Nutrients Uptake and Accumulation in Plant Parts of Fragrant Rosa Species Irrigated with Treated and Untreated Wastewater
Abstract
1. Introduction
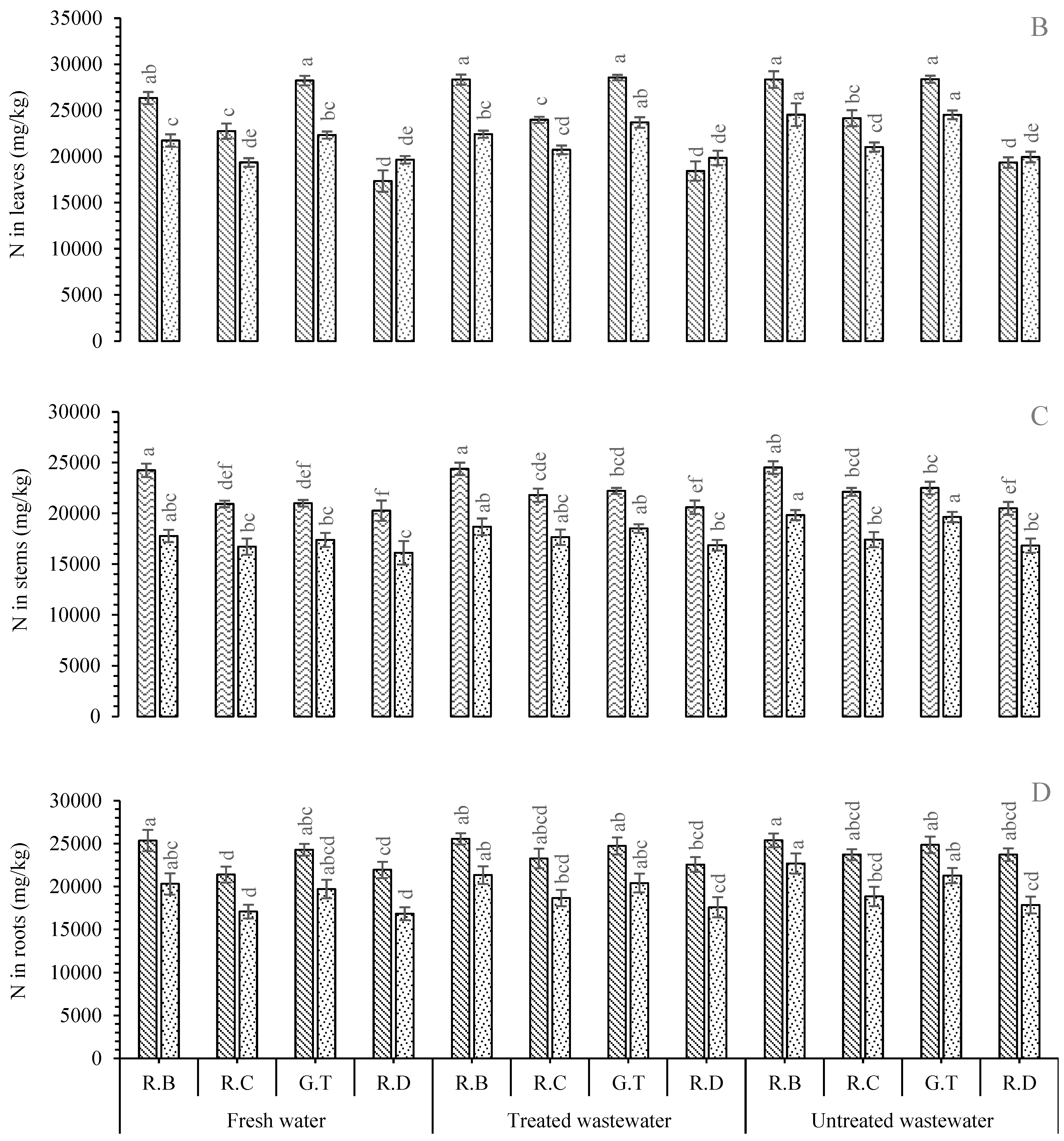
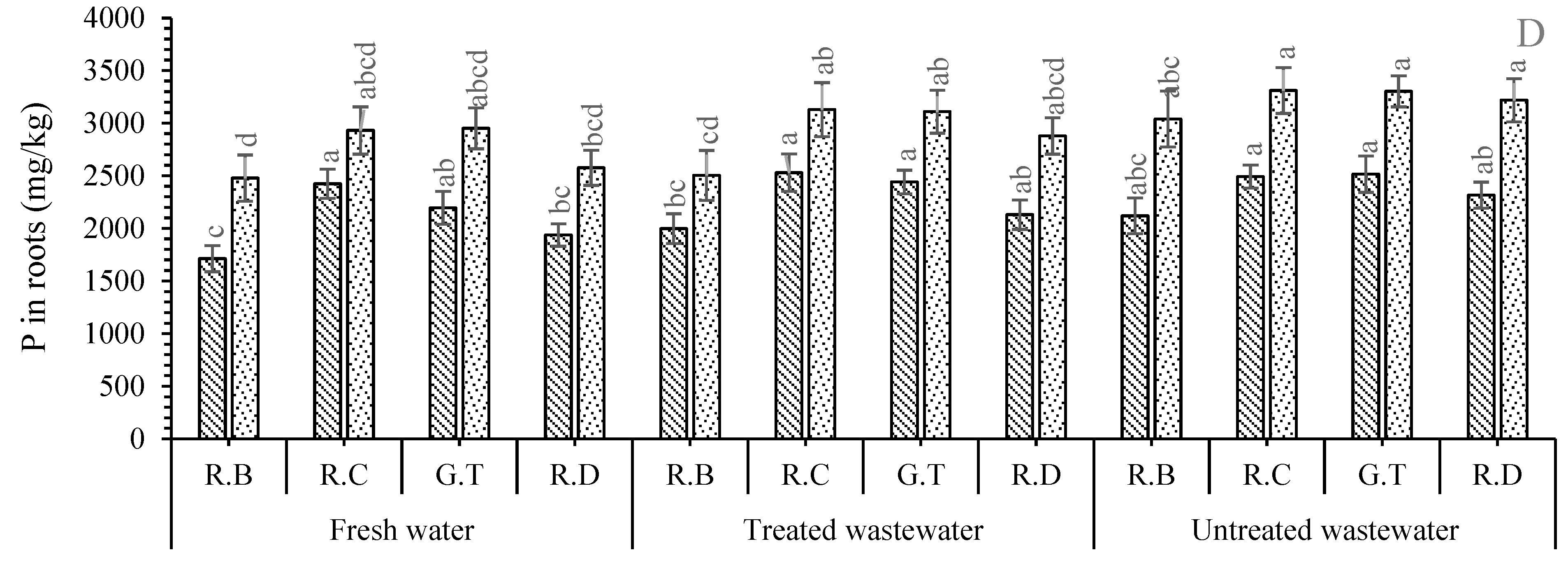
2. Results
Nutrient Concentration in Plant Parts
3. Discussion
4. Materials and Methods
4.1. Research Area Description, Plant Collection, and Experimental Layout
4.2. Decontamination of Wastewater and Analysis
4.3. Determination of Nutrient Elements
Estimation of Nutrients in Plant Parts of Selected Rosa Species
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahsan, M.; Younis, A.; Nafees, M.; Tufail, A.; Shakeel, Q.; Raheel, M.; Nawaz, F.; Jaskani, M.J.; Amin, M.; Sajid, M.; et al. Marginal quality water arbitrated essential oil concentration in metal hoarded flower petals of scented roses. Ecotoxicol. Environ. Saf. 2021, 226, 112853. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.S.; Qi, X. Treated Wastewater Irrigation—A Review. Water 2021, 13, 1527. [Google Scholar] [CrossRef]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.; Perez, J.; Schaar, H.; Fatta-Kassinos, D. Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries. Sci. Total Environ. 2020, 710, 136312. [Google Scholar] [CrossRef] [PubMed]
- Ghernaout, D.; Elboughdiri, N.; Al Arni, S. Water Reuse (WR): Dares, restrictions, and trends. Appl. Eng. 2019, 3, 159–170. [Google Scholar]
- Shuval, H.I.; Adin, A.; Fattal, B.; Rawitz, E.; Yekutiel, P. Wastewater Irrigation in Developing Countries: Health Effects and Technical Solutions; World Bank Report; World Bank: Washington, DC, USA, 1986. [Google Scholar]
- Ahsan, M.; Younis, A.; Jaskani, M.J.; Tufail, A.; Riaz, A.; Schwinghamer, T.; Tariq, U.; Nawaz, F. Heavy metal accumulation imparts structural differences in fragrant Rosa species irrigated with marginal quality water. Ecotoxicol. Environ. Saf. 2018, 159, 240–248. [Google Scholar] [CrossRef]
