Bryophyte Spores Tolerate High Desiccation Levels and Exposure to Cryogenic Temperatures but Contain Storage Lipids and Chlorophyll: Understanding the Essential Traits Needed for the Creation of Bryophyte Spore Banks
Abstract
1. Introduction
2. Results
2.1. Germination of Bryophyte Spores after Desiccation and Freezing
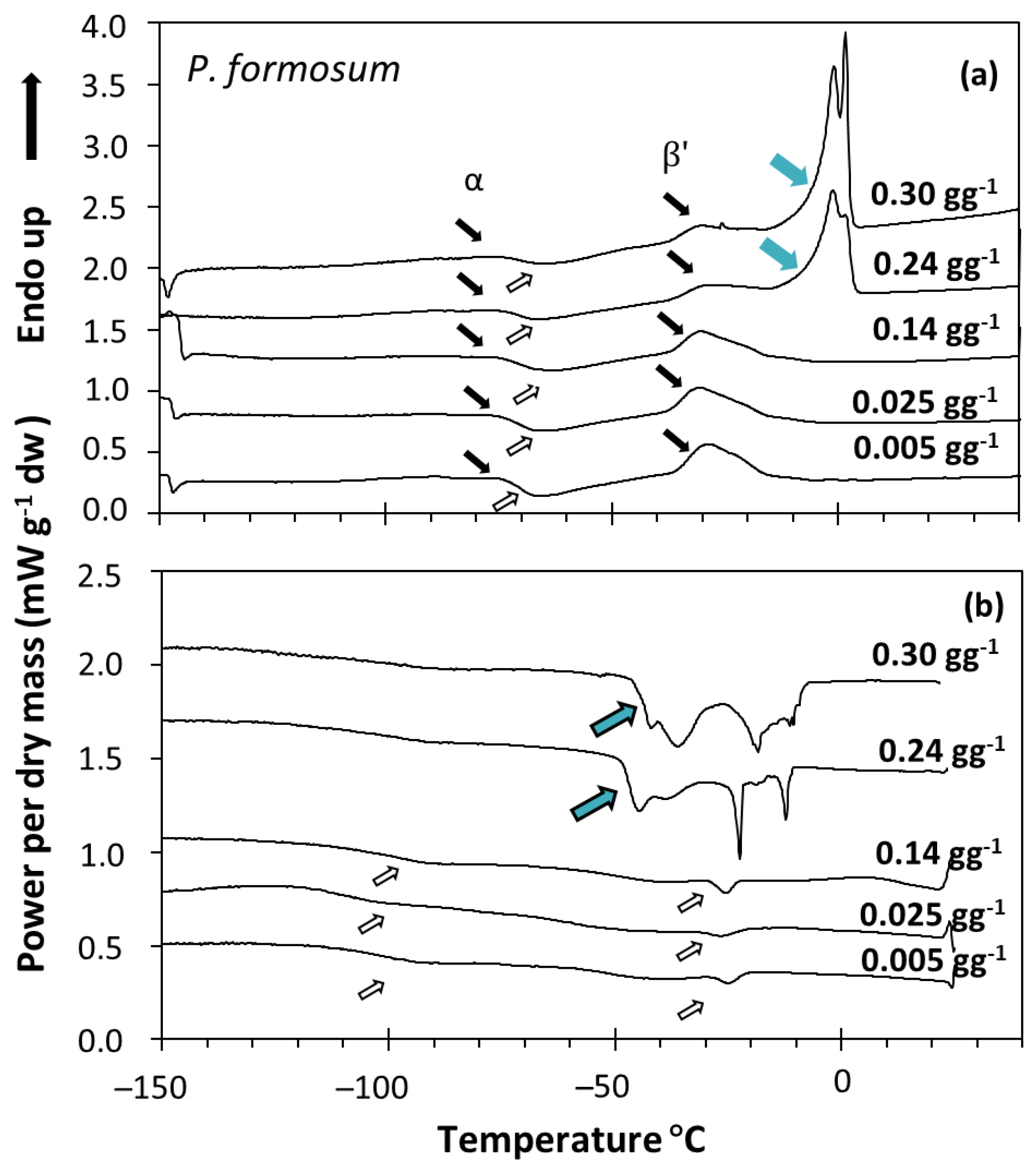
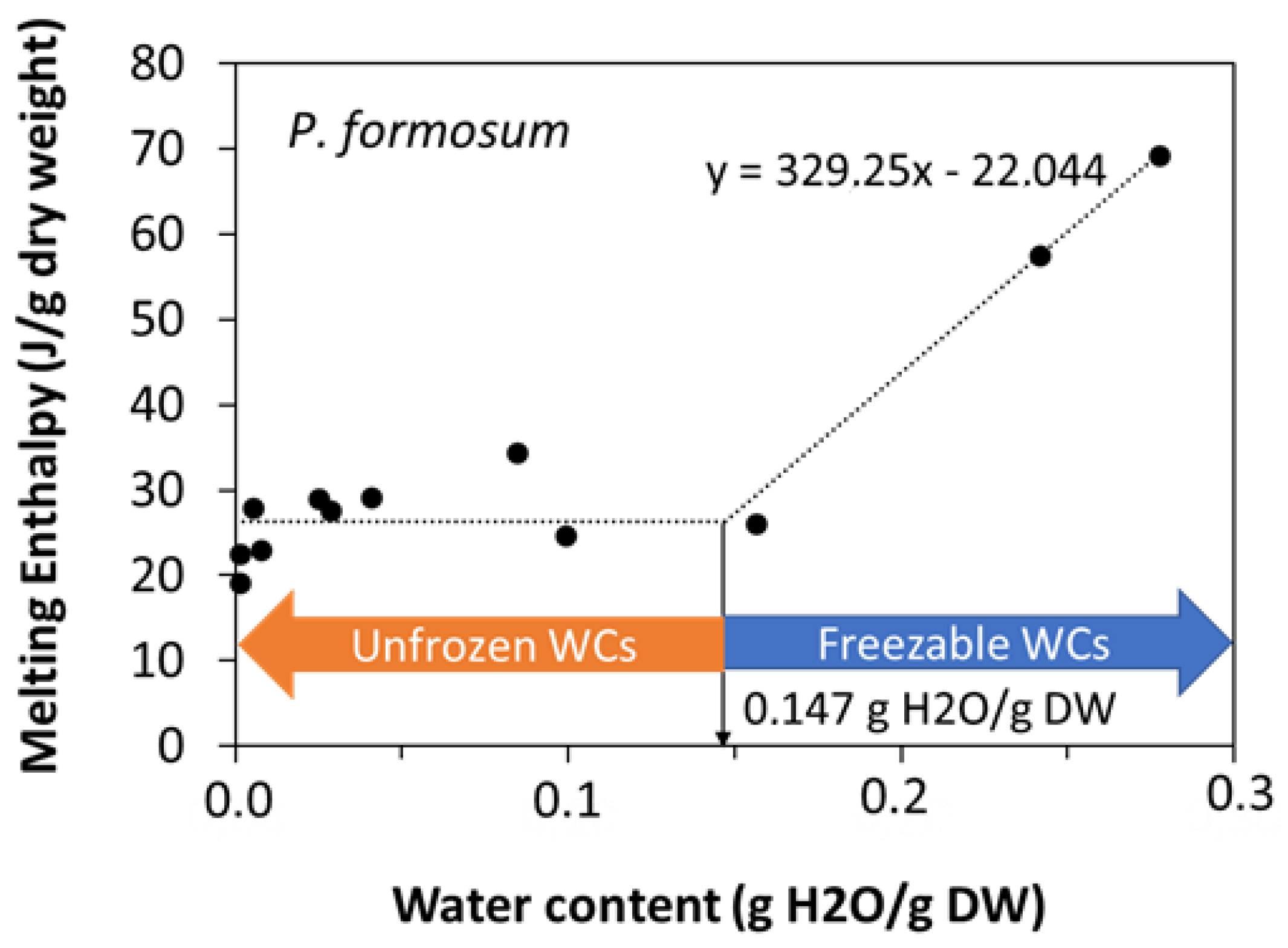
2.2. Calorimetric Properties of Bryophyte Spores in Relation to Storability by Differential Scanning Calorimetry (DSC)
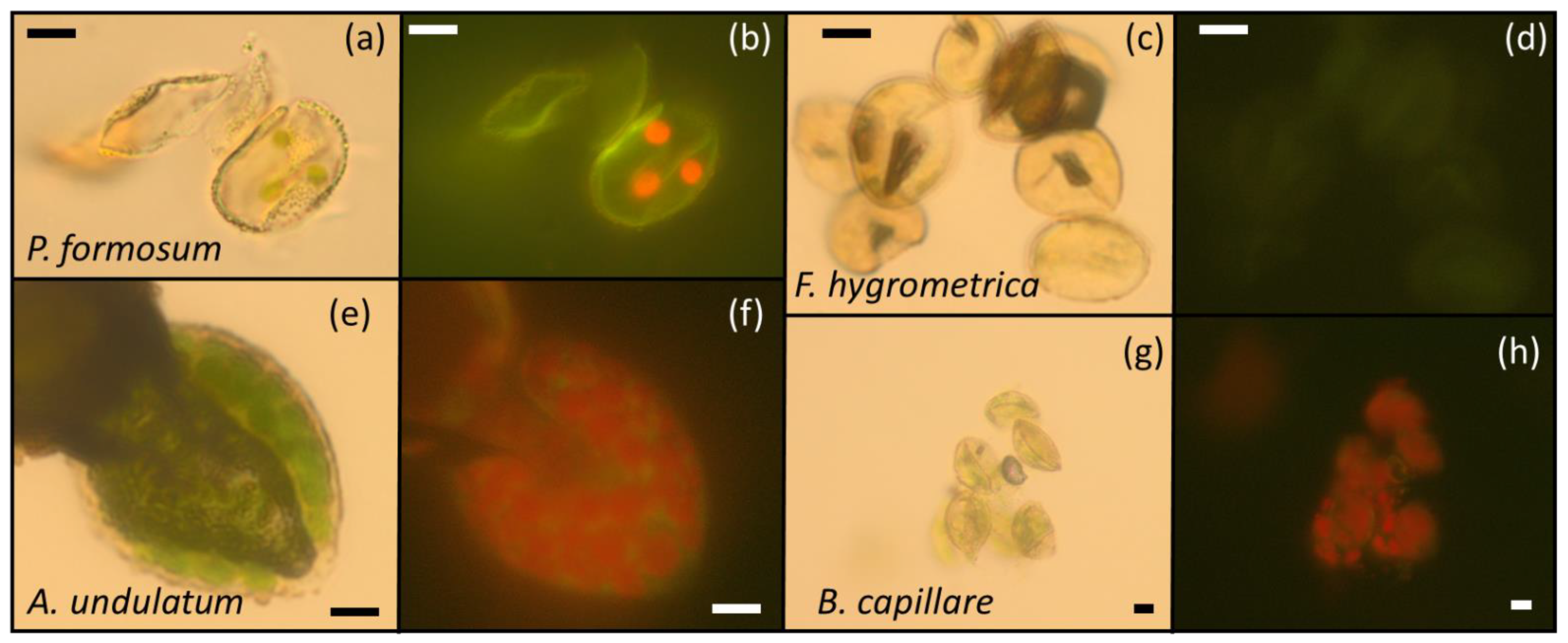
2.3. Chlorophyll and Chloroplast Presence in Spores by Fluorescence and Optical Microscopy
3. Discussion
3.1. Tolerance to Desiccation of Bryophyte Spores
3.2. Tolerance of Bryophyte Spores to Sub-Zero Temperatures
3.3. Calorimetric Properties of Storage Lipids, Chlorophyll Presence and Bryophyte Storability
3.4. Practical Considerations from the Results to Improve the Ex Situ Conservation of Bryophytes
4. Materials and Methods
4.1. Plant Material and Spore Desiccation Treatment
4.2. Germination Tests for Desiccation and Freezing Tolerance Experiments
4.3. Calorimetric Analysis of Bryophyte Spores
4.4. Fluorescence and Optical Microscopy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Preston, J.C.; Sandve, S.R. Adaptation to seasonality and the winter freeze. Front. Plant Sci. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.; Lewis, L.A.; Taylor, W.; Wellman, C.; Cook, M. Early Terrestrialization: Transition from Algal to bryophyte Grade. In Photosynthesis in Bryophytes and Early Land Plants; Advances in Photosynthesis and Respiration (Including Bioenergy and Related Processes); Hanson, D., Rice, S., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 37, pp. 9–28. [Google Scholar]
- de Vries, J.; Curtis, B.A.; Gould, S.B.; Archibald, J.M. Embryophyte stress signaling evolved in the algal progenitors of land plants. Proc. Natl. Am. Sci. USA 2018, 115, E3471–E3480. [Google Scholar] [CrossRef] [PubMed]
- van Zanten, B.O.; Pòcs, T. Distribution and dispersal of bryophytes. Adv. Bryol. 1981, 1, 479–562. [Google Scholar]
- Page, C.N. Ecological strategies in fern evolution: A neopteridological overview. Rev. Palaeobot. Palynol. 2002, 119, 1–33. [Google Scholar] [CrossRef]
- Munoz, J.; Felicisimo, A.M.; Cabezas, F.; Burgaz, A.R.; Martinez, I. Wind as a long-distance dispersal vehicle in the Southern Hemisphere. Science 2004, 304, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, J.L.; Davies, S.J.; Bunyavejchewin, S.; Noor, N.S.M. The role of desiccation tolerance in determining tree species distributions along the Malay–Thai Peninsula. Funct. Ecol. 2008, 22, 221–231. [Google Scholar] [CrossRef]
- Zanne, A.E.; Tank, D.C.; Cornwell, W.K.; Eastman, J.M.; Smith, S.A.; FitzJohn, R.G.; McGlinn, D.J.; O’Meara, B.C.; Moles, A.T.; Reich, P.B.; et al. Three keys to the radiation of angiosperms into freezing environments. Nature 2014, 506, 89–92. [Google Scholar] [CrossRef]
- Li, D.Z.; Pritchard, H.W. The science and economics of ex situ plant conservation. Trends Plant Sci. 2009, 14, 614–621. [Google Scholar] [CrossRef]
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Dixon, K.W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.C. Cryobiotechnologies: Tools for expanding long-term ex situ conservation to all plant species. Biol. Conserv. 2020, 250, 108736. [Google Scholar] [CrossRef]
