Abstract
Water scarcity is a major environmental stress that adversatively impacts wheat growth, production, and quality. Furthermore, drought is predicted to be more frequent and severe as a result of climate change, particularly in arid regions. Hence, breeding for drought-tolerant and high-yielding wheat genotypes has become more decisive to sustain its production and ensure global food security with continuing population growth. The present study aimed at evaluating different parental bread wheat genotypes (exotic and local) and their hybrids under normal and drought stress conditions. Gene action controlling physiological, agronomic, and quality traits through half-diallel analysis was applied. The results showed that water-deficit stress substantially decreased chlorophyll content, photosynthetic efficiency (FV/Fm), relative water content, grain yield, and yield attributes. On the other hand, proline content, antioxidant enzyme activities (CAT, POD, and SOD), grain protein content, wet gluten content, and dry gluten content were significantly increased compared to well-watered conditions. The 36 evaluated genotypes were classified based on drought tolerance indices into 5 groups varying from highly drought-tolerant (group A) to highly drought-sensitive genotypes (group E). The parental genotypes P3 and P8 were identified as good combiners to increase chlorophyll b, total chlorophyll content, relative water content, grain yield, and yield components under water deficit conditions. Additionally, the cross combinations P2 × P4, P3 × P5, P3 × P8, and P6 × P7 were the most promising combinations to increase yield traits and multiple physiological parameters under water deficit conditions. Furthermore, P1, P2, and P5 were recognized as promising parents to improve grain protein content and wet and dry gluten contents under drought stress. In addition, the crosses P1 × P4, P2 × P3, P2 × P5, P2 × P6, P4 × P7, P5 × P7, P5 × P8, P6 × P8, and P7 × P8 were the best combinations to improve grain protein content under water-stressed and non-stressed conditions. Certain physiological traits displayed highly positive associations with grain yield and its contributing traits under drought stress such as chlorophyll a, chlorophyll b, total chlorophyll content, photosynthetic efficiency (Fv/Fm), proline content, and relative water content, which suggest their importance for indirect selection under water deficit conditions. Otherwise, grain protein content was negatively correlated with grain yield, indicating that selection for higher grain yield could reduce grain protein content under drought stress conditions.
1. Introduction
Wheat (Triticum aestivum L.) is one of the most important cereal crops [1,2,3]. Wheat grain is a vital source that supports humans with calories, carbohydrates, protein, and vitamins [4,5]. Moreover, its straw is utilized in animal feeding and other industrial products [6,7]. The global population is expected to continue growing, and wheat demand is expected to rise, particularly in the face of global crises such as pandemics and wars. Consequently, considerable increase in wheat production is required tremendously to ensure food security [8]. Notwithstanding, its production is constrained by recent climate change, particularly in arid regions [9,10]. For instance, extreme climatic events, such as temperature rising and precipitation fluctuations, are expected to become more severe and frequent [11,12,13]. Drought stress is a harsh environmental factor that devastatingly affects global wheat production [14,15]. Nearly more than 50% of the cultivated area of wheat worldwide is subjected to frequent drought stress [16]. Moreover, global urbanization and industrialization have increased the pressure on freshwater resources [17]. Thus, water scarcity and drought problems are expected to worsen, which will negatively impact wheat production [18,19].
Water deficit reduces nutrients uptake, leaf water content, and photosynthesis, which deleteriously reflect on plant growth and productivity [20,21,22]. Furthermore, drought stress induces oxidative stress by raising the production of reactive oxygen species (ROS) [23,24,25]. The ROS damages nucleic acids, photosynthetic pigments, and membrane lipids as well as restricts the metabolism. As a result, there is a substantial reduction in number of leaves, leaf area, plant height, and grain weight, resulting in lower grain yield [26,27,28]. Enzymatic activities as catalase (CAT), peroxides (POD), and superoxide dismutase (SOD) are elevated under water deficit conditions to scavenge ROS and preserve cells from oxidative stress [29,30]. Additionally, increasing proline accumulation has been depicted in the plants that are exposed to drought stress [31,32,33]. It is an important osmoregulator that has an effective role in membrane stabilization to mitigate the injurious effect of water shortage [34,35]. Information on the inter-trait associations among grain yield and other physiological traits enhances the efficacy of breeding programs employing proper traits as selection criteria under drought stress and non-stressed conditions. Traits such as chlorophyll content, relative water content, proline content, and antioxidant activities could be utilized as secondary traits for screening drought-tolerant genotypes in breeding programs [24,36].
Grain quality of bread wheat is essential in terms of nutritional benefit and economics, accordingly, it receives increasing attention [37]. It is affected by genotypes, environments, and their interaction [38,39,40]. Drought is a crucial environmental factor that impacts the quality traits of wheat. Water deficit decreases photosynthesis, promotes leaf senescence, and limits the amount of assimilation, which causes a reduction in carbohydrate contents and total protein [41,42]. It also affects the nitrogen and carbohydrate assimilation rates, which can lead to significant changes in chemical composition, protein content, and starch granule size [42,43]. A negative association was reported between grain yield and grain protein content in wheat [44]. Hence, boosting grain yield and protein content is decisive for developing a high-quality wheat industry and ensuring nutrition and food security [45,46].
Combining the ability and nature of gene action governing agronomic, physiological, and quality traits in wheat could assist in determining merit parents for crossing and promising recombinants in breeding for drought tolerance [47,48,49]. Diallel mating design is a valuable biometric tool to study the general (GCA) and specific (SCA) combining ability effects and gene action in studied traits [50]. It also helps in selecting hybrids or parents for effective breeding either under normal or stressed conditions [51].
The aims of this study were (i) to assess the performance of eight wheat genotypes and their 28 F1 crosses for physiological, agronomic, and quality traits and exploring their diversity in drought tolerance; (ii) to explore the combining ability and type of gene action regulating the inheritance of the evaluated traits under water deficit and well-watered conditions; and (iii) to study the interrelationships among tested traits under drought conditions.
2. Materials and Methods
2.1. Hybridization and Experimental Site
Eight diverse wheat genotypes were selected based on origin diversity and drought tolerance from an earlier screening trial during the growing season of 2018 to 2019 (unpublished data). The used parents comprised four local cultivars, and four exotic genotypes (three from CIMMYT and one genotype from ICARDA). The pedigree of selected parents is shown in Table S1. Half-diallel mating design (8 × 8) was made to generate 28 F1 hybrids during the growing season of 2019 to 2020. The parental genotypes and their cross combinations were assessed in two adjacent irrigation experiments at the Experimental Farm, Faculty of Agriculture, Kafrelsheikh University (31°6′ N, 30°56′ E), Egypt, during the growing season of 2020 to 2021. The 2 experiments were separated by a 5-m wide alley to prevent water leakage. The first experiment (normal condition) was irrigated 5 times throughout the season using the region’s standard practice, totaling approximately 450 mm, while the second experiment was irrigated twice throughout the whole season, with a total of approximately 190 mm, providing water-deficit conditions. The experimental site has an arid climate with an average annual rainfall of ∼80 mm. The meteorological data (i.e., maximum and minimum temperatures, solar radiation, and precipitation) over the growing season are presented in Figure 1. Soil properties of the experimental site are presented in Table S2. The analysis revealed that the soil is clay throughout the profile (15.3% sand, 33.2% silt, and 51.5% clay). Randomized Complete Block Design (RCBD) with three replications was applied for each experiment. Each genotype was sown in 2 rows 2 m-long, with 0.30 m spacing between rows and 0.15 m between the plants. Phosphorus, potassium, and nitrogen fertilizers were performed at rates of 35 kg P2O5 ha−1, 57 kg K2O, and 180 kg N ha−1, respectively. The other agricultural practices comprising sowing date and weed, pest, and disease control were applied following the standard for wheat commercial production.
