Humulus lupulus L. Strobilus In Situ Photosynthesis and Respiration Temperature Responses
Abstract
1. Introduction
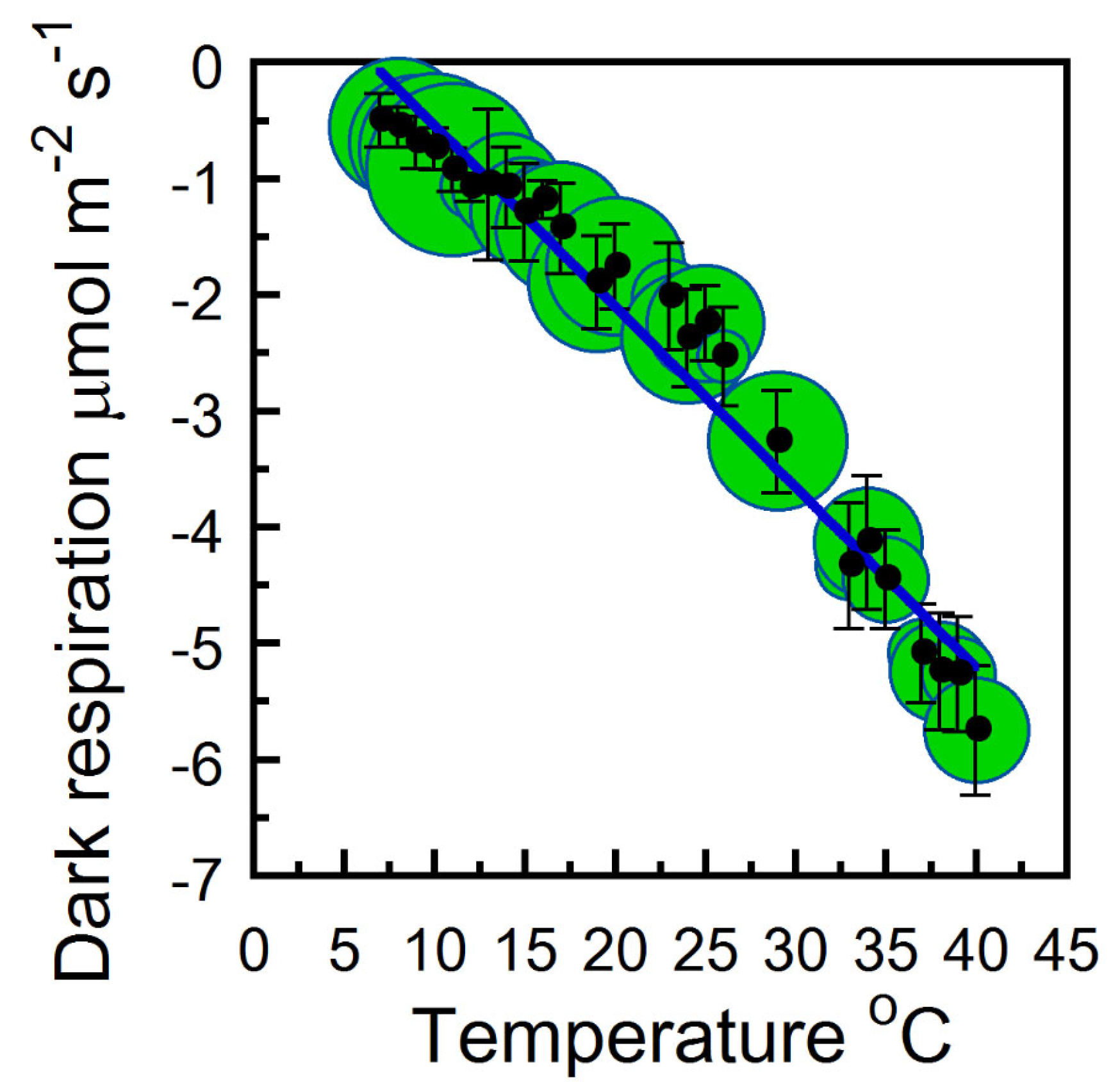
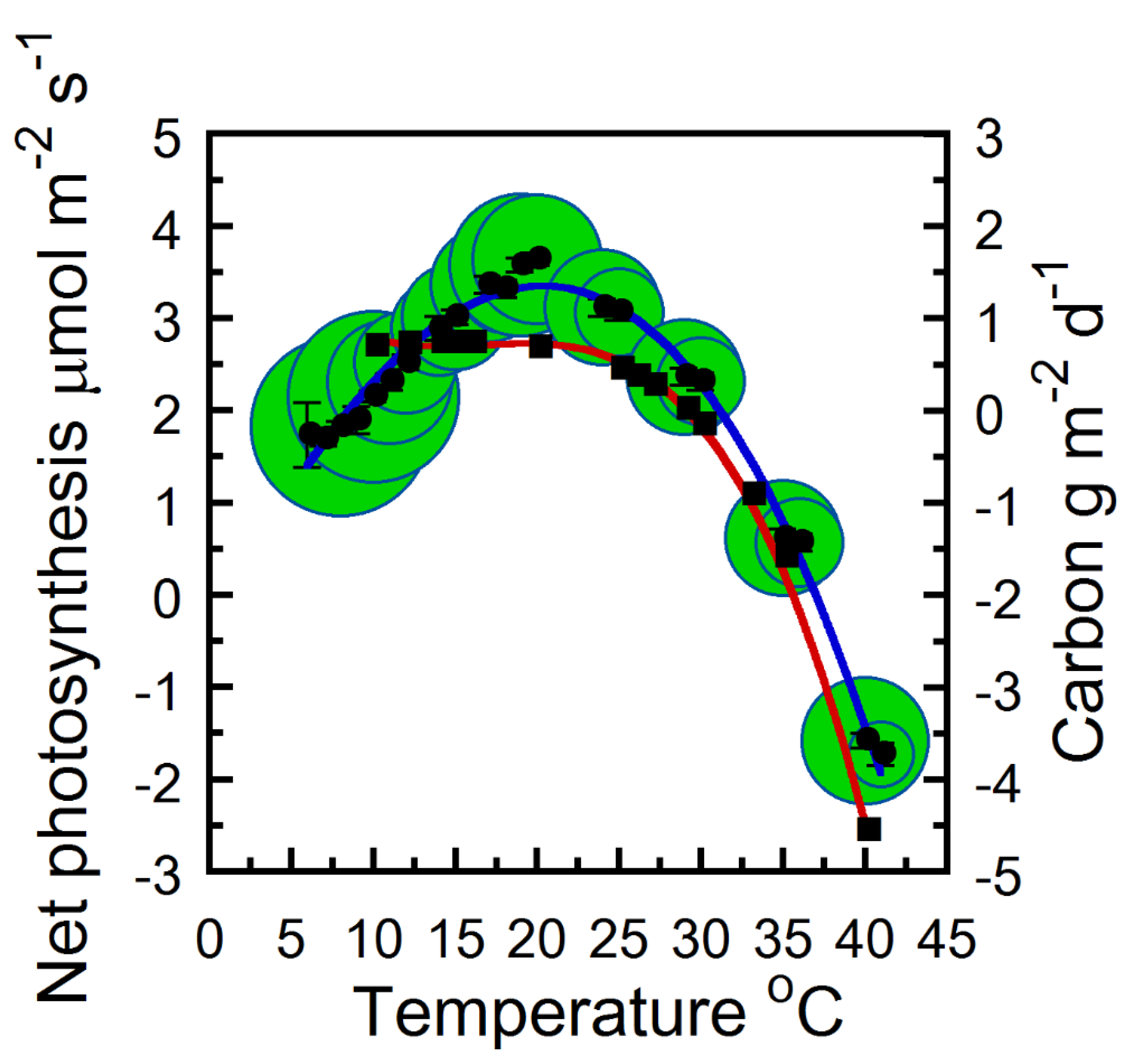
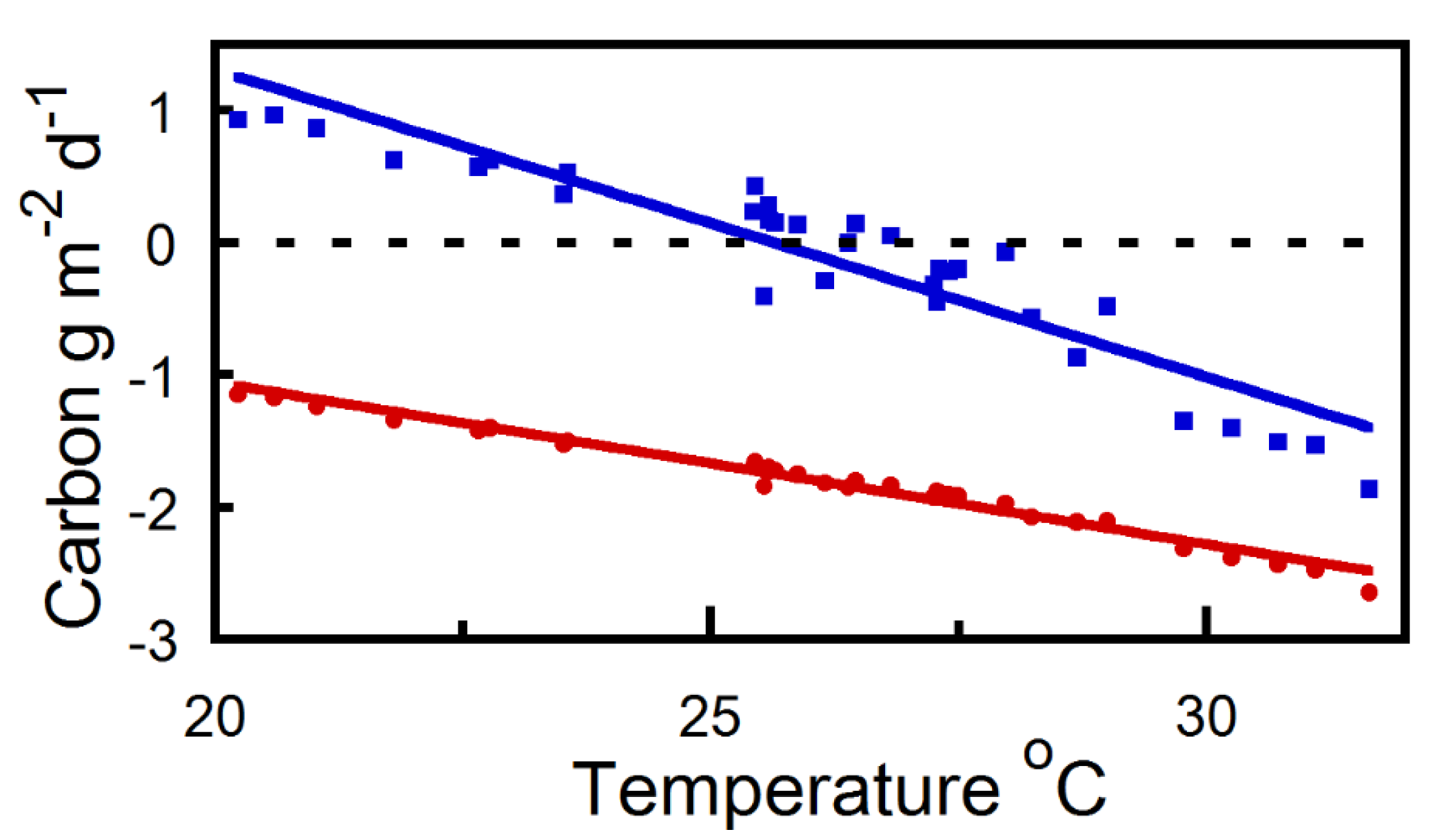
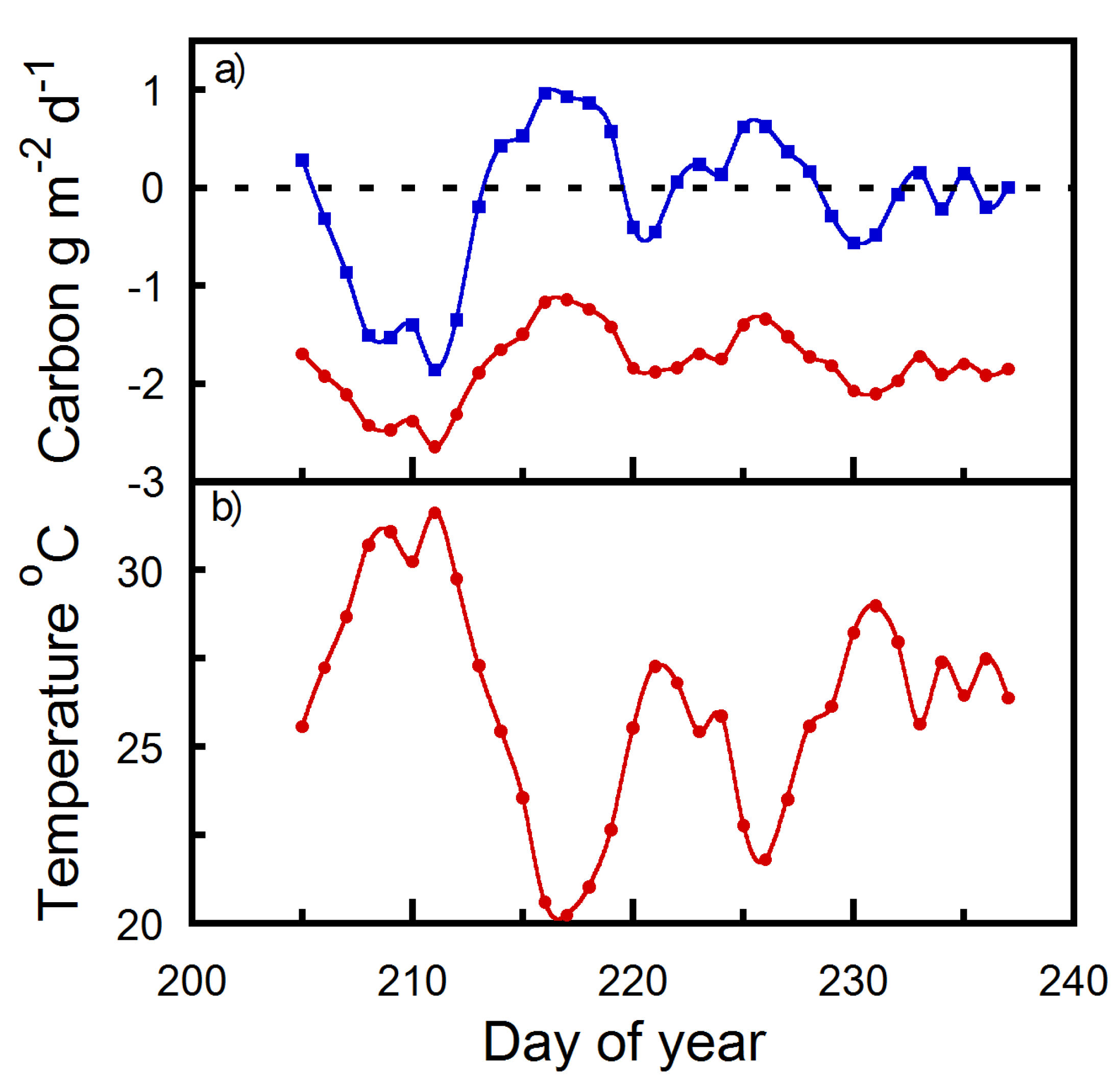
2. Results
2.1. Instantaneous and Daily Strobilus Net Photosynthesis and Respiration Temperature Responses
2.2. Diurnal Strobilus Carbon Balance Estimates
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Environmental Conditions
4.3. Strobili Gas Exchange Measurements
4.4. Diurnal Strobilus Carbon Balance Estimates
4.5. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zattler, F.; Jehl, J. On the influence of atmospheric conditions on yield and quality of the hops in the Hallertau in the period 1926–1961. Hopfen. Rundsch. 1962, 13, 61–64. (In German) [Google Scholar]
- Mozny, M.; Tolasz, R.; Nekovar, J.; Sparks, T.; Trnka, M.; Zalud, Z. The impact of climate change on the yield and quality of Saaz hops in the Czech Republic. Agr. Forest. Meteorol. 2009, 149, 913–919. [Google Scholar] [CrossRef]
- Peat, W.E.; Thomas, G.G. The photosynthetic activity of the developing hop cone. Ann Appl Biol. 1974, 76, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, W.L. Humulus lupulus L. strobilus photosynthetic capacity and carbon assimilation. Plants 2023, 12, 1816. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. Modelling forest growth and carbon storage in response to increasing CO2 and temperature. Tellus 1999, 51B, 871–888. [Google Scholar] [CrossRef]
- Yan-Shih, L.; Medlyn, B.E.; Ellsworth, D.S. Temperature responses of leaf net photosynthesis: The role of component processes. Tree Physiol. 2012, 32, 219–231. [Google Scholar] [CrossRef]
- Dusenge, A.; Duarte, G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Grossman, Y.L.; DeJong, T.M. Carbohydrate requirements for dark respiration by peach vegetative organs. Tree Physiol. 1994, 14, 37–48. [Google Scholar] [CrossRef]
- Neve, R.A. Hops; Chapman and Hall: Edmonds, UK, 1991; pp. 1–20. [Google Scholar]
- Rybaček, V. Hop Production. Development in Crop Science 16; Elsevier Science: Amsterdam, The Netherlands, 1991; pp. 25–120. [Google Scholar]
- Alexander, L.V.; Zhang, X.; Peterson, T.C.; Caesar, J.; Gleason, B.; Tank, A.M.; Haylock, M.; Collins, D.; Trewin, B.; Rahimzadeh, F.; et al. Global observed changes in daily climate extremes of temperature and precipitation. J. Geophys. Res. Atmos. 2006, 111, D05109. [Google Scholar] [CrossRef]
- Dai, A.; Wigley, T.M.L.; Boville, B.A.; Kiehl, J.T.; Buja, L.E. Climates of the twentieth and twenty-first centuries simulated by the NCAR climate system model. J. Clim. 2001, 14, 485–519. [Google Scholar] [CrossRef]
