During Water Stress, Fertility Modulated by ROS Scavengers Abundant in Arabidopsis Pistils
Abstract
1. Introduction
2. Results
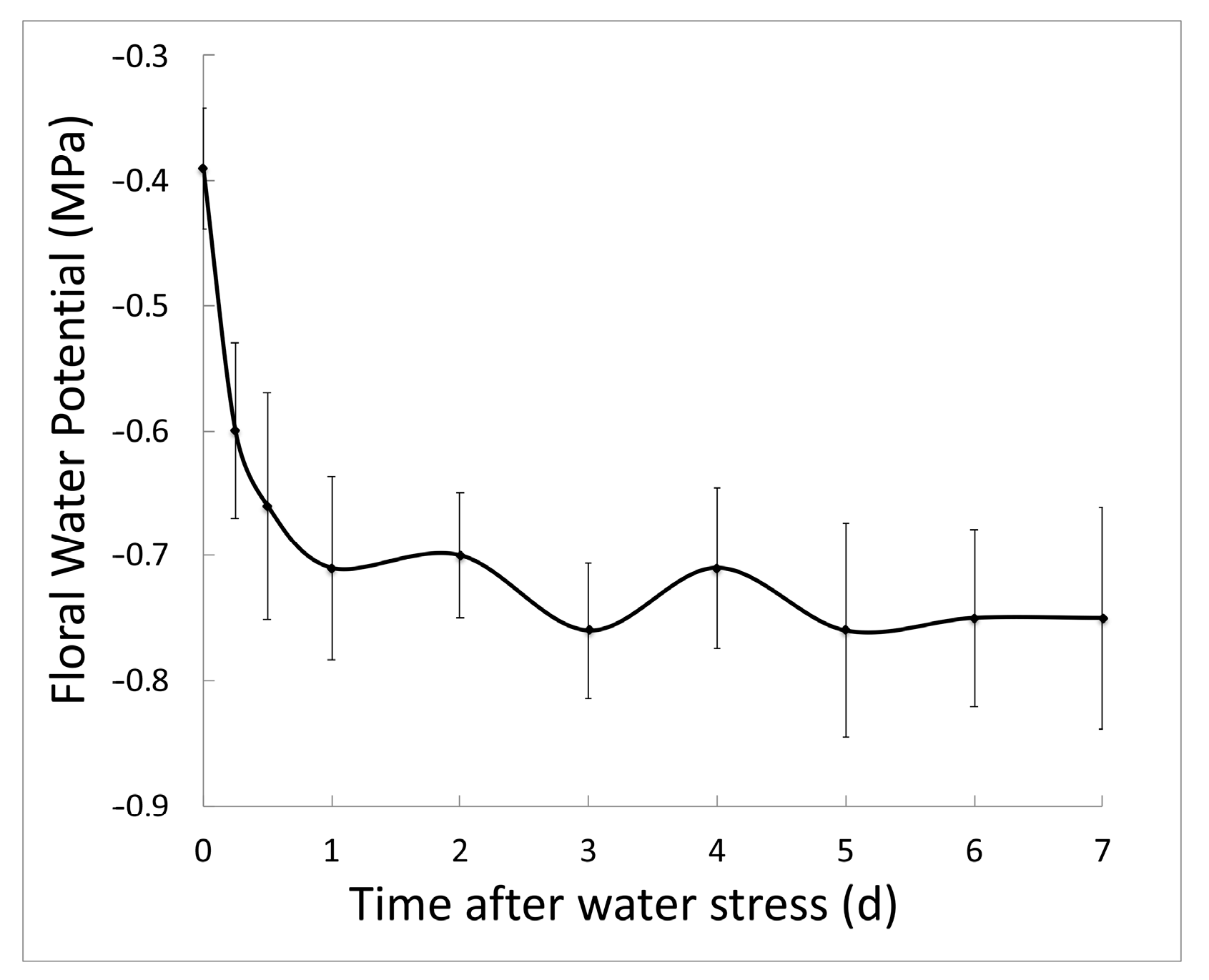
2.1. Salt Stress Lowers Floral Water Potential
2.2. Increased Ovule Abortion Rate in Peroxidase Mutants
2.3. Fertilization Rates and Seed Failure
2.4. H2O2 Levels in Pistils
2.5. Myc-Tagged Peroxidases Exhibited Peroxidase Activity
2.6. Expression and Sub-Cellular Localization of Peroxidases
2.7. Promoter Activity of Peroxidases in Reproductive Tissues
3. Discussion
3.1. Peroxidase Mutants and Fertility
3.2. Stress, Photosynthesis, and ROS Scavengers
3.3. Peroxidases Affecting Fertility
3.4. ROS, Fertility, and PCD
4. Materials and Methods
4.1. Plant Growth and Fertility Measurements
4.2. RNA Extraction and PCR
4.3. Metabolite Assays
4.4. Constructs and Plant Transformants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittler, R.; Vanderwera, S.; Golery, M.; Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Williams, C.E.; Nemacheck, J.A.; Wang, H.; Subramanyam, S.; Zheng, C.; Chen, M.-S. Reactive oxygen species are involved in plant defense against a gall midge. Plant Physiol. 2010, 152, 985–999. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Dat, J.F.; Pellinen, R.; Beeckman, T.; Van De Cotte, B.; Langebartels, C.; Kangasjarvi, J.; Inze, D.; Breusegem, F. Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J. 2003, 33, 621–632. [Google Scholar] [CrossRef]
- Prasad, T.K.; Anderson, M.D.; Martin, B.A.; Stewart, C.R. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 1994, 6, 65–74. [Google Scholar] [CrossRef]
- Bashandy, T.; Guilleminot, J.; Vernoux, T.; Caparros-Ruiz, D.; Ljung, K.; Meyer, Y.; Reichheld, J.P. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 2010, 22, 376–391. [Google Scholar] [CrossRef]
- Iglesias, M.J.; Terrile, M.C.; Bartoli, C.G.; D’Ippólito, S.; Casalongué, C.A. Auxin signaling participates in the adaptative response against oxidative stress and salinity by interacting with redox metabolism in Arabidopsis. Plant Molec. Biol. 2010, 74, 215–222. [Google Scholar] [CrossRef]
- Jabs, T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem. Pharm. 1999, 57, 231–245. [Google Scholar] [CrossRef]
- Pennell, R.I.; Lamb, C. Programmed cell death in plants. Plant Cell 1997, 9, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; van Montagu, M.; Inze, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Vandenabeele, S.; Vanderauwera, S.; Vuylsteke, M.; Tombauts, S.; Langebartels, C.; Seidlitz, H.K.; Zabeau, M.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Catalase deficiency drastically affects high light-induced gene expression in Arabidopsis thaliana. Plant J. 2004, 39, 45–58. [Google Scholar] [CrossRef]
- Foyer, C.H.; Souriau, N.; Perret, S.; Lelandais, M.; Kunert, K.J.; Pruvost, C.; Jouanin, L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol. 1995, 109, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 49, 249–279. [Google Scholar] [CrossRef]
