Enhancing Attention and Interest in Plants to Mitigate Plant Awareness Disparity
Abstract
1. Introduction
2. Results
2.1. Are Individual Plants Perceived More Positively Than Plants in Groups?
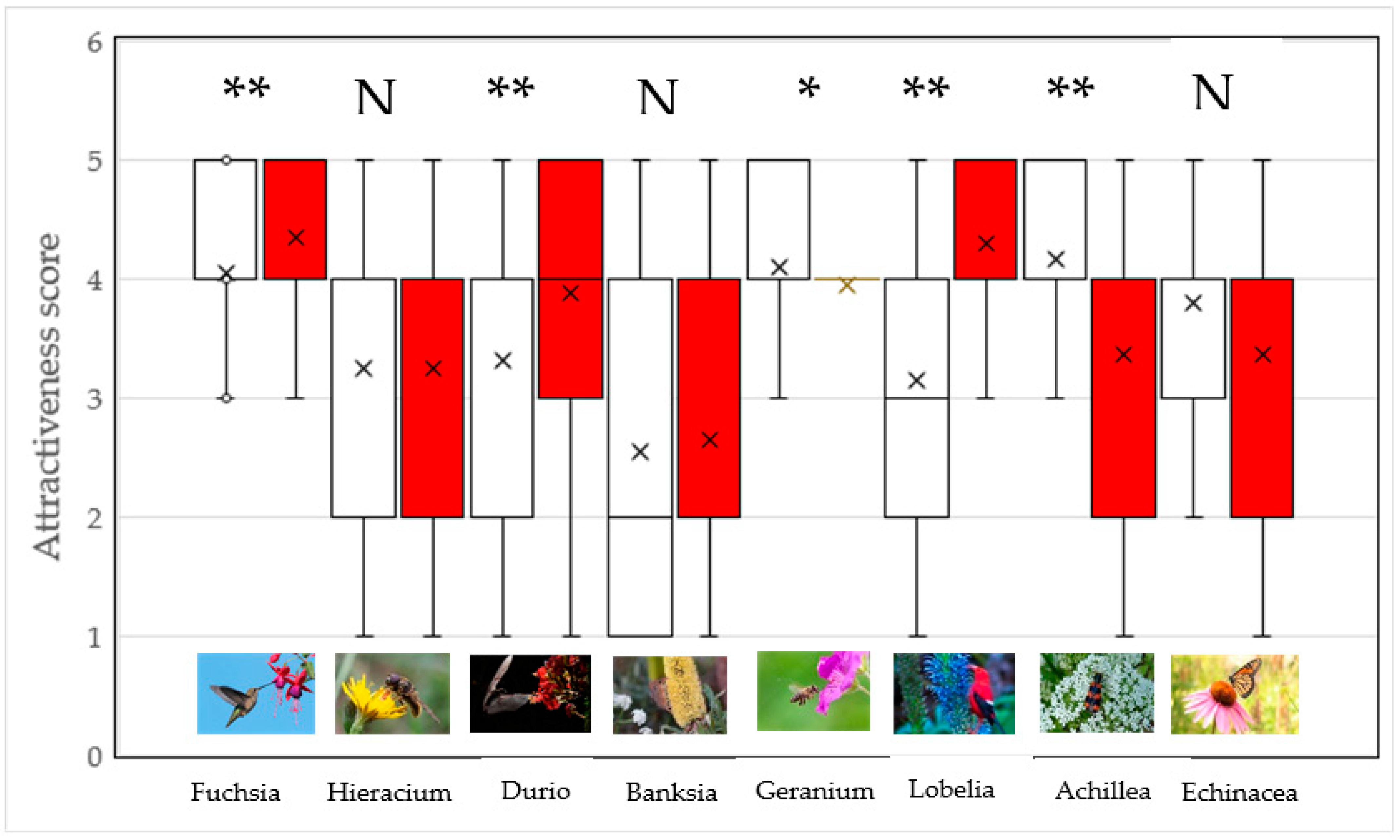
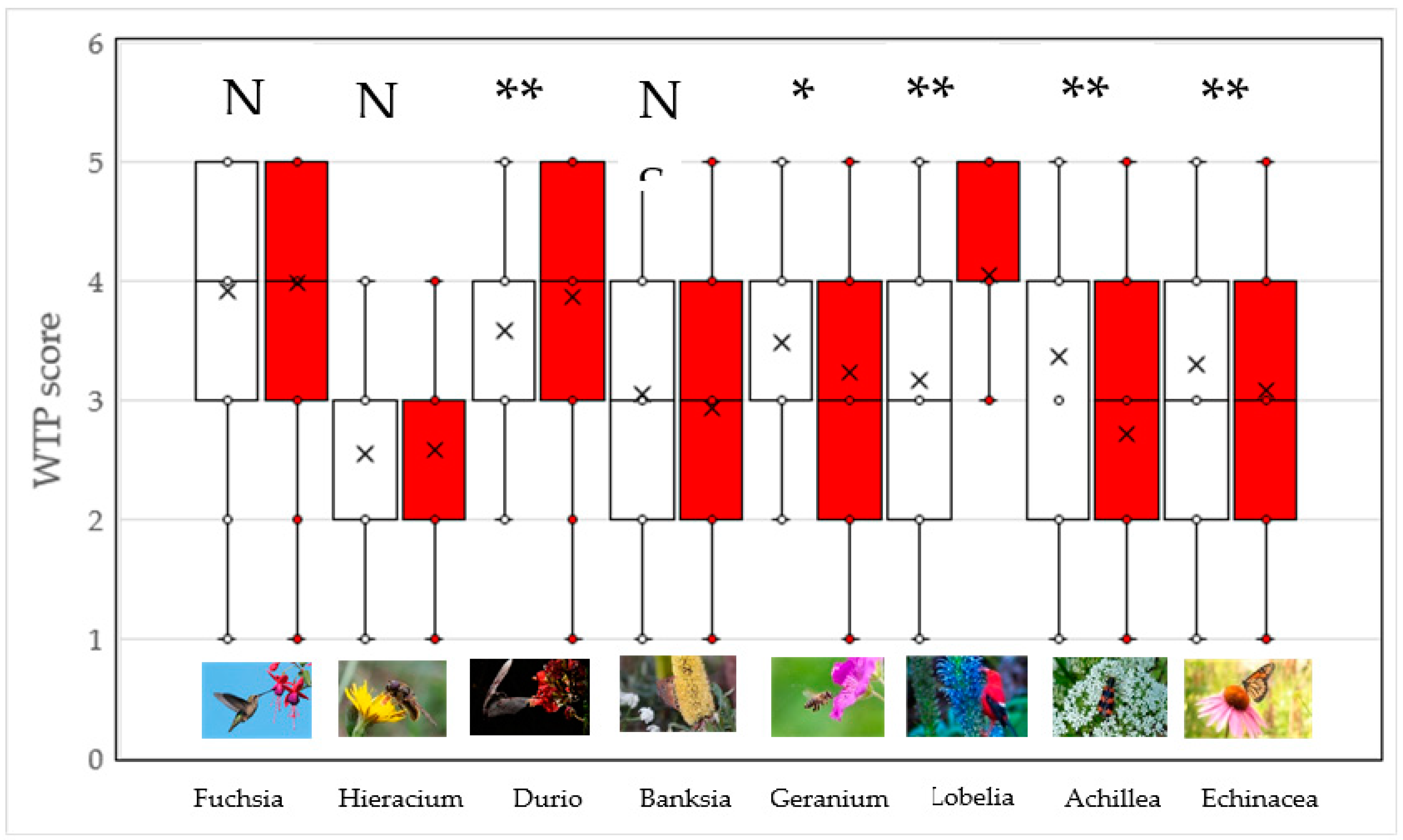
2.2. Are Plants Presented on Their Own Perceived Differently Than Those with Pollinators?
2.3. Are There Differences in the Perception of Plants between Males and Females?
2.4. The Influence of Pollinators on Differences between Males and Females
2.5. Participants’ Preferences for Animal-Plant Interaction Topics
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Selection of Pictures
4.3. Individual Plants vs. Plants in Groups
4.4. Manipulation of the Presence of Pollinators
4.5. Measuring Preferences for Animal–Plant Interactions
4.6. Measures
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birx, H.J. (Ed.) Encyclopedia of Anthropology; SAGE: Thousand Oaks, CA, USA, 2006. [Google Scholar]
- Diamond, J.; Bellwood, P. Farmers and their languages: The first expansions. Science 2003, 300, 597–603. [Google Scholar] [CrossRef]
- Kabir, M.H.; Mimura, M.; Tsai, J.C. Spreading waves in a farmers and hunter-gatherers model of the Neolithic transition in Europe. Bull. Math. Biol. 2018, 80, 2452–2480. [Google Scholar] [CrossRef]
- Cox, P.A. Will tribal knowledge survive the millennium? Science 2000, 287, 44–45. [Google Scholar] [CrossRef]
- Turner, N.J.; Turner, K.L. “Where our women used to get the food”: Cumulative effects and loss of ethnobotanical knowledge and practice; case study from coastal British Columbia. Botany 2008, 86, 103–115. [Google Scholar] [CrossRef]
- DeFries, R.S.; Rudel, T.; Uriarte, M.; Hansen, M. Deforestation driven by urban population growth and agricultural trade in the twenty-first century. Nat. Geosci. 2010, 3, 178–181. [Google Scholar] [CrossRef]
- Krishnan, S.; Moreau, T.; Kuehny, J.; Novy, A.; Greene, S.L.; Khoury, C.K. Resetting the table for people and plants: Botanic gardens and research organizations collaborate to address food and agricultural plant blindness. Plants People Planet 2019, 1, 157–163. [Google Scholar] [CrossRef]
- Pyle, R.M. The Thunder Tree: Lessons from an Urban Wildland; Houghton Mifflin: Boston, MA, USA, 1993. [Google Scholar]
- Louv, R. Last Child in the Woods: Saving Our Children from Nature-Deficit Disorder; Algonquin Books of Chapel Hill: Chapel Hill, NC, USA, 2005. [Google Scholar]
