Stability of Leaf Yerba Mate (Ilex paraguariensis) Metabolite Concentrations over the Time from the Prism of Secondary Sexual Dimorphism
Abstract
1. Introduction
2. Results
2.1. Experiment 1—SSD in Some Leaf Secondary Metabolites of Yerba Mate Observed in Four Provenances during the Winter and Summer Growth Pauses
2.2. Experiment 2—SSD in Some Leaf Metabolites of Yerba Mate Observed in Four Provenances over Four Subsequent Years
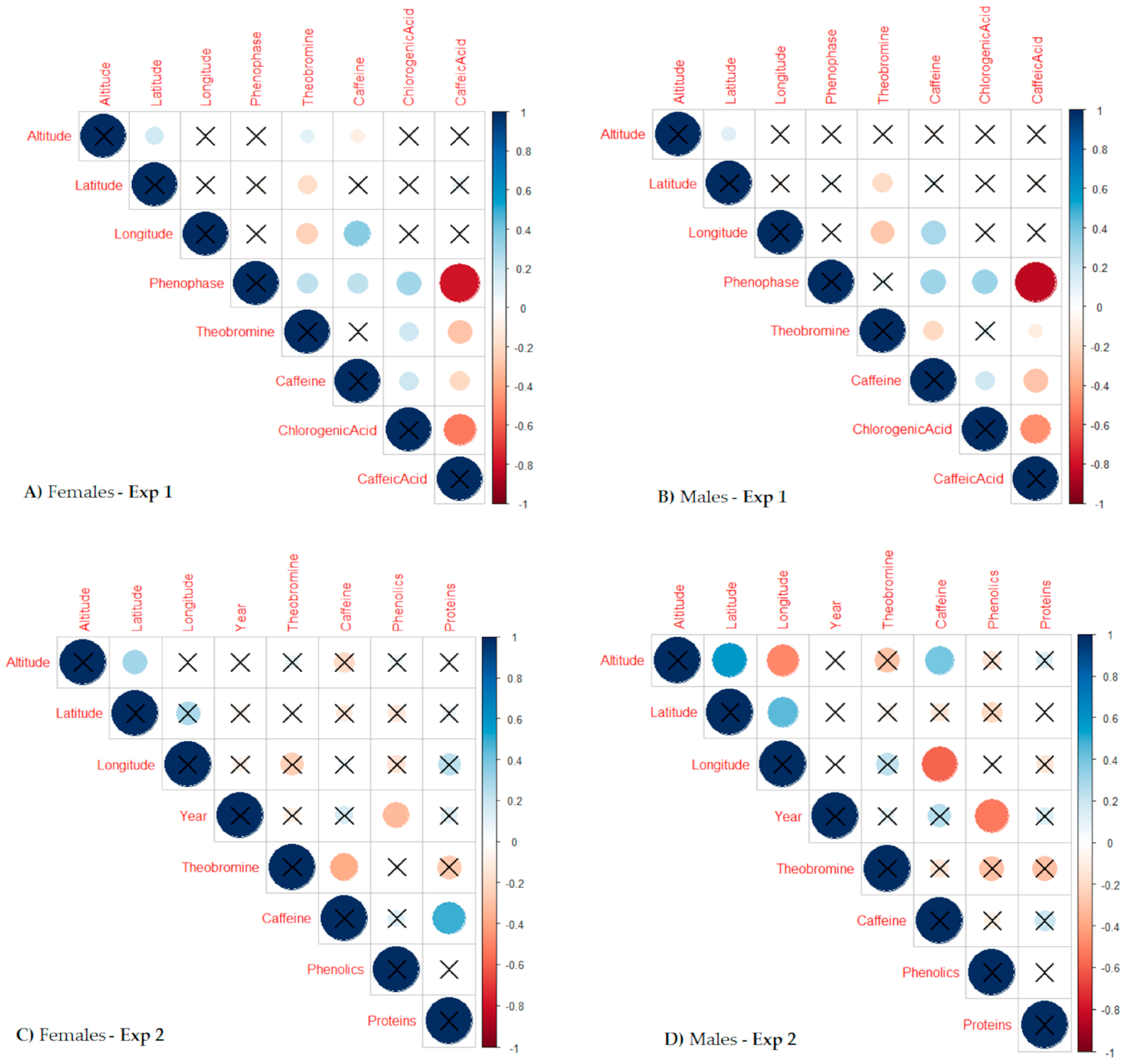
2.3. Metabolite Correlations with Geographical Elements of the Yerba Mate Origin, Leaf Harvest Phenophase, and Plant Age
3. Discussion
4. Materials and Methods
4.1. Experimental Field, Provenances, and Sequence of Harvests in Experiments 1 and 2
4.2. Metabolite Determination
4.3. Experimental Design and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ashihara, H.; Crozier, A. Caffeine: A well known but little mentioned compound in plant science. Trends Plant Sci. 2001, 6, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Herai, Y.; Koizumi, N.; Kusano, T.; Sano, H. 7-methylxanthine methyltransferase of coffee plants: Gene isolation and enzymatic properties. J. Biol. Chem. 2001, 276, 8213–8218. [Google Scholar] [CrossRef]
- Ashihara, H.; Kato, M.; Crozier, A. Distribution, Biosynthesis and Catabolism of Methylxanthines in Plants. Handb. Exp. Pharmacol. 2011, 200, 11–31. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of gallic acid on gut health: Focus on the gut microbiome, immune response, and mechanisms of action. Front. Immunol. 2020, 10, 580208. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef]
- Moreira, E.A.; Pilon, A.C.; Andrade, L.E.; Lopes, N.P. New perspectives on chlorogenic acid accumulation in harvested leaf tissue: Impact on traditional medicine preparations. ACS Omega 2018, 3, 18380–18386. [Google Scholar] [CrossRef]
- Heck, C.I.; Schmalko, M.; de Mejia, E.G. Effect of Growing and Drying Conditions on the Phenolic Composition of Mate Teas (Ilex paraguariensis). J. Agric. Food Chem. 2008, 56, 8394–8403. [Google Scholar] [CrossRef]
- Aseel, D.G.; Rashad, Y.M.; Hammad, S.M. Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against tomato mosaic virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, I.; Kariyat, R. The multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef]
- Fiorentini, R.; Galoppini, C. The proteins from leaves. Plant Foods Hum. Nutr. 1983, 32, 335–350. [Google Scholar] [CrossRef]
- Becker, E. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Tenorio, A.T.; Kyriakopoulou, K.E.; Suarez-Garcia, E.; van den Berg, C.; van der Goot, A.J. Understanding differences in protein fractionation from conventional crops, and herbaceous and aquatic biomass—Consequences for industrial use. Trends Food Sci. Technol. 2018, 71, 235–245. [Google Scholar] [CrossRef]
- van Wijk, K.J.; Kessler, F. Plastoglobuli: Plastid microcompartments with integrated functions in metabolism, plastid developmental transitions, and environmental adaptation. Annu. Rev. Plant Biol. 2017, 68, 253–289. [Google Scholar] [CrossRef] [PubMed]
