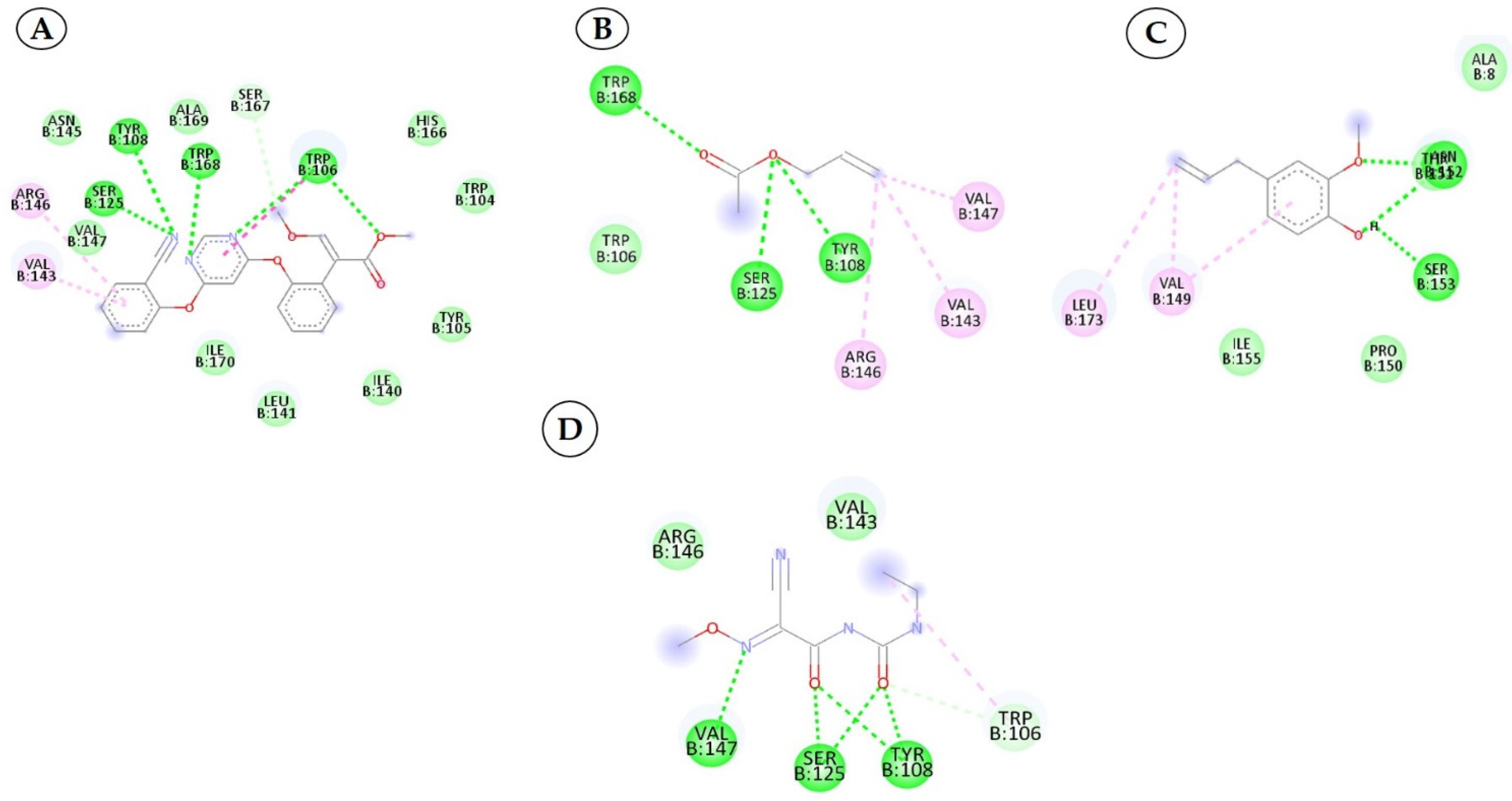

3.3. Interactions between the QNE4 Effector Protein and Phytochemicals, Antimicrobial Compounds, and Chemically Synthesized Compounds
3.4. Interactions between the Cytochrome Oxidase Subunit 1 Protein and Phytochemicals, Antimicrobial Compounds, and Fungicides
| Sl. No. | Compound with PubChem ID | Structural and Chemical Formula | No. of H Bonds | Amino Acid Residue of Cytochrome Oxidase Subunit 1 Protein Involved in Hydrogen Bonding with Ligand |
|---|---|---|---|---|
| 1 | Cucurbitacin-I 5281321 |  C30H42O7 | 5 | ARG461.GLU142, LEU141,TYR108,SER125 |
| 2 | Saponarin 441381 |  C22H30O15 C22H30O15 | 5 | TRP106, SER167,HIS166,SER125,MET127 |
| 3 | Cucurbitacin-D 5281318 |  C32H44O7 | 3 | ARG146, TYR108, SER125 |
| 4 | Cucurbitacin-E 5281319 |  C32H44O8 C32H44O8 | 3 | TRP106, SER167, HIS166 |
| 5 | Swertianolin 5858086 |  C20H20O11 C20H20O11 | 4 | SER125, HIS166, SER167, TRP106 |
| 6 | Cucurbitacin-A 5281315 |  C32H46O9 C32H46O9 | 5 | TRP168, SER125, SER167, HIS166, TRP104 |
| 7 | Cucurbitacin-B 5281316 | 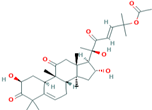 C32H46O8 | 5 | ASN152, ARG146, LEU141, VAL145, VAL147 |
