Cryopreservation of Holm Oak Embryogenic Cultures for Long-Term Conservation and Assessment of Polyploid Stability
Abstract
1. Introduction
2. Results
2.1. Effect of Explant Type
2.2. Effect of PVS2 and LN Cryostorage Period
2.3. Effect of Genotype
2.4. Effect of Long-Term Cryopreservation and Genotype
2.5. Plant Regeneration
2.6. Analysis of Ploidy Stability by Flow Cytometry
3. Discussion
4. Materials and Methods
4.1. Plant Material and Culture Conditions
4.2. Cryopreservation Equipment
4.3. Cryopreservation Experiments
4.3.1. Standard Cryopreservation Procedure
4.3.2. Effect of Explant Type
4.3.3. Effect of PVS2 and LN Storage Time
4.3.4. Effect of Genotype
4.3.5. Effect of Long-Term Cryopreservation and Genotype
4.4. Plant Regeneration
4.5. Ploidy Stability Analysis by Flow Cytometry
4.6. Data Collection and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CV | coefficient of variation |
| DI | DNA index |
| GD | Gresshoff and Doy medium |
| LN | liquid nitrogen |
| MS | Murashige and Skoog medium |
| NES | nodular embryogenic structures |
| PVS2 | plant vitrification solution 2 |
| SE | somatic embryogenesis |
| SH | Schenk and Hildebrandt medium |
References
- De Rigo, D.; Caudullo, G. Quercus ilex in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 152–153. [Google Scholar]
- Peñuelas, J.; Sardans, J.; Filella, I.; Estiarte, M.; Llusià, J.; Ogaya, R.; Carnicer, J.; Bartrons, M.; Rivas-Ubach, A.; Grau, O.; et al. Assessment of the impacts of climate change on Mediterranean terrestrial ecosystems based on data from field experiments and long-term monitored field gradients in Catalonia. Environ. Exp. Bot. 2018, 152, 49–59. [Google Scholar] [CrossRef]
- Natalini, F.; Alejano, R.; Vázquez-Piqué, J.; Cañellas, I.; Gea-Izquierdo, G. The role of climate change in the widespread mortality of holm oak in open woodlands of Southwestern Spain. Dendrochronologia 2016, 38, 51–60. [Google Scholar] [CrossRef]
- IPCC, 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; p. 40. [Google Scholar]
- Trouillier, M.; van der Maaten-Theunissen, M.; Scharnweber, T.; Wilmking, M. A unifying concept for growth trends of trees and forests—The “potential natural forest”. Front. For. Glob. Change 2020, 3, 581334. [Google Scholar] [CrossRef]
- Linnakoski, R.; Kasanen, R.; Dounavi, A.; Forbes, K.M. Editorial: Forest health under climate change: Effects on tree resilience, and pest and pathogen dynamics. Front. Plant Sci. 2019, 10, 1157. [Google Scholar] [CrossRef] [PubMed]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Available online: https://archnetwork.org/lessons-learn-from-the-spanish-dehesa-a-new-model-for-scottish-agriculture-woodland-management (accessed on 11 April 2022).