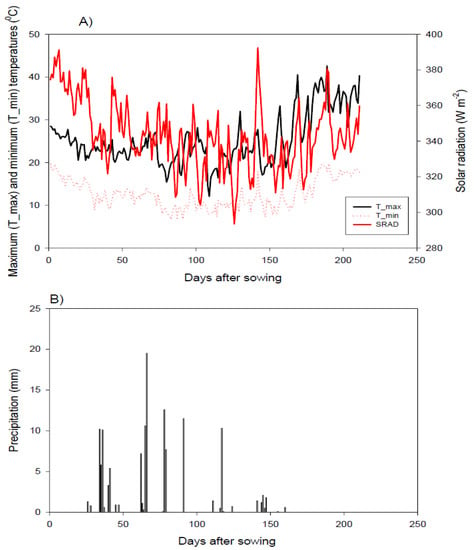
Figure 1.
Daily minimum and maximum temperatures, as well as solar radiation (A) and precipitation (B), at the experimental site.
2.2. Measured Traits
2.2.1. Physiological and Biochemical Traits
Chlorophyll Content and Chlorophyll Fluorescence
About 1 g fresh weight of mixed leaves was homogenized in 5 mL of 85% cold acetone and centrifuged. The extract was diluted to the appropriate volume before the optical density was determined at 663 and 647 nm [52]. The following equations were applied to calculate the chlorophyll content of the samples as mg/g fresh weight:
Chlorophyll a = 11.79 E663 − 2.29 E647, Chlorophyll b = 20.05 E647 − 4.77 E663
The parameters of chlorophyll fluorescence were assessed in upper fully expanded leaf tissue using a portable Optic-Science OS-30p+ fluorometer (Opti-Sciences, Inc., Hudson, NH, USA). Calculations of maximum PS II Fv/Fm quantum yield were performed using the formula of Maxwell and Johnson [53]: Fv/Fm = (Fm − F0)/Fm.
Relative Water Content (RWC)
Leaf RWC was determined as outlined by Barrs and Weatherley [54]. Fresh weight (FW) of leaves was determined, then they were immersed in water for 5 h, and the turgid weights (TW) were recorded. Then in an oven at 80 °C for 24 h, the samples were dried and dry weight (DW) was determined. The RWC was calculated as follows:
RWC = ((FW − DW)/(TW − DW)) × 100
Determination of Proline Content and Antioxidant Enzyme Activities
Proline content was determined as outlined by Bates, et al. [55]. Utilizing mortar and pestle, leaf samples (0.5 g) were homogenized in 5 mL of sulphosalicylic acid (3%). Almost 2 mL of extract was placed in a tube, and then 2 mL of ninhydrin reagent and 2 mL of glacial acetic acid were included. In a water bath at 100 °C for 60 min, the reaction mixture was boiled. After cooling the reaction mixture, 6 mL of toluene was included and then transferred to a separating funnel. After careful mixing, the chromophore including toluene was separated, and absorbance was read at 520 nm in a spectrophotometer against toluene blank. Proline concentration was recorded utilizing a calibration curve and expressed as mg proline g/FW.
The activities of catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) were determined as outlined by Aebi, et al. [56], Vetter, et al. [57], and Beauchamp and Fridovich [58], respectively. Fresh leaf samples (0.5 g) were homogenized in 5 mL of 50 mM cold K-phosphate buffer (pH 7.8). The homogenates were centrifuged for 20 min at 10,000× g at 4 °C. The supernatant was utilized to measure the antioxidant enzyme activity as (Units mg−1 protein).
2.2.2. Agronomic Traits
Plant height (cm) was recorded as the distance from the soil surface to the tip of the spike, excluding awns. Number of grains/spike and spike length (cm) were recorded from 10 main spikes chosen randomly from each plot. Thousand grains weight (g) was evaluated as the weight of 1000 grains. Ten guarded pants from each plot were harvested, dried, and threshed, and the grain yield per plant (g) was determined.
2.2.3. Grain Quality Traits
The grain quality traits were measured on samples taken from the grain bulk of each genotype. The grains samples (~20 g) were grounded to fine powder to pass through 2 mm mesh. Finally, the powder was used in the analysis of total crude protein and carbohydrate content. Total nitrogen content was analyzed utilizing the micro-Kjeldahl method, and then total crude protein % was computed by multiplying total N% by 5.85 [59]. Total carbohydrate in grinded wheat grains was analyzed as described in the method of Dubois, et al. [60]. Wet and dry gluten percentages were determined by hand-washing weighted meal samples according to the standard method of Pleshkov [61] until the starch was not detected in the washing water, then dried and weighed in grams.
2.3. Drought Tolerance Indices
Tolerance indices were calculated to identify potentially drought-tolerant genotypes. Geometric mean productivity (GMP) [62], mean productivity (MP) = (Ys + Yp)/2 [63], yield index (YI) = Ys/Ȳs [64], and stress tolerance index (STI) = (Ys × Yp)/(Ȳp)2 [62]. Cluster analysis was performed based on tolerance indices to discriminate the tested genotypes according to their drought tolerance [65]. The cluster and principal component analyses were applied utilizing R statistical software version 4.4.1, library factoextra [66].
2.4. Statistical Analysis
Combining ability analysis was performed following Griffing’s method-2 model-1 [67]. Principal component analysis (PCA) and heatmap were applied using averages of the physiological, agronomic, and grain quality traits to explore the relationships among them using R statistical software.
3. Results
3.1. Diallel Analysis
Significant differences among genotypes (G), parents (P), F1 crosses (C), and P vs. C were detected for most evaluated traits under both conditions (Table 1). The mean squares of GCA and SCA were highly significant for all studied traits under both well-watered and stressed conditions. The ratio of GCA/SCA was more than the unity for all evaluated traits, except chlorophyll a, proline content, and catalase activity (CAT) under normal conditions; total chlorophyll content; the photosynthetic efficiency (Fv/Fm); peroxidase (POD) and superoxide dismutase (SOD) activities under water deficit conditions; and carbohydrates content under both conditions.

Table 1.
Mean squares from ordinary and combining ability analysis for all investigated traits under well-watered and drought stress conditions.