- Proctor, M.C.F.; Oliver, M.J.; Wood, A.J.; Alpert, P.; Stark, L.R.; Cleavitt, N.L.; Mishler, B.D. Desiccation-Tolerance in bryophytes: A Review. Bryologist 2007, 110, 595–621. [Google Scholar] [CrossRef]
- Nagao, M.; Matsui, K.; Uemura, M. Klebsormidium flaccidum, a charophycean green alga, exhibits cold acclimation that is closely associated with compatible solute accumulation and ultrastructural changes. Plant Cell Environ. 2008, 31, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.J.; Tuba, Z.; Mishler, B.D. The evolution of vegetative desiccation tolerance in land plants. Plant Ecol. 2000, 151, 85–100. [Google Scholar] [CrossRef]
- Oliver, M.J.; Velten, J.E.F.F.; Mishler, B.D. Desiccation tolerance in bryophytes: A reflection of the primitive strategy for plant survival in dehydrating habitats? Integr. Comp. Biol. 2005, 45, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Gaff, D.F.; Oliver, M. The evolution of desiccation tolerance in angiosperm plants: A rare yet common phenomenon. Funct. Plant Biol. 2013, 40, 315–328. [Google Scholar] [CrossRef]
- Tebele, S.M.; Marks, R.A.; Farrant, J.M. Two Decades of Desiccation Biology: A Systematic Review of the Best Studied Angiosperm Resurrection Plants. Plants 2021, 10, 2784. [Google Scholar] [CrossRef]
- Buitink, J.; Walters-Vertucci, C.; Hoekstra, F.A.; Leprince, O. Calorimetric properties of dehydrating pollen (analysis of desiccation-tolerant and an intolerant species). Plant Physiol. 1996, 111, 235–242. [Google Scholar] [CrossRef]
- Leprince, O.; Buitink, J. Desiccation tolerance: From genomics to the field. Plant Sci. 2010, 179, 554–564. [Google Scholar] [CrossRef]
- Dekkers, B.J.; Costa, M.C.D.; Maia, J.; Bentsink, L.; Ligterink, W.; Hilhorst, H.W. Acquisition and loss of desiccation tolerance in seeds: From experimental model to biological relevance. Planta 2015, 241, 563–577. [Google Scholar] [CrossRef]
- Kallow, S.; Longin, K.; Sleziak, N.F.; Janssens, S.B.; Vandelook, F.; Dickie, J.; Swennen, R.; Paofa, J.; Carpentier, S.; Panis, B. Challenges for ex situ conservation of wild bananas: Seeds collected in Papua New Guinea have variable levels of desiccation tolerance. Plants 2020, 9, 1243. [Google Scholar] [CrossRef]
- Smolikova, G.; Leonova, T.; Vashurina, N.; Frolov, A.; Medvedev, S. Desiccation tolerance as the basis of long-term seed viability. Int. J. Mol. Sci. 2021, 22, 101. [Google Scholar] [CrossRef]
- Impe, D.; Ballesteros, D.; Nagel, M. Impact of drying and cooling rate on the survival of the desiccation-sensitive wheat pollen. Plant Cell Rep. 2022, 41, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Segreto, R.; Hassel, K.; Bardal, R.; Stenøien, H.K. Desiccation tolerance and natural cold acclimation allow cryopreservation of bryophyteswithout pretreatment or use of cryoprotectants. Bryologist 2010, 113, 760–769. [Google Scholar] [CrossRef]
- Walters, C.; Pence, V.C. The unique role of seed banking and cryobiotechnologies in plant conservation. Plants People Planet 2021, 3, 83–91. [Google Scholar] [CrossRef]
- FAO. Genebank Standards for Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2014. [Google Scholar]