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Dixon, K.W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.C. Cryobiotechnologies: Tools for expanding long-term ex situ conservation to all plant species. Biol. Conserv. 2020, 250, 108736. [Google Scholar] [CrossRef]
- Ballesteros, D.; Pritchard, H.W. The Cryobiotechnology of Oaks: An Integration of Approaches for the Long-Term Ex Situ Conservation of Quercus Species. Forests 2020, 11, 1281. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Nicolas, J.L.; Heredia, N.; Uscola, M. Quercus ilex L. In Producción y Manejo de Semillas y Plantas Forestales. Tomo II; Pemán, J., Navarro-Cerrillo, R.M., Nicolás, J.L., Prada, M.A., Serrada, R., Eds.; Organismo Autónomo Parques Nacionales: Madrid, Spain, 2013; pp. 226–250. [Google Scholar]
- Walters, C.; Berjak, P.; Pammenter, N.; Kennedy, K.; Raven, P. Preservation of recalcitrant seeds. Science 2013, 339, 915–916. [Google Scholar] [CrossRef]
- Li, J.W.; Ozudogru, E.A.; Li, J.; Wang, M.-R.; Bi, W.-L.; Lambardi, M.; Wang, Q.-C. Cryobiotechnology of forest trees: Recent advances and future prospects. Biodivers. Conserv. 2018, 27, 795–814. [Google Scholar] [CrossRef]
- Salaj, T.; Klubicová, K.; Matusova, R.; Salaj, J. Somatic Embryogenesis in Selected Conifer Trees Pinus nigra Arn. and Abies Hybrids. Front. Plant Sci. 2019, 10, 13. [Google Scholar] [CrossRef]
- Corredoira, E.; Martínez, M.T.; San-José, M.C.; Ballester, A. Conservation of Hardwood Forests species. In Biodiversity and Conservation of Woody Plants; Ahuja, M.R., Jain, S.M., Eds.; Springer: Cham, Switzerland, 2017; pp. 421–453. [Google Scholar]
- Park, Y.S.; Beaulieu, J.; Bousquet, J. Multi-varietal forestry integrating genomic selection and somatic embryogenesis. In Vegetative Propagation of Forest Trees; Park, Y.S., Bonga, J.M., Moon, H.-K., Eds.; National Institute of Forest Science (Nifos): Seoul, Korea, 2016; pp. 302–322. [Google Scholar]
- von Arnold, S.; Sabala, I.; Bozhkov, P.; Dyachok, J.; Filonova, L. Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult. 2002, 69, 233–249. [Google Scholar] [CrossRef]
- Lambardi, M.; Ozudogru, A.E.; Benelli, C. Cryopreservation of embryogenic callus. In Plant Cryopreservation: A Practical Guide; Reed, B., Ed.; Springer: Berlin, Germany, 2008; pp. 177–210. [Google Scholar]
- Guan, Y.; Li, S.-G.; Fan, X.-F.; Su, Z.-H. Application of somatic embryogenesis in woody plants. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. Vitr. Cell. Dev. Biol. Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Fahy, G.M.; MacFarlane, D.R.; Angell, C.A.; Meryman, H.T. Vitrification as an approach to cryopreservation. Cryobiology 1984, 21, 407–426. [Google Scholar] [CrossRef]