| 8 | Cucumerin-A 44257649 |  C29H28O11 | 4 | LEU141, SER125, TRP106, SER167 |
| 9 | Luotonin A 10334120 | 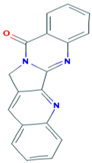 C18H11N3O | 2 | TYR108, SER125 |
| 10 | Cucumerin-B 44257648 | 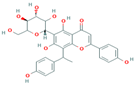 C29H28O11 C29H28O11 | 5 | LEU141, MET127, SER125, TYR108, SER167 |
| Sl. No. | Compound with PubChem ID | Structural and Chemical Formula | No. of H Bonds | Amino Acid Residue of Cytochrome Oxidase Subunit 1 Protein Involved in Hydrogen Bonding with Ligand |
|---|---|---|---|---|
| 1 | Azoxystrobin 3034285 |  C22H17N3O5 | 4 | SER125, TYR108, TRP168, TRP106 |
| 2 | Allyl acetate 11584 | 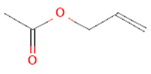 C5H8O2 | 3 | SER125, TYR108 and TRP168 |
| 3 | Kresoxim methyl 6112114 |  C18H19NO4 | 2 | TAM 552, SER153 |
| 4 | Curzate 5364079 |  C7H10N4O3 | 3 | VAL147, SER125, TYR108 |
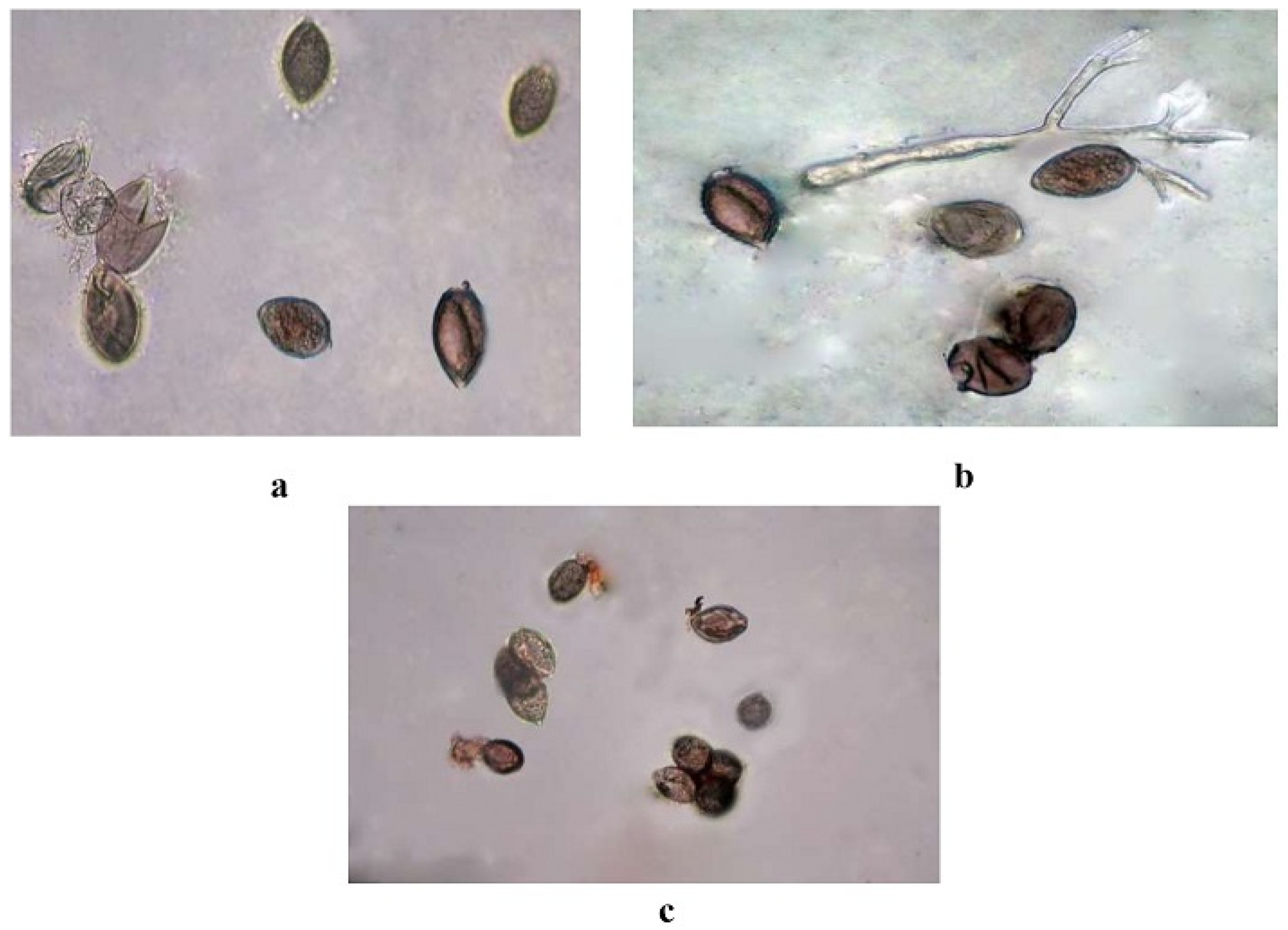
3.5. In Vitro Evaluation of Botanicals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nandkarni, K.M. Indian materia medica. Newsletter 1927, 12, 40. [Google Scholar]
- Mukherjee, P.K.; Nema, N.K.; Maity, N.; Sarkar, B.K. Phytochemical and therapeutic potential of cucumber. Fitoterapia 2013, 84, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Sahu, T.; Sahu, J. Cucumis sativus (cucumber): A review on its pharmacological activity. J. Appl. Pharm. Res. 2015, 3, 4–9. [Google Scholar]
- Ugwu, C.; Suru, S. Cosmetic, Culinary and Therapeutic Uses of Cucumber (Cucumis sativus L.). In Cucumber Economic Values and Its Cultivation and Breeding; IntechOpen: Rijeka, Croatia, 2021; p. 39. [Google Scholar]
- Gocher, D.; Sharma, M.K.; Gurjar, H.R. Management of root-knot nematode (Meloidogyne incognita) in poly house on cucumber (Cucumis sativus L.) as seed soaking treatment. Indian J. Nematol. 2018, 48, 108–112. [Google Scholar]
- FAO. World Food and Agriculture Statistical Yearbook; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Anonymous. Horticulture Statistics Division, Department of Agriculture; Cooperation and Farmers Welfare, Ministry of Agriculture and Farmers Welfare, Government of India: New Delhi, India, 2020; pp. 1–2.
