Evaluation of Antimicrobial Activity of Kitaibelia vitifolia Extract against Proven Antibiotic-Susceptible and Multidrug-Resistant (MDR) Strains of Bacteria of Clinical Origin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Drug Resistance Pattern of the Tested Bacterial Strains
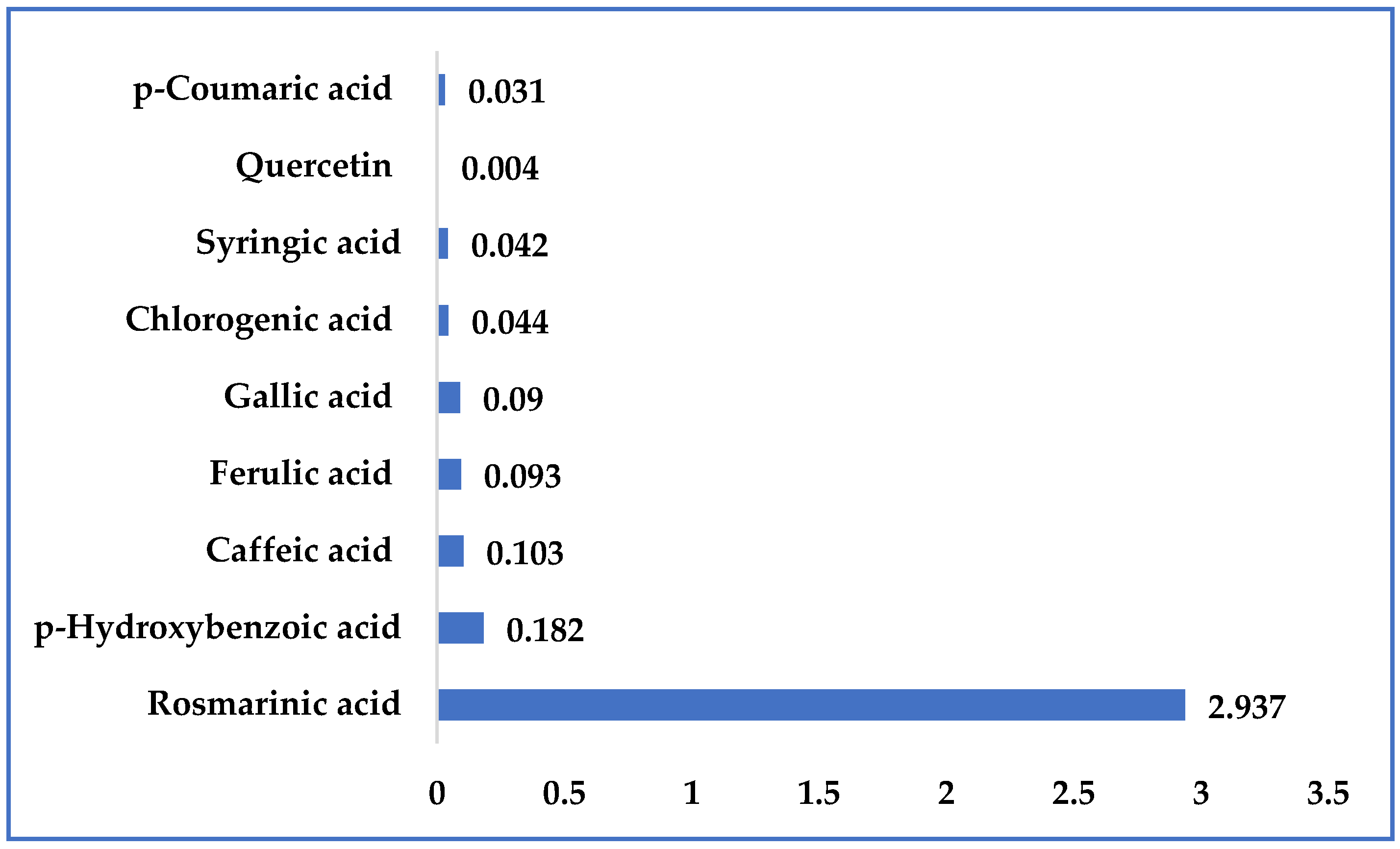
2.2. Characterization of the Kitaibellia Vitifolia Plant Extract
2.3. Antibacterial Activity of the Plant Extract
3. Materials and Methods
3.1. Plant Material
3.2. Preparations of Plant Extract
3.3. Isolation, Identification, and Characterization of Bacterial Isolates Collected from Healthcare-Associated Infections
- Klebsiella spp.—18 isolates (1 from blood, 17 from urine);
- Methicillin-resistant Staphylococcus aureus (MRSA)—3 isolates (1 from nose, 1 from central venous catheter smear, 1 from wound);
- Acinetobacter spp.—3 isolates (2 from bronchial aspirate, 1 from urine);
- Pseudomonas aeruginosa—5 isolates (3 from urine, 1 from ear, 1 from bronchial aspirate);
- Vancomycin-resistant Enterococcus (VRE)—1 isolate (from urine).