- Eichelmann, H.; Laisk, A. Ribulose-1,5-bisphosphate carboxylase/oxygenase content, assimilatory charge, and mesophyll conductance in leaves. Plant Physiol. 1999, 119, 179–190. [Google Scholar] [CrossRef]
- Portis, A.R., Jr.; Parry, M.A.J. Discoveries in Rubisco (Ribulose 1,5-bisphosphate carboxylase/oxygenase): A historical perspective. Photosynth. Res. 2007, 94, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pan, L.; Guo, W.; Li, Y.; Wang, W. A convergent mechanism of sex determination in dioecious plants: Distinct sex-determining genes display converged regulation on floral B-class genes. Front. Plant Sci. 2022, 13, 953445. [Google Scholar] [CrossRef]
- Razumova, O.V.; Alexandrov, O.S.; Bone, K.D.; Karlov, G.I.; Divashuk, M.G. Sex chromosomes and sex determination in dioecious agricultural plants. Agronomy 2023, 13, 540. [Google Scholar] [CrossRef]
- Barrett, S.C.H.; Hough, J. Sexual dimorphism in flowering plants. J. Exp. Bot. 2013, 64, 67–82. [Google Scholar] [CrossRef]
- Álvarez-Cansino, L.; Barradas, M.C.D.; Zunzunegui, M.; Esquivias, M.P.; Dawson, T.E. Gender-specific variation in physiology in the dioecious shrub Corema album throughout its distributional range. Funct. Plant Biol. 2012, 39, 968–978. [Google Scholar] [CrossRef]
- Rakocevic, M.; Maia, A.H.N.; Duarte, M.M.; Wendling, I. Secondary sexual dimorphism in biomass production of Ilex para-guariensis progenies associated to their provenances and morphotypes. Exp. Agric. 2023, 59, e3. [Google Scholar] [CrossRef]
- Palumbo, M.J.; Putz, F.E.; Talcott, S.T. Nitrogen fertilizer and gender effects on the secondary metabolism of yaupon, a caffeine-containing North American holly. Oecologia 2007, 151, 1–9. [Google Scholar] [CrossRef]
- Hallé, F.; Oldeman, R.A.A.; Tomlinson, P.B. Tropical Trees and Forests—An Architectural Analysis; Springer: Berlin, Germany, 1978; 441p. [Google Scholar]
- Matsunaga, F.T.; Rakocevic, M.; Brancher, J.D. Modeling the 3D structure and rhythmic growth responses to environment in dioecious yerba-mate. Ecol. Model. 2014, 290, 34–44. [Google Scholar] [CrossRef]
- Guédon, Y.; Costes, E.; Rakocevic, M. Modulation of the yerba-mate metamer production phenology by the cultivation system and the climatic factors. Ecol. Model. 2018, 384, 188–197. [Google Scholar] [CrossRef]
- Cardozo, E.L., Jr.; Ferrarese-Filho, O.; Filho, L.C.; Ferrarese, M.D.L.L.; Donaduzzi, C.M.; Sturion, J.A. Methylxanthines and phenolic compounds in mate (Ilex paraguariensis St. Hil.) progenies grown in Brazil. J. Food Compos. Anal. 2007, 20, 553–558. [Google Scholar] [CrossRef]
- Gawron-Gzella, A.; Chanaj-Kaczmarek, J.; Cielecka-Piontek, J. Yerba mate—A long but current history. Nutrients 2021, 13, 3706. [Google Scholar] [CrossRef]
- Penteado, J.F., Jr.; Goulart, I.C.G.R. Erva 20: Sistema de Produção Para Erva Mate; Embrapa: Brasília, DF, Brazil, 2019; Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1106677 (accessed on 15 March 2023).