3.2. Mean Performance of the Evaluated Parents and Their Cross Combinations
3.2.1. Physiological and Biochemical Traits
Drought stress significantly reduced chlorophyll a by 31.4% (Figure 2A and Figure 3A). The highest mean values were assigned for P6, P8, P1 × P3, P1 × P4, P1 × P5, P1 × P6, P1 × P8, P2 × P6, P2 × P7, P2 × P8, P3 × P6, P3 × P8, and P4 × P8 under water deficit conditions (Figure S1A). Similarly, a water deficit declined chlorophyll b by 22.9% (Figure 2B and Figure 3A). The parents P3 and P8 and the hybrids P2 × P4, P2 × P5, P2 × P8, P3 × P5, P4 × P5, P3 × P8, P4 × P6, P6 × P7, and P7×P8 proved the highest values under water deficit conditions (Figure S1B). The total chlorophyll content declined by 28.6% due to water limitation (Figure 2C and Figure 3A). The highest values were assigned for P8, P3 × P4, P3 × P5, P3 × P8, and P6 × P7 under stressed conditions (Figure S1C). Likewise, photosynthetic efficiency (Fv/Fm) significantly decreased by 24.1% under water deficit (Figure 2D and Figure 3A). The genotypes P1, P2, P3, P7, P1 × P6, P3 × P5, P3 × P8, and P4 × P8 displayed the highest values under stressed conditions (Figure S1D). Relative water content (RWC) was also depressingly impacted by drought stress; it decreased by 16.3% under water deficit (Figure 2E and Figure 3A). The highest values were given by, P3, P8, P2 × P8, P3 × P8, and P4 × P5 under stress conditions. Otherwise, water scarcity caused a considerable increase in proline content and the activities of antioxidant enzymes: CAT, POD, and SOD by 90.2, 107.5, 155.7, and 47.4%, respectively, compared to well-watered conditions (Figure 2F–I and Figure 3A). The genotypes P3, P8, P3 × P5, P3 × P6, and P3×P8 displayed the maximum values of proline content under stressed conditions (Figure S2A). The highest values of CAT were recorded by P5, P7, P2 × P3, P2 × P8, P5 × P7, and P6 × P7 under water deficit conditions (Figure S2B). Regarding POD, the genotypes P2, P6, P2 × P3, P2 × P6 P5 × P7, and P6 × P8 exhibited the largest values under stressed conditions (Figure S2C). The genotypes P1, P6, P1 × P8, P3 × P6, and P6 × P8 recorded the highest SOD under water deficiency conditions (Figure S2D).

Figure 2.
Boxplots with minimum, median, mean, and maximum values for chlorophyll a (A), chlorophyll b (B), total chlorophyll content (C), photosynthetic efficiency (D), relative water content (E), proline content (F), catalase activity (G), peroxidase activity (H), and superoxide dismutase activity (I).
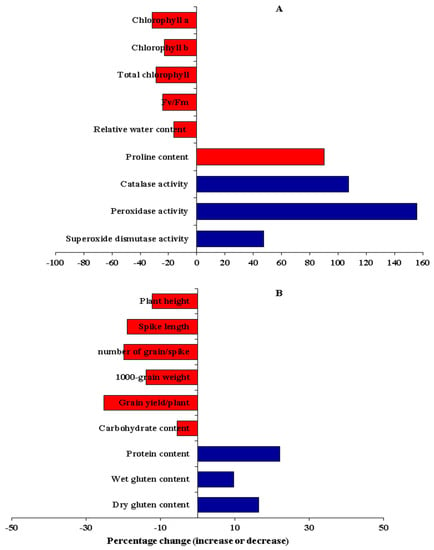
Figure 3.
Percentage change (increase or decrease) in physiological and biochemical traits (A) and agronomic and grain quality traits (B) exposed to drought stress compared with well-watered wheat plants.
3.2.2. Agronomic Traits
Plant height was significantly affected by water deficit; it decreased by 12.3% compared to well-watered conditions (Figure 3B and Figure 4A). The genotypes P1, P3, P4, P8, P1 × P8, P3 × P5, and P6 × P7 possessed the tallest plants, whereas the shortest ones were P2, P5, and P2 × P6 under water scarcity conditions (Figure S3A). Likewise, the spike length was significantly reduced by 19.0% due to the decrease in the amount of irrigation water applied (Figure 3B and Figure 4B). The genotypes P2, P4, P8, P1 × P8, P2 × P8, P3 × P8, P4 × P7, and P4 × P8 had the longest spike under stress conditions (Figure S3B). The number of grains/spike also declined by 20.0% due to water shortage conditions (Figure 3B and Figure 4C). The genotypes P2, P4, P8, P2 × P4, and P3 × P8 had the highest number of grains/spike under water scarcity conditions (Figure S3C). Likewise, the water drought treatment dropped 1000 grain weight by 19.9% (Figure 3B and Figure 4D). The heaviest 1000-grain weight was assigned for P1, P8, P1 × P3, P2 × P5, P3 × P7, and P3 × P8 under water scarcity conditions (Figure S3D). Eventually, grain yield/plant was significantly reduced by 25.3% under water stress conditions (Figure 3B and Figure 4E). The genotypes P3, P8, P1 × P8, P2 × P4, P3 × P5, P3 × P8, P4 × P8, and P5 × P8 displayed the highest grain yield under water deficiency conditions (Figure S3E).

Figure 4.
Boxplots with minimum, median, mean, and maximum values for plant height (A), spike length (B), number of grains per spike (C), 1000-grain weight (D), grain yield per plant (E), carbohydrate content (F), (G) protein content, (H) wet gluten content, and (I) dry gluten content.
3.2.3. Grain Quality Traits
Water deficit significantly reduced carbohydrate content by 5.5% (Figure 3B and Figure 4F). The genotypes P7, P8, P1 × P2, P2 × P8, P3 × P7, P4 × P8 and P6 × P7 recorded the highest values under water scarcity conditions (Figure S4A). Conversely, water deficit treatment significantly increased grain protein content by 22.0% (Figure 3B and Figure 4G). The genotypes P1, P2, P5, P1 × P4, P2 × P5, P2 × P6, P5 × P7, and P5 × P8 had the highest grain protein content under water deficit conditions (Figure S4B). Likewise, wet gluten content was significantly affected by water deficit and also increased by 9.7% under deficit irrigation (Figure 3B and Figure 4H). The genotypes P1, P2, P7, P1 × P7, P1 × P8, P2 × P3, and P2 × P8 had the highest wet gluten content under water deficit conditions (Figure S4C). Similarly, dry gluten content increased by 16.4% under water scarcity conditions (Figure 3B and Figure 4I). The highest values were obtained by the genotypes P2, P5, P1 × P5, P3 × P7, P5 × P6, and P5 × P7 under water deficit conditions (Figure S4D).
3.3. Genotypic Classification Based on Drought Tolerance Indices
The hierarchical cluster classified the 36 evaluated genotypes into 5 groups according to their drought tolerance (Figure 5). Group (A) included two genotypes (P3 × P8 and P4 × P8) that possessed the highest tolerance indices (Table S3); accordingly, they are considered highly drought-tolerant genotypes. The group (B) comprised of four genotypes (P3, P8, P3 × P5, P6 × P7, and P5 × P6) had high values; accordingly, they are deemed drought-tolerant genotypes. Similarly, group (C) consist of 14 genotypes with intermediate values of tolerance indices; hence, they are categorized as moderate drought-tolerant genotypes. Otherwise, eight genotypes in group (D) and seven genotypes in group (E) recorded the lowest values. Consequently, they are considered drought-sensitive and highly drought-sensitive genotypes, in the same order.
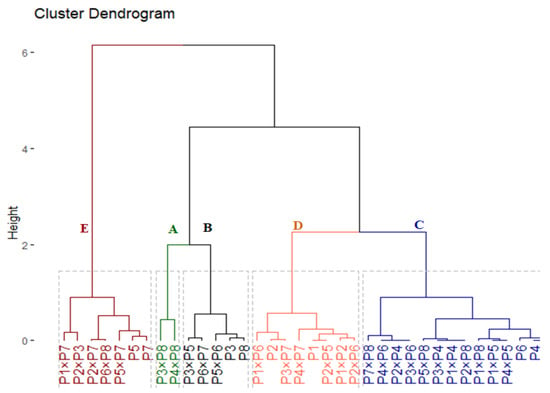
Figure 5.
Dendrogram of the phenotypic distances among eight wheat genotypes and their 28 F1s based on 4 drought tolerance indices (GM, MP, YI, and STI).