3.4. Antimicrobial Susceptibility Testing (AST) Using the Disc Diffusion (DD) Method
3.5. Determination of Minimum Inhibitory Concentration (MIC) Using Micro Dilution Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harbottle, H.; Thakur, S.; Zhao, S.; White, D.G. Genetics of Antimicrobial Resistance. Anim. Biotechnol. 2006, 17, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilvert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad buds, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarthg, S.; Hindlerh, J.F.; Kahlmeteri, G.; Olsson-Liljequistj, B.; et al. MultidruG−resistant, extensively druG−resistant and pandruG−resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit: A critical review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Vikesland, P.J.; Pruden, A.; Alvarez, P.J.J.; Aga, D.; Bürgmann, H.; Li, X.; Manaia, C.M.; Nambi, I.; Wigginton, K.; Zhang, T.; et al. Toward a Comprehensive Strategy to Mitigate Dissemination of Environmental Sources of Antibiotic Resistance. Environ. Sci. Technol. 2017, 51, 13061–13069. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.; Hu, Y.; Bax, R.; Page, C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 2002, 1, 895–910. [Google Scholar] [CrossRef]
- Cantón, R.; Morosini, M.I. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol. Rev. 2011, 35, 977–991. [Google Scholar] [CrossRef]
- Loureiro, R.J.; Roque, F.; Rodrigues, A.T.; Herdeiro, M.T.; Ramalheira, E. O uso de antibióticos e as resistências bacterianas: Breves notas sobre a sua evolução. Rev. Port. De Saúde Pública 2016, 34, 77–84. [Google Scholar] [CrossRef]
- Lima, W.G.; Ramos-Alves, M.C.; Soares, A.C. From psychiatric disorders to antibiotic therapy: Repositioning of chlorpromazine as an antibacterial agent. Rev. Colomb. De Cienc. Químico-Farm. 2019, 48, 5–28. [Google Scholar] [CrossRef]
- Garcia, R.; Garcia, F.A.D.O.; Pereira, P.S.; Coutinho, H.D.M.; Siyadatpanah, A.; Norouzi, R.; Wilairatana, P.; Pereira, M.D.L.; Nissapatorn, V.; Tintino, S.R.; et al. Microbial resistance: The role of efflux pump superfamilies and their respective substrates. Life Sci. 2022, 295, 120391. [Google Scholar] [CrossRef]
- Ren, W.; Yin, J.; Xiao, H.; Chen, S.; Liu, G.; Tan, B.; Li, N.; Peng, Y.; Li, T.; Zeng, B.; et al. Intestinal Microbiota-Derived GABA Mediates Interleukin-17 Expression during Enterotoxigenic Escherichia coli Infection. Front. Immunol. 2017, 7, 685. [Google Scholar] [CrossRef]
- Xu, G.B.; Xiao, Y.H.; Zhang, Q.Y.; Zhou, M.; Liao, S.G. Hepatoprotective natural triterpenoids. Eur. J. Med. Chem. 2018, 145, 691–716. [Google Scholar] [CrossRef]
- Amison, R.T.; O’Shaughnessy, B.G.; Arnold, S.; Cleary, S.J.; Nandi, M.; Pitchford, S.C.; Bragonzi, A.; Page, C.P. Platelet Depletion Impairs Host Defense to Pulmonary Infection with Pseudomonas aeruginosa in Mice. Am. J. Respir. Cell Mol. Biol. 2018, 58, 331–340. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Heleno, S.A.; Alves, M.J.; Ferreira, I.C. Bacterial Resistance: Antibiotics of last generation used in clinical practice and the rise of natural products as new therapeutic alternatives. Curr. Pharm. Des. 2020, 26, 815–837. [Google Scholar] [CrossRef]
- Hobson, C.; Chan, A.N.; Wright, G.D. The Antibiotic Resistome: A Guide for the Discovery of Natural Products as Antimicrobial Agents. Chem. Rev. 2021, 121, 3464–3494. Available online: https://pubs.acs.org/doi/10.1021/acs.chemrev.0c01214 (accessed on 11 July 2023). [CrossRef] [PubMed]
