Antibacterial and Antibiofilm Activity of Different Species of Fabiana sp. Extract Obtained via Maceration and Ultrasound-Assisted Extraction against Staphylococcus epidermidis
Abstract
1. Introduction
2. Results
2.1. Comparison of Ultrasound-Assisted Extraction (UAE) and Maceration
2.2. Antimicrobial Activity
2.2.1. Profile of Antibiotic Resistance of S. epidermidis
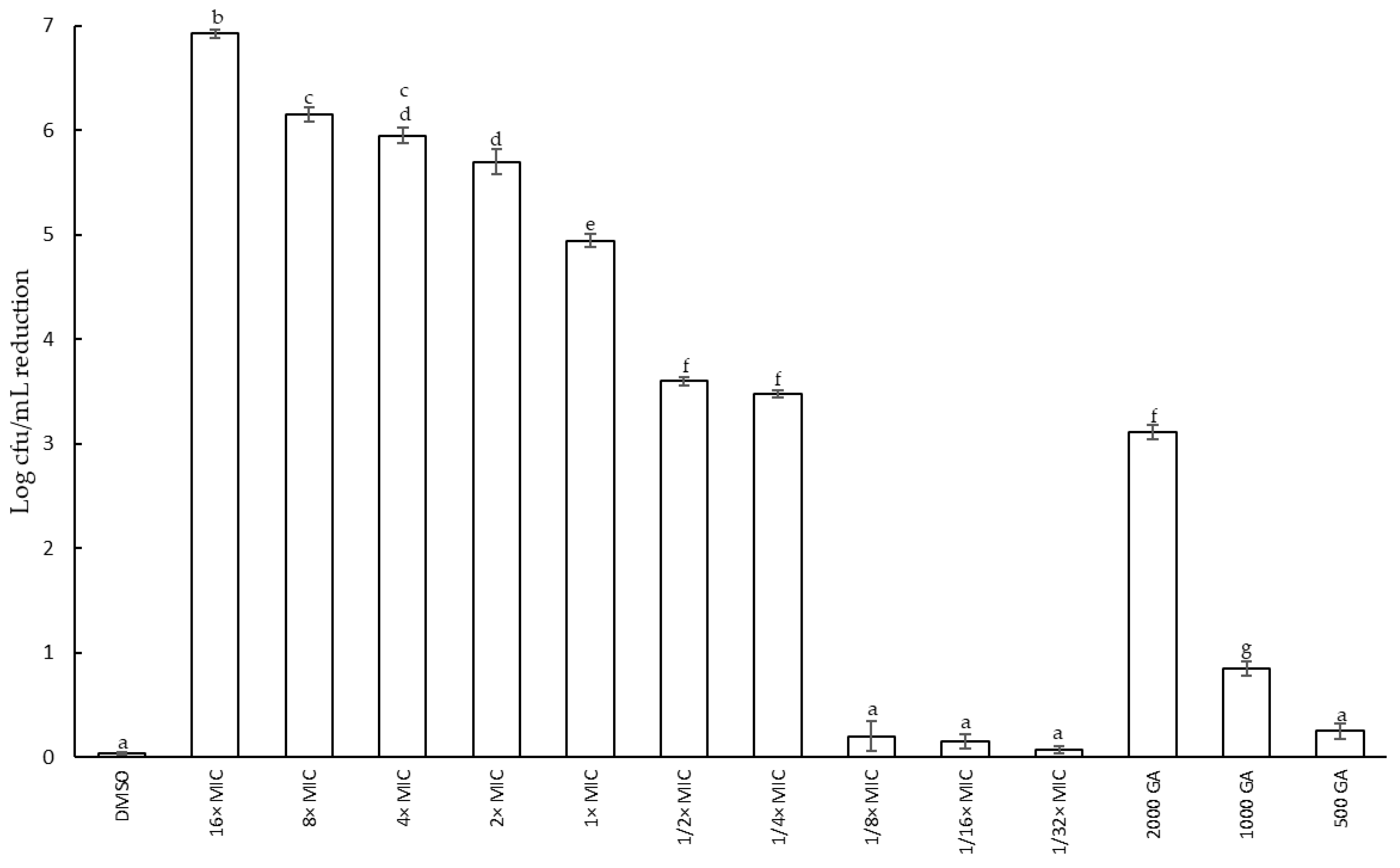
2.2.2. Effect of Fabiana Species on Growth of S. epidermidis: Reduction in Cell Viability and Minimum Inhibitory Concentration (MIC)
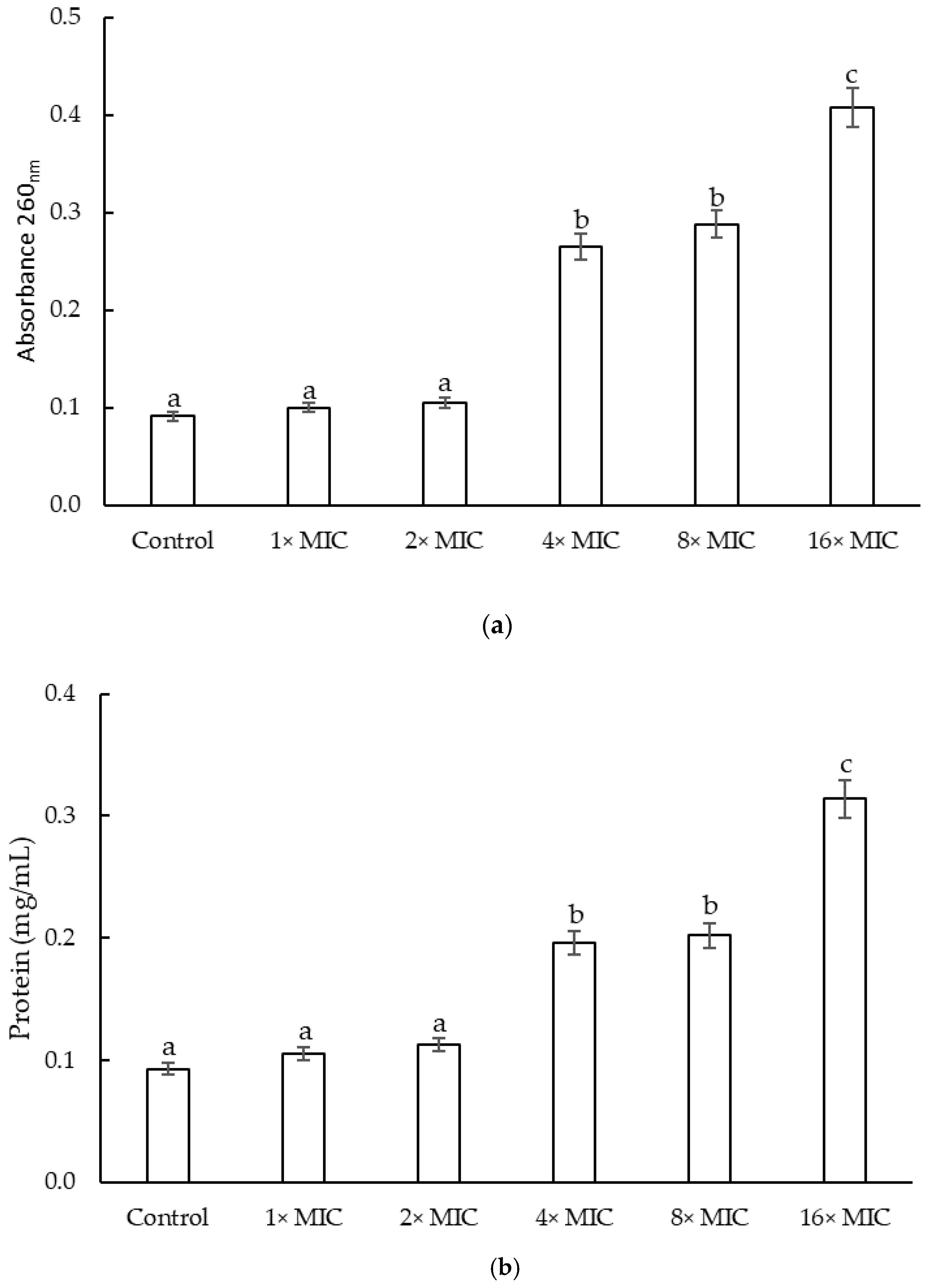
2.2.3. Cell Constituents’ Release of S. epidermidis
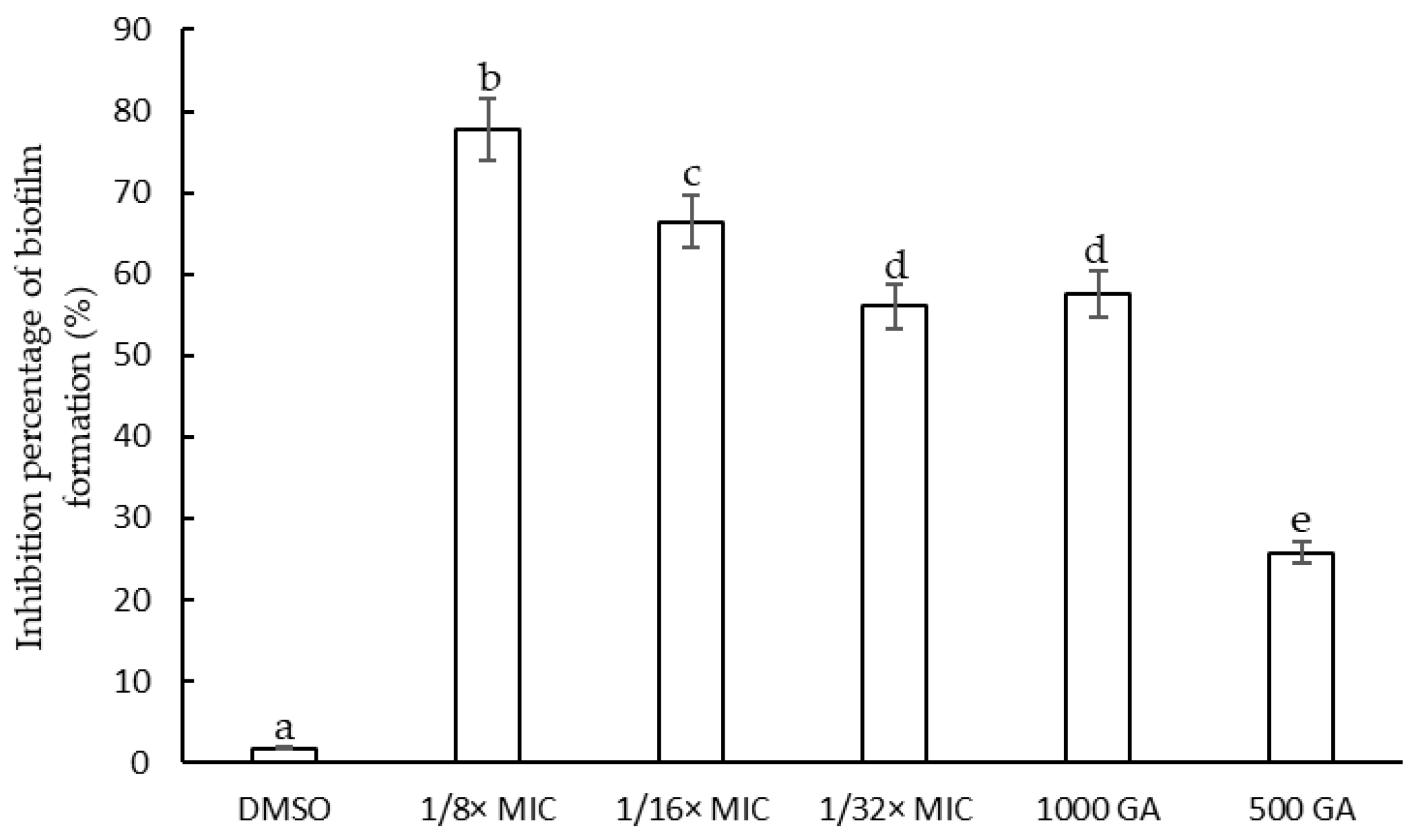
2.3. Antibiofilm Activity of F. densa Extract
2.3.1. Determination of the Minimum Inhibitory Concentration of F. densa Extracts on Biofilm Formation and on Metabolic Activity
2.3.2. Effect of F. densa Extract on Preformed Biofilm and Metabolic Activity
2.3.3. Reduction in Extracellular Polymeric Substances (EPS) and Proteins during Biofilm Formation
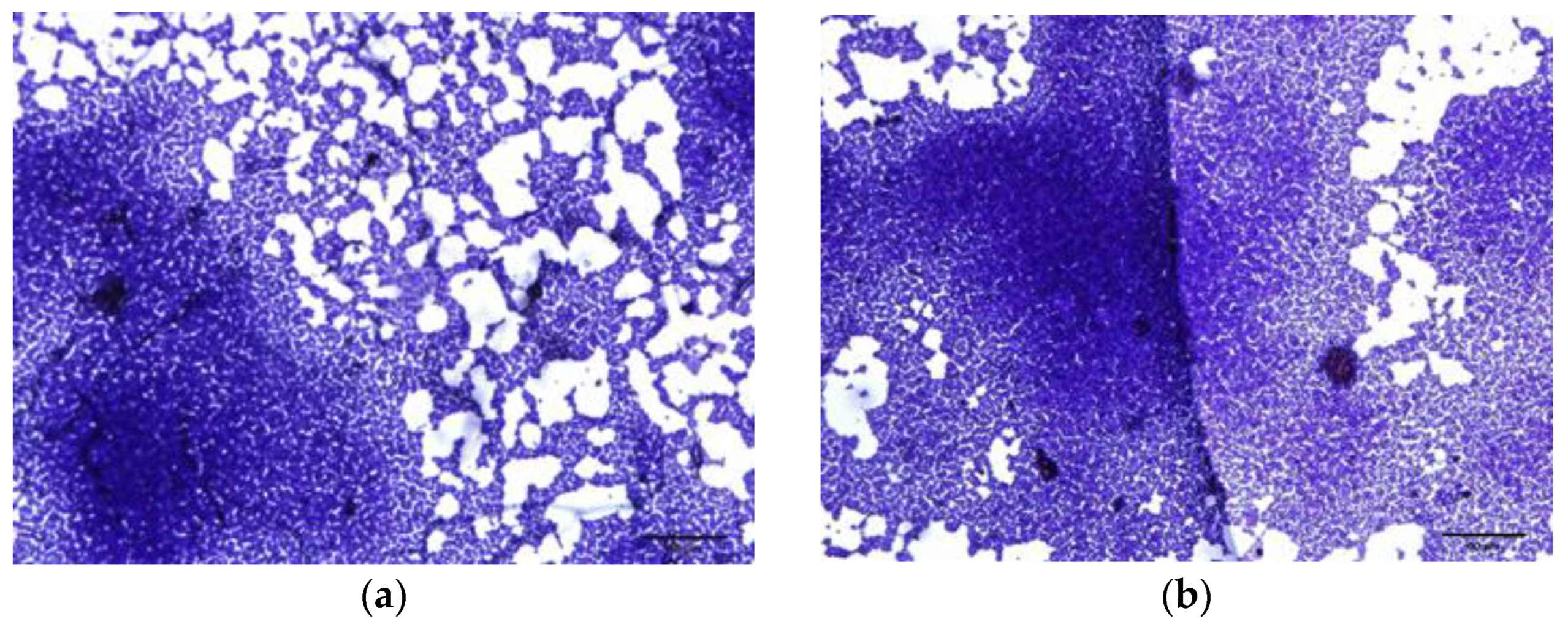
2.3.4. Microscopic Observation of Biofilm Formation
3. Discussion
3.1. Extraction Methods and Chemical Composition of Fabiana Species
3.2. Antimicrobial and Antibiofilm Activity of F. densa against S. epidermidis
4. Materials and Methods
4.1. Plant Material
4.2. Plants Extracts Preparation
4.3. Phytochemical Screening
4.3.1. Phenolic Compound Determination
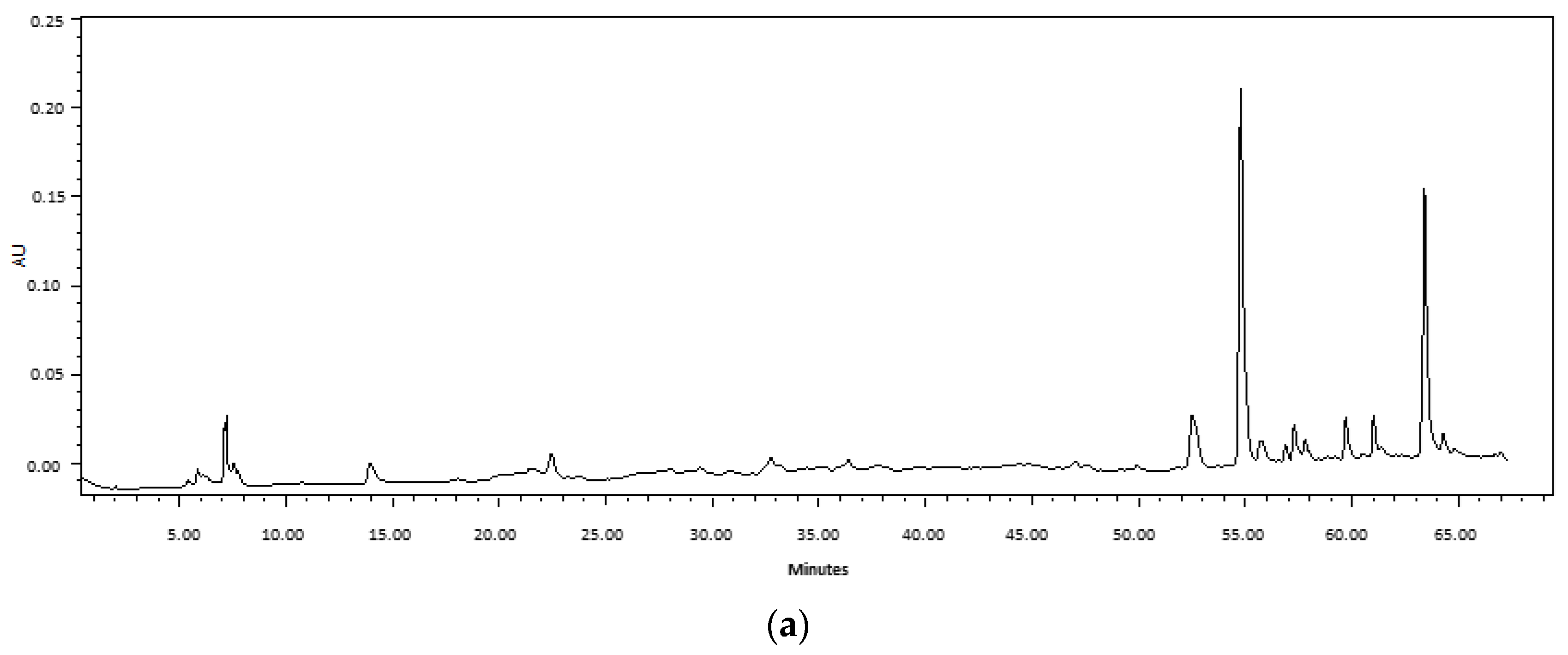
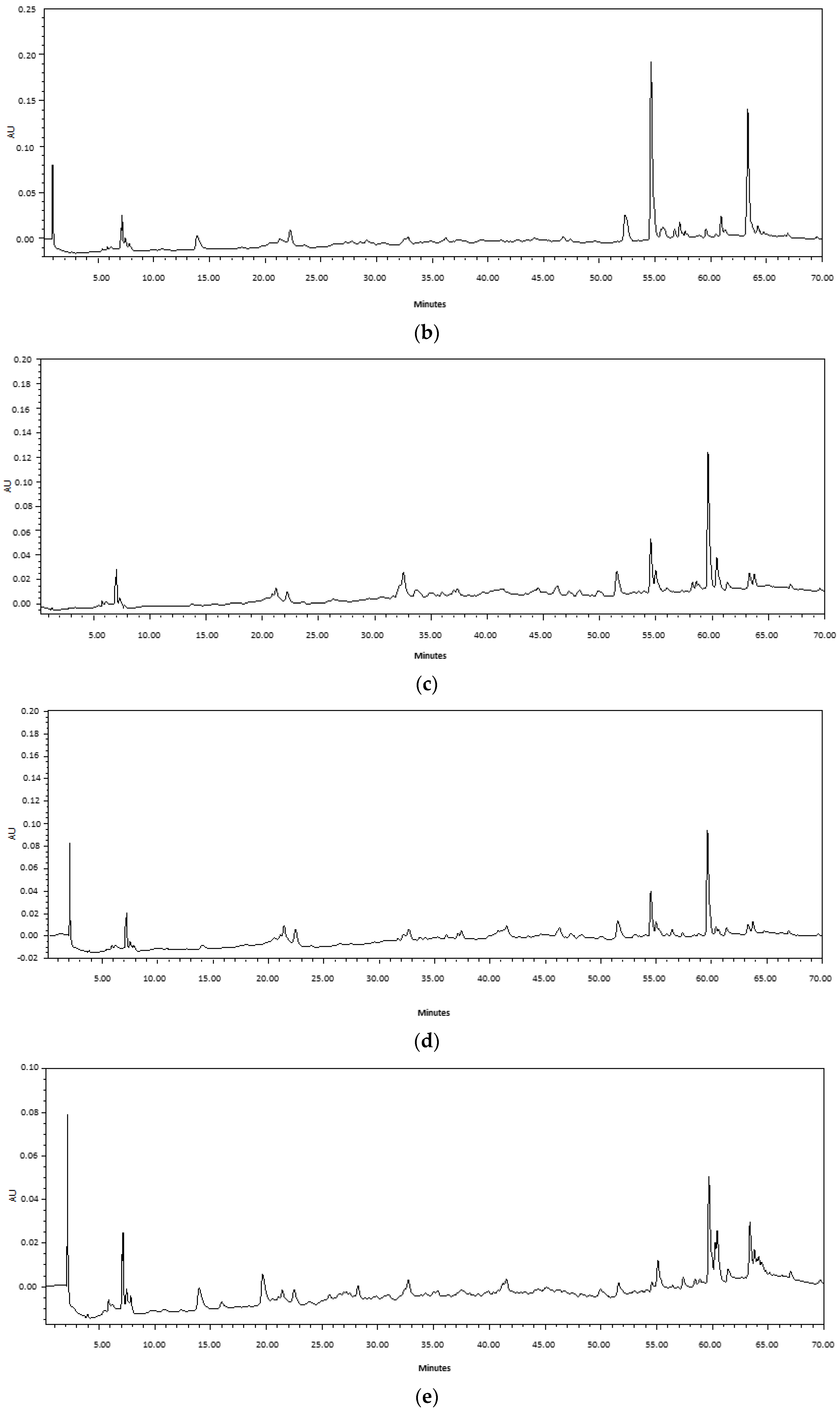
4.3.2. HPLC Analysis
4.4. Bacterial Strain
4.5. Antimicrobial Susceptibility Testing
4.6. Minimal Inhibitory Concentration (MIC)
4.7. Viability Cell Analysis and Release of Cellular Constituents
4.8. Damage to Membrane Integrity
4.9. Determination of the MIC of Biofilm Formation
4.10. Determination of Preformed Biofilm Eradication Capacity
4.11. Measurement of the Biofilm Metabolic Activity of the Biofilm
4.12. Extraction and Quantification of Extracellular Polymeric Substances (EPS)
4.13. Biofilm Inhibition Optical Microscopy Visualization
4.14. Statical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, M.M.; Horswill, A.R. Staphylococcus epidermidis—Skin friend or foe? PLoS Pathog. 2020, 16, e1009026. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis—The “accidental” pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Marchant, E.A.; Boyce, G.K.; Sadarangani, M.; Lavoie, P.M. Neonatal sepsis due to coagulase-negative Staphylococci. Clin. Dev. Immunol. 2013, 2013, 586076. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Speer, C.P.; Glaser, K. Beyond sepsis: Staphylococcus epidermidis is an underestimated but significant contributor to neonatal morbidity. Virulence 2018, 9, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative Staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef]