- Soga, M.; Gaston, K.J. Extinction of experience: The loss of human–nature interactions. Front. Ecol. Environ. 2016, 14, 94–101. [Google Scholar] [CrossRef]
- Parsley, K.M. Plant awareness disparity: A case for renaming plant blindness. Plants People Planet 2020, 2, 598–601. [Google Scholar] [CrossRef]
- Wandersee, J.H.; Schussler, E.E. Preventing plant blindness. Am. Biol. Teach. 1999, 61, 82–86. [Google Scholar] [CrossRef]
- Wandersee, J.H.; Schussler, E. Toward a theory of plant blindness. Plant Sci. Bull. 2001, 47, 2–9. [Google Scholar]
- Krosnick, S.E.; Baker, J.C.; Moore, K.R. The pet plant project: Treating plant blindness by making plants personal. Am. Biol. Teach. 2018, 80, 339–345. [Google Scholar] [CrossRef]
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.K.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’connor, M.I. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 2012, 486, 105–108. [Google Scholar] [CrossRef]
- Oliveira, W.; Silva, J.L.; Porto, R.G.; Cruz-Neto, O.; Tabarelli, M.; Viana, B.F.; Peres, C.A.; Lopes, A.V. 62 signatories. Plant and pollination blindness: Risky business for human food security. BioScience 2020, 70, 109–110. [Google Scholar]
- Balding, M.; Williams, K.J.H. Plant blindness and the implications for plant conservation. Conserv. Biol. 2016, 30, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Kinchin, I. Investigating secondary-school girls’ preferences for animals or plants: A simple ‘head-to-head’ comparison using two unfamiliar organisms. J. Biol. Educ. 1999, 33, 95–99. [Google Scholar]
- Lindemann-Matthies, P. Loveable’ mammals and ‘lifeless’ plants: How children’s interest in common local organisms can be enhanced through observation of nature. Int. J. Sci. Educ. 2005, 27, 655–677. [Google Scholar] [CrossRef]
- Schussler, E.E.; Olzak, L.A. It’s not easy being green: Student recall of plant and animal images. J. Biol. Educ. 2008, 42, 122–219. [Google Scholar] [CrossRef]
- Pany, P.; Meier, F.D.; Dünser, B.; Yanagida, T.; Kiehn, M.; Möller, A. Measuring students’ plant awareness: A prerequisite for effective botany education. J. Biol. Educ. 2022, 1–14. [Google Scholar] [CrossRef]
- Amprazis, A.; Papadopoulou, P. Plant blindness: A faddish research interest or a substantive impediment to achieve sustainable development goals? Environ. Educ. Res. 2020, 26, 1065–1087. [Google Scholar] [CrossRef]
- Barrutia, O.; Ruiz-González, A.; Sanz-Azkue, I.; Díez, J.R. Secondary school students’ familiarity with animals and plants: Hometown size matters. Environ. Educ. Res. 2022, 28, 1564–1583. [Google Scholar] [CrossRef]