- Parmanik, A.; Das, S.; Kar, B.; Bose, A.; Dwivedi, G.R.; Pandey, M.M. Current Treatment Strategies Against MultidruG−Resistant Bacteria: A Review. Curr. Microbiol. 2022, 79, 388. [Google Scholar] [CrossRef]
- Martin, K.W.; Ernst, E. Herbal medicines for treatment of bacterial infections: A review of controlled clinical trials. J. Antimicrob. Chemother. 2003, 51, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.C.; Leite, A.A.M.; Xavier, K.G.S.; Takahashi, J.A.; Bemquere, M.P.; Chartone-Souza, E.; Nascimento, A.M.A. Synergic interaction between pomegranate extracts and antibiotics against Staphylococcus aureus. Can. J. Microbiol. 2005, 51, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Stavri, M.; Piddock, L.J.V.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260. [Google Scholar] [CrossRef]
- Khan, R.; Islam, B.; Akram, M.; Shakil, S.; Ahmad, A.; Manazir Ali, S.; Siddiqui, M.; Khan, A.U. Antimicrobial Activity of Five Herbal Extracts Against Multi Drug Resistant (MDR) Strains of Bacteria and Fungus of Clinical Origin. Molecules 2009, 14, 586–597. [Google Scholar] [CrossRef]
- Chusri, S.; Villanueva, I.; Voravuthikunchai, S.P.; Davies, J. Enhancing antibiotic activity: A strategy to control Acinetobacter infections. J. Antimicrob. Chemother. 2009, 64, 1203–1211. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Lewis, K.; Ausubel, F.M. Prospects of plant derived antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, S.; Ahmet, A.; Sağdiç, O.; Budak, Ü. Phenolic compounds and antioxidant and antimicrobial properties of Helichrysum species collected from eastern Anatolia, Turkey. Turk. J. Biol. 2010, 34, 463–473. [Google Scholar] [CrossRef]
- Amzad, H.M.; Dawood, S.M. A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant Merremia borneensis. Arab. J. Chem. 2015, 8, 66–71. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Lee, S.B.; Cha, K.H.; Kim, S.N.; Altantsetseg, S.; Shatar, S.; Sarangerel, O.; Nho, C.W. The Antimicrobial Activity of Essential Oil from Dracocephalum foetidum against Pathogenic Microorganisms. J. Microbiol. 2007, 45, 53–57. [Google Scholar] [PubMed]
- Cowan, M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Alzoreky, N.S.; Nakahara, K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Iqbal, K.; Nawaz, S.A.; Malik, A.; Riaz, N.; Mukhtar, N.; Mohammad, P.; Choudhary, M.I. Isolation and lipoxygenase-inhibition studies of phenolic constituents from Ehretia obtusifolia. Chem. Biodivers. 2005, 2, 104–111. [Google Scholar] [CrossRef]
- Iqbāl, A.; Farrukh, A. New Strategies Combating Bacterial Infection. In 5.4 Natural Products for MDR Microorganisms; Iqbal, A., Farrukh, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; p. 133. ISBN 978-3-527-32206-0. [Google Scholar]
- Mašković, P.; Solujić, S.; Mihailović, V.; Mladenović, M.; Cvijović, M.; Mladenović, J.; Aćamović-Đoković, G.; Kurćubić, V. Phenolic Compounds and Biological Activity of Kitaibelia vitifolia. J. Med. Food. 2011, 14, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Aboaba, O.O.; Efuwape, B.M. Antibacterial properties of some Nigerian spices. Mol. Biol. Res. Commun. 2001, 13, 183–188. [Google Scholar]
