Humulus lupulus L. Strobilus Photosynthetic Capacity and Carbon Assimilation
Abstract
1. Introduction
2. Results
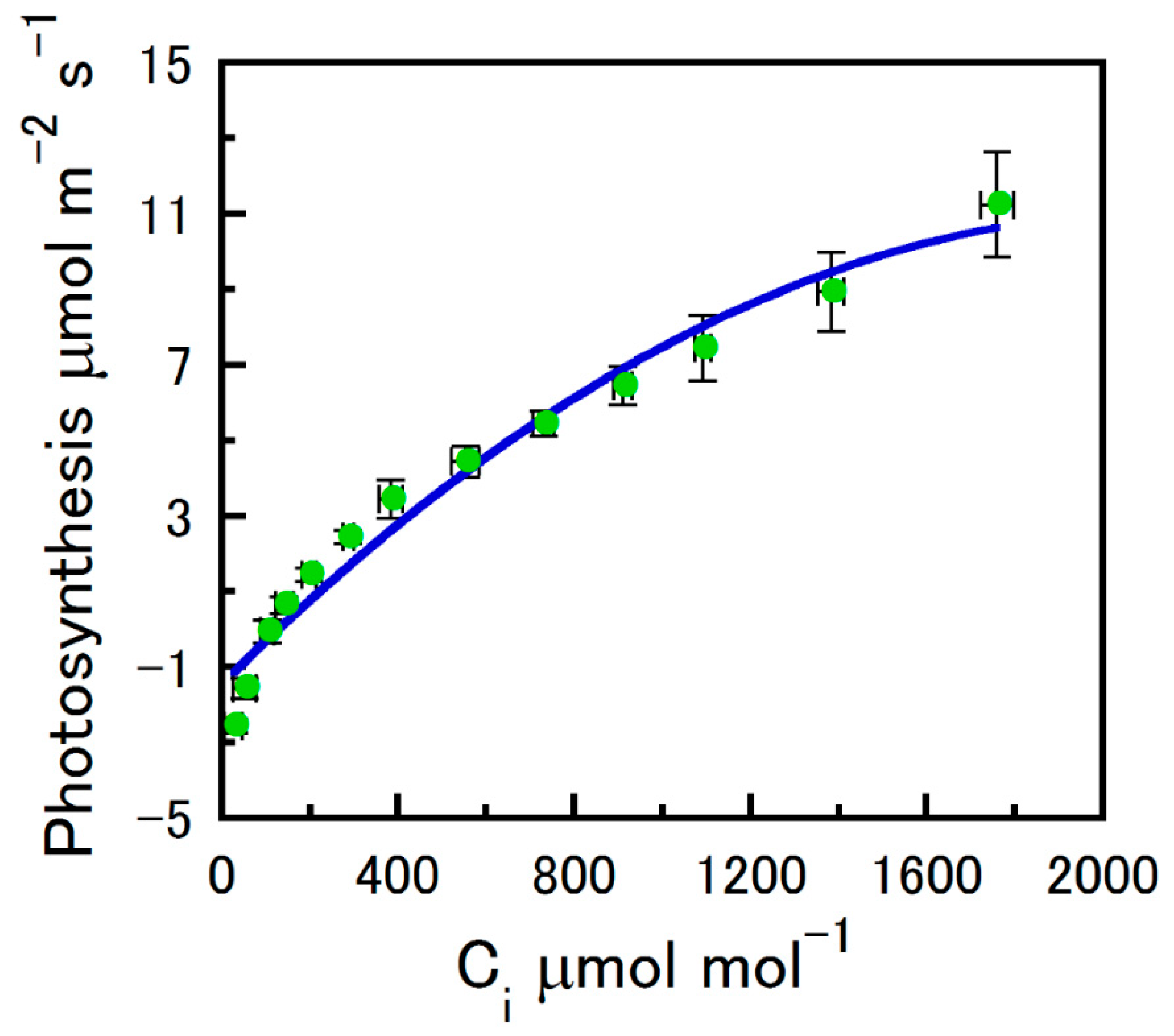
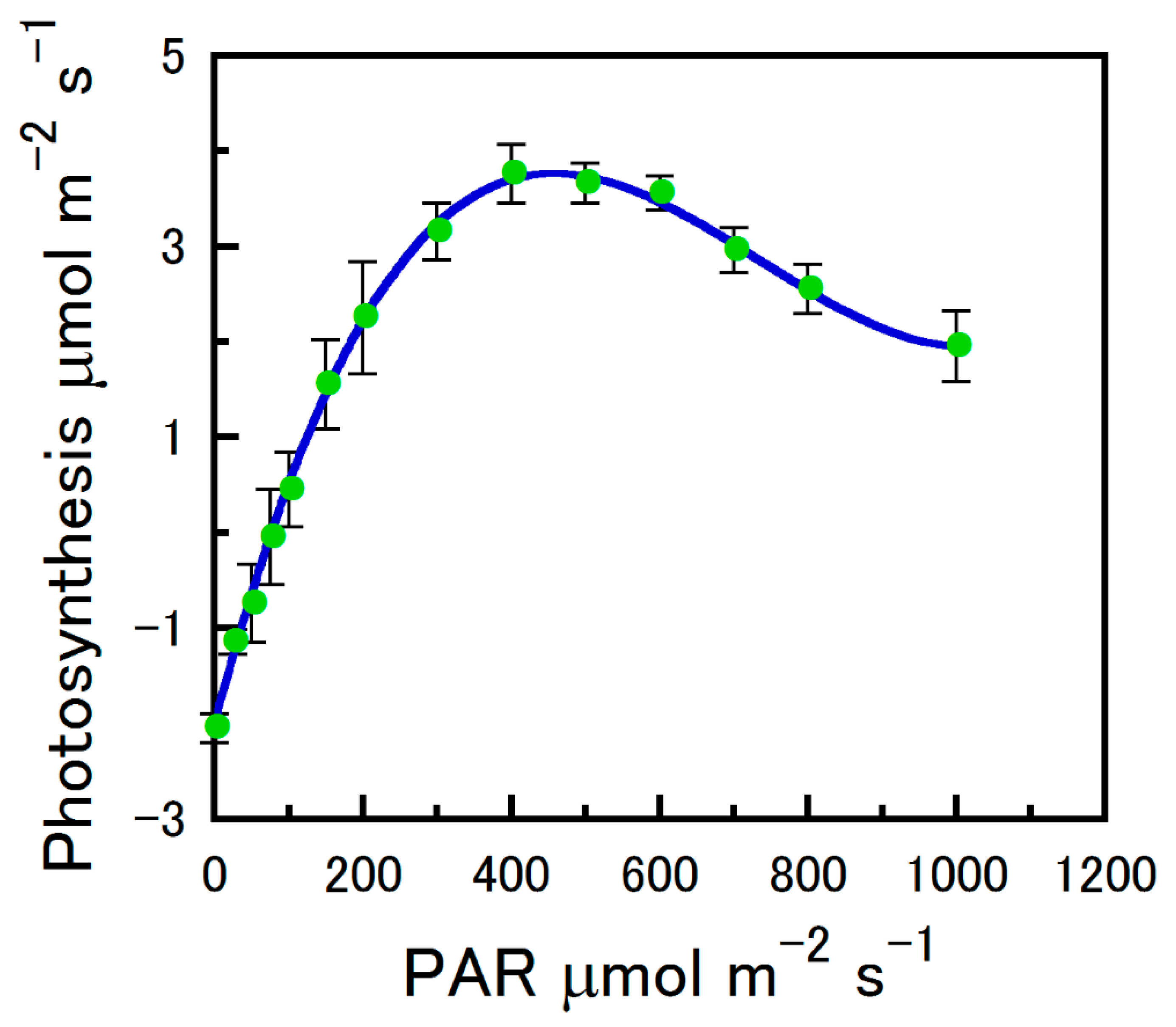
2.1. Strobilus Gas Exchange
Net Photosynthesis Versus CO2 and Photosynthetically Active Radiation
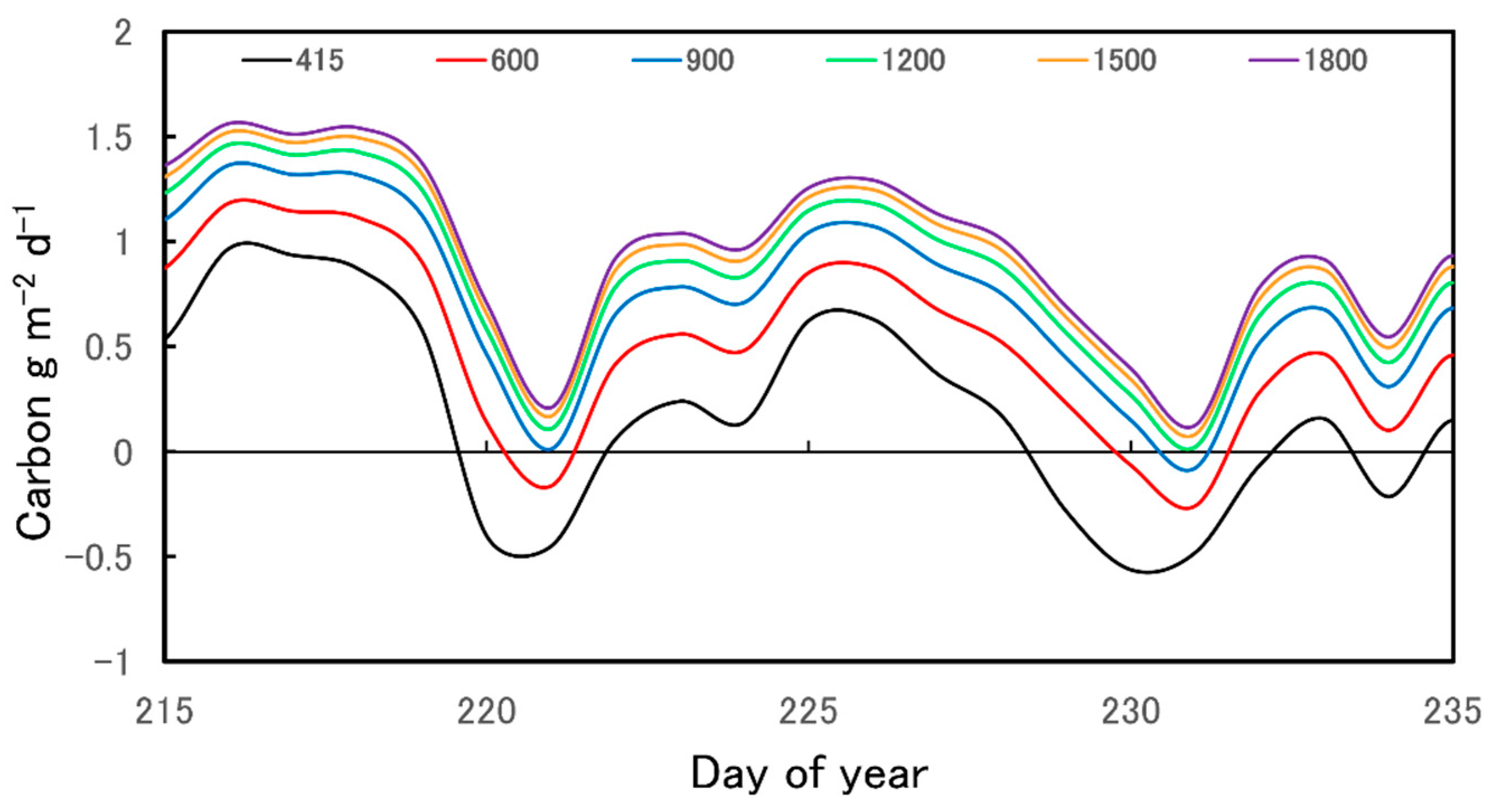
2.2. Diurnal Carbon Balance Estimates
2.3. Leaf Defoliation and Bract Carbon Assimilation Interruption
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Environmental Conditions
4.3. Strobilus Gas Exchange and Light Absorption Measurements
4.4. An/Ci Curve Fitting
4.5. An/PAR Curve Fitting
4.6. Diurnal Strobilus Carbon Balance Estimates
4.7. Bine Defoliation and Bract Photosynthesis Interruption
4.8. Statistical Analysis
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadell, J.G.; Monteiro, P.M.S.; Costa, M.H.; Cotrim da Cunha, L.; Cox, P.M.; Eliseev, A.V.; Henson, S.; Ishii, M.; Jaccard, S.; Koven, C.; et al. Global Carbon and other Biogeochemical Cycles and Feedbacks. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 673–816. [Google Scholar] [CrossRef]
- Brazel, A.J.; Ó’Maoiléidigh, D.S. Photosynthetic activity of reproductive organs. J. Exp. Bot. 2019, 70, 1737–1754. [Google Scholar] [CrossRef] [PubMed]
- Tambussi, E.A.; Bort, J.; Guiamet, J.J.; Nogués, S.; Araus, J.L. The photosynthetic role of ears in C3 cereals: Metabolism, water use efficiency and contribution to grain yield. Crit. Rev. Plant Sci. 2007, 26, 1–6. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Figueroa, M.E.; Davy, A.J. Salt stimulation of growth and photosynthesis in an extreme halophyte, Arthrocnemum macrostachyum. Plant Biol. 2010, 1, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Pengelly, J.J.; Kwasny, S.; Bala, S.; Evans, J.R.; Voznesenskaya, E.V.; Koteyeva, N.K.; Edwards, G.E.; Furbank, R.T.; von Caemmerer, S. Functional analysis of corn husk photosynthesis. Plant Physiol. 2011, 156, 503–513. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Zhang, Y.L.; Luo, H.H.; Li, W.; Oguchi, R.; Fan, D.Y.; Chow, W.S.; Zhang, W.F. Important photosynthetic contribution from the non-foliar green organs in cotton at the late growth stage. Planta 2012, 235, 325–336. [Google Scholar] [CrossRef]