- Obeso, J.R. A hierarchical perspective in allocation to reproduction from whole plant to fruit and seed level. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 217–225. [Google Scholar] [CrossRef]
- Rakocevic, M.; Medrado, M.J.S.; Martim, S.F.; Assad, E.D. Sexual dimorphism and seasonal changes of leaf gas exchange in the dioecious tree Ilex paraguariensis grown in two contrasted cultivation types. Ann. Appl. Biol. 2009, 154, 291–301. [Google Scholar] [CrossRef]
- Rakocevic, M.; Costes, E.; Assad, E. Structural and physiological sexual dimorphism estimated from three-dimensional virtual trees of yerba-mate (Ilex paraguariensis) is modified by cultivation environment. Ann. Appl. Biol. 2011, 159, 178–191. [Google Scholar] [CrossRef]
- Rakocevic, M.; Martim, S.F. Time series in analysis of yerba-mate biennial growth modified by environment. Int. J. Biometeorol. 2011, 55, 161–171. [Google Scholar] [CrossRef]
- Rakocevic, M.; Batista, E.R.; Matsunaga, F.T.; Wendling, I.; Marcheafave, G.G.; Bruns, R.E.; Scarminio, I.S.; Ribeiro, R.V. Canopy architecture and diurnal CO2 uptake in male and female clones of yerba-mate cultivated in monoculture and agroforestry. Ann. Appl. Biol. 2023; in review. [Google Scholar]
- Mazzafera, P. Caffeine, theobromine, and theophylline distribution in Ilex paraguariensis. Rev. Bras. Fisiol. Veg. 1994, 6, 149–151. [Google Scholar]
- Rakocevic, M.; Janssens, M.; Scherer, R. Light responses and gender issues in the domestication process of yerba-mate, a sub-tropical evergreen. In Evergreens: Types, Ecology and Conservation; Bezerra, A.D., Ferreira, T.S., Eds.; Nova Science Publicher: New York, NY, USA, 2012; pp. 63–95. [Google Scholar]
- Dartora, N.; Souza, N.M.; Santana-Filho, A.P.; Iacomini, M.; Valduga, A.T.; Gorin, P.A.J.; Sassaki, G.L. UPLC-PDA–MS evaluation of bioactive compounds from leaves of Ilex paraguariensis with different growth conditions, treatments and ageing. Food Chem. 2011, 129, 1453–1461. [Google Scholar] [CrossRef]
- Pauli, E.D.; Scheel, G.L.; Delaroza, F.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S. Photodiode array chromatographic-spectrophotometric metabolite quantification for yerba-mate plant sexual dimorphism differentiation. Microchem. J. 2019, 151, 104218. [Google Scholar] [CrossRef]
- Tormena, C.D.; Pauli, E.D.; Marcheafave, G.G.; Scheel, G.L.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S. FT-IR biomarkers of sexual dimorphism in yerba-mate plants: Seasonal and light accessibility effects. Microchem. J. 2020, 158, 105329. [Google Scholar] [CrossRef]
- Freddo, N.; Beux, M.T.; Anziliero, E.B.; Peruffo, R.G.; Borges, A.S.; Ribeiro, J.D.A. Benefits of yerba mate reach the cosmetics. BJHR 2023, 6, 4835–4842. [Google Scholar] [CrossRef]
- DeMelo, T.O.; Marques, F.; Wendling, I.; Kopka, J.; Erban, A.; Hansel, F. Compostos Presentes em Extrato Metanólico de Tecido Foliar de Erva Mate, por Meio da Cromatografia Gasosa Acoplada à Espectrometria de Massas; Embrapa Florestas: Colombo, PR, Brazil, 2020; Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1125791 (accessed on 15 March 2023).