- Burke, R.; Sherwood, O.L.; Clune, S.; Carroll, R.; McCabe, P.F.; Kane, A.; Kacprzyk, J. Botanical boom: A new opportunity to promote the public appreciation of botany. Plants People Planet 2022, 4, 326–334. [Google Scholar] [CrossRef]
- Amprazis, A.; Papadopoulou, P.; Malandrakis, G. Plant blindness and children’s recognition of plants as living things: A research in the primary schools context. J. Biol. Educ. 2021, 55, 139–154. [Google Scholar] [CrossRef]
- Balas, B.; Momsen, J.L. Attention “blinks” differently for plants and animals. CBE Life Sci. Educ. 2014, 13, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Kanske, P.; Schönfelder, S.; Wessa, M. Emotional modulation of the attentional blink and the relation to interpersonal reactivity. Front. Hum. Neurosci. 2013, 7, 641. [Google Scholar] [CrossRef]
- Zani, G.; Low, J. Botanical priming helps overcome plant blindness on a memory task. J. Environ. Psychol. 2022, 81, 101808. [Google Scholar] [CrossRef]
- New, J.; Cosmides, L.; Tooby, J. Category-specific attention for animals reflects ancestral priorities, not expertise. Proc. Natl. Acad. Sci. USA 2007, 104, 16598–16603. [Google Scholar] [CrossRef]
- Prokop, P.; Fančovičová, J. Seeing coloured fruits: Utilisation of the theory of adaptive memory in teaching botany. J. Biol. Educ. 2014, 48, 127–132. [Google Scholar] [CrossRef]
- Prokop, P.; Fančovičová, J. The perception of toxic and non-toxic plants by children and adolescents with regard to gender: Implications for teaching botany. J. Biol. Educ. 2019, 53, 463–473. [Google Scholar] [CrossRef]
- Margulies, J.D.; Bullough, L.A.; Hinsley, A.; Ingram, D.J.; Cowell, C.; Goettsch, B.; Kiltgard, B.B.; Lavorgna, A.; Sinovas, P.; Phelps, J. Illegal wildlife trade and the persistence of “plant blindness”. Plants People Planet 2019, 1, 173–182. [Google Scholar] [CrossRef]
- Prokop, P.; Masarovič, R.; Hajdúchová, S.; Ježová, Z.; Zvaríková, M.; Fedor, P. Prioritisation of charismatic animals in major conservation journals measured by the altmetric attention score. Sustainability 2022, 14, 17029. [Google Scholar] [CrossRef]
- Adamo, M.; Chialva, M.; Calevo, J.; Bertoni, F.; Dixon, K.; Mammola, S. Plant scientists’ research attention is skewed towards colourful, conspicuous and broadly distributed flowers. Nat. Plants 2021, 7, 574–578. [Google Scholar] [CrossRef]
- Fukushima, C.S.; Mammola, S.; Cardoso, P. Global wildlife trade permeates the Tree of Life. Biol. Conserv. 2020, 247, 108503. [Google Scholar] [CrossRef] [PubMed]