- Panis, B.; Lambardi, M. Status of cryopreservation technologies in plants (crops and forest trees). In Proceedings of the Role of Biotechnology for the Characterization and Conservation of Crop, Forestry, Animal and Fishery Genetic Resources, Turin, Italy, 5–7 March 2005. [Google Scholar]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tnaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic plant species conservation: Biotechnological approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef]
- Xia, K.; Hill, L.M.; Li, D.-Z.; Walters, C. Factors affecting stress tolerance in recalcitrant embryonic axes from seeds of four Quercus (Fagaceae) species native to the USA or China. Ann. Bot. 2014, 14, 1747–1759. [Google Scholar] [CrossRef]
- Engelmann, F. Cryopreservation of embryos: An overwiew. In Plant Embryo Cultures: Methods and Protocols, Methods in Molecular Biology; Thorpe, T.A., Yeung, E.C., Eds.; Springer: New York, NY, USA, 2011; Volume 710, pp. 155–184. [Google Scholar]
- Corredoira, E.; San-José, M.C.; Ballester, A.; Vieitez, A.M. Cryopreservation of zygotic embryo axes and somatic embryos of European chestnut. Cryo Lett. 2004, 25, 33–42. [Google Scholar]
- Martínez, M.T.; Ballester, A.; Vieitez, A.M. Cryopreservation of embryogenic cultures of Quercus robur using desiccation and vitrification procedures. Cryo Lett. 2003, 46, 182–189. [Google Scholar] [CrossRef]
- Valladares, S.; Toribio, M.; Celestino, C.; Vieitez, A.M. Cryopreservation of embryogenic cultures from mature Quercus suber trees using vitrification. Cryo Lett. 2004, 25, 177–186. [Google Scholar]
- Corredoira, E.; Toribio, M.; Vieitez, A.M. Clonal propagation via somatic embryogenesis in Quercus spp. In Tree Biotechnology; Ramawhat, K.G., Mérillon, J.-M., Ahuja, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 262–302. [Google Scholar]
- Barra-Jiménez, A.; Aronen, T.S.; Alegre, J.; Toribio, M. Cryopreservation of embryogenic tissues from mature holm oak trees. Cryobiology 2015, 70, 217–225. [Google Scholar] [CrossRef] [PubMed]
- González-Benito, M.E.; Prieto, R.M.; Herradon, E.; Martin, C. Cryopreservation of Quercus suber and Quercus ilex embryonic axes: In vitro culture, desiccation and cooling factors. Cryo Lett. 2002, 23, 283–290. [Google Scholar]
- Martínez, M.T.; San-José, M.C.; Vieitez, A.M.; Cernadas, M.J.; Ballester, A.; Corredoira, E. Propagation of mature Quercus ilex L. (holm oak) trees by somatic embryogenesis. Plant Cell Tissue Organ Cult. 2017, 131, 321–333. [Google Scholar] [CrossRef]