- Palti, J.; Cohen, Y. Downy mildew of cucurbits (Pseudoperonospora cubensis): The fungus and its hosts, distribution, epidemiology and control. Phytoparasitica 1980, 8, 109–147. [Google Scholar] [CrossRef]
- Savory, E.A.; Granke, L.L.; Quesada-ocampo, L.M.; Varbanova, M.; Hausbeck, M.K.; Day, B. The cucurbit downy mildew pathogen Pseudoperonospora cubensis. Mol. Plant Pathol. 2011, 12, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Haque, M.N.; Hosen, S.; Akhter, J.; Kamal, U.S.B.; Jahan, E.N.; Hossain, A.S.M.S.; Uddin, M.N.; Islam, M.B.; Islam, M.R.; et al. Comparative evaluation of antimicrobial activity of different parts of Abelmoschus moschatus against multi-resistant pathogens. Int. J. Pharm. Sci. Res. 2017, 8, 1873. [Google Scholar]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants. 2022, 28, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Krajaejun, T.; Khositnithikul, R.; Lerksuthirat, T.; Lowhnoo, T.; Rujirawat, T.; Petchthong, T.; Yingyong, W.; Suriyaphol, P.; Smittipat, N.; Juthayothin, T.; et al. Expressed sequence tags reveal genetic diversity and putative virulence factors of the pathogenic oomycete Pythium insidiosum. Fungal Biol. 2011, 115, 683–696. [Google Scholar] [CrossRef]
- Fawzi, E.M.; Khalil, A.; Afifi, A. Antifungal effect of some plant extracts on Alternaria alternate and Fusarium oxysporum. Afr. J. Biotech. 2009, 2, 2590–2597. [Google Scholar]
- Feng, W.; Zheng, X. Control of Alternaria alternata by cassia oil in combination with potassium chloride or sodium chloride. J. App. Microbiol. 2006, 101, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Cheng, Z.; Ahmad, H.; Ali, M.; Chen, X.; Wang, M. Garlic, from remedy to stimulant: Evaluation of antifungal potential reveals diversity in phytoalexin allicin content among garlic cultivars; allicin containing aqueous garlic extracts trigger antioxidants in cucumber. Front. Plant Sci. 2016, 7, 1235. [Google Scholar] [CrossRef] [PubMed]
- Doshi, P.; Nisha, N.; Yousif, A.I.A.; Korosi, K.; Bán, R.; Turóczi, G. Preliminary investigation of effect of neem-derived pesticides on Plasmopara halstedii pathotype 704 in sunflower under in vitro and in vivo conditions. Plants 2020, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Najdabbasi, N.; Mirmajlessi, S.M.; Dewitte, K.; Landschoot, S.; Mänd, M.; Audenaert, K.; Ameye, M.; Haesaert, G. Biocidal activity of plant-derived compounds against Phytophthora infestans: An alternative approach to late blight management. Crop Prot. 2020, 138, 105315. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. Swiss-model: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, 296–303. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System; DeLano Scientific: San Carlos, CA, USA, 2009. [Google Scholar]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B.; De Bakker, P.I.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Cα geometry: ϕ, ψ and Cβ deviation. Protein J. 2003, 50, 437–450. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Cortes-Rojas, D.F.; De Souza, C.R.F.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef]
