Can Grafting Manage Fusarium Wilt Disease of Cucumber and Increase Productivity under Heat Stress?
Abstract
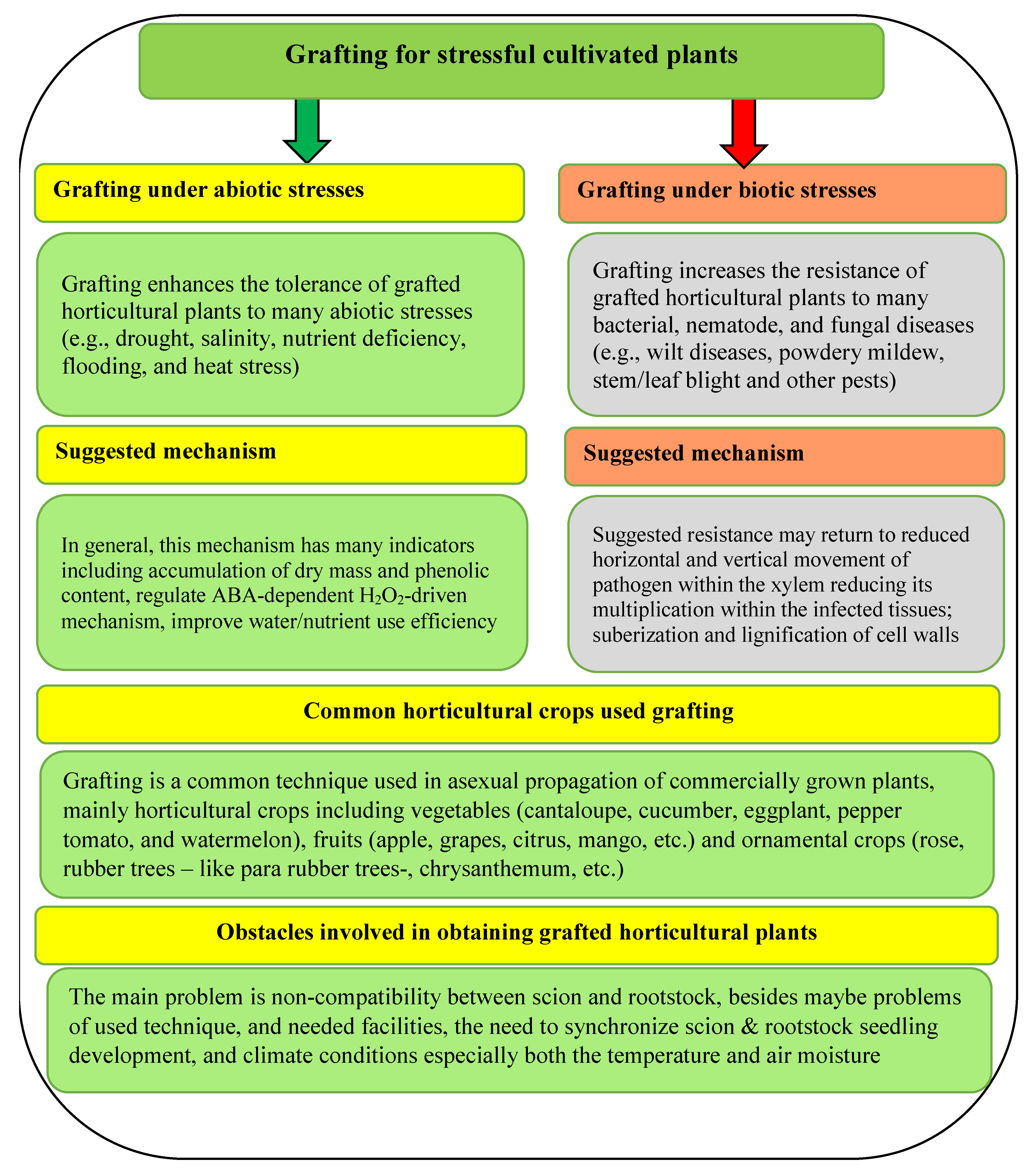
:1. Introduction
2. Results
2.1. Evaluation of Grafted and No-Grafted Cucumber Plants against Fusarium Wilt under Heat Stress
2.2. Anatomical Structure of Grafted Cucumber under Disease Pressure and Heat Stress
2.3. Vegetative Growth in Non-Infected Grafted and Non-Grafted Plants under Heat Stress
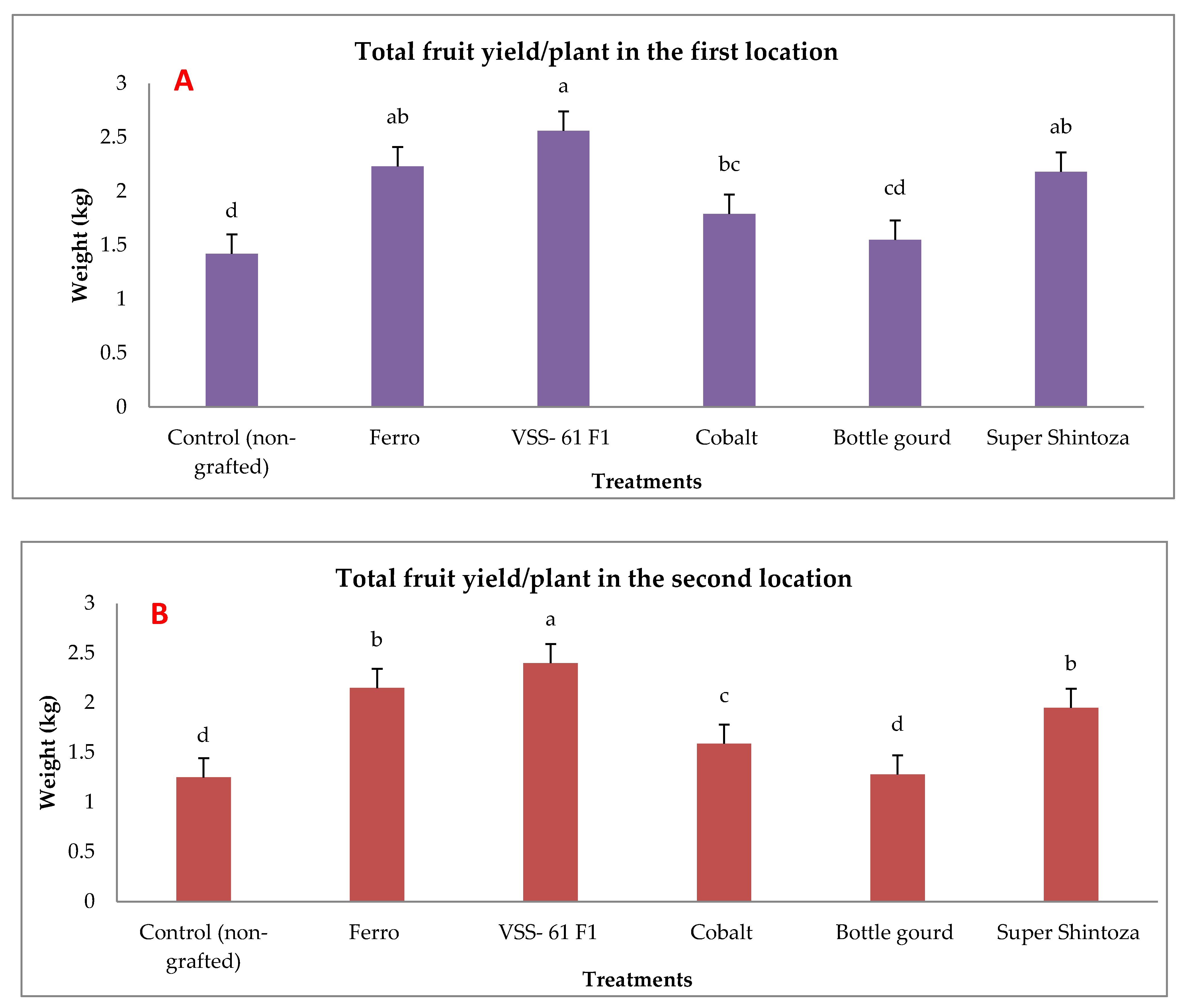
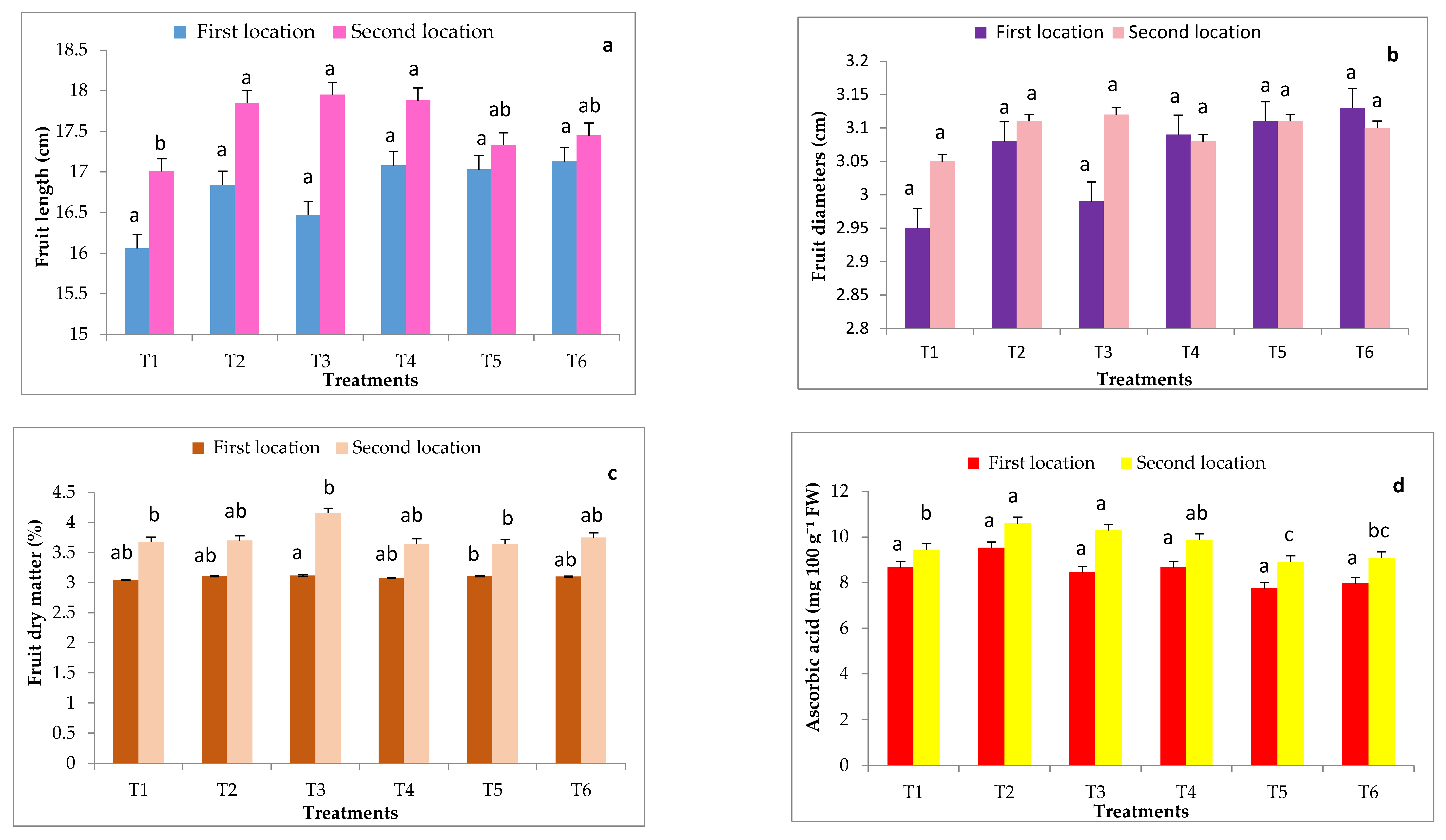
2.4. Fruit Yields and Its Quality in Non-Infected Grafted and Non-Grafted Plants under Heat Stress
3. Discussion
4. Materials and Methods
4.1. Selected Cucumber Scion and Cucurbit Rootstocks
4.2. Growth Conditions
4.2.1. Isolation and Identification of the Fungus
4.2.2. Inoculation Test
4.2.3. Recording Wilt Severity
4.3. Anatomical Structure
4.4. Vegetative Growth, Yield and Its Quality
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shalaby, T.A.; Abd-Alkarim, E.; El-Aidy, F.; Hamed, E.; Sharaf-Eldin, M.; Taha, N.; El-Ramady, H.; Bayoumi, Y.; dos Reis, A.R. Nano-selenium, silicon and H2O2 boost growth and productivity of cucumber under combined salinity and heat stress. Ecotoxicol. Environ. Saf. 2021, 212, 111962. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, Y.; Abd-Alkarim, E.; El-Ramady, H.; El-Aidy, F.; Hamed, E.-S.; Taha, N.; Prohens, J.; Rakha, M. Grafting Improves Fruit Yield of Cucumber Plants Grown under Combined Heat and Soil Salinity Stresses. Horticulturae 2021, 7, 61. [Google Scholar] [CrossRef]
- Debbarma, J.; Sarki, Y.N.; Saikia, B.; Boruah, H.P.D.; Singha, D.L.; Chikkaputtaiah, C. Ethylene response factor (ERF) family proteins in abiotic stresses and CRISPR–Cas9 genome editing of ERFs for multiple abiotic stress tolerance in crop plants: A Review. Mol. Biotechnol. 2019, 61, 153–172. [Google Scholar] [CrossRef]
- Ghani, M.I.; Saleem, S.; Rather, S.A.; Rehmani, M.S.; Alamri, S.; Rajput, V.D.; Kalaji, H.M.; Saleem, N.; Sial, T.A.; Liu, M. Foliar application of zinc oxide nanoparticles: An effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 2022, 289, 133202. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Z.; Zhang, X.; Zheng, S.; Wang, J.; Mo, J. Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci. Hortic. 2020, 262, 109070. [Google Scholar] [CrossRef]
- Amin, B.; Atif, M.J.; Meng, H.; Ghani, M.I.; Ali, M.; Wang, X.; Ding, Y.; Li, X.; Cheng, Z. Biochemical and Physiological Responses of Cucumis sativus Cultivars to Different Combinations of Low-Temperature and High Humidity. J. Plant Growth Regul. 2022, 1–17. [Google Scholar] [CrossRef]
- Gong, W.; Niu, L.; Wang, C.; Wei, L.; Pan, Y.; Liao, W. Hydrogen peroxide is involved in salicylic acid-induced adventitious rooting in cucumber under cadmium stress. J. Plant Biol. 2022, 65, 43–52. [Google Scholar] [CrossRef]
- Basirat, M.; Mousavi, S.M. Effect of foliar application of silicon and salicylic acid on regulation of yield and nutritional responses of greenhouse cucumber under high temperature. J. Plant Growth Regul. 2022, 1–11. [Google Scholar] [CrossRef]