- Foster, T.J. Surface proteins of Staphylococcus epidermidis. Front. Microbiol. 2020, 11, 1829. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Le, K.Y.; Park, M.D.; Otto, M. Immune evasion mechanisms of Staphylococcus epidermidis biofilm infection. Front. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Freitas, A.I.; Lopes, N.; Oliveira, F.; Brás, S.; França, Â.; Vasconcelos, C.; Vilanova, M.; Cerca, N. Comparative analysis between biofilm formation and gene expression in Staphylococcus epidermidis isolates. Future Microbiol. 2018, 13, 415–427. [Google Scholar] [CrossRef]
- Lee, J.Y.H.; Monk, I.R.; Gonçalves da Silva, A.; Seemann, T.; Chua, K.Y.L.; Kearns, A.; Hill, R.; Woodford, N.; Bartels, M.D.; Strommenger, B.; et al. Global spread of three multidrug-resistant lineages of Staphylococcus epidermidis. Nat. Microbiol. 2018, 3, 1175–1185. [Google Scholar] [CrossRef]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.A.; El-Anssary, A.A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. In Herbal Medicine; IntechOpen: London, UK, 2019. [Google Scholar]
- Ruddaraju, L.K.; Pammi, S.V.N.; sankar Guntuku, G.; Padavala, V.S.; Kolapalli, V.R.M. A review on anti-bacterials to combat resistance: From ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J. Pharm. Sci. 2020, 15, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [PubMed]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant secondary metabolites in the battle of drugs and drug-resistant bacteria: New heroes or worse clones of antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Villagrán, C.; Castro, V. Ciencia indígena de los Andes de Chile; Editorial Universitaria: Guadalajara, Mexico, 2000; p. 244. [Google Scholar]
- Villagrán, C.; Romo, M.; Castro, V. Etnobotánica del sur de los Andes de la Primera Región de Chile: Un enlace entre las culturas altiplánicas y las de quebradas altas del Loa superior. Chungará 2003, 35, 73–124. [Google Scholar] [CrossRef]
- Toursarkissian, M. Plantas Medicinales de la Argentina, 1st ed.; Editorial Hemisferio Sur, S.A.: Buenos Aires, Argentina, 1980. [Google Scholar]