- Neve, R.A. Hops; Chapman and Hall: Edmonds, UK, 1991; pp. 1–20. [Google Scholar]
- Rybaček, V. Hop Production; Development in Crop Science 16; Elsevier Science: Amsterdam, The Netherlands; New York, NY, USA, 1991; pp. 25–120. [Google Scholar]
- Peat, W.E.; Thomas, G.G. The photosynthetic activity of the developing hop cone. Ann. Appl. Biol. 1974, 76, 319–324. [Google Scholar] [CrossRef]
- Han, J.; Lei, Z.; Flexas, J.; Zhang, Y.; Carriquí, M.; Zhang, W.; Zhang, Y. Mesophyll conductance in cotton bracts: Anatomically determined internal CO2 diffusion constraints on photosynthesis. J. Exp. Bot. 2018, 69, 5433–5443. [Google Scholar] [CrossRef]
- Bauerle, W.L. Intracanopy CO2 and light interactions on Humulus lupulus L. net canopy carbon gain under current and future atmospheric CO2 concentrations. Agric. For. Meteorol. 2021, 310, 108621. [Google Scholar] [CrossRef]
- Aschan, G.; Pfanz, H. Non-foliar photosynthesis–a strategy of additional carbon acquisition. Flora-Morphol. Distrib. Funct. Ecol. Plants 2003, 198, 81–97. [Google Scholar] [CrossRef]
- Shi, X.; Bloom, A. Photorespiration: The Futile Cycle? Plants 2021, 10, 908. [Google Scholar] [CrossRef]
- Bellasio, C.; Beerling, D.J.; Griffiths, H. An Excel tool for deriving key photosynthetic parameters from combined gas exchange and chlorophyll fluorescence: Theory and practice. Plant Cell Environ. 2016, 39, 1180–1197. [Google Scholar] [CrossRef]
- Aschan, G.; Pfanz, H.; Vodnik, D.; Batič, F. Photosynthetic performance of vegetative and reproductive structures of green hellebore (Helleborus viridis L. agg.). Photosynthetica 2005, 43, 55–64. [Google Scholar] [CrossRef]
- Gnan, S.; Marsh, T.; Kover, P.X. Inflorescence photosynthetic contribution to fitness releases Arabidopsis thaliana plants from trade-off constraints on early flowering. PLoS ONE 2017, 12, e0185835. [Google Scholar] [CrossRef]