- Clifford, M.N.; Ramirez-Martinez, J.R. Chlorogenic acids and purine alkaloids contents of Maté (Ilex paraguariensis) leaf and beverage. Food Chem. 1990, 35, 13–21. [Google Scholar] [CrossRef]
- Duarte, M.M.; Gabira, M.M.; Tomasi, J.D.C.; Amano, E.; Nogueira, A.C.; Wendling, I. Bioactive compounds and leaf anatomy of yerba mate morphotypes. Pesqui. Agropecuária Bras. 2022, 57, e02441. [Google Scholar] [CrossRef]
- Rakocevic, M.; Medrado, M.J.S.; Lavoranti, O.J.; Valduga, A.T. Quality of yerba-mate leaves originating from male and female plants. Pesq. Flor. Bras. 2007, 54, 71–83. Available online: https://pfb.cnpf.embrapa.br/pfb/index.php/pfb/article/view/131 (accessed on 15 March 2023).
- Duarte, M.M.; Tomasi, J.C.; Helm, C.V.; Amano, E.; Lazzarotto, M.; De Godoy, R.C.B.; Nogueira, A.C.; Wendling, I. Caffeinated and decaffeinated mate tea: Effect of toasting on bioactive compounds and consumer acceptance. Rev. Bras. Cienc. Agrar. 2020, 5, e8513. [Google Scholar] [CrossRef]
- Rakocevic, M.; Medrado, M.J.S.; Lucambio, F.; Valduga, A.T. Intensity of bitterness of processed yerba mate leaves originated in two contrasted light environments. Braz. Arch. Biol. Technol. 2008, 51, 569–579. [Google Scholar] [CrossRef]
- Tomasi, J.d.C.; de Lima, G.G.; Duarte, M.M.; de Godoy, R.C.B.; Wendling, I.; Helm, C.V.; Hansel, F.A.; Grunennvaldt, R.L.; Tomazzoli, M.M.; Deschamps, C. Toasted yerba mate: Impact of drying methods on bioactive compounds, antioxidant capacity, and mate tea consumer acceptance. Int. J. Food Sci. Technol. 2021, 45, e15944. [Google Scholar] [CrossRef]
- Park, Y.K.; Paredes-Guzman, J.F.; Aguiar, C.L.; Alencar, S.M.; Fujiwara, F.Y. Chemical constituents in Baccharis dracunculifolia as the main botanical origin of Southeastern Brazilian propolis. J. Agric. Food Chem. 2004, 52, 1100–1103. [Google Scholar] [CrossRef]
- Ogita, S.; Uefuji, H.; Morimoto, M.; Sano, H. Application of RNAi to confirm theobromine as the major intermediate for caffeine biosynthesis in coffee plants with potential for construction of decaffeinated varieties. Plant Mol. Biol. 2004, 54, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Marx, F.; Janssens, M.; Urfer, P.; Scherer, R. Caffeine and theobromine composition of mate (Ilex paraguariensis) leaves in five plantations of Misiones, Argentina. Plant Foods Hum. Nutr. 2003, 58, 1–8. [Google Scholar] [CrossRef]
- Rabska, M.; Pers-Kamczyc, E.; Żytkowiak, R.; Adamczyk, D.; Iszkuło, G. Sexual dimorphism in the chemical composition of male and female in the dioecious tree, Juniperus communis L., growing under different nutritional conditions. Int. J. Mol. Sci. 2020, 21, 8094. [Google Scholar] [CrossRef]
- Paul, C.; Chakraborty, K.; Bhattacharjee, A.; Debnath, B. Gender-specific differences in micro-morphology, secondary metabolites, antioxidant and antimicrobial activity of wild Dioscorea species. Vegetos 2022, 35, 698–706. [Google Scholar] [CrossRef]