- Kaur, P.; Dhull, S.B.; Sandhu, K.S.; Salar, R.K.; Purewal, S.S. Tulsi (Ocimum tenuiflorum) seeds: In vitro DNA damage protection, bioactive compounds and antioxidant potential. J. Food Meas. Charact. 2018, 12, 1530–1538. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, S.; Pu, T.; Fan, L.; Su, F.; Ye, M. Antifungal activity of phenolic monoterpenes and structure related compounds against plant pathogenic fungi. Nat. Prod. Res. 2019, 33, 1423–1430. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, J.; Wang, Z.; Liu, Y.; Li, Y.; Ma, D.; Wang, Q. Discovery of tryptanthrins as novel antiviral and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2020, 68, 5586–5595. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, K.; Wang, Z.; Liu, Y.; Ma, D.; Wang, Q. Luotonin A and its derivatives as novel antiviral and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2020, 68, 8764–8773. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Gao, Y.; Zhang, M.; Ding, X.; Wang, Z.; Ma, D.; Wang, Q. Streptindole and its derivatives as novel antiviral and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2020, 68, 7839–7849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ding, X.; Kang, J.; Gao, Y.; Wang, Z.; Wang, Q. Marine natural product for pesticide candidate: Pulmonarin alkaloids as novel antiviral and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2020, 68, 11350–11357. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Auto Dock4 and Auto Dock Tools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Biovia, D.S. BIOVIA Workbook, Release BIOVIA Pipeline Pilot, Release; Dassault Systemes: San Diego, CA, USA, 2020. [Google Scholar]
- Petrikovszki, R.; Doshi, P.; Turóczi, G.; Tóth, F.; Nagy, P. Investigating the side-effects of neem-derived pesticides on commercial entomopathogenic and slug-parasitic nematode products under laboratory conditions. Plants 2019, 8, 281. [Google Scholar] [CrossRef]
- Yehia, H.M. Methanolic extract of neem leaf (Azadirachta indica) and its antibacterial activity against foodborne and contaminated bacteria on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Am. Eurasian J Agric Environ. Sci. 2016, 16, 598–604. [Google Scholar]
- Bommesh, J.C.; Pitchaimuthu, M.; Sadashiva, A.T.; Sriram, S.; Varalakshmi, B.; Ravishankar, K.V. Identification and confirmation of downy mildew (Pseudoperonospora cubensis Berk. & Curt.) resistance sources in cucumber (Cucumis sativus L.). Indian Phytopathol. 2018, 71, 337–348. [Google Scholar]
- Vincent, J.M. Distortion of fungal hyphae in the presence of certain inhibitors. Nature 1947, 159, 850. [Google Scholar] [CrossRef]
- Sheoran, O.P.; Tonk, D.S.; Kaushik, L.S.; Hasija, R.C.; Pannu, R.S. Statistical Software Package for Agricultural Research Workers. Recent Advances in information theory. In Statistics & Computer Applications; Hooda, D.S., Hasija, R.C., Eds.; Department of Mathematics Statistics, CCS HAU: Hisar, India, 1998; pp. 139–143. [Google Scholar]