- Andrews, A.K.; Svec, L.V. Photosynthetic activity of soybean pods at different growth stages compared to leaves. Can. J. Plant Sci. 1975, 55, 501–505. [Google Scholar] [CrossRef]
- Luthra, Y.P.; Sheoran, I.S.; Rao, A.S.; Singh, R. Ontogenetic changes in the level of ureides and enzymes of their metabolism in various plant parts of pigeonpea (Cajanus cajan). J. Exp. Bot. 1983, 34, 1358–1370. [Google Scholar] [CrossRef]
- Werk, K.S.; Ehleringer, J.R. Photosynthesis by flowers in Encelia farinosa and Encelia californica (Asteraceae). Oecologia 1983, 57, 311–315. [Google Scholar] [CrossRef]
- Heilmeier, H.; Whale, D.M. Carbon dioxide assimilation in the flowerhead of Arctium. Oecologia 1987, 73, 109–115. [Google Scholar] [CrossRef]
- Smillie, R.M. Calvin cycle activity in fruit and the effect of heat stress. Sci. Hortic. 1992, 51, 83–95. [Google Scholar] [CrossRef]
- Leonardos, E.D.; Rauf, S.A.; Weraduwage, S.M.; Marillia, E.-F.; Taylor, D.C.; Micallef, B.J.; Grodzinski, B. Photosynthetic capacity of the inflorescence is a major contributor to daily-C-gain and the responsiveness of growth to elevated CO2 in Arabidopsis thaliana with repressed expression of mitochondrial-pyruvate-dehydrogenase-kinase. Environ. Exp. Bot. 2014, 107, 84–97. [Google Scholar] [CrossRef]
- Sawicki, M.; Courteaux, B.; Rabenoelina, F.; Baillieul, F.; Clement, C.; Ait Barka, E.; Jacquard, C.; Vaillant-Gaveau, N. Leaf vs. inflorescence: Differences in photosynthetic activity of grapevine. Photosynthetica 2017, 55, 58–68. [Google Scholar] [CrossRef]
- Kong, L.; Sun, M.; Xie, Y.; Wang, F.; Zhao, Z. Photochemical and antioxidative responses of the glume and flag leaf to seasonal senescence in wheat. Front. Plant Sci. 2015, 6, 358. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Griffiths, H. Photosynthesis in reproductive structures: Costs and benefits. J. Exp. Bot. 2015, 66, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Noodén, L.D.; Penney, J.P. Correlative controls of senescence and plant death in Arabidopsis thaliana (Brassicaceae). J. Exp. Bot. 2001, 52, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.M.; Vaitheeswaran, V.; Ambrose, S.J.; Purves, R.W.; Page, J.E. Transcriptome analysis of bitter acid biosynthesis and precursor pathways in hop (Humulus lupulus). BMC Plant Biol. 2013, 13, 12. [Google Scholar] [CrossRef]