- Valle, D.L., Jr.; Andrade, J.I.; Puzon, J.J.M.; Cabrera, E.C.; Rivera, W.L. Antibacterial activities of ethanol extracts of Philippine medicinal plants against multidruG−resistant bacteria. Asian Pac. J. Trop. Biomed. 2015, 5, 532–540. [Google Scholar] [CrossRef]
- Askarinia, M.; Ganji, A.; Jadidi-Niaragh, F.; Hasanzadeh, S.; Bahram Mohammadi, B.; Ghalamfarsa, F.; Ghalamfarsa, G.; Mahmoudi, H. A review on medicinal plant extracts and their active ingredients against methicillin-resistant and methicillin-sensitive Staphylococcus aureus. J. Herbmed. Pharmacol. 2019, 8, 173–184. [Google Scholar] [CrossRef]
- Garoy, E.Y.; Gebreab, Y.B.; Achila, O.O.; Tekeste, D.G.; Kesete, R.; Ghirmay, R.; Kiflay, R.; Tesfu, T. Methicillin-resistant Staphylococcus aureus (MRSA): Prevalence and antimicrobial sensitivity pattern among patients—A multicenter study in Asmara, Eritrea. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 8321834. [Google Scholar] [CrossRef]
- Klein, E.; Smith, D.L.; Laxminarayan, R. Hospitalizations and deaths caused by methicillin resistant Staphylococcus aureus, United States, 1999–2005. Emerg. Infect. Dis. 2007, 13, 1840–1846. [Google Scholar] [CrossRef]
- Hoseini Alfatemi, S.M.; Motamedifar, M.; Hadi, N.; Sedigh Ebrahim Saraie, H. Analysis of Virulence Genes Among Methicillin Resistant Staphylococcus aureus (MRSA) Strains. Jundishapur J. Microbiol. 2014, 7, e10741. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holanda, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2022, 30, 46. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; Fernández, J.; Lombó, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef]
- Rama, J.L.R.; Mallo, N.; Biddau, M.; Fernandes, F.; de Miguel, T.; Sheiner, L.; Choupina, A.; Lores, M. Exploring the powerful phytoarsenal of white grape marc against bacteria and parasites causing significant diseases. Environ. Sci. Pollut. Res. Int. 2021, 28, 24270–24278. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial Capacity of Plant Polyphenols against Gram-positive Bacteria: A Comprehensive Review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, X.; Ma, G. Antimicrobial activities and mechanisms of extract and components of herbs in East Asia. RSC Adv. 2022, 12, 29197. [Google Scholar] [CrossRef]
- Jayaraman, R. Antibiotic resistance: An overview of mechanisms and a paradigm shift. Curr. Sci. 2009, 96, 1475–1484. [Google Scholar]
- Lee, J.; Jung, E.; Koh, J.; Kim, Y.S.; Park, D. Effect of rosmarinic acid on atopic dermatitis. J. Dermatol. 2008, 35, 768–771. [Google Scholar] [CrossRef]
- Jones, G.A.; McAllister, T.A.; Muir, A.D.; Cheng, K.J. Effects of sainfoin (Onobrychis viciifolia Scop.) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Appl. Environ. Microbiol. 1994, 60, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.H.; Farag, S.E.; Mousa, L.A.A.; Abo-Zaid, M.A. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios 1998, 93, 43–54. [Google Scholar]
- Sikkema, J.; De Bont, J.A.M.; Poolman, B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994, 269, 8022–8028. [Google Scholar] [CrossRef]
- Hooker, C.W.; Lott, W.B.; Harrich, D. Inhibitors of human immunodeficiency virus type 1 reverse transcriptase target distinct phases of early reverse transcription. J. Virol. 2001, 75, 3095–3104. [Google Scholar] [CrossRef]
- Huang, S.S.; Zheng, R.L. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006, 239, 271–280. [Google Scholar] [CrossRef]