- Ekins, S.; Liebler, J.; Neves, B.J.; Lewis, W.G.; Coffee, M.; Bienstock, R.; Southan, C.; Andrade, C.H. Illustrating and homology modeling the proteins of the Zika virus. F1000 Res. 2016, 5, 275. [Google Scholar] [CrossRef]
- Prajapat, R.; Bajapai, V.; Gaur, R.K. Structural modeling and validation of rep protein of begomovirus strains (TLCBV and TYLCTHV). Asian J. Biol. Sci. 2011, 4, 352–361. [Google Scholar] [CrossRef]
- Klebe, G. Virtual ligand screening: Strategies, perspectives and limitations. Drugs Today 2006, 11, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Sohajda, T.; Puskas, I.; Roewer, N.; Forster, C.; Broscheit, J.A. Ionization states, cellular toxicity and molecular modeling studies of midazolam complexed with trimethyl-Β-cyclodextrin. Molecules 2014, 19, 16861–16876. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Hamza, A.; Derbalah, A. Recent approaches for controlling downy mildew of cucumber under greenhouse conditions. Plant Prot. Sci. 2015, 52, 1–9. [Google Scholar] [CrossRef]
- Rani, J.R.; Aswathy, T.R.; Kumar, M.S.; Nair, A.S.; Soniya, E.V. Screening of phytochemicals from selected plants with antifungal properties against RXLR effector protein Avr3a11 in Phytophthora capsici. Can. J. Biotechnol. 2017, 1, 34. [Google Scholar]
- Herrera-Calderon, O.; Chacaltana-Ramos, L.J.; Huayanca-Gutierrez, I.C.; Algarni, M.A.; Alqarni, M.; Batiha, G.E.S. Chemical constituents, in vitro antioxidant activity and in silico study on NADPH oxidase of Allium sativum L. (garlic) essential oil. Antioxidants 2021, 10, 1844. [Google Scholar] [CrossRef]
- Maximino, S.C.; Dutra, J.A.; Rodrigues, R.P.; Gonçalves, R.C.; Morais, P.A.; Ventura, J.A.; Schuenck, R.P.; Junior, V.L.; Kitagawa, R.R.; Borges, S.W. Synthesis of eugenol derivatives and evaluation of their antifungal activity against Fusarium solani f. sp. piperis. Curr. Pharm. Des. 2020, 26, 1532–1542. [Google Scholar] [CrossRef]
- Chen, D.; Oezguen, N.; Urvil, P.; Ferguson, C.; Dann, S.M.; Savidge, T.C. Regulation of protein-ligand binding affinity by hydrogen bond pairing. Sci. Adv. 2016, 2, 1240. [Google Scholar] [CrossRef]
- Portz, D.; Koch, E.; Slusarenko, A.J. Effects of garlic (Allium sativum) juice containing allicin on Phytophthora infestans and downy mildew of cucumber caused by Pseudoperonospora cubensis. Eur. J. Plant Pathol. 2008, 122, 197–206. [Google Scholar] [CrossRef]
| Group | Sl. No. | Compounds | PubChem/Drug Bank ID | Source |
|---|---|---|---|---|
| Terpenoids | 1 | Cucurbitacin-A | 5281315 | Cucumis sativus L. |
| 2 | Cucurbitacin-B | 5281316 | Cucumis sativus L. | |
| 3 | Cucurbitacin-C | 5281317 | Cucumis sativus L. | |
| 4 | Cucurbitacin-D | 5281318 | Cucumis sativus L. | |
| 5 | Cucurbitacin-E | 5281319 | Cucumis sativus L. | |
| 6 | Cucurbitacin-I | 5281321 | Cucumis sativus L. | |
| Glucosyl flavones | 7 | Cucumerin-A | 44257649 | Cucumis sativus L. |
| 8 | Cucumerin-B | 44257648 | Cucumis sativus L. | |
| Flavonoids | 9 | Vitexin | 5280441 | Cucumis sativus L. |
| 10 | Isovitexin | 162350 | Cucumis sativus L. | |
| 11 | Orientin | 5281675 | Cucumis sativus L. | |
| 12 | Isoorientin | 114776 | Cucumis sativus L. | |