- McDowell, S.C.L.; McDowell, N.G.; Marshall, J.D.; Hultine, K. Carbon and nitrogen allocation to male and female reproduction in rocky mountain Douglas-fir (Pseudotsuga menziesii var. glauca, Pinaceae). Am. J. Bot. 2000, 87, 539–546. [Google Scholar] [CrossRef]
- DeSoto, L.; Olano, J.M.; Rozas, V. Secondary growth and carbohydrate storage patterns differ between sexes in Juniperus thurifera. Front. Plant Sci. 2016, 7, 723. [Google Scholar] [CrossRef]
- Khan, A.; Wang, Z.; Xu, K.; Li, L.; He, L.; Hu, H.; Wang, G. Validation of an enzyme-driven model explaining photosynthetic Rate Responses to Limited Nitrogen in Crop Plants. Front. Plant Sci. 2020, 25, 533341. [Google Scholar] [CrossRef]
- Aguilera, P.M.; Debat, H.J.; Castrillo, M.L.; Bich, G.A.; Grabiele, M. The DNAJ gene family in yerba mate (Ilex paraguariensis): Genome-wide identification, structural characterization, orthology based classification and expression analysis. Rodriguésia 2023, 74, e00492022. [Google Scholar] [CrossRef]
- Dziedzic, K.; Szopa, A.; Waligórski, P.; Ekiert, H.; Ślesak, H. Sex-related differences in the dioecious species Rumex thyrsiflorus Fingerh: Analysis of the content of phenolic constituents in leaf extracts. Acta Biol. Crac. Ser. Bot. 2020, 62, 43–50. [Google Scholar] [CrossRef]
- Struiving, S.; Hacke, A.C.M.; Simionatto, E.L.; Scharf, D.R.; Klimaczewski, C.V.; Besten, M.A.; Heiden, G.; Boligon, A.A.; Rocha, J.B.T.; Vellosa, J.C.R.; et al. Effects of gender and geographical origin on the chemical composition and antiradical activity of Baccharis myriocephala and Baccharis trimera. Foods 2020, 9, 1433. [Google Scholar] [CrossRef]
- He, Y.; Zhu, Z.; Guo, Q.; Xia, Z. Sex-specific interactions affect foliar defense compound accumulation and resistance to her-bivores in Populus cathayana. Sci. Total Environ. 2021, 774, 145819. [Google Scholar] [CrossRef]
- Massei, G.; Watkins, R.; Hartley, S. Sex-related growth and secondary compounds in Juniperus oxycedrus macrocarpa. Acta Oecologica 2006, 29, 135–140. [Google Scholar] [CrossRef]
- Alvares, A.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. MMeteorol. Z. 2014, 22, 711–728. [Google Scholar] [CrossRef]
- EMBRAPA. Sistema Brasileiro de Classificação de Solos, 3rd ed.; Embrapa Solos Rio de Janeiro: Brasília, DF, Brazil, 2013; 306p. [Google Scholar]
- Wendling, I.; Sturion, J.A.; Stuepp, C.A.; Reis, C.A.F.; Ramalho, M.A.P.; de Resende, M.D.V. Early selection and classification of yerba mate progênies. Pesqui. Agropecuária Bras. 2018, 53, 279–286. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybidic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaiphersburg, MD, USA, 2005. [Google Scholar]
- SAS Institute Inc. SAS/STAT® User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2020. [Google Scholar]
- Blanca, M.J.; Alarcón, R.; Arnau, J.; Bono, R.; Bendayan, R. Non-normal data: Is ANOVA still a valid option? Psicothema 2017, 29, 552–557. [Google Scholar] [CrossRef]
- Davis, C. Encyclopedia of Research Design; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2010. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 15 March 2023).