- Ahsan, M.; Younis, A.; Jaskani, M.J.; Tariq, U.; Shaheen, M.R.; Tufail, A.; Sherani, J.; Nawaz, F. Anatomical changes in stem of scented Rosa spp. in response to heavy metal accumulation under wastewater treatment. Int. J. Agric. Biol. 2019, 21, 1159–1165. [Google Scholar]
- Iqbal, S.; Inam, A.; Inam, A.; Ashfaque, F.; Sahay, S. Potassium and waste water interaction in the regulation of photosynthetic capacity, ascorbic acid and capsaicin in chilli (Capsicum annuum L.) plant. Agric. Water Manag. 2017, 184, 201–210. [Google Scholar] [CrossRef]
- Chen, X.W.; Tsz-Fung, W.J.; Mo, W.Y.; Man, Y.B.; Wang-Wai Ng, C.; Wong, M.H. Ecological performance of the restored south east new territories (sent) landfill in Hong Kong (2000–2012). Land Degrad. Dev. 2015, 1, 1–13. [Google Scholar] [CrossRef]
- U.S. Agency for International Development. Guidelines for Water Reuse; U.S. Agency for International Development: Washington, DC, USA, 2004; pp. 180–181.
- Marinho, L.E.O.; Tonetti, A.L.; Stefanutti, R.; Filho, B.C. Application of reclaimed wastewater in the irrigation of rosebushes. Water Air Soil Pollut. 2013, 224, 1669–1675. [Google Scholar] [CrossRef]
- Modanloo, M.; Darvishi, H.H. Nitrogen application and irrigation with purified urban wastewater effect on NPK accumulation in Fenugreek (Trigonella foenum L.). Walia J. 2015, 31, 25–29. [Google Scholar]
- Morgan, K.T.; Wheaton, T.A.; Parsons, L.R.; Castle, W.S. Effects of reclaimed municipal wastewater on horticultural characteristics, fruit quality, and soil and leaf mineral concentration of citrus. HortScience 2008, 43, 459–464. [Google Scholar] [CrossRef]