- Martínez, M.T.; Arrillaga, I.; Sales, E.; Pérez-Oliver, M.A.; González-Mas, M.d.C.; Corredoira, E. Micropropagation, Characterization, and Conservation of Phytophthora cinnamomi-Tolerant Holm Oak Mature Trees. Forests 2021, 12, 1634. [Google Scholar] [CrossRef]
- Vieitez, A.M.; San José, M.C.; Corredoira, E. Cryopreservation of zygotic embryonic axes and somatic embryos of European chestnut. In Plant Embryo Cultures: Methods and Protocols, Methods in Molecular Biology; Thorpe, T.A., Yeung, E.C., Eds.; Springer: New York, NY, USA, 2011; Volume 710, pp. 201–213. [Google Scholar]
- Vieitez, A.M.; Corredoira, E.; Martínez, M.T.; San-José, M.C.; Sánchez, C.; Valladares, S.; Vidal, N.; Ballester, A. Application of biotechnological tools to Quercus improvement. Eur. J. For. Res. 2012, 131, 519–539. [Google Scholar] [CrossRef]
- Normah, N.M.; Sulong, N.; Reed, B.M. Cryopreservation of shoot tips of recalcitrant and tropical species: Advances and strategies. Cryobiology 2019, 87, 1–14. [Google Scholar] [CrossRef]
- Engelmann, F. Plant cryopreservation: Progress and prospects. Vitr. Cell. Dev. Biol. Plant 2004, 5, 427–433. [Google Scholar] [CrossRef]
- Find, J.I.; Kristensen, M.M.H.; Nørgaard, J.V.; Krogstrup, P. Effect of culture period and cell density on regrowth following cryopreservation of embryogenic suspension cultures of Norway spruce and Sitka spruce. Plant Cell Tissue Organ Cult. 1998, 53, 27–33. [Google Scholar] [CrossRef]
- Sánchez-Romero, C.; Swennen, R.; Panis, B. Cryopreservation of olive embryogenic cultures. Cryo Lett. 2009, 30, 359–372. [Google Scholar]
- Guzmán-García, E.; Bradaï, F.; Sánchez-Romero, C. Cryopreservation of avocado embryogenic cultures using the droplet-vitrification method. Acta Physiol. Plant. 2013, 35, 183–193. [Google Scholar] [CrossRef]
- Bradaï, F.; Almagro-Bastante, J.; Sánchez-Romero, C. Cryopreservation of olive somatic embryos using the droplet-vitrification method: The importance of explant culture conditions. Sci. Hortic. 2017, 218, 14–22. [Google Scholar] [CrossRef]
- Martínez, M.T.; Vieitez, F.J.; Solla, A.; Tapias, R.; Ramírez-Martín, N.; Corredoira, E. Vegetative Propagation of Phytophthora cinnamomi-Tolerant Holm Oak Genotypes by Axillary Budding and Somatic Embryogenesis. Forests 2020, 11, 841. [Google Scholar] [CrossRef]
- Holliday, C.P.; Merkle, S. Preservation of American chestnut germplasm by cryostorage of embryogenic cultures. J. Am. Chestnut Found 2000, 14, 46–52. [Google Scholar]
- Vendrame, W.A.; Holliday, C.P.; Montello, P.M.; Smith, D.R.; Merkle, S.A. Cryopreservation of yellow-poplar and sweetgum embryogenic cultures. New Forests 2001, 21, 283–292. [Google Scholar] [CrossRef]