- Sun, L.; Pehlivan, N.; Esmaeili, N.; Jiang, W.; Yang, X.; Jarrett, P.; Mishra, N.; Zhu, X.; Cai, Y.; Herath, M.; et al. Co-overexpression of AVP1 and PP2A-C5 in Arabidopsis makes plants tolerant to multiple abiotic stresses. Plant Sci. 2018, 274, 271–283. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Borhannuddin Bhuyan, M.H.M.; Hasanuzzaman, M.; Parvin, M.; Mohsin, S.M.; Al Mahmud, J.; Nahar, K.; Fujita, M. Nitric oxide and hydrogen sulfide: Two intimate collaborators regulating plant defense against abiotic stress. Plant Growth Regul. 2020, 90, 409–424. [Google Scholar] [CrossRef]
- Ullah, K.A.; Hussain, A.; Shaban, M.; Khan, A.H.; Alariqi, M.; Gul, S.; Jun, Z.; Lin, S.; Li, J.; Jin, S.; et al. Osmotin: A plant defense tool against biotic and abiotic stresses. Plant Physiol. Biochem. 2018, 123, 149–159. [Google Scholar] [CrossRef]
- Zhou, X.; Tan, Z.; Zhou, Y.; Guo, S.; Sang, T.; Wang, Y.; Shu, S. Physiological mechanism of strigolactone enhancing tolerance to low light stress in cucumber seedlings. BMC Plant Biol. 2022, 22, 30. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.S.; Borhannuddin Bhuyan, M.H.M.; Al Mahmud, J.; Nahar, K.; Fujita, N. The Role of Sulfur in Plant Abiotic Stress Tolerance: Molecular Interactions and Defense Mechanisms. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2018; pp. 221–252. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, J.; Liang, Y.; Liu, J.; Jia, J.; Huo, H.; Wu, Z.; Yang, R.; Gong, H. Transcriptomic dynamics provide an insight into the mechanism for silicon-mediated alleviation of salt stress in cucumber plants. Ecotoxicol. Environ. Saf. 2019, 174, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Su, Y.; Zhou, J.; Zhu, H.; Guo, Z.; Huo, H.; Gong, H. Foliar application of silicon and selenium improves the growth, yield and quality characteristics of cucumber in field conditions. Sci. Hortic. 2022, 294, 110776. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Taha, N.A.; Taher, D.I.; Metwaly, M.M.; El-Beltagi, H.S.; Rezk, A.A.; El-Ganainy, S.M.; Shehata, W.F.; El-Ramady, H.R.; Bayoumi, Y.A. Paclobutrazol improves the quality of tomato seedlings to be resistant to Alternaria solani Blight Disease: Biochemical and histological perspectives. Plants 2022, 11, 425. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, F. Growth-Promoting Ability of Rhodopseudomonas palustris G5 and Its Effect on Induced Resistance in Cucumber Against Salt Stress. J. Plant Growth Regul. 2019, 38, 180–188. [Google Scholar] [CrossRef]
- Liu, F.; Fu, X.; Wu, G.; Feng, Y.; Li, F.; Bi, H.; Ai, X. Hydrogen peroxide is involved in hydrogen sulfide-induced carbon assimilation and photoprotection in cucumber seedlings. Environ. Exp. Bot. 2020, 175, 104052. [Google Scholar] [CrossRef]
- Phogat, V.; Mallants, D.; Cox, J.W.; Šimůnek, J.; Oliver, D.P.; Awad, J. Management of soil salinity associated with irrigation of protected crops. Agric. Water Manag. 2020, 227, 105845. [Google Scholar] [CrossRef]
- Reddy, P.P. (Ed.) Grafted Vegetables for Management of Soilborne Pathogens. In Sustainable Crop Protection under Protected Cultivation; Springer Science + Business Media: Singapore, 2016; pp. 83–97. [Google Scholar] [CrossRef]
- Meimandi, M.M.; Kappel, N. Grafting Plants to Improve Abiotic Stress Tolerance. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II; Hasanuzzaman, M., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 477–490. [Google Scholar] [CrossRef]