- Pérez, E.L. Las Plantas Utilizadas por la Comunidad de Antofagasta de la Sierra, Puna Catamarqueña, Argentina. Bachelor’s Thesis, Facultad de Ciencias Naturales e Instituto Miguel Lillo Universidad Nacional de Tucumán, San Miguel de Tucumán, Argentina, 2006. [Google Scholar]
- Barbarán, F.R. Medicinal plants of the Argentinian puna: A common property resource and an opportunity for local people. In Proceedings of the Governing Shared Resources: Connecting Local Experience to Global Challenges 12th Biennial Conference of the International Association for the Study of Commons, Cheltenham, UK, 14–18 July 2008. [Google Scholar]
- Erazo, S.; Zaldívar, M.; Delporte, C.; Backhouse, N.; Tapia, P.; Belmonte, E.; Monarche, F.D.; Negrete, R. Antibacterial diterpenoids from Fabiana densa var. ramulosa. Planta Med. 2002, 68, 361–363. [Google Scholar] [CrossRef]
- Zampini, I.C.; Cuello, S.; Alberto, M.R.; Ordoñez, R.M.; Almeida, R.D.; Solorzano, E.; Isla, M.I. Antimicrobial activity of selected plant species from “the Argentine puna” against sensitive and multi-resistant bacteria. J. Ethnopharmacol. 2009, 124, 499–505. [Google Scholar] [CrossRef]
- Cuello, S.; Alberto, M.R.; Zampini, I.C.; Ordoñez, R.M.; Isla, M.I. Comparative study of antioxidant and anti-inflammatory activities and genotoxicity of alcoholic and aqueous extracts of four Fabiana Species that grow in mountainous area of Argentina. J. Ethnopharmacol. 2011, 137, 512–522. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Theoduloz, C. Fabiana imbricata Ruiz et Pav. (Solanaceae), a Review of an Important Patagonian Medicinal Plant. J. Ethnopharmacol. 2019, 228, 26–39. [Google Scholar] [CrossRef]
- Quaglio, D.; Corradi, S.; Erazo, S.; Vergine, V.; Berardozzi, S.; Sciubba, F.; Cappiello, F.; Crestoni, M.E.; Ascenzioni, F.; Imperi, F.; et al. Structural elucidation and antimicrobial characterization of novel diterpenoids from Fabiana densa var. ramulosa. ACS. Med. Chem. Lett. 2020, 11, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Ojha, K.S.; Aznar, R.; O’Donnell, C.; Tiwari, B.K. Ultrasound Technology for the Extraction of Biologically Active Molecules from Plant, Animal and Marine Sources. TrAC—Trends Analyt. Chem. 2020, 122, 115663. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 24th Informational Supplement. (M100 Ed. 32); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Yong Ryu, S.; Lee, J. Calcium-chelating alizarin and other anthraquinones inhibit biofilm formation and the hemolytic activity of Staphylococcus aureus. Sci. Rep. 2016, 6, 19267. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, A.; Balasubramaniam, B.; Ranjitha, R.; Srinivasan, R.; Packiavathy, I.A.S.V.; Balamurugan, K.; Pandian, S.K.; Ravi, A.V. In vitro and in vivo biofilm inhibitory efficacy of geraniol-cefotaxime combination against Staphylococcus spp. Food Chem. Toxicol. 2019, 125, 322–332. [Google Scholar] [CrossRef]