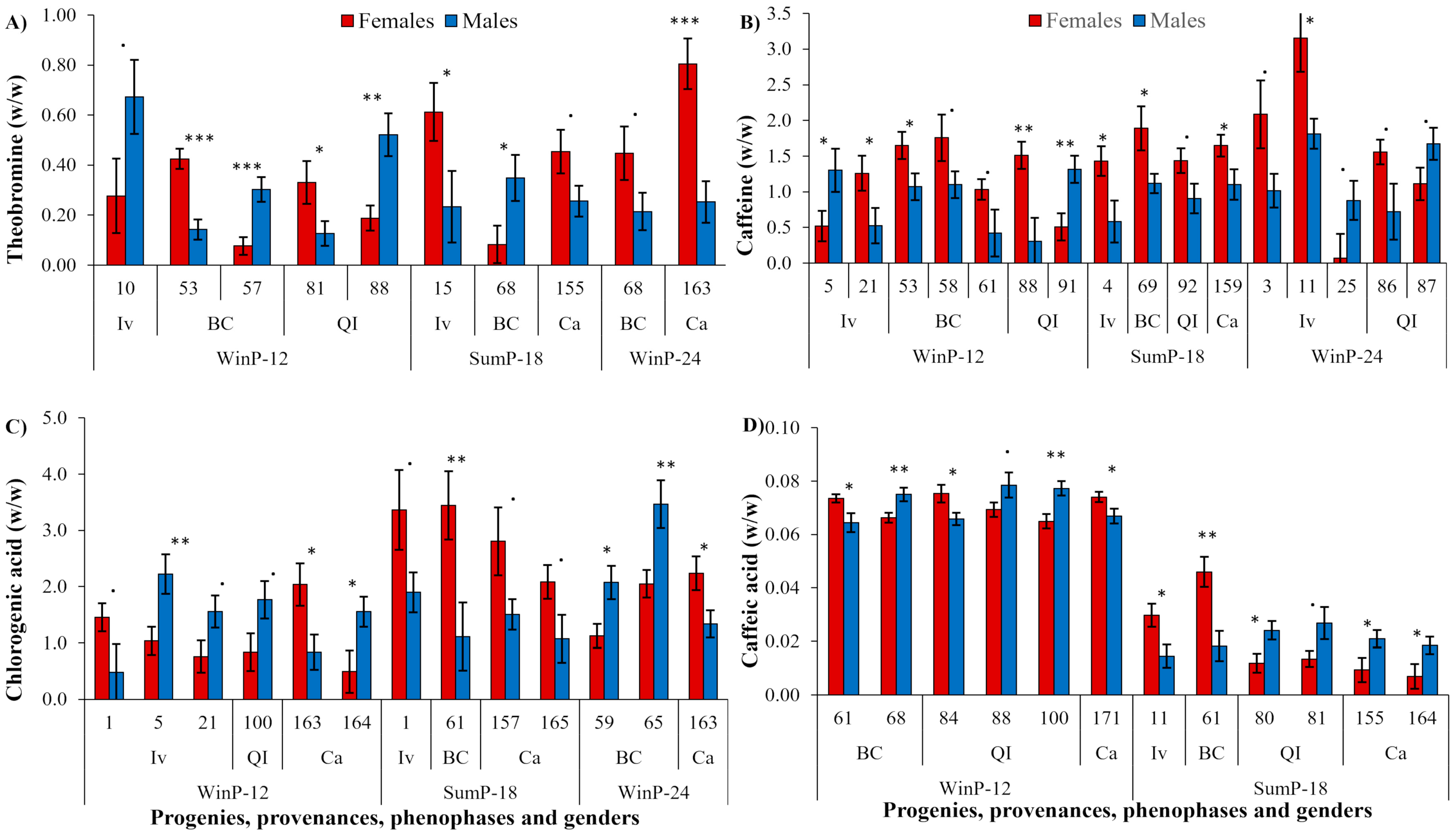


| Estimated Means ± SE Corresponding to Each Provenance | |||||
|---|---|---|---|---|---|
| Metabolite | Phenophase | Ivaí | Barão de Cotegipe | Quedas do Iguaçú | Cascavel |
| Theobromine (%, w/w) | WinP-12 | 0.33 ± 0.02 a | 0.12 ± 0.02 b | 0.16 ± 0.02 b | 0.16 ± 0.02 b |
| SumP-18 | 0.35 ± 0.02 a | 0.23 ± 0.02 a | 0.22 ± 0.02 a | 0.23 ± 0.02 a | |
| WinP-24 | 0.34 ± 0.02 a | 0.20 ± 0.02 a | 0.23 ± 0.02 a | 0.27 ± 0.02 a | |
| Caffeine (%, w/w) | WinP-12 | 0.70 ± 0.06 c | 1.02 ± 0.06 b | 1.18 ± 0.06 b | 1.13 ± 0.06 b |
| SumP-18 | 0.96 ± 0.06 b | 1.30 ± 0.06 a | 1.35 ± 0.06 a | 1.35 ± 0.06 a | |
| WinP-24 | 1.11 ± 0.06 a | 1.31 ± 0.06 a | 1.35 ± 0.06 a | 1.45 ± 0.06 a | |
| Chlorogenic acid (%, w/w) | WinP-12 | 1.09 ± 0.08 c | 1.09 ± 0.08 c | 1.10 ± 0.08 c | 1.13 ± 0.08 c |
| SumP-18 | 2.44 ± 0.08 a | 2.45 ± 0.08 a | 2.60 ± 0.08 a | 2.40 ± 0.08 a | |
| WinP-24 | 1.60 ± 0.08 b | 1.80 ± 0.08 b | 1.66 ± 0.08 b | 1.79 ± 0.09 b | |
| Caffeic acid (%, w/w) | WinP-12 | 0.068 ± 0.001 a | 0.067 ± 0.001 a | 0.068 ± 0.001 a | 0.068 ± 0.001 a |
| SumP-18 | 0.012 ± 0.001 b | 0.013 ± 0.001 b | 0.012 ± 0.001 b | 0.012 ± 0.001 b | |
| WinP-24 | 0.012 ± 0.001 b | 0.015 ± 0.001 b | 0.014 ± 0.001 b | 0.012 ± 0.001 b | |
| Factor | Metabolite | Factor Class | % of Progeny with ‘YES’ Cases | Fisher’s Exact Test p-Value * |
|---|---|---|---|---|
| (A) SSD expression | Theobromine | Winter pause | 9.68 | 0.7513 |
| Summer pause | 6.82 | |||
| Caffeine | Winter pause | 18.09 | 0.4547 | |
| Summer pause | 11.36 | |||
| Chlorogenic acid | Winter pause | 13.83 | 0.7913 | |
| Summer pause | 11.36 | |||
| Caffeic acid | Winter pause | 7.45 | 0.3475 | |
| Summer pause | 13.64 | |||