- Shi, L.; Du, N.; Yuan, Y.; Shu, S.; Sun, J.; Guo, S. Vinegar residue compost as a growth substrate enhances cucumber resistance against the Fusarium wilt pathogen Fusarium oxysporum by regulating physiological and biochemical responses. Environ. Sci. Pollut. Res. 2016, 23, 18277–18287. [Google Scholar] [CrossRef]
- Sallaku, G.; Sandén, H.; Babaj, I.; Kaciu, S.; Balliu, A.; Rewald, B. Specific nutrient absorption rates of transplanted cucumber seedlings are highly related to RGR and influenced by grafting method, AMF inoculation and salinity. Sci. Hortic. 2019, 243, 177–188. [Google Scholar] [CrossRef]
- Ezzo, M.I.; Mohamed, A.S.; Glala, A.A.; Saleh, S.A. Utilization of grafting technique for sustaining cantaloupe productivity and quality under deficit irrigation water. Bull. Natl. Res. Cent. 2020, 44, 23. [Google Scholar] [CrossRef] [Green Version]
- Orosco-Alcalá, B.E.; Núñez-Palenius, H.G.; Díaz-Serrano, F.; Pérez-Moreno, L.; Valencia-Posadas, M.; Trejo-Tellez, L.I.; Cruz-Huerta, N.; Valiente-Banuet, J.I. Grafting improves salinity tolerance of bell pepper plants during greenhouse production. Hortic. Environ. Biotechnol. 2021, 62, 831–844. [Google Scholar] [CrossRef]
- Pal, S.; Rao, E.S.; Hebbar, S.S.; Sriram, S.; Pitchaimuthu, M.; Rao, V.K. Assessment of Fusarium wilt resistant Citrullus sp. rootstocks for yield and quality traits of grafted watermelon. Sci. Hortic. 2020, 272, 109497. [Google Scholar] [CrossRef]
- Nordey, T.; Schwarz, D.; Kenyon, L.; Manickam, R.; Huat, J. Tapping the potential of grafting to improve the performance of vegetable cropping systems in sub-Saharan Africa. A review. Agron. Sustain. Dev. 2020, 40, 23. [Google Scholar] [CrossRef]
- Shi, X.; Qiao, K.; Li, B.; Zhang, S. Integrated management of Meloidogyne incognita and Fusarium oxysporum in cucumber by combined application of abamectin and fludioxonil. Crop Prot. 2019, 126, 104922. [Google Scholar] [CrossRef]
- Punja, Z.K.; Tirajoh, A.; Collyer, D.; Ni, L. Efficacy of Bacillus subtilis strain QST 713 (Rhapsody) against four major diseases of net-house cucumbers. Crop Prot. 2019, 124, 104845. [Google Scholar] [CrossRef]
- Kehoe, M.A.; Webster, C.; Wang, C.; Jones, R.A.C.; Coutts, B.A. Occurrence of cucumber green mottle mosaic virus in Western Australia. Australas. Plant Pathol. 2022, 51, 1–8. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.C.; Baysal-Gure, F. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Rajwade, J.M.; Chikte, R.G.; Paknikar, K.M. Nanomaterials: New weapons in a crusade against phytopathogens. Appl. Microbiol. Biotechnol. 2020, 104, 1437–1461. [Google Scholar] [CrossRef]
- Lee, H.-J.; Cho, I.-S.; Ju, H.-J.; Jeong, R.-D. Rapid and visual detection of tomato spotted wilt virus using recombinase polymerase amplification combined with lateral flow strips. Mol. Cell. Probes 2021, 57, 101727. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.A.; Popoola, A.R.; Enikuomehin, O.A.; Bodunde, J.G. Evaluation of integrated management of bacterial wilt of tomato using grafting, biofumigant and plant resistance activator under field conditions. Australas. Plant Pathol. 2020, 49, 249–255. [Google Scholar] [CrossRef]