- Corredoira, E.; Valladares, S.; Allona, I.; Aragoncillo, C.; Vieitez, A.M.; Ballester, A. Genetic Transformation of European Chestnut Somatic Embryos with a Native Thaumatin-Like Protein (CsTL1) Gene Isolated from Castanea sativa Seeds. Tree Physiol. 2012, 32, 1389–1402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corredoira, E.; San José, M.C.; Vieitez, A.M.; Allona, I.; Cipriano, A.; Ballester, A. Agrobacterium-mediated transformation of European chestnut somatic embryos with a Castanea sativa (Mill.) endochitinase gene. New For. 2016, 47, 669–684. [Google Scholar] [CrossRef]
- Cano, V.; Martínez, M.T.; San José, M.C.; Couselo, J.L.; Varas, E.; Bouza-Morcillo, L.; Toribio, M.; Corredoira, E. Regeneration of Transgenic Plants by Agrobacterium-Mediated Transformation of Quercus ilex L. Somatic Embryos with the Gene CsTL1. New For. 2020, 51, 1003–1021. [Google Scholar] [CrossRef]
- Cano, V.; Martínez, M.T.; Couselo, J.L.; Varas, E.; Vieitez, F.J.; Corredoira, E. Efficient Transformation of Somatic Embryos and Regeneration of Cork Oak Plantlets with A Gene (CsTL1) Encoding a Chestnut Thaumatin-Like Protein. Int. J. Mol. Sci. 2021, 22, 1757. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. Cryo Lett. 2007, 28, 151–172. [Google Scholar]
- Yamuna, G.; Sumathi, V.; Geetha, S.P.; Praveen, K.; Swapna, N.; Nirmal, B.K. Cryopreservation of in vitro grown shoots of ginger (Zingiber officinale Rosc.). Cryo Lett. 2007, 28, 241–252. [Google Scholar]
- Sánchez, C.; Martínez, M.T.; Vidal, N.; San José, M.C.; Valladares, S.; Vieitez, A.M. 2008. Preservation of Quercus robur germplasm by cryostorage of embryogenic cultures derived from mature trees and RAPD analysis of genetic stability. Cryo Lett. 2008, 29, 493–504. [Google Scholar]
- Gallard, A.; Panis, B.; Dorion, N.; Swennen, R.; Grapin, A. Cryopreservation of Pelargonium apices by droplet-vitrification. Cryo Lett. 2008, 29, 243–251. [Google Scholar]
- Uchendu, E.; Lata, H.; Chandra, S.; Khan, I.A.; ElSohly, M.A. Cryopreservation of Shoot Tips of Elite Cultivars of Cannabis sativa L. by Droplet Vitrification. Med. Cannabis Cannabinoids 2019, 2, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Aceves, L.; Portillo, L.; Folgado, R.; de Jesús Romo-Paz, F.; Gonzalez-Arnao, M.T. New Approaches for Micropropagation and Cryopreservation of Agave Peacockii, An Endangered Species. Research Square Platform LLC. Available online: https://www.researchsquare.com/article/rs-1013233/v1 (accessed on 1 November 2021).