| Megastigmane derivatives | 13 | Cucumegastigmane-I | 16105430 | Cucumis sativus L. |
| 14 | Cucumegastigmane-II | 16105434 | Cucumis sativus L. | |
| 15 | (+)-Dehydrovomifoliol | 688492 | Cucumis sativus L. | |
| Indolic secondary metabolites | 16 | Indole-3-aldehyde | 10256 | Cucumis sativus L. |
| 17 | Indole-3-carboxylic acid | 69867 | Cucumis sativus L. | |
| Flavone glucosides | 18 | Isoscoparin | 442611 | Cucumis sativus L. |
| 19 | Saponarin | 441381 | Cucumis sativus L. | |
| 20 | Vicenin-2 | 442664 | Cucumis sativus L. | |
| 21 | Apigenin-7-O-glucoside | 5280746 | Cucumis sativus L. | |
| 22 | Quercetin-3-O-glucoside | 5280804 | Cucumis sativus L. | |
| 23 | Isorhamnetin-3-O-glucoside | 5318645 | Cucumis sativus L. | |
| 24 | Kaemferol-3-O-rhamnoside | 5316673 | Cucumis sativus L. | |
| Polyphenol | 25 | 4-hydroxycinnamic acid | 637542 | Cucumis sativus L. |
| Antimicrobial compounds | 26 | Carrageenan | 71597331 | Acanthophora specifira V. |
| 27 | Acyclovir | 135398513 | Chemically synthesized | |
| 28 | 5-Azacytidine | 9444 | Chemically synthesized | |
| 29 | Cytarabine | 6253 | Chemically synthesized | |
| 30 | Ribavirin | 37542 | Chemically synthesized | |
| 31 | Ridovudine | 35370 | Chemically synthesized | |
| 32 | Ningnanmycin | 44588235 | Streptomyces noursei var. xichangensis | |
| 33 | Vidarabine | 21704 | Chemically synthesized | |
| 34 | Acycloguanosine | 135398513 | Chemically synthesized | |
| 35 | 2-Thiouracil | 1269845 | Chemically synthesized | |
| 36 | Moroxydine hydrochloride | 76621 | Chemically synthesized | |
| 37 | Luotonin A | 10334120 | Peganumnigella strum B. | |
| 38 | Tylophorinine | 264751 | Cynanchum, Pergularia and Tylophora | |
| 39 | Antofine | 639288 | Cynanchum komarovii I. | |
| 40 | Deoxytylophorinine | 6426880 | Cynanchum komarovii I. | |
| 41 | Pyrroloisoquinoline | 86733878 | Cynanchum komarovii I. | |
| 42 | Pulmonarin-A | 76335702 | Synoicum pulmonaria | |
| 43 | Pulmonarin-B | 76313965 | Synoicum pulmonaria | |
| 44 | Streptindole | 135431 | Streptococcus faecium | |
| 45 | Tryptanthrin | 73549 | Indigofera tinctoria L. | |
| 46 | Essramycin | 24829329 | Streptomyces sp. | |
| 47 | Chlorogenic acid | 1794427 | Solanum tuberosum L. | |
| 48 | Peonidin | 441773 | Solanum tuberosum L. | |
| 49 | Swertianolin | 5858086 | Swertia chirayita L., S. macrosperma L., Gentiana campestris L. | |
| 50 | Zidovudine | 35370 | Chemically synthesized | |
| Clove | 51 | Eugenol | 3314 | Syzygium aromaticum |
| 52 | Eugenol acetate | 7136 | Syzygium aromaticum | |
| 53 | (E)-β-Caryophyllene | 5281515 | Syzygium aromaticum | |
| Garlic | 54 | Allyl acetate | 11584 | Allium sativum |
| 55 | Allicin | 65036 | Allium sativum | |
| 56 | Allixin | 86374 | Allium sativum | |
| 57 | Alliin | 87310 | Allium sativum | |
| Neem | 58 | Azadiractin a | 5281303 | Azadirachta indica |
| 59 | Nibolin b | 6443005 | Azadirachta indica | |
| 60 | Azadiractin b | 16126804 | Azadirachta indica | |
| 61 | Nimbin | 108058 | Azadirachta indica | |
| Tulasi | 62 | Gallic acid | 370 | Ocimum tenuiflorum |