3.4. General Combining Ability (GCA) Effects
3.4.1. Physiological and Biochemical Traits
The positive GCA estimates are pivotal for all traits, except negative plant height values are favorable. The GCA effects for measured traits differed significantly among the evaluated parents (Table 2). The desirable GCA effects of chlorophyll a were assigned for P2, P3, and P4 under normal conditions and P1, P2, P6, and P8 under water scarcity conditions. The favorable GCA effects for chlorophyll b and total chlorophyll were obtained by P2 under water deficit conditions, P7 under normal conditions, and P3 and P8 under both conditions. The highest GCA effects for Fv/Fm were expressed by P2 and P7 under non-stressed conditions and P1 and P3 under stress conditions. Moreover, significant GCA effects for RWC were detected for P3, P6, and P8 under both conditions. Additionally, P3 recorded the maximum GCA effects for proline content. The best combiners for CAT activity were P6 under normal conditions and P2, P7, and P8. Meanwhile, the highest GCA values for POD activity were exhibited by P3 and P8 under normal conditions, P2 under water deficit conditions, and P6 under both conditions. Furthermore, the highest GCA estimates for SOD activity were recorded by P8, followed by P1, under normal conditions, while the highest under stress conditions were for P1, followed by P3.

Table 2.
General combining ability estimates (GCA) of the eight parents for all assessed traits under well-watered and drought stress conditions.
3.4.2. Agronomic Traits
The parents P2, P5, and P6 showed negative and significant effects towards dwarfness, while P3, P4, and P8 expressed positive and significant effects under both normal and water-stressed conditions. The highest GCA effects of spike length were recorded by P4 and P8 under both normal and stressful conditions. Positive GCA effects for the number of grains per spike were expressed by P3 under well-watered conditions, P6 and P8 under stress conditions, and P2 and P4 under both conditions. The best combiners for 1000-grain weight were demonstrated by P4 under well-watered conditions, P3 under drought stress conditions, and P8 under both conditions. The highest GCA for grain yield was obtained by P1 under well-watered conditions; P6 under drought stress conditions; and P3, P4, and P8 under both conditions.
3.4.3. Grain Quality Traits
The parents P3 and P4 had the highest GCA effects for carbohydrate content. Under both conditions, P1, P2, and P5 exhibited the greatest positive and significant effects for grain protein content. Similarly, P1, P2, P7, and P8 recorded the highest positive and significant effects for wet gluten content under both conditions. Finally, the highest GCA for dry gluten content was recorded by P1 and P5 under both conditions.
3.5. Specific Combining Ability (SCA) Estimates
3.5.1. Physiological and Biochemical Traits
The SCA values for the cross combinations are presented in Table 3. Out of the 28 crosses, 5 under normal and 18 under stressed conditions recorded significantly positive SCA effects for chlorophyll a. The highest significant and positive SCA effects for chlorophyll b and total chlorophyll were obtained by P2 × P4, P2 × P5, P2 × P8, P3 × P5, P3 × P8, and P6 × P7 under both conditions. Respecting Fv/Fm, the SCA effects were positive and significant for the hybrids P1 × P5 and P2 × P4 under normal conditions, P1 × P6 and P5 × P6 under water scarcity conditions, and P3 × P5 and P3 × P8 under both conditions. The maximum and positively significant SCA values for RWC were exhibited by P1 × P4, P1 × P6, P2 × P4, P2 × P7, P2 × P8, P3 × P5, P3 × P8, P4 × P5, P4 × P8, and P6 × P7 under both normal and water-stressed conditions. For proline content, high SCA effects were obtained by P1 × P6, P3 × P7, P4 × P6, P4 × P8, P5 × P6, and P5 × P8 under stress conditions and P3 × P5 and P3 × P8 under both conditions. In the case of the activities of antioxidant enzymes, the cross combinations P1 × P6, P2 × P3, and P3 × P8 were the best specific combiners for CAT, P1 × P2, P2 × P3, P3 × P8, and P5 × P7 for POD and P1 × P8, P2 × P3, P2 × P4, P2 × P7, P3 × P6, and P6 × P8 for SOD under both conditions.

Table 3.
Specific combining ability effects (SCA) of 28 F1 cross combinations for all studied traits under well-watered and drought stress conditions.
3.5.2. Agronomic Traits
High significant and negative SCA effects for plant height were recorded by P1 × P3, P1 × P4, P1 × P6, P1 × P7, P2 × P3, P2 × P6, P2 × P7, P2 × P8, P3 × P4, P3 × P6, P4 × P5, and P5 × P7. In contrast, the highest positive SCA effects were recorded by P1 × P2, P1 × P8, P2 × P4, and P6 × P7 under both conditions. High significant and positive SCA estimates for spike length were revealed by P3 × P5, P3 × P8, P5 × P6 and P6 × P7 under both conditions. The crosses P1 × P8, P2 × P4, P2 × P7, P3 × P5, P3 × P8, P4 × P5, P5 × P6 and P6 × P7 expressed the largest positive and significant SCA values for number of grains per spike. Regarding 1000-grain weight, the hybrids P2 × P5, P3 × P7, P3 × P8 and P6 × P7 under both conditions displayed the highest positive SCA values. Additionally, the highest significant and positive SCA effects for grain yield per plant were obtained by P1 × P2, P1 × P5, P1 × P8 and P3 × P7 under normal conditions, P4 × P8 and P5 × P6 under water deficit conditions and P2 × P4, P3 × P5, P3 × P8 and P6 × P7 under both conditions.
3.5.3. Grain Quality Traits
The hybrids P1 × P2, P1 × P5, P2 × P8, P3 × P7, P3 × P8, P4 × P5, and P4 × P6 possessed significantly positive SCA effects for carbohydrate content. Similarly, the hybrids P1 × P4, P2 × P3, P2 × P5, P2 × P6, P4 × P7, P5 × P7, P5 × P8, P6 × P8, and P7 × P8 were identified to be good specific combiners for grain protein content. Likewise, the crosses P1 × P6, P1 × P7, P1 × P8, P2 × P3, P2 × P4, P2 × P6, P2 × P8, P3 × P4, P3 × P7, P4 × P6, P5 × P6, P5 × P7, P6 × P7, and P7 × P8 for wet gluten content and P1 × P3, P1 × P5, P1 × P6, P2 × P3, P2 × P4, P3 × P7, P4 × P6, P5 × P6, P5 × P7, and P6 × P8 for dry gluten content displayed the highest positive and significant SCA effects under both normal and water-stress conditions.
3.6. Interrelationship among Physiological, Agronomic, and Quality Traits
The first two principal components (PCAs) explained most of the variability, 60.80% (50.94% and 9.86% by PCA1 and PCA2). Hence, the two PCAs were utilized to construct the PC-biplot (Figure 6). Strong correlation was distinguished between grain yield and each of the following: chlorophyll a, chlorophyll b, total chlorophyll, Fv/Fm, RWC, proline content, plant height, spike length, 1000 grain weight, and number of grains per spike. In contrast, a negative association was detected between yield traits and each of the following: antioxidant enzyme activities (CAT, POD, and SOD), grain protein content, wet gluten content, and dry gluten content. Analogous results were deduced by the association heatmap as presented in Figure S5.