- Patzak, J.; Krofta, K.; Henychová, A.; Nesvadba, V. Number and size of lupulin glands, glandular trichomes of hop (Humulus lupulus L.), play a key role in contents of bitter acids and polyphenols in hop cone. Int. J. Food Sci. Technol. 2015, 50, 1864–1872. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, F.; Liu, B.; Huhman, D.V.; Sumner, L.W.; Dixon, R.A.; Wang, G. Characterization of the formation of branched short-chain fatty acid:CoAs for bitter acid biosynthesis in hop glandular trichomes. Mol. Plant 2013, 6, 1301–1317. [Google Scholar] [CrossRef]
- Kong, L.A.; Xie, Y.; Sun, M.Z.; Si, J.S.; Hu, L. Comparison of the photosynthetic characteristics in the pericarp and flag leaves during wheat (Triticum aestivum L.) caryopsis development. Photosynthetica 2016, 54, 40–46. [Google Scholar] [CrossRef]
- Franks, P.J.; W. Doheny-Adams, T.; Britton-Harper, Z.J.; Gray, J.E. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015, 207, 188–195. [Google Scholar] [CrossRef]
- Denny, M.J. Translocation of nutrients and hormones. In Advanced Plant Physiology; Wilkins, M.B., Ed.; Pitman: London, UK, 1984; pp. 277–296. [Google Scholar]
- Hüve, K.; Bichele, I.; Ivanova, H.; Keerberg, O.; Pärnik, T.; Rasulov, B.; Tobias, M.; Niinemets, Ü. Temperature responses of dark respiration in relation to leaf sugar concentration. Physiol. Plant. 2012, 144, 320–334. [Google Scholar] [CrossRef]
- Kenny, S.T.; Zimmermann, C.E. Registration of Centennial hop. Crop Sci. 1991, 31, 1092–1093. [Google Scholar] [CrossRef]
- Bauerle, W.L. Disentangling photoperiod from hop vernalization and dormancy for global production and speed breeding. Sci. Rep. 2019, 9, 16003. [Google Scholar] [CrossRef]
- Bauerle, W.L. Internode elongation and strobili production of Humulus lupulus cultivars in response to local strain sensing. Sci. Rep. 2021, 11, 9017. [Google Scholar] [CrossRef]