- Walters, C. Orthodoxy, recalcitrance and in-between: Describing variation in seed storage characteristics using threshold responses to water loss. Planta 2015, 242, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Colville, L.; Pritchard, H.W. Seed life span and food security. New Phytol. 2019, 224, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, D.; Pence, V.C. Survival and death of seeds during liquid nitrogen storage: A case study on seeds with short lifespans. Cryoletters 2017, 38, 278–289. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, Y.E. Effect of storage temperature and moisture on the germination of papaya seeds. Seed Sci. Res. 1991, 1, 69–72. [Google Scholar] [CrossRef]
- Seaton, P.T.; Pritchard, H.W. Orchid seed storage: Historical perspective, current status, and future prospects for long-term conservation. Selbyana 1993, 14, 89–104. [Google Scholar]
- Crane, J.; Miller, A.L.; Van Roekel, J.W.; Walters, C. Triacylglycerols determine the unusual storage physiology of Cuphea seed. Planta 2003, 217, 699–708. [Google Scholar] [CrossRef]
- Crane, J.; Kovach, D.; Gardner, C.; Walters, C. Triacylglycerol phase and ‘intermediate’ seed storage physiology: A study of Cuphea carthagenensis. Planta 2006, 223, 1081–1089. [Google Scholar] [CrossRef]
- Chau, M.M.; Chambers, T.; Weisenberger, L.; Keir, M.; Kroessig, T.I.; Wolkis, D.; Kam, R.; Yoshinaga, A.Y. Seed freeze sensitivity and ex situ longevity of 295 species in the native Hawaiian flora. Am. J. Bot. 2019, 106, 1248–1270. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; Hudson, A.R.; Dickie, J.B.; Cook, C.; O’Hara, T.; Trivedi, C. Exploring seed longevity of UK native trees: Implications for ex situ conservation. Seed Sci. Res. 2020, 30, 101–111. [Google Scholar] [CrossRef]
- Ballesteros, D.; Pritchard, H.W.; Walters, C. Dry architecture: Towards the understanding of the variation of longevity in desiccation-tolerant germplasm. Seed Sci. Res. 2020, 30, 142–155. [Google Scholar] [CrossRef]
- López-Pozo, M.; Fernández-Marín, B.; García-Plazaola, J.I.; Ballesteros, D. Desiccation tolerance in ferns: From the unicellular spore to the multi-tissular sporophyte. In Current Advances in Fern Research; Fernández, H., Ed.; Springer: Cham, Switzerland, 2018; pp. 401–426. [Google Scholar]
- Ballesteros, D.; Hill, L.M.; Lynch, R.T.; Pritchard, H.W.; Walters, C. Longevity of preserved germplasm: The temperature dependency of aging reactions in glassy matrices of dried fern spores. Plant Cell Physiol. 2019, 60, 376–392. [Google Scholar] [CrossRef]
- Glime, J.M. Bryophyte Ecology; Michigan Technological University: Houghton, MI, USA, 2021; Available online: https://digitalcommons.mtu.edu/oabooks/4 (accessed on 25 November 2021).
- van Zanten, B.O. Experimental studies on trans-oceanic long-range dispersal of moss spores in the Southern Hemisphere. J. Hatt. Bot. Lab. 1978, 44, 455–482. [Google Scholar]
- Ballesteros, D.; Walters, C. Calorimetric properties of water and triacylglycerols in fern spores relating to storage at cryogenic temperatures. Cryobiology 2007, 55, 1–9. [Google Scholar] [CrossRef]
- Mira, S.; Nadarajan, J.; Liu, U.; González-Benito, M.E.; Pritchard, H.W. Lipid thermal fingerprints of long-term stored seeds of Brassicaceae. Plants 2019, 8, 414. [Google Scholar] [CrossRef]
- Rowntree, J.K.; Pressel, S.; Ramsay, M.M.; Sabovljevic, A.; Sabovljevic, M. In vitro conservation of European bryophytes. Vitr. Cell Dev. Biol. Plant 2011, 47, 55–64. [Google Scholar] [CrossRef]
- Sabovljević, M.; Vujičić, M.; Pantović, J.; Sabovljević, A. Bryophyte conservation biology: In vitro approach to the ex situ conservation of bryophytes from Europe. Plant Biosyst. 2014, 148, 857–868. [Google Scholar] [CrossRef]