- Adamo, M.; Sousa, R.; Wipf, S.; Correia, R.A.; Lumia, A.; Mucciarelli, M.; Mammola, S. Dimension and impact of biases in funding for species and habitat conservation. Biol. Cons. 2022, 272, 109636. [Google Scholar] [CrossRef]
- Nic Lughadha, E.; Bachman, S.P.; Leão, T.C.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.A.; Gâteblé, G.; et al. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- Parsley, K.M.; Daigle, B.J.; Sabel, J.L. Initial development and validation of the Plant Awareness Disparity Index. CBE Life Sci. Educ. 2022, 21, ar64. [Google Scholar] [CrossRef]
- Martín-López, B.; Montes, C.; Benayas, J. The non-economic motives behind the willingness to pay for biodiversity conservation. Biol. Cons. 2007, 139, 67–82. [Google Scholar] [CrossRef]
- Jacobs, M.H.; Harms, M. Influence of interpretation on conservation intentions of whale tourists. Tour. Manag. 2014, 42, 123–131. [Google Scholar] [CrossRef]
- Prokop, P.; Fančovičová, J. Does colour matter? The influence of animal warning coloration on human emotions and willingness to protect them. Anim. Cons. 2013, 16, 458–466. [Google Scholar] [CrossRef]
- Prokop, P.; Fančovičová, J. Animals in dangerous postures enhance learning, but decrease willingness to protect animals. Eur. J. Math. Sci. Technol. Educ. 2017, 13, 6069–6077. [Google Scholar] [CrossRef]
- Castillo-Huitrón, N.M.; Naranjo, E.J.; Santos-Fita, D.; Estrada-Lugo, E. The importance of human emotions for wildlife conservation. Front. Psychol. 2020, 11, 1277. [Google Scholar] [CrossRef]
- Notaro, S.; Grilli, G. How much fear? Exploring the role of integral emotions on stated preferences for wildlife conservation. Environ. Manag. 2022, 69, 449–465. [Google Scholar] [CrossRef]
- Fančovičová, J.; Prokop, P.; Kubíčková, M. The effect of aposematic signals of plants on students’ perception and willingness to protect them. Sustainability 2022, 14, 9121. [Google Scholar] [CrossRef]
- Gunnthorsdottir, A. Physical attractiveness of animal species a decision factor for its preservation. Antrozoös 2001, 14, 204–215. [Google Scholar] [CrossRef]
- Landová, E.; Poláková, P.; Rádlová, S.; Janovcová, M.; Bobek, M.; Frynta, D. Beauty ranking of mammalian species kept in the Prague Zoo: Does beauty of animals increase the respondents’ willingness to protect them? Sci. Nat. 2018, 105, 1–14. [Google Scholar] [CrossRef]