- Takagi, H. Recent developments in cryopreservation of shoot apices of tropical species. In Cryopreservation of Tropical Plant Germplasm. Current Research Progress and Application; JIRCAS International Agriculture Series No. 8; IPGRI: Rome, Italy, 2021; pp. 178–192. [Google Scholar]
- Grenier de March, G.; de Boucaud, M.T.; Chmielarz, P. Cryopreservation of Prunus avium L. embryogenic tissues. Cryo Lett. 2005, 26, 341–348. [Google Scholar]
- San José, M.C.; Corredoira, E.; Oliveira, H.; Santos, C. Cryopreservation of somatic embryos of Alnus glutinosa (L.) Gaertn. and confirmation of ploidy stability by flow cytometry. Plant Cell Tissue Organ Cult. 2015, 123, 489–499. [Google Scholar] [CrossRef]
- O’Brien, C.; Constantin, M.; Walia, A.; Lim Yuan Yiing, J.; Mitter, N. Cryopreservation of somatic embryos for avocado germplasm conservation. Sci. Hortic. 2016, 211, 328–335. [Google Scholar] [CrossRef]
- Bradaï, F.; Sánchez-Romero, C. Effect of Cryopreservation on Olive (Olea europaea L.) Plant Regeneration via Somatic Embryogenesis. Plants 2021, 10, 34. [Google Scholar] [CrossRef]
- Harding, K. Genetic integrity of cryopreserved plant cells: A review. Cryo Lett. 2004, 25, 3–22. [Google Scholar]
- Nunes, S.; Marum, L.; Farinha, N.; Tolentino Pereira, V.; Almeida, T.; Dias, M.C.; Conceição Santos, C. Plant regeneration from ploidy-stable cryopreserved embryogenic lines of the hybrid Pinus elliottii × P. caribaea. Ind. Crops Prod. 2017, 105, 215–224. [Google Scholar] [CrossRef]
- Fernandes, P.; Rodriguez, E.; Pinto, G.; Roldán-Ruiz, I.; Loose, M.D.; Santos, C. Cryopreservation of Quercus suber somatic embryos by encapsulation-dehydration and evaluation of genetic stability. Tree Physiol. 2008, 28, 1841–1850. [Google Scholar] [CrossRef]
- Popova, E.V.; Lee, E.J.; Wu, C.H.; Hahn, E.J.; Paek, K.Y. A simple method for cryopreservation of Ginkgo biloba callus. Plant Cell Tissue Organ Cult. 2009, 97, 337–343. [Google Scholar] [CrossRef]
- Ahn, C.H.; Heo, K.; Park, H.S.; Choi, Y.E. In vitro propagation and cryopreservation of Thuja koraiensis Nakai via somatic embryogenesis. Vitro Cell. Dev. Biol. Plant 2019, 55, 605–614. [Google Scholar] [CrossRef]
- Barra-Jiménez, A.; Blasco, M.; Ruiz-Galea, M.; Celestino, C.; Alegre, J.; Arrillaga, I.; Toribio, M. Cloning mature holm oak trees by somatic embryogenesis. Trees 2014, 28, 657–667. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrand, A.C. Medium and techniques for induction of growth of monocotyledonous and dicotyledonous plant cell culture. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gresshoff, P.M.; Doy, C.H. Development and differentiation of haploid Lycopersicon esculentum. Planta 1972, 107, 161–170. [Google Scholar] [CrossRef]