- Sillmann, J.; Kharin, V.V.; Zwiers, F.W.; Zhang, X.; Bronaugh, D. Climate extremes indices in the CMIP5 multi-model ensemble: Part 2. Future climate projections. J. Geophys. Res. Atmos. 2013, 118, 2473–2493. [Google Scholar] [CrossRef]
- Xu, J.; Misra, G.; Sreenivasulu, N.; Henry, A. What happens at night? Physiological mechanisms related to maintaining grain yield under high night temperature in rice. Plant Cell Environ. 2021, 7, 2245–2261. [Google Scholar] [CrossRef]
- Welch, J.R.; Vincent, J.R.; Auffhammer, M.; Moya, P.F.; Dobermann, A.; Dawe, D. Rice yields in tropical/subtropical Asia exhibit large but opposing sensitivities to minimum and maximum temperatures. Proc. Natl. Acad. Sci. USA 2010, 107, 14562–14567. [Google Scholar] [CrossRef]
- Bahuguna, R.N.; Solis, C.A.; Shi, W.; Jagadish, K.S. Post-flowering night respiration and altered sink activity account for high night temperature-induced grain yield and quality loss in rice (Oryza sativa L.). Physiol. Plant. 2017, 159, 59–73. [Google Scholar] [CrossRef] [PubMed]
- García, G.A.; Dreccer, M.F.; Miralles, D.J.; Serrago, R.A. High night temperatures during grain number determination reduce wheat and barley grain yield: A field study. Glob. Chang. Biol. 2015, 21, 4153–4164. [Google Scholar] [CrossRef] [PubMed]