- Kumbar, S.; Narayanankutty, C.; Kurian, P.S.; Sreelatha, U.; Barik, S. Evaluation of eggplant rootstocks for grafting eggplant to improve fruit yield and control bacterial wilt disease. Eur. J. Plant Pathol. 2021, 161, 73–90. [Google Scholar] [CrossRef]
- Nakaho, K. Mechanisms of resistance to Ralstonia solanacearum in tomato rootstocks and integrated management of bacterial wilt using high grafting. J. Gen. Plant Pathol. 2021, 87, 398–402. [Google Scholar] [CrossRef]
- Ogundeji, A.O.; Li, Y.; Liu, X.; Meng, L.; Sang, P.; Mu, Y.; Wu, H.; Ma, Z.; Hou, J.; Li, S. Eggplant by grafting enhanced with biochar recruits specific microbes for disease suppression of Verticillium wilt. Appl. Soil Ecol. 2021, 163, 103912. [Google Scholar] [CrossRef]
- Sabry, S.; Ali, A.Z.; Abdel-Kader, D.A.; Abou-Zaid, M.I. Histopathological and biochemical aspects of grafted and non-grafted cucumber infected with stem rot caused by Fusarium spp. Saudi. J. Biol. Sci. 2022, 29, 1770–1780. [Google Scholar] [CrossRef]
- Kashyap, A.; Planas-Marquès, M.; Capellades, M.; Valls, M.; Coll, N.S. Blocking intruders: Inducible physico-chemical barriers against plant vascular wilt pathogens. J. Exp. Bot. 2021, 72, 184–198. [Google Scholar] [CrossRef]
- Li, M.; Ma, G.; Lian, H.; SU, X.; Tian, Y.; Huang, W.; Mie, J.; Jiang, X. The effects of Trichoderma on preventing cucumber Fusarium wilt and regulating cucumber physiology. J. Integr. Agric. 2019, 18, 607–617. [Google Scholar] [CrossRef] [Green Version]
- Cong, Y.; Fan, H.; Ma, Q.; Lu, Y.; Xu, L.; Zhang, P.; Chen, K. Mixed culture fermentation between Rhizopus nigricans and Trichoderma pseudokoningii to control cucumber Fusarium wilt. Crop Prot. 2019, 124, 104857. [Google Scholar] [CrossRef]
- Han, L.; Wang, Z.; Li, N.; Wang, Y.; Feng, J.; Zhang, X. Bacillus amyloliquefaciens B1408 suppresses Fusarium wilt in cucumber by regulating the rhizosphere microbial community. Appl. Soil Ecol. 2019, 136, 55–66. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, J.; Shi, Y.; Wu, F.; Zhou, X. Green manures of Indian mustard and wild rocket enhance cucumber resistance to Fusarium wilt through modulating rhizosphere bacterial community composition. Plant Soil 2019, 441, 283–300. [Google Scholar] [CrossRef]
- Papadaki, A.M.; Bletsos, F.A.; Menexes, G.; Moustafa Ismail, A.M.; Lagopodi, A.L. Effectiveness of six rootstocks for Fusarium wilt control in cucumber, and their influence on growth, yield and fruit quality characteristics. J. Plant Pathol. 2017, 99, 643–650. [Google Scholar]
- Pouralibaba, H.R.; Pérez-De-Luque, A.; Rubiales, D. Histopathology of the infection on resistant and susceptible lentil accessions by two contrasting pathotypes of Fusarium oxysporum f. sp. lentis. Eur. J. Plant Pathol. 2017, 148, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Taha, N.; Abdalla, N.; Bayoumi, Y.; El-Ramady, H. Management of net-house cucumber production under arid environments: A Review. Envion. Biodiv. Soil Secur. 2020, 4, 123–136. [Google Scholar] [CrossRef]