- Narendra, D.; Venkataramani, S.; Shen, G.; Wang, J.; Pasapula, V.; Lin, Y.; Kornyeyev, D.; Holaday, A.S.; Zhang, H. The Arabidopsis ascorbate peroxidase 3 is a peroxisomal membrane-bound antioxidant enzyme and is dispensable for Arabidopsis growth and development. J. Exp. Bot. 2006, 57, 3033–3042. [Google Scholar] [CrossRef]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A family of class III plant peroxidases. Plant Cell Physiol. 2001, 42, 462–468. [Google Scholar] [CrossRef]
- Tognolli, M.; Penel, C.; Greppin, H.; Simon, P. Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene 2002, 288, 129–138. [Google Scholar] [CrossRef]
- Cosio, C.; Dunand, C. Specific functions of individual class III peroxidase genes. J. Exp. Bot. 2009, 60, 391–408. [Google Scholar] [CrossRef]
- Welinder, K.G.; Justesen, A.F.; Kjaersgard, I.V.H.; Jensen, R.B.; Rasmussen, S.K.; Jespersen, H.M.; Duroux, L. Structural diversity and transcription of class III peroxidases from Arabidopsis thaliana. Eur. J. Biochem. 2002, 269, 6063–6081. [Google Scholar] [CrossRef]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Lam, E. Controlled cell death, plant survival and development. Nat. Rev. Mol. Cell Biol. 2004, 5, 305–315. [Google Scholar] [CrossRef]
- Gechev, T.S.; van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Wojtaszek, P. Mechanisms for the generation of reactive oxygen species in plant defence—Broad perspective. Physiol. Mol. Plant Pathol. 2004, 51, 347–366. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2004, 51, 463–499. [Google Scholar] [CrossRef]
- Sun, K.; Hunt, K.; Hauser, B.A. Ovule abortion in Arabidopsis triggered by stress. Plant Physiol. 2004, 135, 2358–2367. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Yang, Y.L.; Wu, H.; Wang, D.; Liu, J.Q. Involvement of hydrogen peroxide and nitric oxide in salt resistance in the calluses from Populus euphratica. Plant Cell Environ. 2007, 30, 775–785. [Google Scholar] [CrossRef]
- Sun, K.; Cui, Y.; Hauser, B.A. Environmental stress alters gene expression and induces ovule abortion: Reactive oxygen species appear as ovules commit to abort. Planta 2005, 222, 632–642. [Google Scholar] [CrossRef]
- Schneitz, K.; Hulskamp, M.; Pruitt, R.E. Wild-type ovule development in Arabidopsis thaliana—A light microscope study of cleared whole-mount tissue. Plant J. 1995, 7, 731–749. [Google Scholar] [CrossRef]
- Seltman, H.J. Two-way ANOVA. In Experimental Design and Analysis; Carnegie Mellon Press: Pittsburgh, PA, USA, 2012; pp. 267–292. [Google Scholar]
- Neuberger, G.; Maurer-Stroh, S.; Eisenhaber, B.; Hartig, A.; Eisenhaber, F. Prediction of peroxisomal targeting signal 1 containing proteins from amino acid sequence. J. Mol. Biol. 2003, 328, 581–592. [Google Scholar] [CrossRef]
- Winter, D.; Vinegar, B.; Nahal, H.; Ammar, R.; Wilson, G.V.; Provart, N.J. An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2007, 2, e718. [Google Scholar] [CrossRef]
- Hauser, B.A.; Sun, K.; Oppenheimer, D.G.; Sage, T. Changes in mitochondrial membrane potential and accumulation of reactive oxygen species precede ultrastructural changes during ovule abortion. Planta 2006, 223, 292–299. [Google Scholar] [CrossRef]