- Loureiro, J.; Rodríguez, E.; Dolezel, J.; Santos, C. Two new nuclear isolation buffers for plant DNA flow cytometry. A test with 37 species. Ann Bot. 2007, 100, 875–888. [Google Scholar] [CrossRef]
- Dolezel, J.; Bartos, J.; Voglmayr, H.; Greilhuber, J. Nuclear DNA content and genome size of trout and human. Cytometry 2003, 51A, 127–128. [Google Scholar]
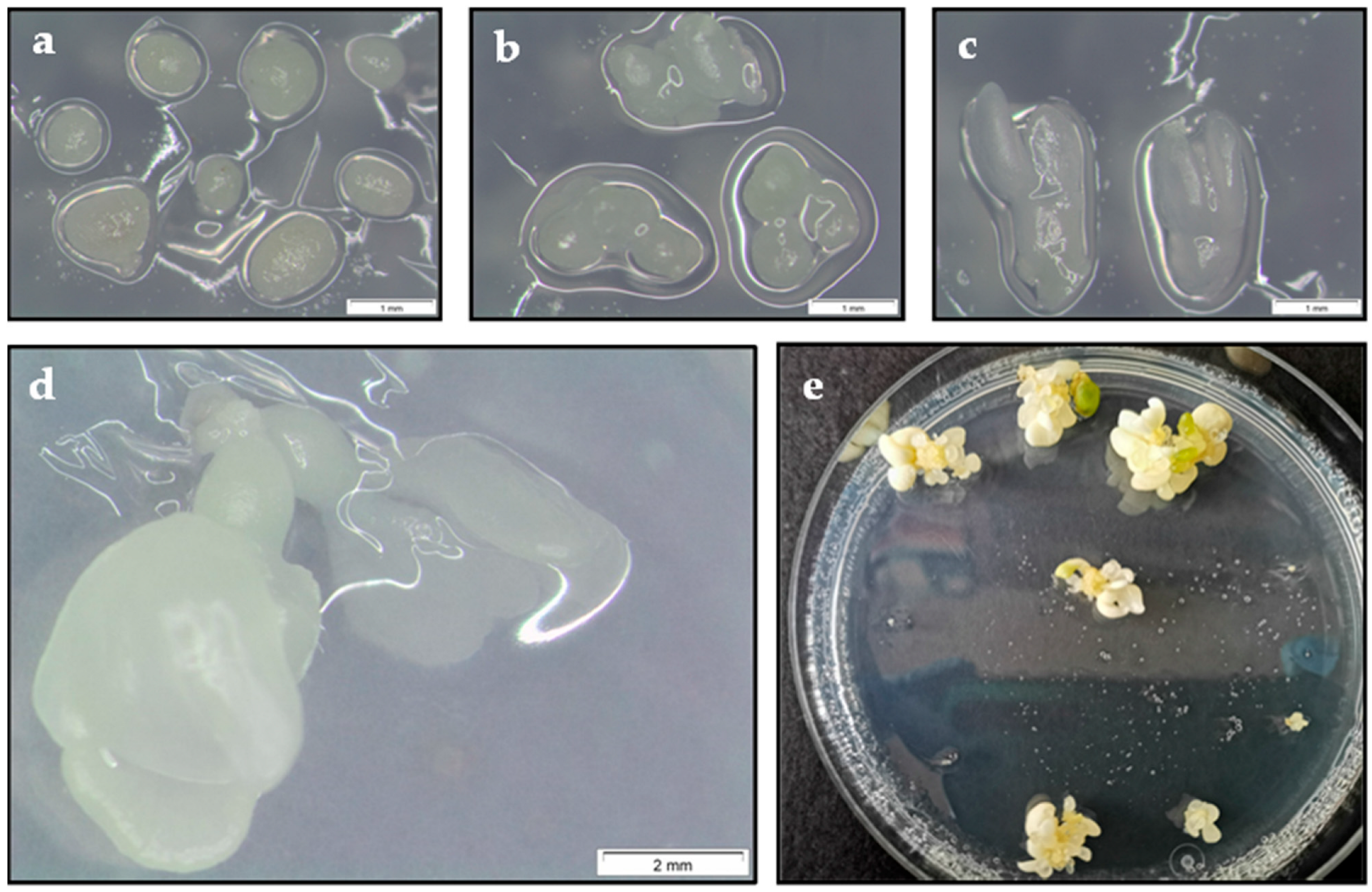

| Developmental Stage of Somatic Embryos | Survival (%) | Embryo Recovery (%) | ||||
|---|---|---|---|---|---|---|
| Line Q8 | −LN | +LN | Mean | −LN | +LN | Mean |
| NES | 100 ± 0.0 a | 88.3 ± 2.8 a | 94.1 a | 96.7 ± 2.8 a | 56.7 ± 2.8 b | 76.7 a |
| Globular-heart | 100 ± 0.0 a | 3.3 ± 2.8 b | 51.7 b | 66.7 ± 2.8 b | 3.3 ± 2.8 c | 35.0 b |
| Cotyledonary | 100 ± 0.0 a | 0.0 ± 0.0 b | 50.0 b | 3.3 ± 2.8 c | 0.0 ± 0.0 c | 1.65 c |
| Mean | 100 a | 30.5 b | 55.6 a | 30.0 b | ||
| ANOVA II | ||||||
| Cryostorage in LN (A) | p ≤ 0.001 | p ≤ 0.001 | ||||
| Explant (B) | p ≤ 0.001 | p ≤ 0.001 | ||||
| A × B | p ≤ 0.001 | p ≤ 0.01 | ||||
| Line E2 | −LN | +LN | Mean | −LN | +LN | Mean |
| NES | 100 ± 0.0 a | 80.0 ± 0.0 b | 90.0 a | 93.3 ± 2.8 a | 46.7 ± 2.8 c | 70.0 a |
| Globular-heart | 100 ± 0.0 a | 0.0 ± 0.0 c | 50.0 b | 66.7 ± 2.8 b | 0.0 ± 0.0 d | 33.4 b |
| Cotyledonary | 100 ± 0.0 a | 0.0 ± 0.0 c | 50.0 b | 3.3 ± 2.8 d | 0.0 ± 0.0 d | 1.65 c |
| Mean | 100 a | 26.7 b | 54.4 a | 15.6 b | ||
| ANOVA II | ||||||
| Cryostorage in LN (A) | p ≤ 0.001 | p ≤ 0.001 | ||||
| Explant (B) | p ≤ 0.001 | p ≤ 0.001 | ||||
| A × B | p ≤ 0.001 | p ≤ 0.01 | ||||
| (a) | ||||
| Embryogenic Line/Time in PVS2 (min) | Control PVS2−LN | Storage Time in LN (Months) | ||
| Q8 | 1 | 6 | 12 | |
| PVS2 15 | 96.7 ± 3.2 | 90.0 ± 5.6 | 56.7 ± 3.2 | 63.3 ± 3.2 |
| PVS2 30 | 90.0 ± 5.6 | 80.0 ± 0.0 | 60.0 ± 5.6 | 66.7 ± 3.2 |
| E2 | 1 | 6 | 12 | |
| PVS2 15 | 90.0 ± 5.6 | 66.7 ± 8.5 | 63.3 ± 11.6 | 66.7 ± 3.2 |
| PVS2 30 | 60.0 ± 5.6 | 53.3 ± 8.5 | 43.3 ± 3.2 | 40.0 ± 5.6 |
| ANOVA II | ||||
| Genotype (A) | p ≤ 0.05 | p ≤ 0.01 | ns | ns |
| PVS2 time (B) | p ≤ 0.05 | ns | ns | ns |
| A × B | ns | ns | ns | p ≤ 0.05 |
| (b) | ||||
| Embryogenic Line/Time in PVS2 (min) | Control PVS2−LN | Storage Time in LN (Months) | ||
| Q8 | 1 | 6 | 12 | |
| PVS2 15 | 93.3 ± 3.2 | 83.3 ± 8.5 | 53.3 ± 3.2 | 60.0 ± 0.0 |
| PVS2 30 | 86.7 ± 6.4 | 50.0 ± 5.6 | 60.0 ± 5.6 | 56.7 ± 8.5 |
| E2 | 1 | 6 | 12 | |
| PVS2 15 | 90.0 ± 5.6 | 56.7 ± 11.6 | 53.3 ± 6.4 | 60.0 ± 5.6 |
| PVS2 30 | 60.0 ± 5.6 | 40.0 ± 0.0 | 40.0 ± 0.0 | 40.0 ± 5.6 |
| ANOVA II | ||||
| Genotype (A) | ns | ns | ns | ns |
| PVS2 time (B) | ns | p ≤ 0.05 | ns | ns |