- Cui, B.J.; Niu, W.Q.; Du, Y.D.; Zhang, Q. Response of yield and nitrogen use efficiency to aerated irrigation and N application rate in net-house cucumber. Sci. Hortic. 2020, 265, 109220. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Li, X.; Tang, Y.; Zhang, P.; Duan, Z. Sustainable vegetable production under changing climate: The impact of elevated CO2 on yield of vegetables and the interactions with environments-A review. J. Clean. Prod. 2020, 253, 119920. [Google Scholar] [CrossRef]
- Pardo-Alonso, J.-L.; Carreño-Ortega, Á.; Martínez-Gaitán, C.-C.; Fatnassi, H. Behavior of different grafting strategies using automated technology for splice grafting technique. Appl. Sci. 2020, 10, 2745. [Google Scholar] [CrossRef]
- Usanmaz, S.; Abak, K. Plant growth and yield of cucumber plants grafted on different commercial and local rootstocks grown under salinity stress. Saudi J. Biol. Sci. 2019, 26, 1134–1139. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; Omar, S.A.; John, S.V.S.; Zabochnicka-Swiątek, M.; Kalaji, H.M.; Rastogi, A. Physiological and molecular mechanisms of salinity tolerance in grafted cucumber. S. Afr. J. Bot. 2020, 130, 90–102. [Google Scholar] [CrossRef]
- Cao, P.; Li, C.; Wang, H.; Yu, Z.; Xu, X.; Wang, X.; Zhao, J.; Xiang, W. Community structures and antifungal activity of root-associated Endophytic Actinobacteria in healthy and diseased cucumber plants and Streptomyces sp. HAAG3-15 as a Promising Biocontrol Agent. Microorganisms 2020, 8, 236. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Lv, J.; Xie, J.; Feng, Z.; Ma, N.; Li, J.; Yu, J.; Calderón-Urrea, A. Transcriptome analysis reveals the different response to toxic stress in rootstock grafted and non-grafted cucumber seedlings. Int. J. Mol. Sci. 2020, 21, 774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Men, L.; Gao, L.; Tian, Y. Effect of grafting and gypsum application on cucumber (Cucumis sativus L.) growth under saline water irrigation. Agric. Water Manag. 2017, 188, 79–90. [Google Scholar] [CrossRef]
- Al-Harbi, A.R.; Al-Omran, A.M.; Alharbi, K. Grafting improves cucumber water stress tolerance in Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Li, F.; Ai, X.; Bi, H. H2O2 participates in ABA regulation of grafting-induced chilling tolerance in cucumber. Plant Cell Rep. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.F.; Hassan, H.A.; Abdel-Wahab, A.A.; Gebrael, A.A. Effect of grafting on the cucumber yield and quality under high and low temperatures. J. Plant Prod. Mansoura Univ. 2014, 5, 443–456. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Hussein, M.N.E.; Abd-El-Hamid, K.E.; Elwan, M.W.M. Response of watermelon plants grafted onto different cucurbit rootstocks to sub-optimal growing temperature. Hortscience J. Suez Canal Univ. 2018, 7, 25–34. [Google Scholar]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A Technique to modify ion accumulation in horticultural crops. Front. Plant Sci. 2016, 7, 1457. [Google Scholar] [CrossRef] [Green Version]