- Ren, Y.; Ding, C.; Zhang, Y.; Qing, B.; Duan, W. Public attitudes and willingness to pay toward the conservation of Crested Ibis: Insights for management. J. Nat. Cons. 2022, 66, 126118. [Google Scholar] [CrossRef]
- Strgar, J. Increasing the interest of students in plants. J. Biol. Educ. 2007, 42, 19–23. [Google Scholar] [CrossRef]
- Kubiatko, M.; Fančovičová, J.; Prokop, P. Factual knowledge of students about plants is associated with attitudes and interest in botany. Int. J. Sci. Educ. 2021, 43, 1426–1440. [Google Scholar] [CrossRef]
- Gatt, S.; Tunnicliffe, S.D.; Borg, K.; Lautier, K. Young Maltese children’s ideas about plants. J. Biol. Educ. 2007, 41, 117–122. [Google Scholar] [CrossRef]
- Lindemann-Matthies, P.; Briegel, R.; Schüpbach, B.; Junge, X. Aesthetic preference for a Swiss alpine landscape: The impact of different agricultural land-use with different biodiversity. Lands. Urban Plan. 2010, 98, 99–109. [Google Scholar] [CrossRef]
- Stagg, B.C.; Dillon, J. Plant awareness is linked to plant relevance: A review of educational and ethnobiological literature (1998–2020). Plants People Planet 2022, 4, 579–592. [Google Scholar] [CrossRef]
- Hůla, M.; Flegr, J. Habitat selection and human aesthetic responses to flowers. Evol. Hum. Sci. 2021, 3, e5. [Google Scholar] [CrossRef]
- Prokop, P.; Fančovičová, J. Beautiful fruits taste good: The aesthetic influences of fruit preferences in humans. Anthropol. Anz. 2012, 69, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Żmihorski, M.; Dziarska-Pałac, J.; Sparks, T.H.; Tryjanowski, P. Ecological correlates of the popularity of birds and butterflies in Internet information resources. Oikos 2013, 122, 183–190. [Google Scholar] [CrossRef]
- Herzog, H.A., Jr.; Burghardt, G.M. Attitudes toward animals: Origins and diversity. Anthrozoös 1988, 1, 214–222. [Google Scholar] [CrossRef]
- Prokop, P.; Randler, C. Biological predispositions and individual differences in human attitudes toward animals. In Ethnozoology: Animals in Our Lives; Alves, R.R.N., de Albuquerque, A.P.D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 447–466. [Google Scholar]
- Clucas, B.; McHugh, K.; Caro, T. Flagship species on covers of US conservation and nature magazines. Biodivers. Conserv. 2008, 17, 1517–1528. [Google Scholar] [CrossRef]
- Frynta, D.; Šimková, O.; Lišková, S.; Landová, E. Mammalian collection on Noah’s ark: The effects of beauty, brain and body size. PLoS ONE 2013, 8, e63110. [Google Scholar] [CrossRef]
- Small, E. The new Noah’s Ark: Beautiful and useful species only. Part 2. The chosen species. Biodiversity 2012, 13, 37–53. [Google Scholar] [CrossRef]