- Deng, Q.; Zinoviadou, K.G.; Galanakis, C.M.; Orlien, V.; Grimi, N.; Vorobiev, E.; Lebovka, N.; Barba, F.J. The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: Extraction, degradation, and applications. Food Eng. Rev. 2015, 7, 357–381. [Google Scholar] [CrossRef]
- Heng, M.Y.; Tan, S.N.; Yong, J.W.H.; Ong, E.S. Emerging green technologies for the chemical standardization of botanicals and herbal preparations. TrAC—Trends Analyt. Chem. 2013, 50, 1–10. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Comparison of conventional extraction technique with ultrasound assisted extraction on recovery of phenolic compounds from lemon scented tea tree (Leptospermum Petersonii) leaves. Heliyon 2020, 6, e03666. [Google Scholar] [CrossRef]
- Monschein, M.; Jaindl, K.; Buzimkić, S.; Bucar, F. Content of phenolic compounds in wild populations of Epilobium angustifolium growing at different altitudes. Pharm. Biol. 2015, 53, 1576–1582. [Google Scholar] [CrossRef]
- Cirak, C.; Radusiene, J.; Jakstas, V.; Ivanauskas, L.; Seyis, F.; Yayla, F. Altitudinal changes in secondary metabolite contents of Hypericum androsaemum and Hypericum polyphyllum. Biochem. Syst. Ecol. 2017, 70, 108–115. [Google Scholar] [CrossRef]
- Hashim, A.M.; Alharbi, B.M.; Abdulmajeed, A.M.; Elkelish, A.; Hassan, H.M.; Hozzein, W.N. Oxidative stress responses of some endemic plants to high altitudes by intensifying antioxidants and secondary metabolites content. Plants 2020, 9, 869. [Google Scholar] [CrossRef]
- Hasni, S.; Rigane, G.; Ghazghazi, H.; Riguene, H.; Bouallegue, A.; Khedher, O.; Oueslati, M.A.; ben Salem, R. Optimum conditions and LC-ESI-MS analysis of phenolic rich extract from Eucalyptus marginata L. under maceration and ultrasound-assisted extraction methods using response surface methodology. J. Food Qual. 2021, 2021, 5591022. [Google Scholar] [CrossRef]
- Gajic, I.S.; Savic, I.; Boskov, I.; Žerajić, S.; Markovic, I.; Gajic, D. Optimization of ultrasound-assisted extraction of phenolic compounds from black locust (Robiniae pseudoacaciae) flowers and comparison with conventional methods. Antioxidants 2019, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Gan, R.Y.; Ge, Y.Y.; Zhang, D.; Corke, H. Ultrasonic treatment increases extraction rate of common bean (Phaseolus vulgaris L.) Antioxidants. Antioxidants 2019, 8, 83. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Görgüç, A.; Uygun, Ö.; Bircan, C. Steviol glycosides and polyphenols extraction from Stevia rebaudiana Bertoni leaves using maceration, microwave-, and ultrasound-assisted techniques. Sep. Sci. Technol. 2021, 56, 936–948. [Google Scholar] [CrossRef]