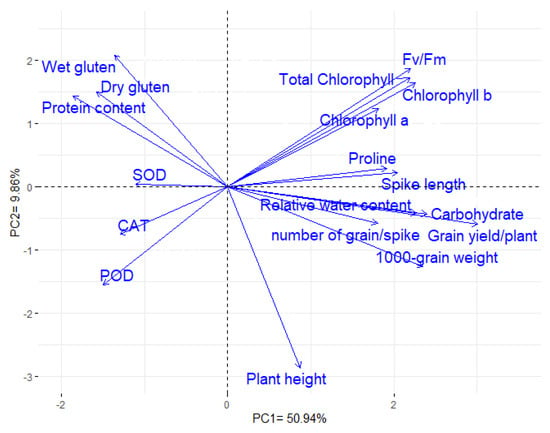
Figure 6.
PC-biplot presents the relationship among the evaluated physiological, agronomic, and quality traits.
4. Discussion
Breeding drought-tolerant and high-yielding wheat genotypes has become increasingly important in order to sustain production in the face of continued population growth and climate change threats [68,69,70]. In the present study, highly significant variations were observed among the parental genotypes and their cross combinations for all studied physiological, agronomic, and quality traits under water deficit conditions. These findings revealed the existence of wide genetic variability in the evaluated materials, which could be exploited for developing drought-tolerant genotypes. This is in consonance with previous reports that demonstrated genetic variability in wheat genotypes for physiological [20,71,72,73], agronomic [51,74,75,76], and quality traits [77,78,79] under water-deficit stress conditions.
Drought is one of the most significant abiotic stresses limiting wheat production, particularly in arid environments [80]. The results elucidated that water scarcity caused substantial reductions in all assessed traits compared to well-watered treatment, except proline content, antioxidant enzyme activities (CAT, POD, and SOD), grain protein content, wet gluten content, and dry gluten content which significantly increased. Chlorophyll a, chlorophyll b, total chlorophyll content, photosynthetic efficiency (Fv/Fm), and relative water content of all tested genotypes were considerably reduced due to water deficit compared to the well-irrigated treatment. In this context, Arjenaki, et al. [81] and [82] deduced that drought stress declines water uptake from root system to the leaves [83]. Accordingly, it decreases the water-holding capacity and stomatal movement, which constrains chlorophyll synthesis, CO2 influx to leaves, and photosynthesis [76,84]. Furthermore, water deficit leads to the production of reactive oxygen species (ROS), including O2−, OH−, H2O2, and O2, in the plants, which causes oxidative damage and impairs cell functions. In addition, the accumulation of ROS causes chlorophyll degradation, the destruction of chloroplasts, and a reduction in photosystem II activity [85,86]. On the other hand, proline accumulation and the induction of CAT, POD, and SOD activities considerably increased in stressed wheat plants in comparison to non-stressed plants. These parameters are important defense mechanisms under drought stress [87,88]. Proline is an important osmolyte that protects plant cells against oxidative stress by osmotic adjustment, regulating cell redox balance, and protein stabilization [31,33]. SOD is the first defense wall in oxidative damage in the cells and plays a key role in the alteration of O2− radicals to H2O2 and O2 [89]. POD converts H2O2 into H2O and oxygen, accordingly, assists the wheat plants in the detoxification mechanism against ROS species [14]. CAT participates in the conversion of H2O2 and plays a pivotal role in metabolism and signal recognition.
Yield-contributing traits are the final products of physiological processes that occur at various development stages. The remarkable reductions in yield traits under water scarcity were caused by lack of absorbed water and inhibition of cell elongation and division [90]. Furthermore, the reduction in the number of grains/spike could be a result of sterility [91] or the abortion of immature embryos [92]. The decline in grain yield and its attributes in the present study was also elucidated by Morsy, et al. [75], Grzesiak, et al. [93], Mujtaba, et al. [94], Shamsi, et al. [95], Qayyum, et al. [23], and Sallam, et al. [15]
Grain quality is essential in terms of the nutritional benefit and economics of wheat. It varies according to genotypes, environments, and their interaction. Ozturk, et al. [77] elucidated that the environmental influences display great impacts on the variations of quality parameters of wheat grains than the genetic factors. The results indicated that water deficit conditions decreased carbohydrates content but increased grain protein content, wet gluten content, and dry gluten content compared to well-irrigated conditions. Generally, there is a negative association between grain yield and grain protein content, the reduction in irrigation reduces grain yield but increases the protein content. The improved protein content could be due to high accumulation rates of grain nitrogen and reduced rates of carbohydrates accumulation. Moreover, water deficiency diminishes carbohydrate synthesis and storage in the grain, enabling more protein accumulation per unit of starch [41,42,43]. In this respect, Elbasyoni, et al. [78], Saint Pierre, et al. [96], Ozturk, et al. [77], and Liu, et al. [97] reported enhanced grain protein and gluten content under water deficit compared to well-watered conditions in wheat.
A successful breeding program depends on the selection of suitable parental genotypes [98,99,100]. The GCA effects display valuable importance in identifying potential parents that could be employed in developing improved genotypes [101,102,103]. The results indicated that the parents P3 and P7 were recognized as promising parents for improving proline content under water deficit conditions. Moreover, P2, P3, P6, and P8 are good parents for most of the studied antioxidant enzymes, chlorophyll content, and relative water content. Additionally, the parents P2, P5, and P6 could be important sources of favorable genes for reducing plant height under both normal and stressed conditions. Furthermore, improving grain yield and its components could be achieved by exploiting P3, P4, and P8, which had constantly significant and positive GCA estimates under both conditions. Subsequently, these parents could inherit beneficial alleles to their progeny and improve grain yield under water-stressed conditions. Similarly, earlier reports stated the significance of employing parents with positive and high GCA estimates for improving grain yield and contributed traits under drought stress conditions [51,103,104]. Interestingly, the parent P8 exhibited favorable GCA estimates for grain yield and was also an excellent combiner for chlorophyll b, total chlorophyll content, relative water content, CAT activity, spike length, and 1000-grain weight. In consequence, it can be employed for breeding drought-tolerant and high-yielding genotypes in the wheat breeding program. The parents P1, P2, and P5 were the best combiners for improving grain protein content, and P1 was the best for improving wet and dry glutens under both conditions. Subsequently, the valuable alleles of these genotypes could be employed in breeding programs for improving grain quality under well-watered and stressed conditions. Parental genotypes with desirable GCA effects for specific traits could be valuable for providing pure lines for breeding purposes.
Hybrids with significant SCA effects are great choices for selection. The result revealed that the crosses P2 × P4, P3 × P5, P3 × P8, and P6 × P7 were identified as excellent combiners for developing high-yielding genotypes under stressed conditions. Out of the aforementioned crosses, three hybrids, P2 × P4, P3 × P5 and P3 × P8, exhibited desirable SCA and high grain yield. The relationship between high yield performance and desired SCA effects was also deduced by Semahegn, et al. [51] and Kamara, et al. [101]. Grain quality is considered by wheat breeders alongside grain yield to meet production demands and market needs [105]. Grain protein content affects the nutritional value and processing qualities of wheat [42,106]. The results demonstrated that the combinations P1 × P4, P2 × P3, P2 × P5, P2 × P6, P4 × P7, P5 × P7, P5 × P8, P6 × P8, and P7 × P8 were the most merit-specific combiners for protein content. Additionally, P1 × P6, P2 × P3, P2 × P4, P3 × P7, P4 × P6, P5 × P6, and P5 × P7 appeared as the best specific combiners for wet and dry gluten under drought and non-stressed conditions. These crosses could be used to develop new genotypes with higher protein and gluten levels for specific industrial applications, as suggested by Joshi, et al. [107]. The majority of specific crosses for grain yield and protein content included high×high and high×poor general combiners, which implies increasing the favorable alleles. Evidently, none of the assessed crosses displayed significant SCA effects for all evaluated traits. Nonetheless, P3 × P8 was a good combiner for chlorophyll b, total chlorophyll content, Fv/Fm, RWC, proline content, CAT, POD, spike length, number of grain per spike, 1000-grain weight, and grain yield per plant. Moreover, P3 × P5 and P6 × P7 were the best combiners for chlorophyll b, total chlorophyll content, RWC, spike length, number of grain per spike, grain yield per plant, and wet gluten content. Subsequently, these crosses could be effectively exploited in wheat breeding programs to ameliorate these traits under drought stress and normal conditions [108].