- García, G.A.; Serrago, R.A.; Dreccer, M.F.; Miralles, D.J. Post-anthesis warm nights reduce grain weight in field-grown wheat and barley. Field Crop Res. 2016, 195, 50–59. [Google Scholar] [CrossRef]
- Hein, N.T.; Wagner, D.; Bheemanahalli, R.; Šebela, D.; Bustamante, C.; Chiluwal, A.; Neilsen, M.L.; Jagadish, S.K. Integrating field-based heat tents and cyber-physical system technology to phenotype high nighttime temperature impact on winter wheat. Plant Methods 2019, 15, 41. [Google Scholar] [CrossRef]
- Lesjak, J.; Calderini, D.F. Increased night temperature negatively affects grain yield, biomass and grain number in Chilean quinoa. Front. Plant Sci. 2017, 8, 352. [Google Scholar] [CrossRef]
- Loka, D.A.; Oosterhuis, D.M. Effect of high night temperatures on cotton respiration, ATP levels and carbohydrate content. Environ. Exp. Bot. 2010, 68, 258–263. [Google Scholar] [CrossRef]
- Bauerle, W.L. Intracanopy CO2 and light interactions on Humulus lupulus L. net canopy carbon gain under current and future atmospheric CO2 concentrations. Agric. For. Meteorol. 2021, 310, 108621. [Google Scholar] [CrossRef]
- Eriksen, R.L.; Rutto, L.K.; Dombrowski, J.E.; Henning, J.A. Photosynthetic cativity of six hop (Humulus lupulus L.) cultivars under different temperature treatments. Hort. Sci. 2020, 55, 403–409. [Google Scholar] [CrossRef]