- Ivanov, M.; Kostić, M.; Stojković, D.; Soković, M. Rosmarinic acid–Modes of antimicrobial and antibiofilm activities of a common plant polyphenol. S. Afr. J. Bot. 2022, 146, 521–527. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Lakušić, B.S.; Ristić, M.S.; Slavkovska, V.N.; Stojanović, D.L.; Lakušić, D.V. Variations in essential oil yields and compositions of Salvia officinalis (Lamiaceae) at different developmental stages. Bot. Serb. 2013, 37, 127–139. [Google Scholar]
- Heipieper, H.J.; Keweloh, H.; Rehm, H.J. Influence of phenols on growth and membrane permeability of free and immobilized E. coli. Appl. Environ. Microbiol. 1991, 57, 1213–1217. [Google Scholar] [CrossRef]
- Song, L.; Hu, X.; Ren, X.; Liu, J.; Liu, X. Antibacterial Modes of Herbal Flavonoids Combat Resistant Bacteria. Front. Pharmacol. 2022, 13, 873374. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Stajić, S.B.; Dmitrić, M.P.; Miletić, N.M. Food safety assessment of burger patties with added herbal plant material. Fleischwirtschaft 2022, 11, 73–78. [Google Scholar]
- Shan, B.; Yi-Zhong, C.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Duffy, C.F.; Power, R.F. Antioxidant and antimicrobial properties of some Chinese plant extracts. Int. J. Antimicrob. Agents 2001, 17, 527–529. [Google Scholar] [CrossRef]
- Fullerton, M.; Khatiwada, J.; Johnson, J.U.; Davis, S.; Williams, L.L. Determination of antimicrobial activity of sorrel (Hibiscus sabdariffa) on Escherichia coli O157:H7 isolated from food, veterinary, and clinical samples. J. Med. Food 2011, 14, 950–956. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Venkateshwaran, K.; Vanitha, J.; Ganesh, M.; Vasudevan, M.; Sivakumar, T. Evaluation of antibacterial activity, phenol and flavonoid contents of Thespesia populnea flower extracts. Pak. J. Pharm. Sci. 2009, 22, 282–286. [Google Scholar] [PubMed]
- Seukep, J.A.; Fankam, A.G.; Djeussi, D.E.; Voukeng, I.; Tankeo, S.B.; Noumdem, J.A.K.; Kuete, A.H.L.N.; Kuete, V. Antibacterial activities of the methanol extracts of seven Cameroonian dietary plants against bacteria expressing MDR phenotypes. SpringerPlus 2013, 2, 363. Available online: http://www.springerplus.com/content/2/1/363 (accessed on 11 July 2023). [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in food—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement. In CLSI Document M100-S21; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011; ISBN 1-56238-742-1. [Google Scholar]
- Satyajit, D.; Sarker, L.N.; Kumarasamy, Y. Microtitre plate based antibacterial assay incorporating resazurin as indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar]

| Strain Designation | AM Agents | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gen | Ami | Net | Tob | Pip | Ert | Imi | Mer | Can | Cux | Cer | Cef | Cep | Cek | Cip | Tri | Tig | Azt | Amp | Acl | Chl | Col | Tet | Dox | Min | |
| 69 | R | S | R | X | R | R | S | S | X | R | R | R | R | X | R | S | R | X | R | S | R | X | R | R | R |
| 736 | R | R | R | X | R | R | S | S | X | R | R | R | R | X | R | R | R | X | R | R | S | X | R | R | R |
| 215 | R | R | R | X | S | S | S | S | X | R | R | R | R | X | R | R | S | X | R | S | S | X | S | S | S |
| 30 | R | R | R | X | S | R | S | S | X | R | R | R | R | X | R | R | S | X | R | S | S | X | R | R | R |