- Khaleel, R.I.; Ismail, N.; Ibrahim, M.H. The impact of wastewater treatments on seed germination and biochemical parameter of Abelmoschus esculentus L. Soc. Behav. Sci. 2013, 91, 453–460. [Google Scholar] [CrossRef]
- Tymchuk, I.; Shkvirko, O.; Sakalova, H.; Malovany, M.; Dabizhuk, T.; Shevchuk, O.; Matviichuk, O.; Vasylinych, T. Wastewater a Source of Nutrients for Crops Growth and Development. J. Ecol. Eng. 2020, 21, 88–96. [Google Scholar] [CrossRef]
- Gryshko, V.M.; Korinovskaya, O.N. Influence of organo-mineral fertilizers on the basis of precipitation of sewage on micromycetes cenosis. Gruntoznavstvo 2015, 16, 75–81. [Google Scholar] [CrossRef][Green Version]
- Aftab, N.; Saleem, K.; Khan, A.H.A.; Butt, T.A.; Mirza, C.R.; Hussain, J.; Farooq, G.; Tahir, A.; Yousaf, S.; Zafar, M.I.; et al. Cosmos sulphureus Cav. is more tolerant to lead than copper and chromium in hydroponics system. Int. J. Environ. Sci. Technol. 2021, 18, 2325–2334. [Google Scholar] [CrossRef]
- Ali, S.; Zohaib, A.; Muhammad, R.; Ihsan, E.Z.; Ilkay, Y.; Aydın, Ü.; Mohamed, M.; Abdel, D.; May, B.J.; Mirza, H.; et al. Application of floating aquatic plants in phytoremediation of heavy metals polluted water: A review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef]
- Karahan, F.; Ozyigit, I.I.; Saracoglu, I.A.; Yalcin, I.E.; Ozyigit, A.H.; Ilcim, A. Heavy metal levels and mineral nutrient status in different parts of various medicinal plants collected from Eastern Mediterranean Region of Turkey. Biol. Trace Elem. Res. 2020, 197, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Leblebici, Z.; Kar, M. Heavy metals accumulation in vegetables irrigated with different water sources and their human daily intake in Nevsehir. J. Agric. Sci. Technol. 2020, 20, 401–415. [Google Scholar]