- Cai, C.; Yin, X.; He, S.; Jiang, W.; Si, C.; Struik, P.C.; Luo, W.; Li, G.; Xie, Y.; Xiong, Y.; et al. Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Glob. Chang. Biol. 2016, 22, 856–874. [Google Scholar] [CrossRef]
- Cannell, M.G.R.; Thornley, J.H.M. Modelling the components of plant respiration: Some guiding principles. Ann. Bot. 2000, 85, 45–54. [Google Scholar] [CrossRef]
- Atkin, O.K.; Bruhn, D.; Hurry, V.M.; Tjoelker, M.G. Evans Review No. 2: The hot and the cold: Unravelling the variable response of plant respiration to temperature. Funct. Plant Biol. 2005, 32, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Atkin, O.K.; Scheurwater, I.; Pons, T.L. Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. New Phytol. 2007, 174, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Sadok, W.; Jagadish, S.V.K. The hidden costs of nighttime warming on yields. Trends Plant Sci. 2020, 25, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Amthor, J.S.; Bar-Even, A.; Hanson, A.D.; Millar, A.H.; Stitt, M.; Sweetlove, L.J.; Tyerman, S.D. Engineering strategies to boost crop productivity by cutting respiratory carbon loss. Plant Cell 2019, 31, 297–314. [Google Scholar] [CrossRef]
- Li, G.; Chen, T.; Feng, B.; Peng, S.; Tao, L.; Fu, G. Respiration, rather than photosynthesis, determines rice yield loss under moderate high-temperature conditions. Front. Plant Sci. 2021, 12, 678653. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Kenny, S.T.; Zimmermann, C.E. Registration of Centennial hop. Crop Sci. 1991, 31, 1092–1093. [Google Scholar] [CrossRef]
- Bauerle, W.L. Disentangling photoperiod from hop vernalization and dormancy for global production and speed breeding. Sci. Rep. 2019, 9, 16003. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, W.L. Internode elongation and strobili production of Humulus lupulus cultivars in response to local strain sensing. Sci. Rep. 2021, 11, 9017. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauerle, W.L.; Hazlett, M. Humulus lupulus L. Strobilus In Situ Photosynthesis and Respiration Temperature Responses. Plants 2023, 12, 2030. https://doi.org/10.3390/plants12102030
Bauerle WL, Hazlett M. Humulus lupulus L. Strobilus In Situ Photosynthesis and Respiration Temperature Responses. Plants. 2023; 12(10):2030. https://doi.org/10.3390/plants12102030
Chicago/Turabian StyleBauerle, William L., and Michael Hazlett. 2023. "Humulus lupulus L. Strobilus In Situ Photosynthesis and Respiration Temperature Responses" Plants 12, no. 10: 2030. https://doi.org/10.3390/plants12102030
APA StyleBauerle, W. L., & Hazlett, M. (2023). Humulus lupulus L. Strobilus In Situ Photosynthesis and Respiration Temperature Responses. Plants, 12(10), 2030. https://doi.org/10.3390/plants12102030