- Alonso, J.M.; Stepanova, A.N.; Leisse, T.J. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef]
- Wagner, A. Distributed robustness versus redundancy as causes of mutational robustness. BioEssays 2005, 27, 176–188. [Google Scholar] [CrossRef]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hecker, A.G.; Hauser, B.A. The APX4 locus regulates seed vigor and seedling growth in Arabidopsis thaliana. Planta 2014, 239, 909–919. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, C.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Botany 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Myouga, F.; Hosoda, C.; Umezawa, T.; Iizumi, H.; Kuromori, T.; Motohashi, R.; Shono, Y.; Nagata, N.; Ikeuchi, M.; Shinozaki, K. A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell 2008, 20, 3148–3162. [Google Scholar] [CrossRef]
- Tung, C.W.; Dwyer, K.G.; Nasrallah, M.E.; Nasrallah, J.B. Genome-wide identification of genes expressed in Arabidopsis pistils specifically along the path of pollen tube growth. Plant Physiol. 2005, 138, 977–989. [Google Scholar] [CrossRef]
- Blee, K.A.; Jupe, S.C.; Richard, G.; Davies, D.R.; Bolwell, G.P. Molecular identification and expression of the peroxidase responsible for the oxidative burst in French bean (Phaseolus vulgaris L.) and related members of the gene family. Plant Mol. Biol. 2001, 47, 607–620. [Google Scholar] [CrossRef]
- Liszkay, A.; Kenk, B.; Schopfer, P. Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta 2003, 217, 658–667. [Google Scholar] [CrossRef]
- Mei, W.; Qin, Y.; Song, W.; Li, J.; Zhu, Y. Cotton GhPOX1 encoding plant class III peroxidase may be responsible for the high level of reactive oxygen species production that is related to cotton fiber elongation. J. Genet. Genomics 2009, 36, 141–150. [Google Scholar] [CrossRef]
- Potocky, M.; Jones, M.A.; Bezvoda, R.; Smirnoff, N.; Zarsky, V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007, 174, 742–751. [Google Scholar] [CrossRef]
- Kandasamy, M.K.; Nasrallah, J.B.; Nasrallah, M.E. Pollen-pistil interactions and developmental regulation of pollen tube growth in Arabidopsis. Development 1994, 120, 3405–3418. [Google Scholar] [CrossRef]
- Kámán-Tóth, E.; Dankó, T.; Gullner, G.; Bozsó, Z.; Palkovics, L.; Pogány, M. Contribution of cell wall peroxidase- and NADPH oxidase-derived reactive oxygen species to Alternaria brassicicola-induced oxidative burst in Arabidopsis. Mol. Plant Pathol. 2019, 20, 485–499. [Google Scholar] [CrossRef]
- Escobar-Restrepo, J.M.; Huck, N.; Kessler, S.; Gagliardini, V.; Gheyselinck, J.; Yang, W.-C.; Grossniklaus, U. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 2007, 317, 656–660. [Google Scholar] [CrossRef]
- Völz, R.; Harris, W.; Hirt, H.; Lee, Y.W. ROS homeostasis mediated by MPK4 and SUMM2 determines synergid cell death. Nat. Commun. 2022, 13, 1746–1758. [Google Scholar] [CrossRef]
- Baranova, E.N.; Chaban, I.A.; Kurenina, L.V.; Konovalova, L.N.; Varlamova, N.V.; Khaliluev, M.R.; Gulevich, A.A. Possible role of crystal-bearing cells in tomato fertility and formation of seedless fruits. Int. J. Mol. Sci. 2020, 13, 9480. [Google Scholar] [CrossRef]