- Bjerke, T.; Østdahl, T. Animal-related attitudes and activities in an urban population. Anthrozoös 2004, 17, 109–129. [Google Scholar] [CrossRef]
- Borgi, M.; Cirulli, F. Attitudes toward animals among kindergarten children: Species preferences. Anthrozoös 2015, 28, 45–59. [Google Scholar] [CrossRef]
- Kellert, S.R. Values and perceptions of invertebrates. Conserv. Biol. 1993, 7, 845–855. [Google Scholar] [CrossRef]
- Schlegel, J.; Rupf, R. Attitudes towards potential animal flagship species in nature conservation: A survey among students of different educational institutions. J. Nat. Conserv. 2010, 18, 278–290. [Google Scholar] [CrossRef]
- Prokop, P.; Tunnicliffe, S.D. Effects of having pets at home on children’s attitudes toward popular and unpopular animals. Anthrozoös 2010, 23, 21–35. [Google Scholar] [CrossRef]
- Lišková, S.; Landová, E.; Frynta, D. Human preferences for colorful birds: Vivid colors or pattern? Evol. Psychol. 2015, 13, 147470491501300203. [Google Scholar] [CrossRef]
- Lišková, S.; Frynta, D. What determines bird beauty in human eyes? Anthrozoös 2013, 26, 27–41. [Google Scholar] [CrossRef]
- Golick, D.; Dauer, J.; Lynch, L.; Ingram, E. A framework for pollination systems thinking and conservation. Environ. Educ. Res. 2018, 24, 1143–1158. [Google Scholar] [CrossRef]
- Sumner, S.; Law, G.; Cini, A. Why we love bees and hate wasps. Ecol. Entomol. 2018, 43, 836–845. [Google Scholar] [CrossRef]
- Gerdes, A.B.; Uhl, G.; Alpers, G.W. Spiders are special: Fear and disgust evoked by pictures of arthropods. Evol. Hum. Behav. 2009, 30, 66–73. [Google Scholar] [CrossRef]
- Breuer, G.B.; Schlegel, J.; Kauf, P.; Rupf, R. The importance of being colorful and able to fly: Interpretation and implications of children’s statements on selected insects and other invertebrates. Int. J. Sci. Educ. 2015, 37, 2664–2687. [Google Scholar] [CrossRef]
- Sieg, A.-K.; Teibtner, R.; Dreesmann, D. Don’t know much about bumblebees? A Study about secondary school students’ knowledge and attitude shows educational demand. Insects 2018, 9, 40. [Google Scholar] [CrossRef]
- Lipták, B.; Kouba, A.; Patoka, J.; Paunović, M.; Prokop, P. Biological invasions and invasive species in freshwaters: Perception of the general public. Hum. Dim. Wildl. 2023, 1–16. [Google Scholar] [CrossRef]
- Fukano, Y.; Soga, M. Why do so many modern people hate insects? The urbanization–disgust hypothesis. Sci. Total Environ. 2021, 777, 146229. [Google Scholar] [CrossRef]