- Mounce, R.; Smith, P.; Brockington, S. Ex situ conservation of plant diversity in the world’s botanic gardens. Nat. Plants 2017, 3, 795–802. [Google Scholar] [CrossRef]
- Pritchard, H.W.; Nadarajan, J.; Ballesteros, D.; Thammasiri, K.; Prasongsom, S.; Malik, S.K.; Chaudhury, R.; Kim, H.H.; Lin, L.; Li, W.Q.; et al. Cryobiotechnology of tropical seeds-scale, scope and hope. Acta Hortic. 2017, 1167, 37–48. [Google Scholar] [CrossRef]
- Walters, C.; Landré, P.; Hill, L.; Corbineau, F.; Bailly, C. Organization of lipid reserves in cotyledons of primed and aged sunflower seeds. Planta 2005, 222, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, D.; Hill, L.M.; Walters, C. Variation of desiccation tolerance and longevity in fern spores. J. Plant. Physiol. 2017, 211, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M. The Physical Chemistry of Lipids: From Alkanes to Phospholipids; Plenum Press: New York, NY, USA, 1986. [Google Scholar]
- Malcolm, B.; Malcolm, N. Mosses and Other Bryophytes, an Illustrated Glossary; Micro-Optics Press: Nelson, New Zealand, 2000; p. 220. [Google Scholar]
- Van Zanten, B.O. Some considerations on the feasibility of long-distance transport in bryophytes. Acta Bot. Neer. 1984, 33, 231–232. [Google Scholar] [CrossRef]
- Van Zanten, B.O.; Gradstein, S.R. Experimental Dispersal Geography of Neotropical Liverworts. Nova Hedwig. 1988, 90, 41–94. [Google Scholar]
- Li, Q.-P.; Jiang, L.-J.; Lu, B.-B.; Liu, C.; Xiao, T.-Z.; Wu, S.-P.; Wang, J.; Zhu, R.-L. Cryopreservation of spores of Haplocladium microphyllum (Hedw.). Broth. J. East China Norm. Univ. Natur. Sci. Ed. 2014, 2014, 121–125. [Google Scholar]
- Sun, L.-W.; Gao, X.-D.; Xu, D.-E.; Wang, S.-Q.; Yang, Z.-J.; Wang, J. Variation in germination rates of moss spores using a cryopreservation technique: A case study of spores from six moss species. J. East China Norm. Univ. Natur. Sci. Ed. 2019, 2019, 138–143. [Google Scholar]
- Malta, N. Versuche über die Widerstandsfähigkeit der Moose gegen Austrocknung. Acta Univ. Larviensis 1921, 1, 125–129. [Google Scholar]
- Meyer, S.L. Physiological Studies on Mosses. II. Spore Longevity in Physcomitrium turbinatum and Funaria hygrometrica. Bryologist 1941, 44, 69–75. [Google Scholar] [CrossRef]
- Hoekstra, F.A. Differential longevities in desiccated anhydrobiotic plant systems. Integr. Comp. Biol. 2005, 45, 725–733. [Google Scholar] [CrossRef]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Ann. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Buitink, J.; Leprince, O. Glass formation in plant anhydrobiotes: Survival in the dry state. Cryobiology 2004, 48, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.; Hill, L.M.; Wheeler, L.J. Dying while dry: Kinetics and mechanisms of deterioration in desiccated organisms. Integr. Comp. Biol. 2005, 45, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.; Ballesteros, D.; Vertucci, V.A. Structural mechanics of seed deterioration: Standing the test of time. Plant. Sci. 2010, 179, 565–573. [Google Scholar] [CrossRef]
- Medina, N.G.; Estébanez, B. Does spore ultrastructure mirror different dispersal strategies in mosses? A study of seven Iberian Orthotrichum species. PLoS ONE 2014, 9, e112867. [Google Scholar] [CrossRef]