- Aguilar-Villalva, R.; Molina, G.A.; España-Sánchez, B.L.; Díaz-Peña, L.F.; Elizalde-Mata, A.; Valerio, E.; Azanza-Ricardo, C.; Estevez, M. Antioxidant capacity and antibacterial activity from Annona cherimola phytochemicals by ultrasound-assisted extraction and its comparison to conventional methods. Arab. J. Chem. 2021, 14, 103239. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding Pitfalls in Determining Antimicrobial Activity of Plant Extracts and Publishing the Results. BMC Complement. Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J. In Vitro Antimicrobial Activity of Natural Products Using Minimum Inhibitory Concentrations: Looking for New Chemical Entities or Predicting Clinical Response. Med. Aromat. Plants 2012, 1, 7. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal Plants and Antimicrobial Activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, K.P.; Zhang, X.; Pan, D.D.; Sun, Y.Y.; Cao, J.X. Antibacterial activity and mechanism of action of black pepper essential oil on meat-borne Escherichia coli. Front. Microbiol. 2017, 7, 2094. [Google Scholar]
- Trentin, D.D.; Giordani, R.B.; Zimmer, K.R.; da Silva, A.G.; da Silva, M.V.; Correia, M.T.D.S.; Baumvol, I.J.R.; MacEdo, A.J. Potential of medicinal plants from the brazilian semi-arid region (caatinga) against Staphylococcus epidermidis planktonic and biofilm lifestyles. J. Ethnopharmacol. 2011, 137, 327–335. [Google Scholar] [CrossRef]
- Chusri, S.; Sompetch, K.; Mukdee, S.; Jansrisewangwong, S.; Srichai, T.; Maneenoon, K.; Limsuwan, S.; Voravuthikunchai, S.P. Inhibition of Staphylococcus epidermidis biofilm formation by traditional thai herbal recipes used for wound treatment. Evid. Based Complement. Altern. Med. 2012, 2012, 159797. [Google Scholar] [CrossRef] [PubMed]
- di Lodovico, S.; Menghini, L.; Ferrante, C.; Recchia, E.; Castro-Amorim, J.; Gameiro, P.; Cellini, L.; Bessa, L.J. Hop Extract: An efficacious antimicrobial and anti-biofilm agent against multidrug-resistant Staphylococci strains and Cutibacterium acnes. Front. Microbiol. 2020, 11, 1852. [Google Scholar]