- Sarwar, T.; Muhammad, S.; Natasha; Khalid, S.; Shah, A.H.; Ahmad, N.; Naeem, M.A.; ul Haq, Z.; Murtaza, B.; Bakhat, H.F. Quantification and risk assessment of heavy metal build-up in soil–plant system after irrigation with untreated city wastewater in Vehari, Pakistan. Environ. Geochem. Health 2020, 42, 4281–4297. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Amna, K.; Cyrus, R.M.; Tayyab, A.B.; Rocío, B.; Basit, A.; Mazhar, I.; Sohail, Y. Ornamental plants for the phytoremediation of heavy metals: Present knowledge and future perspectives. Environ. Res. 2021, 195, 110780. [Google Scholar] [CrossRef]
- Bernstein, N.; Chaimovitch, D.; Dudai, N. Effect of irrigation with secondary treated effluent on essential oil, antioxidant activity, and phenolic compounds in oregano and rosemary. Agron. J. 2009, 101, 1–10. [Google Scholar] [CrossRef]
- Darvishi, H.H.; Manshouri, M.; Sedghi, H.; Jahromi, S.H.M. Irrigation influence by treated domestic wastewater instead of agronomical water on essential oil yield of basil (Ocimum basilicum L.). Afr. J. Microbiol. Res. 2010, 4, 475–479. [Google Scholar]
- Elsokkary, I.H.; Aboukila, A.F. Beneficial additive values of wastewater irrigation of two aromatic plants grown in low fertile soil. Water Sci. 2020, 34, 132–142. [Google Scholar] [CrossRef]
- Mahmood, S.; Reza, M.R.; Hossain, M.G.; Hauser, B. Response of cytokinins on in vitro shoot multiplication of Rose cv. Frisco. J. Agric. Sci. Technol. 2018, 5, 8–12. [Google Scholar]
- Kotuby-Amacher, J.; Koeing, R.; Kitchen, B. Salinity and Plant Tolerance. Utah State Uni. Ext. 2000. Available online: http://www.extension.usu.edu/publica/agpubs/agso03.pdf (accessed on 15 May 2013).
- Western Australia Department of Agriculture. Salinity Tolerance Chart. 2003. Available online: http://www.staneyo.com/news_files/water/salinity_chart.html (accessed on 27 December 2014).