| (B) Mean male over mean female superiority | Theobromine | WinP-12 | 60.00 | 0.7143 |
| SumP-18 | 33.33 | |||
| WinP-24 | 00.00 | |||
| Caffeine | WinP-12 | 28.57 | 0.6154 | |
| SumP-18 | 0.00 | |||
| WinP-24 | 40.00 | |||
| Chlorogenic acid | WinP-12 | 66.67 | ||
| SumP-18 | 0.00 | 0.1072 | ||
| WinP-24 | 66.67 | |||
| WinP-12 | 50.00 | |||
| Caffeic acid | SumP-18 | 66.67 | 1.0000 |
| Metabolite | Provenance | Overall Model (a) | Gender-Specific Model (b) | |||
|---|---|---|---|---|---|---|
| Parameter Estimate | p-Value (c) | Gender | Parameter Estimate | p-Value | ||
| Theobromine | Iv | −0.04 ± 0.03 | 0.1459 | F | −0.04 ± 0.03 | 0.2138 |
| M | −0.04 ± 0.04 | 0.3991 | ||||
| BC | −0.02 ± 0.03 | 0.4987 | F | −0.01 ± 0.05 | 0.8537 | |
| M | −0.03 ± 0.03 | 0.2899 | ||||
| QI | −0.01 ± 0.04 | 0.7485 | F | −0.03 ± 0.01 | 0.0165 | |
| M | 0.01 ± 0.09 | 0.8967 | ||||
| Ca | −0.02 ± 0.06 | 0.6904 | F | −0.04 ± 0.08 | 0.6018 | |
| M | 0.04 ± 0.04 | 0.3680 | ||||
| Caffeine | Iv | 0.19 ± 0.13 | 0.1499 | F | 0.28 ± 0.21 | 0.1850 |
| M | 0.13 ± 0.18 | 0.4706 | ||||
| BC | 0.17 ± 0.08 | 0.0493 | F | 0.17 ± 0.15 | 0.2594 | |
| M | 0.17 ± 0.10 | 0.1104 | ||||
| QI | 0.06 ± 0.08 | 0.4465 | F | 0.11 ± 0.09 | 0.2556 | |
| M | 0.00 ± 0.15 | 0.9943 | ||||
| Ca | 0.07 ± 0.16 | 0.6618 | F | 0.17 ± 0.21 | 0.4202 | |
| M | −0.10 ± 0.19 | 0.7319 | ||||
| Phenolics | Iv | −0.58 ± 0.12 | <0.0001 | F | −0.41 ± 0.19 | 0.0450 |
| M | −0.75 ± 0.15 | <0.0001 | ||||
| BC | −0.33 ± 0.15 | 0.0308 | F | −0.27 ± 0.21 | 0.2126 | |
| M | −0.37 ± 0.21 | 0.0904 | ||||
| QI | −0.40 ± 0.14 | 0.0084 | F | −0.55 ± 0.20 | 0.0142 | |
| M | −0.23 ± 0.18 | 0.2422 | ||||
| Ca | −0.20 ± 0.12 | 0.1155 | F | −0.22 ± 0.17 | 0.2180 | |
| M | −0.16 ± 0.09 | 0.1426 | ||||