- Bauerle, W.L.; Weston, D.J.; Bowden, J.D.; Dudley, J.B.; Toler, J.E. Leaf absorptance of photosynthetically active radiation in relation to chlorophyll meter estimates among woody plant species. Sci. Hortic. 2004, 101, 169–178. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Duursma, R.A. Plantecophys—An R package for analyzing and modelling leaf gas exchange data. PLoS ONE 2015, 10, e0143346. [Google Scholar] [CrossRef]
- Wullschleger, S.D. Biochemical limitations of carbon assimilation in C3 plants—A retrospective analysis of the A/Ci curves of 109 species. J. Exp. Bot. 1993, 44, 907–920. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D.; Berry, J.A. Biochemical model of C3 photosynthesis. In Photosynthesis In Silico: Understanding Complexity from Molecules to Ecosystems; Laisk, A., Nedbal, L., Govindjee, G., Eds.; Spinger Science & Business Media, B.V.: Dordrecht, The Netherlands, 2009; pp. 209–230. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Evans, J.R.; Hudson, G.S.; Andrews, T.J. The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta 1994, 195, 88–97. [Google Scholar] [CrossRef]
- Prioul, J.L.; Chartier, P. Partitioning of transfer and carboxylation components of intercellular resistance to photosynthetic CO2 fixation: A critical analysis of the methods used. Ann. Bot. 1977, 41, 789–800. [Google Scholar] [CrossRef]
- Parsons, R.; Weyers, J.D.B.; Lawson, T.; Godber, I.M. Rapid and straightforward estimates of photosynthetic characteristics using a portable gas exchange system. Photosynthetica 1997, 34, 265–279. [Google Scholar] [CrossRef]
- Sharp, R.E.; Matthews, M.A.; Boyer, J.S. Kok effect and the quantum yield of photosynthesis: Light partially inhibits dark respiration. Plant Physiol. 1984, 75, 95–101. [Google Scholar] [CrossRef]