The cluster analysis classified the assessed genotypes into five groups (A–E) varied from highly tolerant to highly sensitive genotypes. The genotypes P3, P8, P3 × P8, P4 × P8, P3 × P5, P6 × P7, and P5 × P6 were classified to be drought-tolerant (Figure 5). These genotypes displayed higher agronomic performance compared with the sensitive ones. This superiority in agronomic performance was reflected by their superior efficiency in chlorophyll a, chlorophyll b, Fv/Fm, relative water content, proline content, and enzymatic antioxidants. Thereupon, these tolerant genotypes could be utilized in wheat breeding programs for boosting grain yield under water deficit conditions. Respectively, several previous reports applied tolerance indices and cluster analysis to classify wheat genotypes under drought stress conditions [109,110,111,112,113].
Both additive and non-additive gene actions were included in the inheritance of all evaluated traits under both treatments, as evidenced by the highly significant GCA and SCA effects for all the evaluated traits. However, the GCA/SCA ratio was greater than unity for the majority of the evaluated characteristics in both stressed and non-stressed conditions. This implies the preponderance of additive gene effects in controlling the inheritance of these traits. These findings coincide with those of Sinolinding and Chowdhry [114], Farshadfar, et al. [47], El-Maghraby, et al. [115], Rad, et al. [116], and Semahegn, et al. [51]. They demonstrated that the additive gene actions mainly contributed to the inheritance of several physiological and agronomic traits in wheat under drought and normal conditions. Otherwise, Mwadzingeni, et al. [103] manifested that non-additive gene action was more predominant in the inheritance of grain yield and other agronomic traits in a diallel study investigated under drought stress conditions.
Understanding the associations among physiological, agronomic and quality traits could enhance breeding programs efficiency. The PC-biplot is a suitable statistical approach to assess the interrelationships among evaluated traits. The results displayed that grain yield was positively correlated with chlorophyll a, chlorophyll b, Fv/Fm, relative water content, and proline content under drought stress. Accordingly, selection for improving these physiological traits under water deficit conditions will result in improving the grain yield. These findings coincide with previous reports that reflected the importance of physiological attributes as indicators for grain yield under drought stress [117,118,119]. Strong positive relationships were detected between the plant height, the number of grains per spike, and the 1000-grain weight with grain yield, which implies their importance as valuable traits for indirect selection under drought stress [75,120]. On the other hand, grain yield was negatively associated with grain protein content, wet gluten content, and dry gluten content. Similarly, Ozturk and Aydin [41] Tari [121], Thungo, et al. [122], and Šíp, et al. [123] depicted a significant negative association between grain yield and grain protein, wet gluten content, and dry gluten content.
5. Conclusions
Water-deficit stress substantially reduced all assessed traits except proline content, antioxidant enzyme activities (CAT, POX, and SOD), grain protein content, wet gluten content, and dry gluten content, which were significantly increased. The parental genotypes P3 and P8 and their cross combination are proposed for breeding high-yielding and drought-tolerant wheat genotypes. Additionally, the parental genotypes P1, P2, and P5, as well as the hybrid combinations P1 × P4, P2 × P3, P2 × P5, P2 × P6, P4 × P7, P5 × P7, P5 × P8, P6 × P8, and P7 × P8, were the most promising genotypes for improving grain quality traits under drought-stressed conditions. The tolerance indices and cluster analysis provide valuable information on classifying the genotypes based on their tolerance to drought stress. Chlorophyll a, chlorophyll b, photosynthetic efficiency, proline content, and relative water content displayed highly positive associations with grain yield and its contributing traits under drought stress. These findings suggest the importance of these traits for indirect selection under water deficit conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11070952/s1, Table S1. Code, name, pedigree and source of the eight parental wheat genotypes used in the present study, Table S2. Main soil physico-chemical analysis before wheat cultivation at the experimental site, Table S3. Drought tolerance indices for wheat parental genotypes and their corresponding F1s crosses. Figure S1. Mean performance of the thirty-six wheat genotypes for (A) chlorophyll a, (B) chlorophyll b, (C) total chlorophyll content, (D) photosynthetic efficiency, and (E) relative water content. Figure S2. Mean performance of the thirty-six wheat genotypes for (A) proline content, (B) catalase activity, (C) peroxidase activity, and (D) superoxide dismutase activity. Figure S3. Mean performance of the thirty-six wheat genotypes for (A) plant height, (B) spike length, (C) number of grains per spike, (D) 1000-grain weight, and (E) grain yield per plant. Figure S4. Mean performance of the thirty-six wheat genotypes for (A) carbohydrate content, (B) protein content, (C) wet gluten content, and (D) dry gluten content. Figure S5. Correlation heatmap of the studied physiological, agronomic, and quality traits.
Author Contributions
Conceptualization, M.M.K., A.M.M., R.F.E.M., A.M.S.K. and E.M.; methodology, M.M.K., A.M.M., R.F.E.M., A.M.S.K., M.R., M.M.A.A. and E.M.; software, M.M.K., D.A.E.-M., F.A.S., S.M.A., M.R., E.M.H., S.I.B., M.M.A.A. and E.M.; formal analysis, M.M.K., A.M.M., R.F.E.M., A.M.S.K., M.R., M.M.A.A. and E.M.; investigation, M.M.K., A.M.M., R.F.E.M., A.M.S.K., M.R., M.M.A.A. and E.M.; resources, M.M.K., A.M.M., R.F.E.M., A.M.S.K., D.A.E.-M., F.A.S. and S.M.A.; data curation, M.M.K., A.M.M., R.F.E.M., A.M.S.K., D.A.E.-M., F.A.S., S.M.A., E.M.H., S.I.B. and E.M.; writing—original draft preparation, M.M.K., A.M.M., R.F.E.M., D.A.E.-M., F.A.S., S.M.A., M.M.A.A. and E.M.; writing—review and editing, M.M.K., D.A.E.-M., M.M.A.A. and E.M.; visualization, M.M.K., A.M.M., R.F.E.M., A.M.S.K., M.R., M.M.A.A. and E.M.; supervision, M.M.K., A.M.M., R.F.E.M., A.M.S.K. and E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors are grateful to the Faculty of Agriculture, Kafrelsheikh University, Egypt, Agricultural Research Center, Egypt, for their support provided to conduct this work. Special thanks to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R318), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Statistical Database. 2021. Available online: http://www.fao.org/faostat/en/#data (accessed on 9 September 2021).