- Vertucci, C.W.; Roos, E.E.; Crane, J. Theoretical basis of protocols for seed storage III. Optimum moisture contents for pea seeds stored at different temperatures. Ann. Bot. 1994, 74, 531–540. [Google Scholar] [CrossRef]
- Linder, C.R. Adaptive evolution of seed oils in plants: Accounting for the biogeographic distribution of saturated and unsaturated fatty acids in seed oils. Am. Natur. 2000, 156, 442–458. [Google Scholar] [CrossRef]
- Walters, C.; Wheeler, L.M.; Grotenhuis, J.M. Longevity of seeds stored in a genebank: Species characteristics. Seed Sci. Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Probert, R.J.; Daws, M.I.; Hay, F.R. Ecological correlates of ex situ seed longevity: A comparative study on 195 species. Ann. Bot. 2009, 104, 57–69. [Google Scholar] [CrossRef]
- Hodgetts, N.; Cálix, M.; Englefield, E.; Fettes, N.; García Criado, M.; Patin, L.; Nieto, A.; Bergamini, A.; Bisang, I.; Baisheva, E.; et al. A Miniature World in Decline: European Red List of Mosses, Liverworts and Hornworts; IUCN: Brussels, Belgium, 2019; p. 100. [Google Scholar]
- Pence, V.C. Cryopreservation of bryophytes and Ferns. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; p. 534. [Google Scholar]
- Ballesteros, D.; Pence, V. Fern Conservation: Spore, Gametophyte, and Sporophyte Ex situ Storage, In vitro Culture, and Cryopreservation. In Current Advances in Fern Research; Fernández, H., Ed.; Springer: Cham, Switzerland, 2018; pp. 227–249. [Google Scholar]
- Nebot, A.; Philpott, V.J.; Pajdo, A.; Ballesteros, D. Cryopreservation of Fern Spores and Pollen. In Cryopreservation and Freeze-Drying Protocols. Methods in Molecular Biology; Wolkers, W.F., Oldenhof, H., Eds.; Humana: New York, NY, USA, 2021; Volume 2180, pp. 623–637. [Google Scholar]
- North, T.; Chong, C.; Cross, A.; van der Walt, K.; Ballesteros, D. Special collections and under-represented taxa in Australasian ex situ conservation programs. In Plant Germplasm Conservation in Australia (3rd Edn). Strategies and Guidelines for Developing, Managing and Utilising Ex Situ Collections; Martyn Yenson, A.J., Offord, C.A., Meagher, P.F., Auld, T., Bush, D., Coates, D.J., Commander, L.E., Guja, L.K., Norton, S.L., Makinson, R.O., Eds.; Australian Network for Plant Conservation: Canberra, Australia, 2021; pp. 403–439. [Google Scholar]
- Reski, R.; Abel, W.O. Induction of budding on chloronemata and caulonemata of the moss, Physcomitrella patens, using isopentenyladenine. Planta 1985, 165, 354–358. [Google Scholar] [CrossRef]
- Leprince, O.; Walters-Vertucci, C. A calorimetric study of the glass transition behaviors in axes of bean seeds with relevance to storage stability. Plant. Physiol. 1995, 109, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- International Seed Testing Association (ISTA). International Rules for Seed Testing; ISTA: Bassersdorf, Switzerland, 2021. [Google Scholar]
- Sundue, M.; Vasco, A.; Moran, R.C. Cryptochlorophyllous spores in ferns: Nongreen spores that contain chlorophyll. Int. J. Plant. Sci. 2011, 172, 1110–1119. [Google Scholar] [CrossRef]
- Maciel-Silva, A.S.; Silva, F.C.D.; Valio, I.F. All green, but equal? Morphological traits and ecological implications on spores of three species of mosses in the Brazilian Atlantic forest. An. Acad. Bras. Cienc. 2014, 86, 1249–1262. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Species Name | Germination (Above: Final %, Below: Rate as t50 [Days]) | |
|---|---|---|