- Dyubeni, L.; Mayekiso, B.; Magwa, M.L. A comparative study on essential oil yield and composition of rose-scented geranium (P.c.v. Rose) commercially grown on three different sites of the Amathole region in the Eastern Cape, South Africa. Afr. J. Agric. Res. 2012, 7, 5842–5848. [Google Scholar]
- Alghobar, M.A.; Suresha, S. Evaluation of metal accumulation in soil and tomatoes irrigated with sewage water from Mysore city, Karnataka, India. J. Saudi Soc. Agri. Sci. 2015, 16, 49–59. [Google Scholar] [CrossRef]
- Salehi, A.; Tabari, M. Nutritional properties of soil and leaves of Tehran pine trees irrigated with urban sewage. Environ. Sci. Technol. 2014, 16, 262–274. [Google Scholar]
- De Carlo, L.; Battilani, A.; Solimando, D.; Caputo, M.C. Application of time-lapse ERT to determine the impact of using brackish wastewater for maize irrigation. J. Hydrol. 2020, 582, 124465. [Google Scholar] [CrossRef]
- Disciglio, G.; Gatta, G.; Libutti, A.; Gagliardi, A.; Carlucci, A.; Lops, F.; Cibelli, F.; Tarantino, A. Effects of irrigation with treated agro-industrial wastewater on soil chemical characteristics and fungal populations during processing tomato crop cycle. J. Soil Sci. Plant Nutr. 2015, 15, 765–780. [Google Scholar] [CrossRef]
- Smirnoff, N.; Stewart, G.R. Nitrate assimilation and translocation by higher plants: Comparative physiology and ecological consequences. Physiol. Plant. 1985, 64, 133–140. [Google Scholar] [CrossRef]
- Edelstein, M.; Ben-Hur, M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 2018, 234, 431–444. [Google Scholar] [CrossRef]
- Soufan, W.; Okla, M.K.; Al-Ghamdi, A.A. Effects of irrigation with treated wastewater or well water on the nutrient concentration of two alfalfa (Medicago sativa L.) cultivars in Riyadh, Saudi Arabia. Agronomy 2019, 9, 729. [Google Scholar] [CrossRef]
- Mok, H.-F.; Barker, S.F.; Hamilton, A.J. A probabilistic quantitative microbial risk assessment model of norovirus disease burden from wastewater irrigation of vegetables in Shepparton, Australia. Water Res. 2014, 54, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, H.; Vandana, S.; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. [Google Scholar]
- Rajasekar, M.; Udhaya-Nandhini, D.; Swaminathan, V.; Balakrishnan, K. A review on role of macro nutrients on production and quality of vegetables. Int. J. Chem. Stud. 2017, 5, 304–309. [Google Scholar]
- Yuan, H.; Lu, T.; Wang, Y.; Chen, Y.; Lei, T. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Utilization of treated sewage wastewater for green forage production in a hydroponic system. Emir. J. Food Agric. 2011, 32, 80–94. [Google Scholar] [CrossRef]
- Shahbazzadeh, R.; Amirinejad, A.A. Effects of raw municipal wastewater on soil physical quality and biological yield of wheat (case study: Harsin). Iran. J. Soil Water Res. 2018, 49, 83–90. [Google Scholar]
- Chiou, R.J. Risk assessment and loading capacity of reclaimed wastewater to be reused for agricultural irrigation. Environ. Monit. Assess. 2008, 142, 255–262. [Google Scholar] [CrossRef]
- Arienzo, M.; Christen, E.W.; Quayle, W.; Kumar, A. A review of the fate of potassium in the soil-plant system after land application of wastewaters. J. Hazard. Mat. 2009, 164, 415–422. [Google Scholar] [CrossRef]
- Al-Suhaibani, N.; Seleiman, M.F.; El-Hendawy, S.; Abdella, K.; Alotaibi, M.; Alderfasi, A. Integrative effects of treated wastewater and synthetic fertilizers on productivity, energy characteristics, and elements uptake of potential energy crops in an arid agro-ecosystem. Agronomy 2021, 11, 2250. [Google Scholar] [CrossRef]
- Tak, H.I.; Babalola, O.O.; Huyser, M.H.; Inam, A. Urban wastewater irrigation and its effect on growth: Photosynthesis and yield of chickpea under different doses of potassium. Soil Sci. Plant Nutr. 2013, 59, 156–167. [Google Scholar] [CrossRef]
- Mkhinini, M.; Boughattas, I.; Alphonse, V.; Livet, A.; Gıustı-Mıller, S.; Bannı, M.; Bousserrhıne, N. Heavy metal accumulation and changes in soil enzymes activities and bacterial functional diversity under long-term treated wastewater irrigation in East Central region of Tunisia (Monastir governorate). Agric. Water Manag. 2020, 235, 106150. [Google Scholar] [CrossRef]