- Asseng, S.; Kheir, A.M.S.; Kassie, B.T.; Hoogenboom, G.; Abdelaal, A.I.N.; Haman, D.Z.; Ruane, A.C. Can Egypt become self-sufficient in wheat? Environ. Res. Lett. 2018, 13, 094012. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Babar, M.A.; et al. Climate change impact and adaptation for wheat protein. Glob. Change Biol. 2019, 25, 155–173. [Google Scholar] [CrossRef]
- Tufail, T.; Saeed, F.; Afzaal, M.; Ain, H.B.U.; Gilani, S.A.; Hussain, M.; Anjum, F.M. Wheat straw: A natural remedy against different maladies. Food Sci. Nutr. 2021, 9, 2335–2344. [Google Scholar] [CrossRef]
- Swailam, M.; Mowafy, S.; El-Naggar, N.; Mansour, E. Agronomic responses of diverse bread wheat genotypes to phosphorus levels and nitrogen forms in a semiarid environment. SABRAO J. Breed. Genet. 2021, 53, 592–608. [Google Scholar] [CrossRef]
- Berners-Lee, M.; Kennelly, C.; Watson, R.; Hewitt, C.; Kapuscinski, A.R.; Locke, K.A.; Peters, C.J. Current global food production is sufficient to meet human nutritional needs in 2050 provided there is radical societal adaptation. Elem. Sci. Anth. 2018, 6, 52. [Google Scholar] [CrossRef]
- Polade, S.D.; Gershunov, A.; Cayan, D.R.; Dettinger, M.D.; Pierce, D.W. Precipitation in a warming world: Assessing projected hydro-climate changes in California and other Mediterranean climate regions. Sci. Rep. 2017, 7, 10783. [Google Scholar] [CrossRef]
- Ding, Z.; Ali, E.F.; Elmahdy, A.M.; Ragab, K.E.; Seleiman, M.F.; Kheir, A.M.S. Modeling the combined impacts of deficit irrigation, rising temperature and compost application on wheat yield and water productivity. Agric. Water Manag. 2021, 244, 106626. [Google Scholar] [CrossRef]
- Han, B.; Benner, S.G.; Flores, A.N. Evaluating impacts of climate change on future water scarcity in an intensively managed semi-arid region using a coupled model of biophysical processes and water rights. Hydrol. Earth Syst. Sci. Discuss. 2018, 5194, 1–53. [Google Scholar]
- Attia, A.; El-Hendawy, S.; Al-Suhaibani, N.; Tahir, M.U.; Mubushar, M.; dos Santos Vianna, M.; Ullah, H.; Mansour, E.; Datta, A. Sensitivity of the DSSAT model in simulating maize yield and soil carbon dynamics in arid Mediterranean climate: Effect of soil, genotype and crop management. Field Crops Res. 2021, 260, 107981. [Google Scholar] [CrossRef]
- Mansour, E.; Moustafa, E.S.; Qabil, N.; Abdelsalam, A.; Wafa, H.A.; El Kenawy, A.; Casas, A.M.; Igartua, E. Assessing different barley growth habits under Egyptian conditions for enhancing resilience to climate change. Field Crops Res. 2018, 224, 67–75. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. Sustain. Agric. 2009, 29, 185–212. [Google Scholar]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Leng, G.; Hall, J. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci. Total Environ. 2019, 654, 811–821. [Google Scholar] [CrossRef]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.-P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2007, 58, 147–159. [Google Scholar] [CrossRef]
- Senapati, N.; Stratonovitch, P.; Paul, M.J.; Semenov, M.A. Drought tolerance during reproductive development is important for increasing wheat yield potential under climate change in Europe. J. Exp. Bot. 2019, 70, 2549–2560. [Google Scholar] [CrossRef]
- Tardieu, F.; Parent, B.; Caldeira, C.F.; Welcker, C. Genetic and physiological controls of growth under water deficit. Plant Physiol. 2014, 164, 1628–1635. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; Mansour, E.; El-Sobky, E.-S.E.; Abdul-Hamid, M.I.; Taha, T.F.; Elakkad, H.A.; Arnaout, S.M.; Eid, R.S.; El-Tarabily, K.A.; Yasin, M.A. Physio-biochemical and agronomic responses of faba beans to exogenously applied nano-silicon under drought stress conditions. Front. Plant Sci. 2021, 12, 637783. [Google Scholar] [CrossRef]
- Sakran, R.M.; Ghazy, M.I.; Rehan, M.; Alsohim, A.S.; Mansour, E. Molecular genetic diversity and combining ability for some physiological and agronomic traits in rice under well-watered and water-deficit conditions. Plants 2022, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.; Al Ayoubi, S.; Sher, A.; Bibi, Y.; Ahmad, S.; Shen, Z.; Jenks, M.A. Improvement in drought tolerance in bread wheat is related to an improvement in osmolyte production, antioxidant enzyme activities, and gaseous exchange. Saudi J. Biol. Sci. 2021, 28, 5238–5249. [Google Scholar] [CrossRef] [PubMed]
- Desoky, E.-S.M.; Mansour, E.; Ali, M.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; Rady, M.M.; Ali, E.F. Exogenously used 24-epibrassinolide promotes drought tolerance in maize hybrids by improving plant and water productivity in an arid environment. Plants 2021, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Mansour, E.; Moustafa, E.S.; Desoky, E.-S.M.; Ali, M.; Yasin, M.A.; Attia, A.; Alsuhaibani, N.; Tahir, M.U.; El-Hendawy, S. Multidimensional evaluation for detecting salt tolerance of bread wheat genotypes under actual saline field growing conditions. Plants 2020, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- El-Mageed, A.; Taia, A.; Belal, E.E.; Rady, M.O.; El-Mageed, A.; Shimaa, A.; Mansour, E.; Awad, M.F.; Semida, W.M. Acidified biochar as a soil amendment to drought stressed (Vicia faba L.) plants: Influences on growth and productivity, nutrient status, and water use efficiency. Agronomy 2021, 11, 1290. [Google Scholar] [CrossRef]
- El-Sanatawy, A.M.; Ash-Shormillesy, S.M.; Qabil, N.; Awad, M.F.; Mansour, E. Seed halo-priming improves seedling vigor, grain yield, and water use efficiency of maize under varying irrigation regimes. Water 2021, 13, 2115. [Google Scholar] [CrossRef]
- Mannan, M.; Tithi, M.A.; Islam, M.R.; Al Mamun, M.; Mia, S.; Rahman, M.; Awad, M.F.; ElSayed, A.I.; Mansour, E.; Hossain, M. Soil and foliar applications of zinc sulfate and iron sulfate alleviate the destructive impacts of drought stress in wheat. Cereal Res. Commun. 2022. [Google Scholar] [CrossRef]
- Nasirzadeh, L.; Sorkhilaleloo, B.; Hervan, E.M.; Fatehi, F. Changes in antioxidant enzyme activities and gene expression profiles under drought stress in tolerant, intermediate, and susceptible wheat genotypes. Cereal Res. Commun. 2021, 49, 83–89. [Google Scholar] [CrossRef]
- Mansour, E.; Mahgoub, H.A.; Mahgoub, S.A.; El-Sobky, E.-S.E.; Abdul-Hamid, M.I.; Kamara, M.M.; AbuQamar, S.F.; El-Tarabily, K.A.; Desoky, E.-S.M. Enhancement of drought tolerance in diverse Vicia faba cultivars by inoculation with plant growth-promoting rhizobacteria under newly reclaimed soil conditions. Sci. Rep. 2021, 11, 24142. [Google Scholar] [CrossRef]
- Hong-Bo, S.; Xiao-Yan, C.; Li-Ye, C.; Xi-Ning, Z.; Gang, W.; Yong-Bing, Y.; Chang-Xing, Z.; Zan-Min, H. Investigation on the relationship of proline with wheat anti-drought under soil water deficits. Colloids Surf. B. 2006, 53, 113–119. [Google Scholar] [CrossRef]