| Before LN Exposure | After LN Exposure | |
| Atrichum undulatum (acc. 1) | 25 a 3.20 | 2 b 4.85 |
| Bryum capillare (acc. 1) | 100 3.10 | 100 5.60 |
| B. capillare (acc. 2) | 100 2.60 | 100 2.60 |
| B. capillare (acc. 4) | 100 a 2.3 | 84 b 2.3 |
| B. capillare (acc. 5) | 100 2.55 | 100 2.6 |
| B. capillare (acc. 7) | 100 4.8 | 100 5.2 |
| Ceratodonpurpureus (acc. 1) | 100 a 3 | 71 b 2.8 |
| Dicranum scoparium (acc. 4) | 39 a 4.6 | 42 b 6.5 |
| Funaria hygrometrica (acc. 1) | 100 2.7 | 100 4 |
| F. hygrometrica (acc. 3) | 100 2.5 | 100 3 |
| F. hygrometrica (acc. 7) | 75 4.7 | 74 3.45 |
| F. hygrometrica (acc. 8) | 100 4.9 | 100 6.75 |
| Kindbergia praelonga (acc. 2) | 100 a 6.25 | 60 b 7.1 |
| K. praelonga (acc. 3) | 21 5 | 0 - |
| Leptobryum pyriforme | 100 a 4.85 | 56 b 5.95 |
| Marchantia polymorpha (acc. 1) | 7 2.4 | 0 - |
| Mnium hornum (acc. 2) | 100 5.61 | 100 5.95 |
| Orthodontium lineare (acc. 3) | 79 a 3.9 | 73 b 6.3 |
| O. lineare (acc. 3, test 2) | 54 10.65 | 57 6.2 |
| Polytrichum formosum (acc. 1) | 100 a 6 | 76 b 8.2 |
| P. formosum (acc. 1, test 2) | 100 5.75 | 100 5.65 |
| P. formosum (acc. 2) | 100 5.45 | 100 5.6 |
| Tortula muralis (acc. 2) | 100 6.4 | 100 6.25 |
| Component | Parameter | B. capillare | O. lineare | P. formosum | D. scoparium |
|---|---|---|---|---|---|
| Lipid | Melting temperature (°C) | −35.12 ± 0.01 | −34.43 ± 0.04 | −38.60 ± 4.61 | - |
| Melting enthalpy (J g−1 DW) | 10.1 ± 0.4 | 3.6 ± 3.0 | 25.8 ± 4.2 | 0 | |
| Predominant fatty acid (a) | Linolenic | Linolenic | Linolenic | - | |
| Enthalpy of melt (a) | 88 | 88 | 88 | - | |
| Lipid content% | 11.5 | 4.1 | 29.3 | 0 | |
| Water | ∆HH2O (J g−1 H2O) | - | - | 329 | - |
| Unfrozen WC (g H2O gDW−1) | - | - | 0.147 | - |
| Species Name | Accession | Location | Exposition | Coordinates | Collection Date | Ecology * | Chloroplasts/ Chlorophyll ++ |
|---|---|---|---|---|---|---|---|
| Atrichum undulatum (Hedw.) P. Beauv. (a) | 1 (a) | Wakehurst Place, way to Westwood Lake | 22 N | 51°4′13″N 0°5′32″W | 30 June 2021 | Terrestrial, terricolous | Y/Y |
| 2 | Wakehurst Place, Mansion | 348 N | N 51°3′58″ W 0°5′29″ | 1 July 2021 | - | ||
| 3 | Ardingly reservoir | 305 NW | N 51°3′36″ W 0°5′55″ | 22 July 2021 | - | ||
| Barbula unguiculata Hedw. | - | Wakehurst Place, water garden | 341 N | N 51°3′48′′ W 0°5′24′′ | 5 July 2021 | Aquatic, saxicolous | Y/Y |
| Brachytheciastrum velutinum (Hedw.) Ignatov & Huttunen. | - | Ardingly reservoir | 201 S | N 51°3′0.9″ W 0°6′7″ | 20 July 2021 | Epiphytic | Y/Y |
| Brachythecium rutabulum (Hedw.) Schimp. | 1 | Wakehurst Place, water garden | 359 N | N 51°3′49″ W 0°5′25″ | 5 July 2021 | Ubiquitous | Y/Y |
| 2 | 310 NW | N 51°3′59″ W 0°5′23″ | - | ||||
| Bryum argenteum Hedw. | 1 | Wakehurst Place, water garden | 226 SW | N 51°3′59″ W 0°4′56″ | 26 July 2021 | Terricolous | N/N |
| Bryum capillare Hedw. (a), (b) | 1 (a) | Wakehurst Place, MSB greenhouse | 161 S | N 51°4′7.9″ W 0°5′25″ | 30 July 2021 | Terricolous | Y/Y |
| 2 (a), (b) | 179 S | N 51°4′7.9″ W 0°5′24″ | Y/Y | ||||
| 3 | Wakehurst Place, water garden | 79 E | N 51°3′50″ W 0°4′56″ | 5 July, 2021 | Y/Y | ||
| 4 (a) | Wakehurst Place, greenhouses | 265 O | N 51°4′3.2″ W 0°5′24″ | 6 July 2021 | Y/Y | ||
| 5 (a) | 245 SW | N 51°4′4.4″ W 0°5′24″ | N/N | ||||
| 6 | 232 SW | N 51°4′3.8″ W 0°5′25″ | - | ||||
| 7 (a) | Wakehurst Place, Mansion | 10 N | N 51°3′59″ W 0°4′56″ | 26 July 2021 | - | ||