- Khaliluev, M.R.; Chaban, I.A.; Kononenko, N.V.; Baranova, E.N.; Dolgov, S.V.; Kharchenko, P.N.; Poliakov, V.I. Abnormal floral meristem development in transgenic tomato plants do not depend on the expression of genes encoding defense-related PR-proteins and antimicrobial peptides. Ontogenez 2014, 45, 28–41. [Google Scholar] [CrossRef]
- Hu, C.H.; Zeng, Q.D.; Tai, L.; Li, B.B.; Zhang, P.P.; Nie, X.M.; Wang, P.Q.; Liu, W.T.; Li, W.Q.; Kang, Z.S.; et al. Interaction between TaNOX7 and TaCDPK13 contributes to plant fertility and drought tolerance by regulating ROS production. J. Agric. Food Chem. 2020, 68, 7333–7347. [Google Scholar] [CrossRef]
- Gibeaut, D.M.; Hulett, J.; Cramer, G.R.; Seemann, J.R. Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol. 1997, 115, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Pratt, L.H.; McCurdy, D.W.; Shimazaki, Y.; Cordonnier, M.-M. Immunodetection of phytochrome: Immunocytochemistry, immunoblotting, and immunoquantitation. In Immunology in Plant Science; Linskens, H.F., Jackson, J.F., Eds.; Springer: New York, NY, USA, 1986; pp. 50–74. [Google Scholar]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: B-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Park, S.O.; Zheng, Z.; Oppenheimer, D.G.; Hauser, B.A. The PRETTY FEW SEEDS2 gene encodes an Arabidopsis homeodomain protein that regulates ovule development. Development 2005, 132, 841–849. [Google Scholar] [CrossRef] [PubMed]





| Abortion Rate (%) | ||
|---|---|---|
| Genotype | Healthy 1 | Stressed 1 |
| Wild type | 12.9 ± 2.0 | 26.9 ± 4.6 |
| per17 | 29.6 ± 5.8 b | 44.6 ± 6.0 a |
| per28 | 23.2 ± 3.3 b | 34.6 ± 4.4 |
| fsd2 | 35.2 ± 6.8 c | 29.0 ± 4.2 |
| apx4 | 21.4 ± 4.2 | 23.2 ± 4.0 |
| Wild type | 18.0 ± 1.7 | 33.4 ± 3.3 |
| per29 | 50.8 ± 3.5 e | 41.6 ± 4.8 |
| Wild type | 14.9 ± 1.3 | 33.1 ± 2.7 |
| per17 per28 | 39.7 ± 6.3 c | 64.0 ± 6.6 e |
| Wild type | 23.9 ± 3.1 | 49.5 ± 4.5 |
| per17 per29 | 35.6 ± 6.0 | 67.7 ± 6.9 b |
| Wild type | 21.6 ± 1.5 | 39.5 ± 3.6 |
| per28 per29 | 33.7 ± 4.0 b | 40.0 ± 2.9 |
| Wild type | 5.4 ± 0.7 | 25.4 ± 4.6 |
| per17 per28 per29 | 31.5 ± 5.2 e | 66.3 ± 5.6 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-Y.; Head, D.J.; Hauser, B.A. During Water Stress, Fertility Modulated by ROS Scavengers Abundant in Arabidopsis Pistils. Plants 2023, 12, 2182. https://doi.org/10.3390/plants12112182
Wang Y-Y, Head DJ, Hauser BA. During Water Stress, Fertility Modulated by ROS Scavengers Abundant in Arabidopsis Pistils. Plants. 2023; 12(11):2182. https://doi.org/10.3390/plants12112182
Chicago/Turabian StyleWang, Ya-Ying, Donald J. Head, and Bernard A. Hauser. 2023. "During Water Stress, Fertility Modulated by ROS Scavengers Abundant in Arabidopsis Pistils" Plants 12, no. 11: 2182. https://doi.org/10.3390/plants12112182
APA StyleWang, Y.-Y., Head, D. J., & Hauser, B. A. (2023). During Water Stress, Fertility Modulated by ROS Scavengers Abundant in Arabidopsis Pistils. Plants, 12(11), 2182. https://doi.org/10.3390/plants12112182