- Nicolas, E.; Alarcón, J.J.; Mounzer, O.; Pedrero, F.; Nortes, P.A.; Alcobendas, R.; Romero-Trigueros, C.; Bayona, J.M.; MaestreValero, J.F. Long-term physiological and agronomic responses of mandarin trees to irrigation with saline reclaimed water. Agric. Water Manag. 2016, 166, 1–8. [Google Scholar] [CrossRef]
- Chojnacka, K.; Witek-Krowiak, A.; Moustakas, K.; Skrzypczak, D.; Mikula, K.; Loizidou, M. A transition from conventional irrigation to fertigation with reclaimed wastewater: Prospects and challenges. Renew. Sustain. Energy Rev. 2020, 130, 109959. [Google Scholar] [CrossRef]
- Mansir, I.; Oertlé, E.; Choukr-Allah, R. Evaluation of the Performance and Quality of Wastewater Treated by M’zar Plant in Agadir, Morocco. Water 2021, 13, 954. [Google Scholar] [CrossRef]
- Dysko, J.; Kaniszewski, S.; Kowalczyk, W. Lignite as a new medium in soilless cultivation of tomato. J. Elem. 2015, 20, 559–569. [Google Scholar]
- Jilani, M.I.; Ahmad, M.I.; Hanif, R.; Nadeem, R.; Hanif, M.A.; Khan, M.A.; Ahmad, I.; Iqbal, T. Proximate analysis and mineral profile of three elite cultivars of Rosa hybrida flowers. Pak. J. Bot. 2012, 44, 1711–1714. [Google Scholar]
- Manohara, B.; Belagali, S.L. Characterization of essential nutrients and heavy metals during municipal solid waste composting. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 9664–9672. [Google Scholar]
- Akhtar, N.; Inam, A.; Inam, A.; Khan, N.A. Effects of city wastewater on the characteristics of wheat with varying doses of nitrogen, phosphorus, and potassium. Recent Res. Sci. Technol. 2012, 4, 18–29. [Google Scholar]
- Ibekwe, A.M.; Gonzalez-Rubio, A.; Suarez, D.L. Impact of treated wastewater for irrigation on soil microbial communities. Sci. Total Environ. 2018, 622, 1603–1610. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Lopes, A.R.; Vaz-Moreira, I.; Silva, E.F.; Manaia, C.M.; Nunes, O.C. Wastewater reuse in irrigation: A microbiological perspective on implications in soil fertility and human and environmental health. Environ. Int. 2015, 75, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elwahed, M.S. Influence of long-term wastewater irrigation on soil quality and its spatial distribution. Ann. Agric. Sci. 2018, 63, 191–199. [Google Scholar] [CrossRef]
- Chen, L.; Feng, Q.; Li, C.; Wei, Y.; Zhao, Y.; Feng, Y.; Zheng, H.; Li, F.; Li, H. Impacts of aquaculture wastewater irrigation on soil microbial functional diversity and community structure in arid regions. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, F.; Lu, H.; Liu, Y.; Mao, C. Phosphate uptake and transport in plants: An elaborate regulatory system. Plant Cell Physiol. 2021, 62, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, S.; Jiao, W.; Wang, M.; Chang, A.C. Reclaimed water: A safe irrigation water source? Environ. Dev. 2013, 8, 74–83. [Google Scholar] [CrossRef]
- Al-Absi, K.M.; Al-Nasir, F.M.; Mahadeen, A.Y. Mineral content of three olive cultivars irrigated with treated industrial wastewater. Agric. Water Manag. 2009, 96, 616–626. [Google Scholar] [CrossRef]
- McDonnell, R.P.; Staines, M.H.; Bolland, H.S. Determining the critical plant test potassium concentration for annual and Italian ryegrass on dairy pastures in south-western Australia. Grass Forage Sci. 2018, 73, 112–122. [Google Scholar] [CrossRef]
- Bedbabis, S.; Ferrara, G.; Ben-Rouina, B.; Boukhris, M. Effects of irrigation with treated wastewater on olive tree growth, yield and leaf mineral elements at short term. Sci. Hortic. 2010, 126, 345–350. [Google Scholar] [CrossRef]
- Batarseh, M.; Rawajfeh, A.; Ioannis, K.; Prodromos, K. Treated municipal wastewater irrigation impact on olive trees (Olea Europaea L.) at Al-Tafilah, Jordan. Water Air Soil Pollut. 2011, 217, 185–196. [Google Scholar] [CrossRef]
- Alghobar, M.A.; Suresha, S. Effect of wastewater irrigation on growth and yield of rice crop and uptake and accumulation of nutrient and heavy metals in soil. App. Ecol. Environ. Sci. 2016, 4, 53–60. [Google Scholar]
- United States Salinity Laboratory Staff. Diagnosis and Improvement of Saline and Alkali Soils; USDA Hand Book No. 60; United States Government Publishing Office: Washington, DC, USA, 1954.