| Ceratodon purpureus (Hedw.) Brid. (a) | 1 (a) | Wakehurst Place, Mansion | 261 W | N 51°4′1.69″ W 0°5′25″ | 1 July 2021 | Terricolous | Y/Y |
| 2 | 56 NE | N 51°3′56″ W 0°5′25″ | - | ||||
| 3 | Wakehurst Place, greenhouses | 255 W | N 51°4′4.2″W 0°5′26″ | 6 July 2021 | - | ||
| Dicranella heteromalla (Hedw.) Schimp. | 1 | Wakehurst Place, water garden | 322 NW | N 51°3′49″ W 0°5′35″ | 5 July 2021 | Terrestrial | - |
| 2 | Wakehurst Place, greenhouses | 265 W | N 51°4′2.3″ W 0°5′24″ | 6 July 2021 | Y/Y | ||
| 3 | Ardingly reservoir | 305 NW | N 51°3′36″ W 0°5′55″ | 22 July 2021 | Y/Y | ||
| 4 | Wakehurst Place, Himalayan Glade | 21 N | N 51°3′52″ W 0°5′41″ | 26 July 2021 | - | ||
| Dicranum scoparium Hedw. (a), (b) | 1 | Wakehurst Place, MSB greenhouse | 192 S | N 51°4′7.3″ W 0°5′24″ | 30 June 2021 | Ubiquitous | - |
| 2 | Chidingly Wood | 216 SW | N 51°3′59″ W 0°4′56″ | 8 July 2021 | - | ||
| 3 | 216 SW | N 51°3′59″ W 0°4′56″ | - | ||||
| 4 (a), (b) | Wakehurst Place, water garden | 303 NW | N 51°3′51″ W 0°5′21″ | 26 July 2021 | Y/Y | ||
| Fissidens taxifolius Hedw. | - | Ardingly reservoir | 274 W | N 51°3′9.1″ W 0°7′10″ | 22 July 2021 | Terricolous | Y/Y |
| Funaria hygrometrica Hedw. (a) | 1 (a) | Wakehurst Place, MSB greenhouse | 281 W | N 51°4′8.18″ W 0°5′24.9″ | 30 July 2021 | Terricolous | N/N |
| 2 | Wakehurst Place, Wall Garden | 207 S | N 51°3′59.9″ W 0°4′57″ | 1 July 2021 | - | ||
| 3 (a) | Wakehurst Place, Mansion | 348 N | N 51°3′58″ W 0°5′29″ | 1 July 2021 | N/N | ||
| 4 | Wakehurst Place, greenhouses | 146 SE | N 51°3′48″ W 0°5′28″ | 5 July 2021 | - | ||
| 5 | 182 S | N 51°4′2.5″ W 0°5′25″ | 6 July 2021 | - | |||
| 6 | 204 SW | N 51°4′2.3″ W 0°5′24″ | - | ||||
| 7 (a) | 245 SW | N 51°4′4.4″ W 0°5′24″ | N/N | ||||
| 8 (a) | Ardingly reservoir | 134 SE | N 51°3′9.1″ W 0°7′10″ | 20 July 2021 | Y/Y | ||
| Hypnum cupressiforme Hedw. | 1 | Wakehurst Place, way to Westwood Lake | 27 NE | N 51°4′9.5″ W 0°5′30″ | 30 June 2021 | Epiphytic and epiphyllous | - |
| 2 | 113 SE | N 51°4′0.17″ W 0°5′1.43″ | - | ||||
| 3 | 205 SW | N 51°4′2.7″ W 0°6′0.26″ | Y/Y | ||||
| 4 | Wakehurst Place, Himalayan Glade | 214 SW | N 51°3′47″ W 0°5′41.97″ | 30 June 2021 | - | ||
| 5 | Wakehurst Place, Mansion | 154 SE | N 51°4′1.65″ W 0°5′25″ | 1 July 2021 | Y/Y | ||
| 6 | Wakehurst Place, water garden | 219 SW | N 51°3′49″ W 0°5′21″ | 5 July 2021 | - | ||
| 7 | 259 W | N 51°4′20″ W 0°5′59″ | - | ||||
| 8 | 358 N | N 51°3′48″ W 0°5′24″ | Y/Y | ||||
| Isothecium myosuroides Brid. | - | Ardingly reservoir | 250 W | N 51°3′39″ W 0°7′10″ | 22 July 2021 | Epiphytic on bark | Y/Y |
| Kindbergia praelonga (Hedw.) Ochyra (a) | 1 | Wakehurst Place, Westwood Lake | 357 N | N 51°3′13″ W 0°7′8.3″ | 30 July 2021 | Terricolous | Y/Y |
| 2 (a) | Wakehurst Place, water garden | 356 N | N 51°3′55″ W 0°4′56″ | 5 July 2021 | - | ||
| 3 (a) | Wakehurst Place, Westwood Lake | 135 SE | N 51°4′48″ W 0°6′2.48″ | 19 July 2021 | Y/Y | ||
| Leptobryum pyriforme (Hedw.) Wilson (a) | - (a) | Wakehurst Place, greenhouses | 182 S | N 51°4′2.5″ W 0°5′25″ | 6 July 2021 | Terricolous | - |
| Marchantia polymorpha L. (a) | 1 (a) | Wakehurst Place, MSB greenhouse | 216 SW | N 51°4′7.8″ W 0°5′24″ | 30 July 2021 | Terricolous, sometimes submerged | - |
| 2 | Wakehurst Place, greenhouses | 182 S | N 51°4′2.5″ W 0°5′25″ | 6 July 2021 | - | ||