| 369 | R | R | R | X | S | S | S | S | X | R | R | R | R | X | S | R | S | X | R | S | S | X | S | S | S |
| 319 | R | R | R | X | S | S | S | S | X | R | R | S | S | X | S | R | S | X | R | S | S | X | S | S | S |
| 374 | S | S | R | X | S | S | S | S | X | R | R | R | R | X | R | R | S | X | R | S | S | X | R | R | R |
| 535 | R | S | S | X | R | S | S | S | X | R | R | R | R | X | R | R | R | X | R | R | R | X | R | R | R |
| 539 | R | R | R | X | R | R | S | R | X | R | R | R | R | X | R | R | R | X | R | S | R | X | R | R | R |
| 233 | R | R | R | X | R | R | S | S | X | R | R | R | R | X | R | R | S | X | R | R | S | X | R | R | R |
| 220 | R | R | S | X | R | S | S | S | X | R | R | R | R | X | R | R | R | X | R | R | R | X | R | R | R |
| 047 | R | R | S | X | R | S | S | S | X | R | R | R | R | X | R | R | S | X | R | R | R | X | R | R | R |
| 304 | R | S | R | X | S | S | S | S | X | R | R | R | R | X | R | R | R | X | R | R | R | X | R | R | R |
| 033 | R | R | R | X | S | S | S | S | X | R | R | R | R | X | R | R | R | X | R | S | R | X | R | R | R |
| 376 | R | R | R | X | R | S | S | S | X | R | R | R | R | X | R | R | S | X | R | R | R | X | R | R | R |
| 729 | R | R | R | X | S | S | S | S | X | R | R | R | R | X | R | R | R | X | R | S | S | X | S | S | S |
| 361 | R | R | R | X | R | R | R | R | X | R | R | R | R | X | R | R | S | X | R | R | R | X | R | R | R |
| 073 | R | R | R | X | R | R | S | S | X | R | R | R | R | X | R | R | R | X | R | R | R | X | R | R | R |
| E. coli ATCC 25922 | S | S | S | X | S | S | S | S | X | S | S | S | S | X | S | S | S | X | S | S | S | X | S | S | S |
| Strain Designation | AM Agents | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gen | Rif | Cel | Cen | Cip | Cef | Cep | Cet | Cer | Fua | Tri | Van | Tei | Tig | Tet | Dox | Min | Dap | Ery | Lin | Chl | Pho | Qin | |
| 1726 | R | S | X | R | R | R | R | R | R | S | S | S | X | S | R | R | S | X | R | S | R | S | X |
| 1063 | R | S | X | R | R | R | R | R | R | S | S | S | X | S | S | S | S | X | R | S | S | S | X |
| 2056 | R | R | X | R | R | R | R | R | R | S | R | S | X | S | R | R | R | X | R | S | R | S | X |
| Staphylococcus aureus ATCC 25923 | S | S | X | S | S | S | S | S | S | S | S | S | X | S | S | S | S | X | S | S | S | S | X |
| Strain Designation | AM Agents | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gen | Ami | Net | Tob | Pip | Tic | Dor | Imi | Mer | Cef | Cep | Cet | Cer | Cip | Lev | Tri | AmpS | Col | Pol | Tet | Dox | Min | |
| 1578 | R | R | R | R | R | X | X | R | R | R | R | R | R | R | R | R | R | X | X | R | R | R |
| 1577 | R | R | S | R | R | X | X | R | R | R | R | R | R | R | R | S | R | X | X | R | S | S |
| 6401 | R | R | R | R | R | X | X | R | R | R | R | R | R | R | R | R | R | X | X | S | S | S |
| Strain Designation | AM Agents | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gen | Ami | Net | Tob | Pip | Tic | Dor | Imi | Mer | Cef | Cep | Cip | Lev | Azt | Col | Pol | |
| 5067 | R | R | R | R | R | X | X | R | R | R | R | R | R | X | S | X |
| 5414 | R | R | R | R | R | X | X | R | R | R | R | R | R | X | R | X |
| 1913 | R | R | R | R | R | X | X | R | R | R | R | R | R | X | S | X |
| 1874 | R | R | R | R | R | X | X | R | R | R | R | R | R | X | S | X |
| 6315 | R | R | R | R | R | X | X | R | R | R | R | R | R | X | S | X |