- Alloway, B.J. Heavy Metals in Soils; John Wiley and Sons: New York, NY, USA, 1990. [Google Scholar]
- WHO. Guidelines for the Safe Use of Wastewater and Food Stuff; Report of the Joint WHO/FAO: Geneva, Switzerland, 2013. [Google Scholar]
- Environmental Protection Agency. Water Quality Standards; Government of Pakistan: Islamabad, Pakistan, 2007.
- Kiziloglu, F.M.; Turan, M.; Sahin, U.; Kuslu, Y.; Dursun, A. Effects of untreated and treated wastewater irrigation on some chemical properties of cauliflower (Brassica olerecea L. var. Botrytis) and red cabbage (Brassica olerecea L. var. Rubra) grown on calcareous soil in Turkey. Agric. Water Manag. 2008, 95, 716–724. [Google Scholar] [CrossRef]
- Pescod, M.B. Wastewater Treatment and Use in Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1992; p. 47. [Google Scholar]
- Eaton, A.D.; Glescer, L.S.; Rice, E.W.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Chapman, H.D.; Parker, F. Determination of NPK, Methods of Analysis for Soil, Plant and Water, 2nd ed.; University of California, Agriculture Division: Riverside, CA, USA, 1961; pp. 150–179. [Google Scholar]
- Anonymous. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemist: Arlington, VA, USA, 1990. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometric Approach, 3rd ed.; McGraw Hill: New York, NY, USA, 1997. [Google Scholar]






| Sr. No. | Characteristics | Unit | Value | IASS * | |
|---|---|---|---|---|---|
| 1 | Texture | Clay loam soil | |||
| 00–15cm | 15–30 cm | ||||
| 2 | pH | 8.2 | 8.2 | 4–8.5 | |
| 3 | EC | dS m−1 | 2.54 | 2.49 | 4 |
| 4 | Organic Matter | % | 1.12 | 1.18 | >0.86 |
| 5 | Nitrogen | mg/kg | 0.041 | 0.041 | --- |
| 6 | Phosphorus | mg/kg | 10.5 | 9.5 | >7.0 |
| 7 | Potassium | mg/kg | 194 | 134 | >80 |
| 8 | Lead | mg/kg | 3.16 | 3.32 | 6.0 |
| 9 | Cadmium | mg/kg | 0.04 | 0.05 | 1.0 |
| 10 | Nickel | mg/kg | 0.36 | 0.34 | 20 |
| 11 | Zinc | mg/kg | 5.28 | 3.6 | 10 |
| 12 | Copper | mg/kg | 3.04 | 2.3 | 6.0 |
| Sr. No. | Parameters | Untreated Wastewater | Treated Wastewater | Freshwater | NEQS |
|---|---|---|---|---|---|
| 1 | EC (dS/m) | 2.13 | 1.43 | 0.13 | 1.5 |
| 2 | pH | 8.31 | 7.58 | 7.22 | 6–9.2 |
| 3 | Color | Greyish | Rust Brown | - | - |
| 4 | Turbidity | 155 | 29.12 | 43 | - |
| 5 | Hardness (mg/dm3) | 536 | 416 | 184 | - |
| 6 | DO (mg/dm3) | 1.36 | 2.38 | 4 | - |
| 7 | BOD (mg/dm3) | 432 | 267 | - | 260 |
| 8 | COD (mg/dm3) | 558 | 481 | - | 450 |