| Proteins | Iv | 0.45 ± 0.32 | 0.1623 | F | 0.55 ± 0.46 | 0.2423 |
| M | 0.39 ± 0.45 | 0.3932 | ||||
| BC | 0.37 ± 0.33 | 0.2598 | F | 0.47 ± 0.56 | 0.4195 | |
| M | 0.31 ± 0.41 | 0.4615 | ||||
| QI | 0.13 ± 0.31 | 0.6877 | F | −0.17 ± 0.34 | 0.6307 | |
| M | 0.55 ± 0.58 | 0.3591 | ||||
| Ca | 0.06 ± 0.35 | 0.8694 | F | −0.07 ± 0.44 | 0.8775 | |
| M | 0.23 ± 0.56 | 0.6986 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakocevic, M.; Maia, A.d.H.N.; de Liz, M.V.; Imoski, R.; Helm, C.V.; Cardozo Junior, E.L.; Wendling, I. Stability of Leaf Yerba Mate (Ilex paraguariensis) Metabolite Concentrations over the Time from the Prism of Secondary Sexual Dimorphism. Plants 2023, 12, 2199. https://doi.org/10.3390/plants12112199
Rakocevic M, Maia AdHN, de Liz MV, Imoski R, Helm CV, Cardozo Junior EL, Wendling I. Stability of Leaf Yerba Mate (Ilex paraguariensis) Metabolite Concentrations over the Time from the Prism of Secondary Sexual Dimorphism. Plants. 2023; 12(11):2199. https://doi.org/10.3390/plants12112199
Chicago/Turabian StyleRakocevic, Miroslava, Aline de Holanda Nunes Maia, Marcus Vinicius de Liz, Rafaela Imoski, Cristiane Vieira Helm, Euclides Lara Cardozo Junior, and Ivar Wendling. 2023. "Stability of Leaf Yerba Mate (Ilex paraguariensis) Metabolite Concentrations over the Time from the Prism of Secondary Sexual Dimorphism" Plants 12, no. 11: 2199. https://doi.org/10.3390/plants12112199
APA StyleRakocevic, M., Maia, A. d. H. N., de Liz, M. V., Imoski, R., Helm, C. V., Cardozo Junior, E. L., & Wendling, I. (2023). Stability of Leaf Yerba Mate (Ilex paraguariensis) Metabolite Concentrations over the Time from the Prism of Secondary Sexual Dimorphism. Plants, 12(11), 2199. https://doi.org/10.3390/plants12112199