- Al-Tuwaijri, M.Y. Studies on Fusarium wilt disease of cucumber. J. Appl. Pharm. Sci. 2015, 5, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, M.; Li, Y.; Gu, Z.; Ling, N.; Shen, Q.; Guo, S. Wilted cucumber plants infected by Fusarium oxysporum f. sp. cucumerinum do not suffer from water shortage. Ann. Bot. 2017, 120, 427–436. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, S.; Wang, L.; Wang, L.; He, X.; Shu, S.; Sun, J.; Lu, N. Identification of microRNAs associated with the exogenous spermidine-mediated improvement of high-temperature tolerance in cucumber seedlings (Cucumis sativus L.). BMC Genom. 2018, 19, 285. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Hashem, F.A.; Maze, M.; Shalaby, T.A.; Shehata, W.F.; Taha, N.M. Control of gas emissions (N2O and CO2) associated with applied different rates of nitrogen and their influences on growth, productivity, and physio-biochemical attributes of green bean plants grown under different irrigation methods. Agronomy 2022, 12, 249. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wang, P.; Chen, J.; Cao, Y. Gene expression, hormone signaling, and nutrient uptake in the root regermination of grafted watermelon plants with different pumpkin rootstocks. J. Plant Growth Regul. 2022, 1–16. [Google Scholar] [CrossRef]
- Kissoudis, C.; Sunarti, S.; van de Wiel, C.; Visser, R.G.F. Gerard van der Linden, Yuling Bai. J. Exp. Bot. 2016, 67, 5119–5132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelínek, T.; Koudela, M.; Kofránková, V.; Salava, J. Effect of temperature on severity of Fusarium wilt of cabbage caused by Fusarium oxysporum f. sp. conglutinans. Eur. J. Plant Pathol. 2019, 155, 1277–1286. [Google Scholar] [CrossRef]
- Li, H.; Yuan, G.; Zhu, C.; Zhao, T.; Zhang, R.; Wang, X.; Yang, J.; Ma, J.; Zhang, Y.; Zhang, X. Soil fumigation with ammonium bicarbonate or metam sodium under high temperature alleviates continuous cropping-induced Fusarium wilt in watermelon. Sci. Hortic. 2019, 246, 979–986. [Google Scholar] [CrossRef]
- Alsamir, M.; Ahmad, N.M.; Mahmood, T.; Trethowan, R. Impact of heat Stress on Fusarium Wilt (F. solani) incidence in cultivated tomato and related species. Aust. J. Crop Sci. 2017, 11, 997–1004. [Google Scholar] [CrossRef]
- Sefloo, N.G.; Steinkellner, S.; Hage-Ahmed, K. The bioprotective effect of root endophytic Serendipita herbamans against Fusarium wilt in tomato and its impact on root traits are determined by temperature. Rhizosphere 2021, 20, 100453. [Google Scholar] [CrossRef]
- Balfagón, D.; Rambla, J.L.; Granell, A.; Arbona, V.; Gomez-Cadenas, A. Grafting improves tolerance to combined drought and heat stresses by modifying metabolism in citrus scion. Environ. Exp. Bot. 2022, 195, 104793. [Google Scholar] [CrossRef]
- De Oliveira, M.M.T.; Lu, S.; Zurgil, U.; Raveh, E.; Tel-Zur, N. Grafting in Hylocereus (Cactaceae) as a tool for strengthening tolerance to high temperature stress. Plant Physiol. Biochem. 2021, 160, 94–105. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, D. Transcriptome analysis of scions grafted to potato rootstock for improving late blight resistance. BMC Plant Biol. 2021, 21, 272. [Google Scholar] [CrossRef]
- Yin, J.; Yan, J.; Hou, L.; Jiang, L.; Xian, W.; Guo, Q. Identification and functional deciphering suggested the regulatory roles of long intergenic ncRNAs (lincRNAs) in increasing grafting pepper resistance to Phytophthora capsici. BMC Genom. 2021, 22, 868. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Tai, P.; Li, X.; Jia, C.; Yuan, H.; He, L.; Sun, L. A reduction in cadmium accumulation and sulphur containing compounds resulting from grafting in eggplants (Solanum melogena) is associated with DNA methylation. Plant Soil 2021, 468, 183–196. [Google Scholar] [CrossRef]