| 9 | TDS (mg/dm3) | 1678 | 1281 | 218 | 3000 |
| 10 | SS (mg/dm3) | 1.1 | 0.15 | 0.8 | - |
| 11 | Total Solids (mg/dm3) | 1372 | 982 | 218 | - |
| 12 | TSS (mg/dm3) | 194 | 63 | 17 | 400 |
| 13 | Chlorides (mg/dm3) | 436 | 290 | 138 | 1000 |
| 14 | Cadmium (mg/dm3) | 0.013 | 0.01 | 0.001 | 0.01 |
| 15 | Nickel (mg/dm3) | 0.12 | 0.08 | 0.10 | 1.0 |
| 16 | Arsenic (mg/dm3) | 0.005 | 0.004 | ND | 1.0 |
| 17 | Zinc (mg/dm3) | 3.48 | 2.62 | 0.18 | 5.0 |
| 18 | Potassium (mg/dm3) | 34.6 | 21.71 | 7.61 | - |
| 19 | Lead (mg/dm3) | 0.66 | 0.42 | 0.021 | 0.5 |
| 20 | Iron (mg/dm3) | 4.82 | 3.47 | 0.32 | 5.0 |
| 21 | Cobalt (mg/dm3) | 0.079 | 0.029 | 0.17 | 0.05 |
| 22 | Copper (mg/dm3) | 0.24 | 0.13 | 0.05 | 0.5 |
| 23 | Chromium (mg/dm3) | 0.98 | 0.73 | 0.04 | 1.0 |
| 24 | Calcium (mg/dm3) | 57.35 | 39.72 | 28.1 | 200 |
| 26 | Sodium (mg/dm3) | 236.91 | 178.23 | 36.47 | 250 |
| 27 | Magnesium (mg/dm3) | 63 | 47 | 30 | 150 |
| 28 | Phosphorus (mg/dm3) | 2.49 | 1.76 | 0.39 | 15 |
| 29 | Total Nitrogen (mg/dm3) | 6.10 | 4.87 | 3.84 | 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahsan, M.; Nafees, M.; Amin, M.; Nawaz, F.; Tufail, A.; Sardar, H.; Shokralla, S.; Mahmoud, E.A.; El-Sabrout, A.M.; Elansary, H.O. Nutrients Uptake and Accumulation in Plant Parts of Fragrant Rosa Species Irrigated with Treated and Untreated Wastewater. Plants 2022, 11, 1260. https://doi.org/10.3390/plants11091260
Ahsan M, Nafees M, Amin M, Nawaz F, Tufail A, Sardar H, Shokralla S, Mahmoud EA, El-Sabrout AM, Elansary HO. Nutrients Uptake and Accumulation in Plant Parts of Fragrant Rosa Species Irrigated with Treated and Untreated Wastewater. Plants. 2022; 11(9):1260. https://doi.org/10.3390/plants11091260
Chicago/Turabian StyleAhsan, Muhammad, Muhammad Nafees, Muhammad Amin, Fahim Nawaz, Aasma Tufail, Hasan Sardar, Shadi Shokralla, Eman A. Mahmoud, Ahmed M. El-Sabrout, and Hosam O. Elansary. 2022. "Nutrients Uptake and Accumulation in Plant Parts of Fragrant Rosa Species Irrigated with Treated and Untreated Wastewater" Plants 11, no. 9: 1260. https://doi.org/10.3390/plants11091260
APA StyleAhsan, M., Nafees, M., Amin, M., Nawaz, F., Tufail, A., Sardar, H., Shokralla, S., Mahmoud, E. A., El-Sabrout, A. M., & Elansary, H. O. (2022). Nutrients Uptake and Accumulation in Plant Parts of Fragrant Rosa Species Irrigated with Treated and Untreated Wastewater. Plants, 11(9), 1260. https://doi.org/10.3390/plants11091260