- Ernst, J.; Theimer, S. Evaluating the effects of environmental education programming on connectedness to nature. Environ. Educ. Res. 2011, 17, 577–598. [Google Scholar] [CrossRef]
- Mayer, F.S.; Frantz, C.M. The connectedness to nature scale: A measure of individuals’ feeling in community with nature. J. Environ. Psychol. 2004, 24, 503–515. [Google Scholar] [CrossRef]
- Brownlee, K.; Parsley, K.M.; Sabel, J.L. An analysis of plant awareness disparity within introductory biology textbook images. J. Biol. Educ. 2021, 57, 422–431. [Google Scholar] [CrossRef]
- Schussler, E.E.; Link-Pérez, M.A.; Weber, K.M.; Dollo, V.H. Exploring plant and animal content in elementary science textbooks. J. Biol. Educ. 2010, 44, 123–128. [Google Scholar] [CrossRef]
- Charness, G.; Gneezy, U.; Kuhn, M.A. Experimental methods: Between-subject and within-subject design. J. Econ. Behav. Org. 2012, 81, 1–8. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 26.0; Released; IBM Corp: Armonk, NY, USA, 2019. [Google Scholar]
| Saffron (Crocus sp.) | Dog Rose (Rosa canina Linnaeus, 1753) | Spruce (Picea abies Linnaeus, 1753) | Beech Tree (Fagus sylvatica Linnaeus, 1753) | ||
|---|---|---|---|---|---|
| Attractiveness | Individually | 4 (4.13, 4.33) | 4 (3.32, 3.61) | 4 (3.38, 3.65) | 4 (4.22, 4.42) |
| In group | 5 (4.31, 4.5) | 4 (3.04, 3.35) | 4 (3.72, 3.98) | 4 (4.15, 4.37) | |
| Wilcoxon Z | 3.25 | 4.32 | 4.47 | 0.90 | |
| p | 0.001 | <0.001 | <0.001 | 0.37 | |
| WTP | Individually | 4 (3.92, 4.18) | 3 (2.68, 2.99) | 3 (2.66, 2.99) | 4 (3.49, 3.81) |
| In group | 4 (3.8, 4.07) | 3 (2.61, 2.92) | 3 (3.12, 3.46) | 4 (3.34, 3.68) | |
| Wilcoxon Z | 1.44 | 1.12 | 5.5 | 1.24 | |
| p | 0.15 | 0.26 | <0.001 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokop, P.; Fančovičová, J. Enhancing Attention and Interest in Plants to Mitigate Plant Awareness Disparity. Plants 2023, 12, 2201. https://doi.org/10.3390/plants12112201
Prokop P, Fančovičová J. Enhancing Attention and Interest in Plants to Mitigate Plant Awareness Disparity. Plants. 2023; 12(11):2201. https://doi.org/10.3390/plants12112201
Chicago/Turabian StyleProkop, Pavol, and Jana Fančovičová. 2023. "Enhancing Attention and Interest in Plants to Mitigate Plant Awareness Disparity" Plants 12, no. 11: 2201. https://doi.org/10.3390/plants12112201
APA StyleProkop, P., & Fančovičová, J. (2023). Enhancing Attention and Interest in Plants to Mitigate Plant Awareness Disparity. Plants, 12(11), 2201. https://doi.org/10.3390/plants12112201