- Singsaas, E.L.; Ort, D.R.; DeLucia, E.H. Variation in measured values of photosynthetic quantum yield in ecophysiological studies. Oecologia 2001, 128, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Leuning, R.; Kelliher, F.M.; De Pury, D.G.G.; Schulze, E.D. Leaf nitrogen, photosynthesis, conductance and transpiration: Scaling from leaves to canopies. Plant Cell Environ. 1995, 18, 1183–1200. [Google Scholar] [CrossRef]
- Leuning, R. A critical appraisal of a combined stomatal photosynthesis model for C3 plants. Plant Cell Environ. 1995, 18, 339–355. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Duursma, R.A.; Eamus, D.; Ellsworth, D.E.; Prentice, I.C.; Barton, C.V.M.; Crous, K.Y.; De Angelis, P.; Freeman, M.; Wingate, L. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob. Chang. Biol. 2012, 17, 2134–2144. [Google Scholar] [CrossRef]
- Kavalier, A.R.; Litt, A.; Ma, C.; Pitra, N.J.; Coles, M.C.; Kennelly, E.J.; Matthews, P.D. Phytochemical and morphological characterization of hop (Humulus lupulus L.) cones over five developmental stages using high performance liquid chromatography coupled to time-of-flight mass spectrometry, ultrahigh performance liquid chromatography photodiode array detection, and light microscopy techniques. J. Agric. Food Chem. 2011, 59, 4783–4793. [Google Scholar] [CrossRef]


| Parameter | Mean ± SE |
|---|---|
| Rd | −2.1 ± 0.3 |
| Amax | 4.2 ± 0.3 |
| ϕ | 0.033 ± 0.002 |
| Ls | 341.0 ± 16.5 |
| Lc | 76.7 ± 12.6 |
| Vcmax | 14.1 ± 1.1 |
| Γ | 110.3 ± 13.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauerle, W.L. Humulus lupulus L. Strobilus Photosynthetic Capacity and Carbon Assimilation. Plants 2023, 12, 1816. https://doi.org/10.3390/plants12091816
Bauerle WL. Humulus lupulus L. Strobilus Photosynthetic Capacity and Carbon Assimilation. Plants. 2023; 12(9):1816. https://doi.org/10.3390/plants12091816
Chicago/Turabian StyleBauerle, William L. 2023. "Humulus lupulus L. Strobilus Photosynthetic Capacity and Carbon Assimilation" Plants 12, no. 9: 1816. https://doi.org/10.3390/plants12091816
APA StyleBauerle, W. L. (2023). Humulus lupulus L. Strobilus Photosynthetic Capacity and Carbon Assimilation. Plants, 12(9), 1816. https://doi.org/10.3390/plants12091816