| Mnium hornum Hedw. (a) | 1 | Wakehurst Place, water garden | 55 E | N 51°3′59″ W 0°4′57″ | 5 July 2021 | Terricolous | - |
| 2 (a) | Wakehurst Place, MSB greenhouse | 192 S | N 51°4′7.3″ W 0°5′24.9″ | 16 July 2021 (a) | Y/Y | ||
| Orthodontium lineare Schwägr. (a), (b) | 1 | Chidingly Wood | 53 NE | N 51°4′23″ W 0°4′39″ | 8 July 2021 | Saxicolous | - |
| 2 | 79 E | N 51°3′59″ W 0°4′56″ | Y/Y | ||||
| 3 (a), (b) | Wakehurst Place, Himalayan Glade | 170 S | N 51°3′51″ W 0°5′42″ | 26 July 2021 | N/N | ||
| Orthotrichum affine Schrader ex Bridel | 1 | Wakehurst Place, way to Mansion | 33 NE | N 51°3′13″ W 0°5′41″ | 30 June 2021 | Epiphytic on bark | - |
| 2 | 240 S | N 51°3′59″ W 0°4′57″ | 1 July 2021 | Y/Y | |||
| 3 | Wakehurst Place, Himalayan Glade | 33 NE | N 51°3′13″ W 0°5′41″ | 30 July 2021 | Y/Y | ||
| Oxyrrhynchium hians (Hedw.) Loeske | 1 | Wakehurst Place, water garden | 359 N | N 51°3′59″ W 0°4′57″ | 5 July 2021 | Terrestrial | - |
| 2 | Ardingly reservoir | 140 SE | N 51°3′38″ W 0°5′54″ | 22 July 2021 | - | ||
| Plagiomnium undulatum (Hedw.) T.J.Kop. | - | Wakehurst Place, water garden | 297 NW | N 51°3′48″ W 0°4′57″ | 5 July 2021 | Saxicolous | - |
| Polytrichum formosum (Hedw.) G.L. Sm. (a), (b) | 1 (a), (b) | Wakehurst Place, Westwood Lake | 328 NW | N 51°3′47″ W 0°5′57″ | 30 July 2021 | Terricolous | Y/Y |
| 2 (a), (b) | Wakehurst Place, winter garden | 11 N | N 51°3′59″ W 0°4′57″ | 1 July 2021 | Terricolous | Y/Y | |
| Tortula muralis Hedw. (a) | 1 | Wakehurst Place, Mansion | 360 N | N 51°3′58″ W 0°5′22″ | 30 June 2021 | Saxicolous | Y/Y |
| 2 (a) | 72 E | N 51°3′59″ W 0°4′57″ | 1 July 2021 | N/Y | |||
| Ulota bruchii Hornsch. ex Brid. | 1 | Wakehurst Place, Himalayan Glade | 160 S | N 51°3′17″ W 0°7′2.14″ | 30 July 2021 | Epiphytic | - |
| 2 | Wakehurst Place, Westwood Lake | 358 N | N 51°4′26.7″ W 0°5′12.6″ | 19 July 2021 | Y/Y | ||
| Ulota crispa (Hedw.) Brid. | - | Wakehurst Place, Japan garden | 195 N | N 51°3′45″ W 0°4′59″ | 5 July 2021 | Epiphytic | Y/Y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiloca, G.; Brundu, G.; Ballesteros, D. Bryophyte Spores Tolerate High Desiccation Levels and Exposure to Cryogenic Temperatures but Contain Storage Lipids and Chlorophyll: Understanding the Essential Traits Needed for the Creation of Bryophyte Spore Banks. Plants 2022, 11, 1262. https://doi.org/10.3390/plants11091262
Tiloca G, Brundu G, Ballesteros D. Bryophyte Spores Tolerate High Desiccation Levels and Exposure to Cryogenic Temperatures but Contain Storage Lipids and Chlorophyll: Understanding the Essential Traits Needed for the Creation of Bryophyte Spore Banks. Plants. 2022; 11(9):1262. https://doi.org/10.3390/plants11091262
Chicago/Turabian StyleTiloca, Giuseppe, Giuseppe Brundu, and Daniel Ballesteros. 2022. "Bryophyte Spores Tolerate High Desiccation Levels and Exposure to Cryogenic Temperatures but Contain Storage Lipids and Chlorophyll: Understanding the Essential Traits Needed for the Creation of Bryophyte Spore Banks" Plants 11, no. 9: 1262. https://doi.org/10.3390/plants11091262
APA StyleTiloca, G., Brundu, G., & Ballesteros, D. (2022). Bryophyte Spores Tolerate High Desiccation Levels and Exposure to Cryogenic Temperatures but Contain Storage Lipids and Chlorophyll: Understanding the Essential Traits Needed for the Creation of Bryophyte Spore Banks. Plants, 11(9), 1262. https://doi.org/10.3390/plants11091262