| A × B | ns | ns | ns | ns |
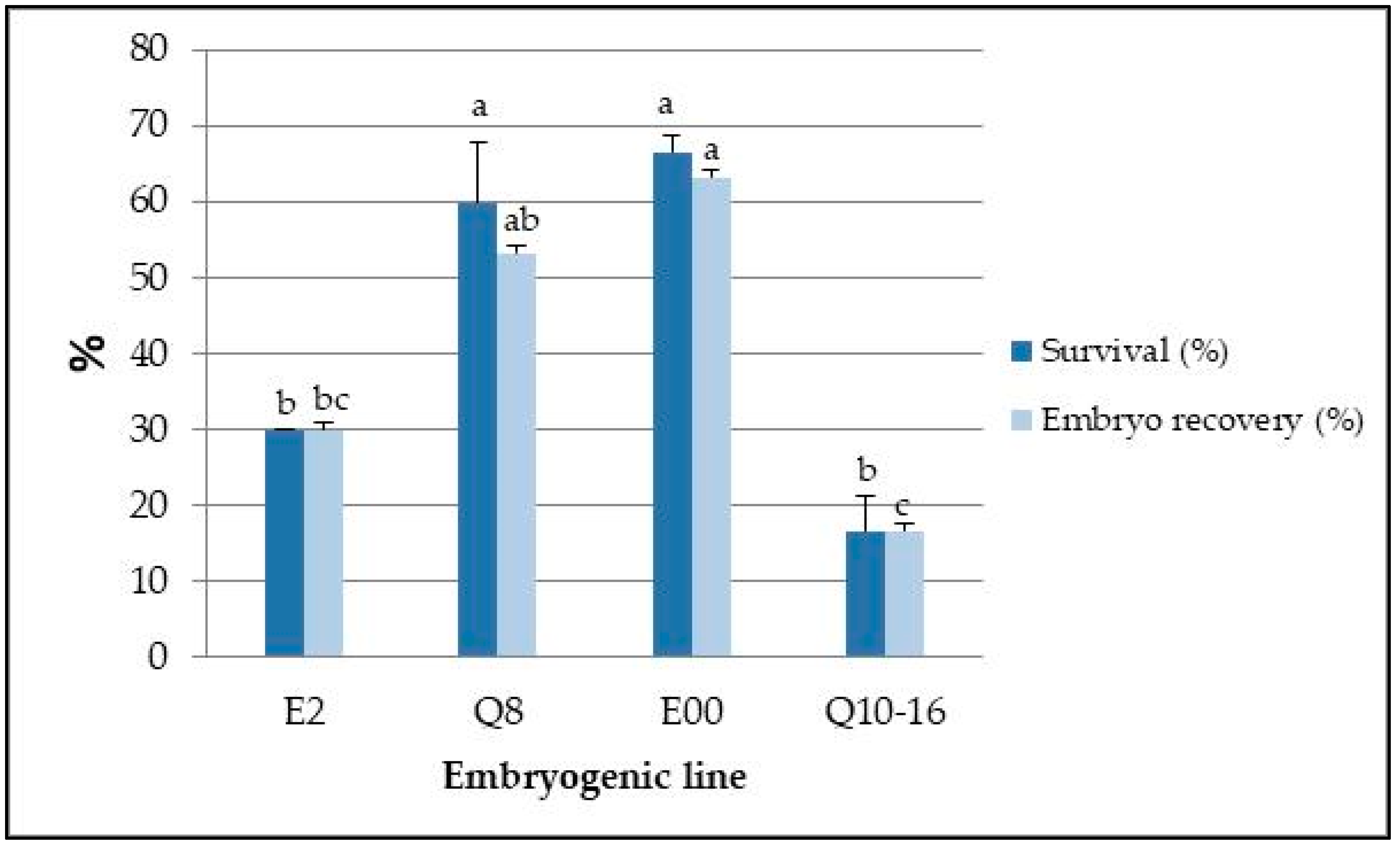
| Embryogenic Line | Survival (%) | Embryo Recovery (%) | ||
|---|---|---|---|---|
| −LN | +LN | −LN | +LN | |
| Q8 | 86.7 ± 3.2 a | 80.0 ± 0.0 a | 80.0 ± 6.4 | 60.0 ± 5.6 |
| E2 | 86.7 ± 6.4 a | 56.7 ± 3.2 b | 80.0 ± 0.0 | 43.3 ± 8.5 |
| E00 | 90.0 ± 0.0 a | 40.0 ± 5.6 c | 80.0 ± 0.0 | 36.7 ± 3.2 |
| Q10–16 | 86.7 ± 6.4 a | 46.7 ± 8.5 bc | 80.0 ± 9.6 | 43.3 ± 6.4 |
| Mean | 87.5 ± 0.7 a | 55.8 ± 7.6 b | 80.0 ± 0.0 a | 45.8 ± 4.3 b |
| ANOVA II | ||||
| Genotype (A) | ns | ns | ||
| Cryostorage in LN (B) | p ≤ 0.001 | p ≤ 0.001 | ||
| A × B | p ≤ 0.05 | ns | ||
| Embryogenic Lines | Plant Regeneration (%) | Root Length (mm) | Shoot Length (mm) | Leaf Number |
|---|---|---|---|---|
| Q8 + LN | 54.2 ± 3.0 | 52.3 ± 4.5 | 11.2 ± 0.9 | 7.0 ± 0.4 |
| E2 + LN | 45.8 ± 7.4 | 60.4 ± 3.5 | 16.4 ± 1.8 | 9.0 ± 0.4 |
| Embryogenic Line | Tissue | Treatment | Ploidy | DNA Index | nDNA Content (pg/2C) | CV (%) | n | Statistics |
|---|---|---|---|---|---|---|---|---|
| E2 | ||||||||
| Embryos | −LN | 2C | 0.244 ± 0.002 | 2.22 ± 0.02 | 5.27 | 4 | ns | |
| +LN | 2C | 0.243 ± 0.008 | 2.12 ± 0.07 | 5.68 | 4 | ns | ||
| Plantlets | −LN | 2C | 0.237 ± 0.006 | 2.15 ± 0.06 | 5.19 | 4 | ns | |
| +LN | 2C | 0.247 ± 0.005 | 2.24 ± 0.04 | 5.41 | 2 | ns | ||
| Q8 | ns | |||||||
| Embryos | −LN | 2C | 0.216 ± 0.004 | 1.97 ± 0.04 | 5.27 | 4 | ns | |
| +LN | 2C | 0.219 ± 0.003 | 1.99 ± 0.03 | 5.68 | 4 | ns | ||
| Plantlets | −LN | 2C | 0.226 ± 0.004 | 2.06 ± 0.04 | 5.19 | 3 | ns | |
| +LN | 2C | 0.233 ± 0. 007 | 2.12 ± 0.07 | 5.41 | 3 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, M.T.; Suárez, S.; Moncaleán, P.; Corredoira, E. Cryopreservation of Holm Oak Embryogenic Cultures for Long-Term Conservation and Assessment of Polyploid Stability. Plants 2022, 11, 1266. https://doi.org/10.3390/plants11091266
Martínez MT, Suárez S, Moncaleán P, Corredoira E. Cryopreservation of Holm Oak Embryogenic Cultures for Long-Term Conservation and Assessment of Polyploid Stability. Plants. 2022; 11(9):1266. https://doi.org/10.3390/plants11091266
Chicago/Turabian StyleMartínez, Maria Teresa, Sonia Suárez, Paloma Moncaleán, and Elena Corredoira. 2022. "Cryopreservation of Holm Oak Embryogenic Cultures for Long-Term Conservation and Assessment of Polyploid Stability" Plants 11, no. 9: 1266. https://doi.org/10.3390/plants11091266
APA StyleMartínez, M. T., Suárez, S., Moncaleán, P., & Corredoira, E. (2022). Cryopreservation of Holm Oak Embryogenic Cultures for Long-Term Conservation and Assessment of Polyploid Stability. Plants, 11(9), 1266. https://doi.org/10.3390/plants11091266