- Carvalho, R.S.; Carollo, C.A.; de Magalhães, J.C.; Palumbo, J.M.; Boaretto, A.G.; Nunes e Sá, I.C.; Ferraz, A.; Lima, W.G.; de Siqueira, J.M.; Ferreira, J.M. Antibacterial and antifungal activities of phenolic compound-enriched ethyl acetate fraction from Cochlospermum regium (Mart. Et. Schr.) Pilger roots: Mechanisms of action and synergism with tanin and gallic Acid. S. Afr. J. Bot. 2018, 114, 181–187. [Google Scholar]
- Liu, M.; Wu, X.; Li, J.; Liu, L.; Zhang, R.; Shao, D.; Du, X. The specific anti-biofilm effect of gallic acid on Staphylococcus aureus by regulating the expression of the ica operon. Food Control 2017, 73, 613–618. [Google Scholar]
- Passos, M.R.; Almeida, R.S.; Lima, B.O.; Rodrigues, J.Z.; Macêdo Neres, N.S.; Pita, L.S.; Marinho, P.D.; Santos, I.A.; da Silva, J.P.; Oliveira, M.C.; et al. Anticariogenic activities of Libidibia ferrea, gallic acid and ethyl gallate against Streptococcus mutans in biofilm model. J. Ethnopharmacol. 2021, 274, 114059. [Google Scholar] [PubMed]
- Shao, D.; Li, J.; Li, J.; Tang, R.; Liu, L.; Shi, J.; Huang, Q.; Yang, H. Inhibition of gallic acid on the growth and biofilm formation of Escherichia coli and Streptococcus mutans. J. Food. Sci. 2015, 80, M1299–M1305. [Google Scholar] [CrossRef]
- Abdel Bar, F.M.; Alossaimi, M.A.; Elekhnawy, E.; Alzeer, M.A.A.; Abo Kamer, A.; Moglad, E.; ElNaggar, M.H. Anti-quorum sensing and anti-biofilm activity of Pelargonium × hortorum root extract against Pseudomonas aeruginosa: Combinatorial effect of catechin and gallic acid. Molecules 2022, 27, 7841. [Google Scholar]
- dos Santos Ramos, F.; Martins, D.M.; Nunes Sagini, J.P.; Quines, C.B.; de Oliveira Pereira, F.S.; de Ávila, D.S.; Zanzarin, D.; Pilau, E.J.; de Lima Postiga, I.A.; Tostes, J.; et al. Soybeans agroindustrial residues as Staphylococcus epidermidis and S. aureus biofilm inhibitors. Ind. Crops Prod. 2021, 170, 113713. [Google Scholar]
- Sivaranjani, M.; Prakash, M.; Gowrishankar, S.; Rathna, J.; Pandian, S.K.; Ravi, A.V. In vitro activity of alpha-mangostin in killing and eradicating Staphylococcus epidermidis RP62A biofilms. Appl. Microbiol. Biotechnol. 2017, 101, 3349–3359. [Google Scholar]
- Swetha, T.K.; Pooranachithra, M.; Subramenium, G.A.; Divya, V.; Balamurugan, K.; Pandian, S.K. Umbelliferone impedes biofilm formation and virulence of methicillin-resistant Staphylococcus epidermidis via impairment of initial attachment and intercellular adhesion. Front. Cell. Infect. Microbiol. 2019, 9, 357. [Google Scholar] [PubMed]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2018, 124, 382–388. [Google Scholar]