| Pseudomonas aeruginosa ATCC 27853 | S | S | S | S | S | X | X | S | S | S | S | S | S | X | S | X |
| Strain Designation | AM Agents | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gen | Str | Cip | Lev | Dor | Imi | Mer | Van | Tei | Tig | Dox | Min | Lin | Amp | |
| 30 VRE | R | R | R | R | X | R | R | R | X | R | R | R | S | R |
| Enterococcus faecalis ATCC 29212 | S | S | S | S | X | S | S | S | X | S | S | S | S | S |
| № | Strain Designation | MIC (µg/mL) |
|---|---|---|
| 1 | 69 Klebsiella spp. | <2.44 |
| 2 | 736 Klebsiella spp. | <2.44 |
| 3 | 215 Klebsiella spp. | <2.44 |
| 4 | 30 Klebsiella spp. | <2.44 |
| 5 | 369 Klebsiella spp. | <2.44 |
| 6 | 319 Klebsiella spp. | <2.44 |
| 7 | 374 Klebsiella spp. | <2.44 |
| 8 | 535 Klebsiella spp. | <2.44 |
| 9 | 539 Klebsiella spp. | <2.44 |
| 10 | 233 Klebsiella spp. | <2.44 |
| 11 | 220 Klebsiella spp. | <2.44 |
| 12 | 047 Klebsiella spp. | <2.44 |
| 13 | 304 Klebsiella spp. | <2.44 |
| 14 | 033 Klebsiella spp. | <2.44 |
| 15 | 376 Klebsiella spp. | <2.44 |
| 16 | 729 Klebsiella spp. | <2.44 |
| 17 | 361 Klebsiella spp. | 625 |
| 18 | 073 Klebsiella spp. | 625 |
| 19 | E. coli ATCC 25922 | <2.44 |
| 20 | 1726 MRSA | <2.44 |
| 21 | 1063 MRSA | <2.44 |
| 22 | 2056 MRSA | 4.88 |
| 23 | Staphylococcus aureus ATCC 25923 | 2.44 |
| 24 | 5067 Pseudomonas aeruginosa | 2.44 |
| 25 | 5414 Pseudomonas aeruginosa | 2.44 |
| 26 | 1913 Pseudomonas aeruginosa | 156.25 |
| 27 | 1874 Pseudomonas aeruginosa | 156.25 |
| 28 | 6315 Pseudomonas aeruginosa | 156.25 |
| 29 | Pseudomonas aeruginosa ATCC 27853 | 156.25 |
| 30 | 1578 Acinetobacter spp. | <2.44 |
| 31 | 1577 Acinetobacter spp. | 1250 |
| 32 | 6401 Acinetobacter spp. | 1250 |
| 33 | 30 vankomycin-resistant Enterococcus (VRE) | <2.44 |
| 34 | Enterococcus faecalis ATCC 29212 | <2.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurćubić, V.S.; Raketić, S.V.; Mašković, J.M.; Mašković, P.Z.; Kurćubić, L.V.; Heinz, V.; Tomasevic, I.B. Evaluation of Antimicrobial Activity of Kitaibelia vitifolia Extract against Proven Antibiotic-Susceptible and Multidrug-Resistant (MDR) Strains of Bacteria of Clinical Origin. Plants 2023, 12, 3236. https://doi.org/10.3390/plants12183236
Kurćubić VS, Raketić SV, Mašković JM, Mašković PZ, Kurćubić LV, Heinz V, Tomasevic IB. Evaluation of Antimicrobial Activity of Kitaibelia vitifolia Extract against Proven Antibiotic-Susceptible and Multidrug-Resistant (MDR) Strains of Bacteria of Clinical Origin. Plants. 2023; 12(18):3236. https://doi.org/10.3390/plants12183236
Chicago/Turabian StyleKurćubić, Vladimir S., Svetlana V. Raketić, Jelena M. Mašković, Pavle Z. Mašković, Luka V. Kurćubić, Volker Heinz, and Igor B. Tomasevic. 2023. "Evaluation of Antimicrobial Activity of Kitaibelia vitifolia Extract against Proven Antibiotic-Susceptible and Multidrug-Resistant (MDR) Strains of Bacteria of Clinical Origin" Plants 12, no. 18: 3236. https://doi.org/10.3390/plants12183236
APA StyleKurćubić, V. S., Raketić, S. V., Mašković, J. M., Mašković, P. Z., Kurćubić, L. V., Heinz, V., & Tomasevic, I. B. (2023). Evaluation of Antimicrobial Activity of Kitaibelia vitifolia Extract against Proven Antibiotic-Susceptible and Multidrug-Resistant (MDR) Strains of Bacteria of Clinical Origin. Plants, 12(18), 3236. https://doi.org/10.3390/plants12183236