| 63 | Catechol | 289 | Ocimum tenuiflorum | |
| 64 | Cinnamic acid | 444539 | Ocimum tenuiflorum | |
| Pudina | 65 | Menthol | 1254 | Mentha spicata subsp. spicata |
| Chemically synthesized compounds | 66 | Azoxystrobin | 3034285 | Chemically synthesized |
| 67 | Ridomil | 3036793 | Chemically synthesized | |
| 68 | Kresoxim methyl | 6112114 | Chemically synthesized | |
| 69 | Curzate | 5364079 | Chemically synthesized | |
| 70 | Oxalic acid | 971 | Chemically synthesized | |
| 71 | Salicylic acid | 338 | Chemically synthesized |
| Sl. No. | Protein | Template | Query Coverage (%) | Per Cent Similarities (%) | GMQE | QMEAN |
|---|---|---|---|---|---|---|
| 1 | Cytochrome oxidase subunit 1 | 7 jro 1. B | 99 | 99.0 | 0.77 | 0.67 |
| 2 | QNE 4 | 5 gnc 1. A | 100 | 93.94 | 0.16 | 0.43 +/− 0.05 |
| Secondary Structures | QNE4 | Cytochrome Oxidase Subunit 1 |
|---|---|---|
| Alpha helix % | 42.36 | 44.77 |
| Extended strand % | 12.38 | 21.17 |
| Beta turn % | 8.70 | 8.27 |
| Random coil % | 36.56 | 25.79 |
| Treatment | % Inhibition of Sporangial Germination | ||
|---|---|---|---|
| Concentration | |||
| 5% | 10% | 15% | |
| Clove | 47.41 (6.95) ** | 57.14 (7.62) | 64.51 (8.09) |
| Garlic | 57.14 (7.62) | 61.9 (7.93) | 71.42 (8.51) |
| Tulsi | 38.09 (6.25) | 47.61 (6.97) | 52.38 (7.30) |
| Pudina | 38.09 (6.25) | 47.41 (6.95) | 57.14 (7.62) |
| Neem | 33.33 (5.85) | 42.85 (6.62) | 57.14 (7.62) |
| Control | 16 (4.12) | 16 (4.12) | 16 (4.12) |
| Mean | 42.11 (6.45) | 46.43 (6.78) | 51.91 (7.15) |
| Treatment | Concentration | Treatment X Concentration | |
| S.Em± | 0.271 | 0.177 | 0.469 |
| CD (p < 0.05) | 0.776 | 0.508 | 1.344 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhansirani, N.; Devappa, V.; Sangeetha, C.G.; Sridhara, S.; Shankarappa, K.S.; Mohanraj, M. Identification of Potential Phytochemical/Antimicrobial Agents against Pseudoperonospora cubensis Causing Downy Mildew in Cucumber through In-Silico Docking. Plants 2023, 12, 2202. https://doi.org/10.3390/plants12112202
Jhansirani N, Devappa V, Sangeetha CG, Sridhara S, Shankarappa KS, Mohanraj M. Identification of Potential Phytochemical/Antimicrobial Agents against Pseudoperonospora cubensis Causing Downy Mildew in Cucumber through In-Silico Docking. Plants. 2023; 12(11):2202. https://doi.org/10.3390/plants12112202
Chicago/Turabian StyleJhansirani, Nagaraju, Venkatappa Devappa, Chittarada Gopal Sangeetha, Shankarappa Sridhara, Kodegandlu Subbanna Shankarappa, and Mooventhiran Mohanraj. 2023. "Identification of Potential Phytochemical/Antimicrobial Agents against Pseudoperonospora cubensis Causing Downy Mildew in Cucumber through In-Silico Docking" Plants 12, no. 11: 2202. https://doi.org/10.3390/plants12112202
APA StyleJhansirani, N., Devappa, V., Sangeetha, C. G., Sridhara, S., Shankarappa, K. S., & Mohanraj, M. (2023). Identification of Potential Phytochemical/Antimicrobial Agents against Pseudoperonospora cubensis Causing Downy Mildew in Cucumber through In-Silico Docking. Plants, 12(11), 2202. https://doi.org/10.3390/plants12112202