- Arteaga-Crespo, Y.; Radice, M.; Bravo-Sanchez, L.R.; García-Quintana, Y.; Scalvenzi, L. Optimisation of ultrasound-assisted extraction of phenolic antioxidants from Ilex guayusa Loes. leaves using response surface methodology. Heliyon 2020, 6, e03043. [Google Scholar] [PubMed]
- Ciğeroğlu, Z.; Aras, Ö.; Pinto, C.A.; Bayramoglu, M.; Kırbaşlar, İ.; Lorenzo, J.M.; Barba, F.J.; Saraiva, J.A.; Şahin, S. Optimization of ultrasound-assisted extraction of phenolic compounds from grapefruit (Citrus paradisi Macf.) leaves via D-optimal design and artificial neural network design with categorical and quantitative variables. J. Sci. Food Agric. 2018, 98, 4584–4596. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Torres-Carro, R.; Ana, Y.; Rojas-Márquez, J.D.; Stempin, C.C.; Zampini, I.C.; Isla, M.I.; Alberto, M.R. Argentinean Puna plants with in vitro antioxidant and anti-inflammatory activities as a potential nutraceutical. J. Food Sci. 2019, 84, 3352–3363. [Google Scholar]
- Murray, P.; Baron, E.; Faller, M.; Tenover, F.; Yolken, R. Manual of Clinical Microbiology, 7th ed.; ASM: Washington, DC, USA, 1999. [Google Scholar]
- Guo, Y.; Ding, Y.; Liu, L.; Shen, X.; Hao, Z.; Duan, J.; Jin, Y.; Chen, Z.; Yu, F. Antimicrobial susceptibility, virulence determinants profiles and molecular characteristics of Staphylococcus epidermidis isolates in Wenzhou, eastern China. BMC Microbiol. 2019, 19, 157. [Google Scholar]
- Shi, C.; Zhang, X.; Guo, N. The antimicrobial activities and action-mechanism of tea tree oil against food-borne bacteria in fresh cucumber juice. Microb. Pathog. 2018, 125, 262–271. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant natural products targeting bacterial virulence factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef]
- Selvaraj, A.; Valliammai, A.; Premika, M.; Priya, A.; Bhaskar, J.P.; Krishnan, V.; Pandian, S.K. Sapindus Mukorossi Gaertn. and its bioactive metabolite oleic acid impedes methicillin-resistant Staphylococcus aureus biofilm formation by down regulating adhesion genes expression. Microbiol. Res. 2021, 242, 126601. [Google Scholar] [CrossRef] [PubMed]
- ben Abdallah, F.; Lagha, R.; Gaber, A. Biofilm inhibition and eradication properties of medicinal plant essential oils against methicillin-resistant Staphylococcus aureus clinical isolates. Pharmaceuticals 2020, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.A.; Rossatto, F.C.; Medeiros, A.W.; Correa, A.P.; Brandelli, A.; Frazzon, A.P.; da Motta, A.D. Evaluation antibacterial and antibiofilm activity of the antimicrobial peptide p34 against Staphylococcus aureus and Enterococcus faecalis. An. Acad. Bras. Cienc. 2018, 90, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, M.M.; Rohloff, J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control 2016, 61, 156–164. [Google Scholar] [CrossRef]