- Yuan, H.; Tai, P.; Gustav, W.; Xue, F.; Sun, L. Grafting as a mitigation strategy to reduce root-to-shoot cadmium translocation in plants of Solanaceae family. J. Clean. Prod. 2021, 319, 128708. [Google Scholar] [CrossRef]
- Afify, A.E.M.M.; El-Beltagi, H.S. Effect of insecticide cyanophos on liver function in adultmale rats. Fresen Environ Bull 2011, 20, 1084–1088. [Google Scholar]
- Mohamed, A.A.; El-Beltagi, H.S.; Rashed, M.M. Cadmium stress induced change in some hydrolytic enzymes, free radical formation and ultrastructural disorders in radish plant. Electron. J. Environ. Agric. Food Chem. 2009, 8, 969–983. [Google Scholar]
- Booth, C. The Genus Fusarium; The Eastern Press: London, UK, 1971; pp. 147–149. [Google Scholar]
- Liu, L.; Kloepper, J.W.; Tuzun, S. Induction of systemic resistance in Cucumber against Fusarium wilt by plant growth-promoting rhizobacteri. Phytopathology 1995, 85, 695–698. [Google Scholar] [CrossRef]
- Nassar, M.A.; El-Sahhar, K.F. Botanical Preparations and Microscopy (Microtechnique); Academic Bookshop: Giza, Egypt, 1998; 219p. (In Arabic) [Google Scholar]
- Joel, D.M. AGS (Alcian Green Safranin): A simple differential staining of plant material for the light microscope. Proc. R. Microsc. Soc. 1983, 18, 149–151. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2020, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
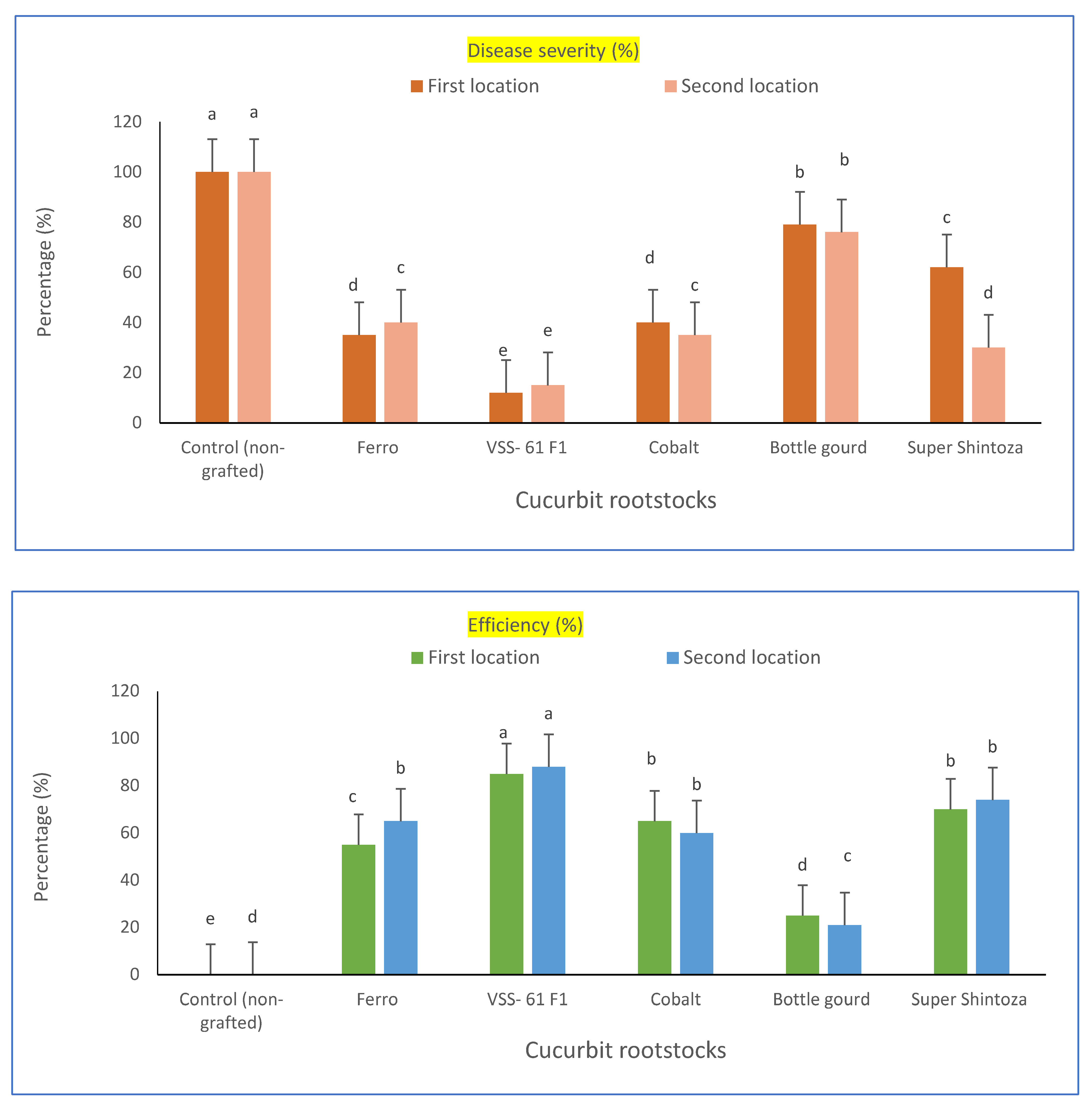
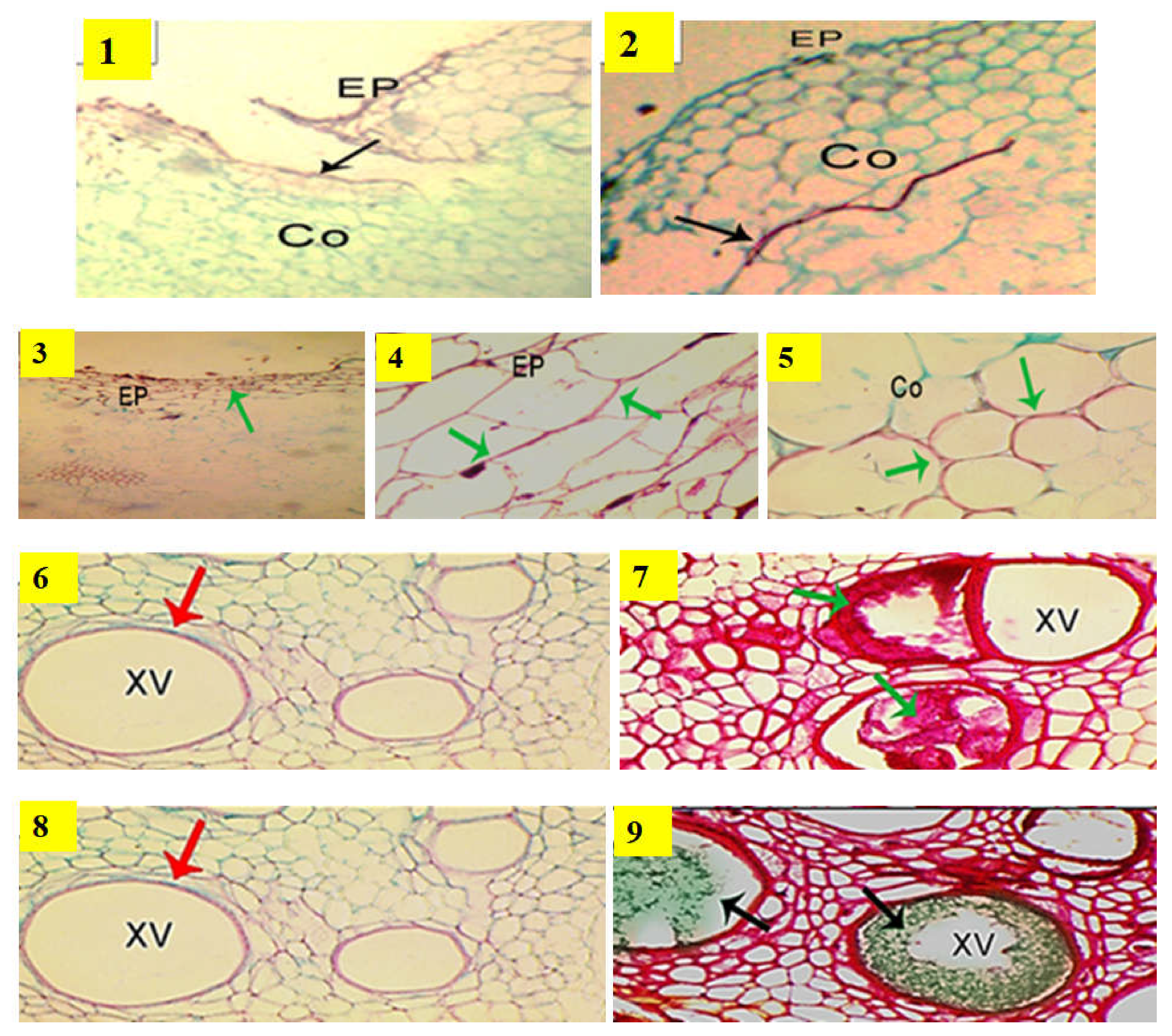

| Cucurbit Rootstocks | Disease Incidence (%) | |||
|---|---|---|---|---|
| Days after Transplanting | ||||
| 30 | 45 | 60 | 75 | |
| First location | ||||
| Control (non-grafted) | 50.0 a | 83.3 a | 100 a | 100 a |
| Ferro | 16.6 c | 20.5 de | 30.3 de | 35.0 c |
| VSS-61 F1 | 8.3 d | 16.6 e | 20.8 f | 20.8 d |
| Cobalt | 16.6 c | 33.3 c | 41.6 c | 41.6 c |
| Bottle gourd | 33.3 b | 50.0 b | 66.6 b | 79.1 b |
| Super Shintoza | 8.3 d | 20.8 de | 25.0 ef | 33.3 c |
| F-test | * | ** | ** | ** |
| Second location | ||||
| Control (non-grafted) | 40.0 a | 65.3 a | 85.5 a | 100 a |
| Ferro | 12.4 c | 15.5 de | 25.6 d | 31.5 c |
| VSS-61 F1 | 5.0 d | 10.8 e | 16.6 e | 16.6 d |
| Cobalt | 12.6 c | 24.6 c | 30.3 c | 35.9 c |
| Bottle gourd | 30.6 b | 50.0 b | 66.6 b | 75.4 b |
| Super Shintoza | 5.3 d | 15.6 de | 25.0 d | 31.2 c |
| F-test | * | ** | ** | ** |
| Treatments | After 30-Day Transplanting | After 70-Day Transplanting | |||
|---|---|---|---|---|---|
| Main Stem Diameter (mm) | Stem Length (cm) | Chlorophyll Fluorescence | |||
| Rootstock | Scion | FV/FM | FV/F0 | ||
| First location | |||||
| Control (non-grafted) | 10.17 c | 9.38 c | 217.25 b | 0.61 c | 2.07 c |
| Ferro | 12.56 a | 10.84 ab | 256.87 ab | 0.75 ab | 2.48 a |
| VSS—61 F1 | 12.45 a | 11.13 a | 295.16 a | 0.83 a | 2.55 a |
| Cobalt | 12.09 ab | 9.95 bc | 248.18 ab | 0.72 ab | 2.22 b |
| Bottle gourd | 11.53 b | 9.78 c | 240.09 ab | 0.68 b | 2.15 b |
| Super Shintoza | 12.23 ab | 9.84 bc | 293.65 a | 0.74 ab | 2.33 ab |
| F-test | ** | ** | * | ** | ** |
| Second location | |||||
| Control (non-grafted) | 10.40 c | 9.40 c | 222.30 b | 0.60 c | 2.02 c |
| Ferro | 12.64 a | 10.20 ab | 265.80 ab | 0.79 ab | 2.49 b |
| VSS—61 F1 | 12.55 a | 10.80 a | 304.05 a | 0.90 a | 2.67 a |
| Cobalt | 12.33 a | 9.90 bc | 254.20 ab | 0.77 ab | 2.48 b |
| Bottle gourd | 12.02 b | 9.80 bc | 248.40 ab | 0.73 b | 2.24 bc |
| Super Shintoza | 12.09 b | 9.90 bc | 299.95 a | 0.78 ab | 2.47 b |
| F-test | * | * | ** | ** | ** |
| Item | The Experiment Details |
|---|---|
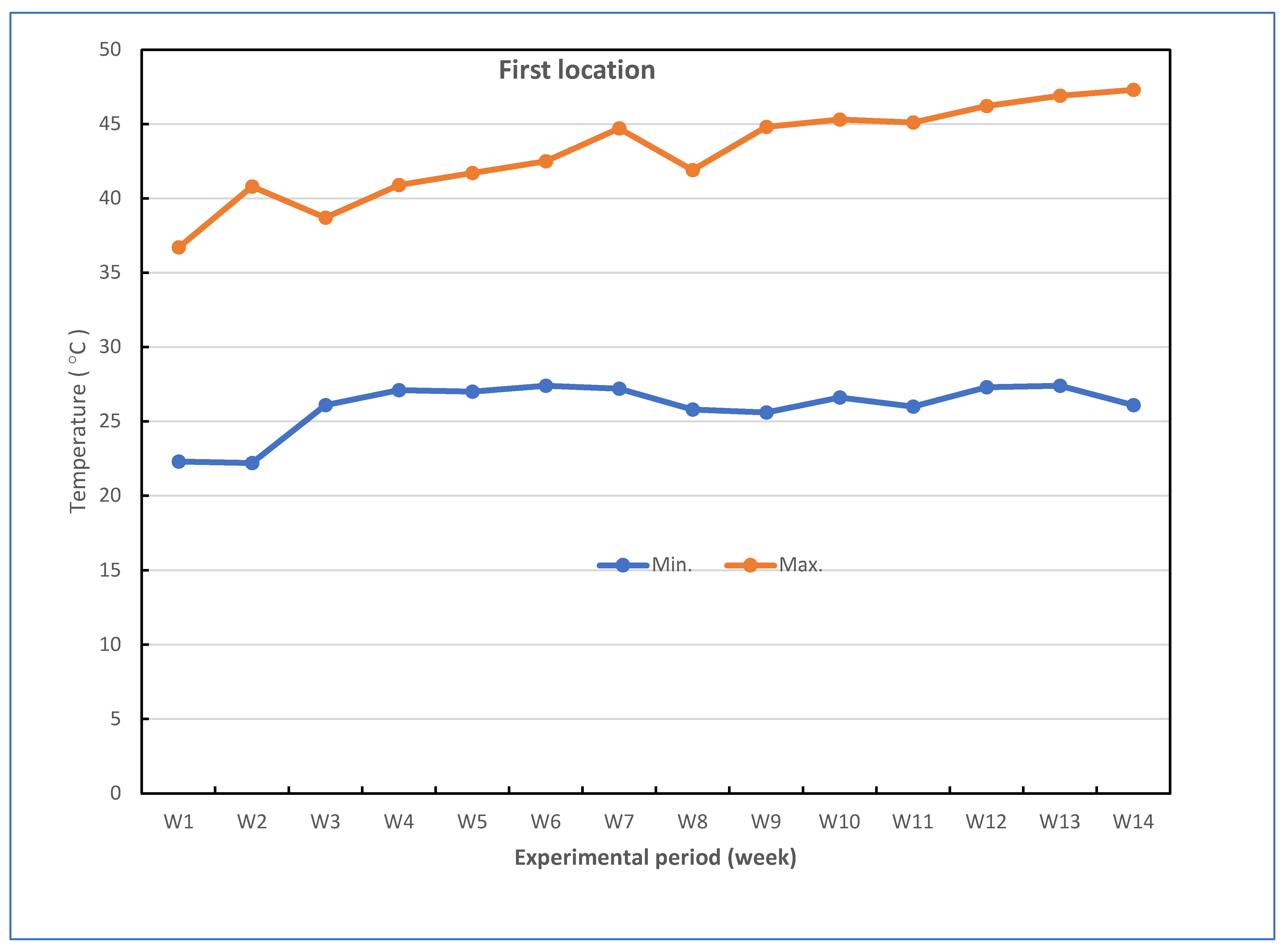
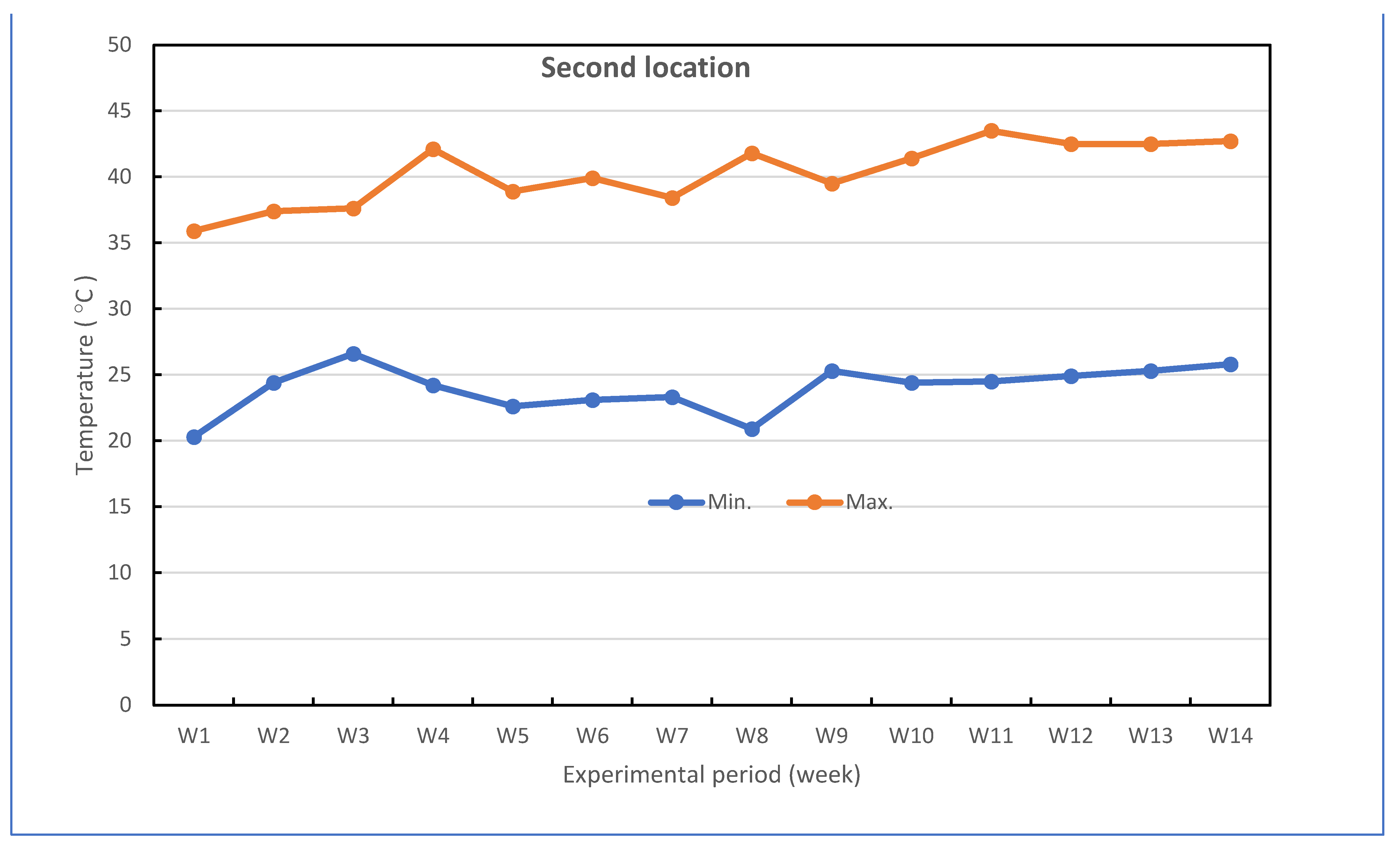
| Studied stress | Combined stress including Fusarium wilt disease (biotic) and heat stress (abiotic) |
| Growth media | Sandy loam soil infested with and without F. oxysporum f. sp. cucumerinum infection (the soil pH was 7.80 and soil salinity was 3.32 dS m−1 used in the two locations) |
| Experiment duration | 105 days for each location |
| Experimental location | Two locations (i.e., Kafr El-Sheikh and Sidi Salem) |
| Grafting details | Cucumber hybrid grafted onto five cucurbit rootstocks |
| Measured parameters | Fusarium wilt disease parameters and evidence of resistance to Fusarium by anatomical study for grafted plants infested with Fusarium, and growth, yield and fruit quality Parameters for grafted plants without infested Fusarium |
| Commercial and Scientific Name | Place of Seeds | Description |
|---|---|---|
| Ferro, Cucurbita maxima × C. moschata | Rijk Zwaan Company, Cairo, Egypt | Highly resistant to wilt of Fusarium and Verticillium |
| Cobalt, Cucurbita maxima × C. moschata | Rijk Zwaan Company, Cairo, Egypt | Tolerant to high temperature, Proof against Fusarium wilt |
| VSS-61 F1, C. pepo (Summer squash) | Techno green seed Company, Cairo, Egypt | Proof against both of Fusarium wilt and nematode |
| Bottle gourd, Lagenaria siceraria L. | Al-Reda Nurseries, Cairo, Egypt | Proof against Fusarium wilt |
| Super Shintoza, Cucurbita maxima × C. moschata | Al-Reda Nurseries, Cairo, Egypt | Tolerant to high temperature and impervious to Fusarium wilt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalaby, T.A.; Taha, N.A.; Rakha, M.T.; El-Beltagi, H.S.; Shehata, W.F.; Ramadan, K.M.A.; El-Ramady, H.; Bayoumi, Y.A. Can Grafting Manage Fusarium Wilt Disease of Cucumber and Increase Productivity under Heat Stress? Plants 2022, 11, 1147. https://doi.org/10.3390/plants11091147
Shalaby TA, Taha NA, Rakha MT, El-Beltagi HS, Shehata WF, Ramadan KMA, El-Ramady H, Bayoumi YA. Can Grafting Manage Fusarium Wilt Disease of Cucumber and Increase Productivity under Heat Stress? Plants. 2022; 11(9):1147. https://doi.org/10.3390/plants11091147
Chicago/Turabian StyleShalaby, Tarek A., Naglaa A. Taha, Mohamed T. Rakha, Hossam S. El-Beltagi, Wael F. Shehata, Khaled M. A. Ramadan, Hassan El-Ramady, and Yousry A. Bayoumi. 2022. "Can Grafting Manage Fusarium Wilt Disease of Cucumber and Increase Productivity under Heat Stress?" Plants 11, no. 9: 1147. https://doi.org/10.3390/plants11091147
APA StyleShalaby, T. A., Taha, N. A., Rakha, M. T., El-Beltagi, H. S., Shehata, W. F., Ramadan, K. M. A., El-Ramady, H., & Bayoumi, Y. A. (2022). Can Grafting Manage Fusarium Wilt Disease of Cucumber and Increase Productivity under Heat Stress? Plants, 11(9), 1147. https://doi.org/10.3390/plants11091147