| Plant | Total Phenolics Compounds (µg GAE1/mL) | Total Flavonoids (µg QE2/mL) | Yield of Dry Extract (mg/mL) | |||
|---|---|---|---|---|---|---|
| Maceration | UAE | Maceration | UAE | Maceration | UAE | |
| F. densa | 1615.41 ± 61.42 aA | 1586.03± 86.47 aA | 220.53 ± 6.76 aA | 143.59 ± 10.88 aB | 16.80 ± 0.43 aA | 13.43 ± 0.05 aB |
| F. patagonica | 2101.11 ± 28.82 bA | 1824.17 ± 33.26 bB | 165.06 ± 3.53 bA | 86.24 ± 5.29 bB | 15.20 ± 0.62 bA | 8.43 ± 0.05 bB |
| F. punensis | 1930.38 ± 22.17 cA | 1872.06 ± 44.35 bA | 234.76 ± 5.00 cA | 180.35 ± 2.94 cB | 12.86 ± 0.60 cA | 10.53 ± 0.4 cB |
| Treatment | Biofilm Formation Inhibition (%) | Biofilm Preformed Eradication (%) |
|---|---|---|
| DMSO | 5.89 ± 2.57 a | 0.24 ± 0.44 a |
| 1/8× MIC | 66.56 ± 0.43 b | 22.49 ± 7.14 b |
| 1/16× MIC | 42.53 ± 2.26 c | 19.38 ± 7.15 b |
| 1/32× MIC | 30.33 ± 5.18 d | 10.15 ± 3.51 c |
| 1000 GA | 67.04 ± 3.60 b | 40.46 ± 4.50 d |
| 500 GA | 42.95 ± 1.27 c | 26.59 ± 2.55 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez Chamás, J.; Isla, M.I.; Zampini, I.C. Antibacterial and Antibiofilm Activity of Different Species of Fabiana sp. Extract Obtained via Maceration and Ultrasound-Assisted Extraction against Staphylococcus epidermidis. Plants 2023, 12, 1830. https://doi.org/10.3390/plants12091830
Martínez Chamás J, Isla MI, Zampini IC. Antibacterial and Antibiofilm Activity of Different Species of Fabiana sp. Extract Obtained via Maceration and Ultrasound-Assisted Extraction against Staphylococcus epidermidis. Plants. 2023; 12(9):1830. https://doi.org/10.3390/plants12091830
Chicago/Turabian StyleMartínez Chamás, José, María Inés Isla, and Iris Catiana Zampini. 2023. "Antibacterial and Antibiofilm Activity of Different Species of Fabiana sp. Extract Obtained via Maceration and Ultrasound-Assisted Extraction against Staphylococcus epidermidis" Plants 12, no. 9: 1830. https://doi.org/10.3390/plants12091830
APA StyleMartínez Chamás, J., Isla, M. I., & Zampini, I. C. (2023). Antibacterial and Antibiofilm Activity of Different Species of Fabiana sp. Extract Obtained via Maceration and Ultrasound-Assisted Extraction against Staphylococcus epidermidis. Plants, 12(9), 1830. https://doi.org/10.3390/plants12091830