- Farooq, M.; Nawaz, A.; Chaudhry, M.; Indrasti, R.; Rehman, A. Improving resistance against terminal drought in bread wheat by exogenous application of proline and gamma—Aminobutyric acid. J. Agron. Crop Sci. 2017, 203, 464–472. [Google Scholar] [CrossRef]
- Mwadzingeni, L.; Shimelis, H.; Tesfay, S.; Tsilo, T.J. Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front. Plant Sci. 2016, 7, 1276. [Google Scholar] [CrossRef] [PubMed]
- ŽIVČÁK, M.; Repkova, J.; OLŠOVSKÁ, K.; BRESTIČ, M. Osmotic adjustment in winter wheat varieties and its importance as a mechanism of drought tolerance. Cereal Res. Commun. 2009, 37, 569–572. [Google Scholar]
- Desoky, E.-S.M.; Elrys, A.S.; Mansour, E.; Eid, R.S.; Selem, E.; Rady, M.M.; Ali, E.F.; Mersal, G.A.; Semida, W.M. Application of biostimulants promotes growth and productivity by fortifying the antioxidant machinery and suppressing oxidative stress in faba bean under various abiotic stresses. Sci. Hortic. 2021, 288, 110340. [Google Scholar] [CrossRef]
- Mansour, E.; Desoky, E.-S.M.; Ali, M.M.; Abdul-Hamid, M.I.; Ullah, H.; Attia, A.; Datta, A. Identifying drought-tolerant genotypes of faba bean and their agro-physiological responses to different water regimes in an arid Mediterranean environment. Agric. Water Manag. 2021, 247, 106754. [Google Scholar] [CrossRef]
- Nuttall, J.; O’leary, G.; Panozzo, J.; Walker, C.; Barlow, K.; Fitzgerald, G. Models of grain quality in wheat-A review. Field Crops Res. 2017, 202, 136–145. [Google Scholar] [CrossRef]
- Zhao, C.-X.; He, M.-R.; Wang, Z.-L.; Wang, Y.-F.; Lin, Q. Effects of different water availability at post-anthesis stage on grain nutrition and quality in strong-gluten winter wheat. C. R. Biol. 2009, 332, 759–764. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Hernandez-Espinosa, N.; Peña, R.J. The influence of drought and heat stress on the expression of end-use quality parameters of common wheat. J. Cereal Sci. 2013, 57, 73–78. [Google Scholar] [CrossRef]
- Begcy, K.; Walia, H. Drought stress delays endosperm development and misregulates genes associated with cytoskeleton organization and grain quality proteins in developing wheat seeds. Plant Sci. 2015, 240, 109–119. [Google Scholar] [CrossRef]
- Ozturk, A.; Aydin, F. Effect of water stress at various growth stages on some quality characteristics of winter wheat. J. Agron. Crop Sci. 2004, 190, 93–99. [Google Scholar] [CrossRef]
- Balla, K.; Rakszegi, M.; Li, Z.; Bekes, F.; Bencze, S.; Veisz, O. Quality of winter wheat in relation to heat and drought shock after anthesis. Czech J. Food Sci. 2011, 29, 117–128. [Google Scholar] [CrossRef]
- Panozzo, J.; Eagles, H. Cultivar and environmental effects on quality characters in wheat. II. Protein. Aust. J. Agric. Res. 2000, 51, 629–636. [Google Scholar] [CrossRef]
- Blanco, A.; Mangini, G.; Giancaspro, A.; Giove, S.; Colasuonno, P.; Simeone, R.; Signorile, A.; De Vita, P.; Mastrangelo, A.; Cattivelli, L. Relationships between grain protein content and grain yield components through quantitative trait locus analyses in a recombinant inbred line population derived from two elite durum wheat cultivars. Mol. Breed. 2012, 30, 79–92. [Google Scholar] [CrossRef]
- Ma, J.; Xiao, Y.; Hou, L.; He, Y. Combining protein content and grain yield by genetic dissection in bread wheat under low-input management. Foods 2021, 10, 1058. [Google Scholar] [CrossRef] [PubMed]
- Abaza, G.M.S.M.; Awaad, H.A.; Attia, Z.M.; Abdel-lateif, K.S.; Gomaa, M.A.; Abaza, S.M.S.M.; Mansour, E. Inducing potential mutants in bread wheat using different doses of certain physical and chemical mutagens. Plant Breed. Biotech. 2020, 8, 252–264. [Google Scholar] [CrossRef]
- Farshadfar, E.; Rafiee, F.; Hasheminasab, H. Evaluation of genetic parameters of agronomic and morpho-physiological indicators of drought tolerance in bread wheat (Triticum aestivum L.) using diallel mating design. Aust. J. Crop Sci. 2013, 7, 268–275. [Google Scholar]
- Kamara, M.M.; Ghazy, N.A.; Mansour, E.; Elsharkawy, M.M.; Kheir, A.; Ibrahim, K.M. Molecular genetic diversity and line× tester analysis for resistance to late wilt disease and grain yield in maize. Agronomy 2021, 11, 898. [Google Scholar] [CrossRef]
- ElShamey, E.A.; Sakran, R.M.; ElSayed, M.A.; Aloufi, S.; Alharthi, B.; Alqurashi, M.; Mansour, E.; Abd El-Moneim, D. Heterosis and combining ability for floral and yield characters in rice using cytoplasmic male sterility system. Saudi J. Biol. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Salem, T.; Rabie, H.; Mowafy, S.; Eissa, A.; Mansour, E. Combining ability and genetic components of egyptian cotton for earliness, yield, and fiber quality traits. SABRAO J. Breed Genet. 2020, 52, 369–389. [Google Scholar]
- Semahegn, Y.; Shimelis, H.; Laing, M.; Mathew, I. Combining ability of bread wheat genotypes for yield and yield-related traits under drought-stressed and non-stressed conditions. S. Afr. J. Plant Soil. 2021, 38, 171–179. [Google Scholar] [CrossRef]
- Fadeel, A.A. Location and Properties of Chloroplasts and Pigment Determination in Roots. Physiol. Plant. 1962, 15, 130–146. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Aebi, H.; Wyss, S.R.; Scherz, B.; SKVARIL, F. Heterogeneity of erythrocyte catalase II: Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur. J. Biochem. 1974, 48, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J.; Steinberg, M.; Nelson, A. Enzyme assay, quantitative determination of peroxidase in sweet corn. J. Agric. Food Chem. 1958, 6, 39–41. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; Choice Reviews Online: Washington, DC, USA, 1999; p. 35. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Pleshkov, B.P. Biochemistry of Agricultural Plants; Kolos: Moscow, Russia, 1965; p. 447. [Google Scholar]
- Fernandez, G.C. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress, Shanhua, Taiwan, 13–16 August 1992; pp. 257–270. [Google Scholar]
- Rosielle, A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environment 1. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Gavuzzi, P.; Rizza, F.; Palumbo, M.; Campanile, R.; Ricciardi, G.; Borghi, B. Evaluation of field and laboratory predictors of drought and heat tolerance in winter cereals. Can. J. Plant Sci. 1997, 77, 523–531. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Package ‘Factoextra’. Extract and Visualize the Results of Multivariate Data Analyses. 2017. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 25 December 2021).
- Griffing, B. Concept of general and specific combining ability in relation to diallel crossing systems. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- Chowdhury, M.K.; Hasan, M.; Bahadur, M.; Islam, M.; Hakim, M.; Iqbal, M.A.; Javed, T.; Raza, A.; Shabbir, R.; Sorour, S. Evaluation of drought tolerance of some wheat (Triticum aestivum L.) genotypes through phenology, growth, and physiological indices. Agronomy 2021, 11, 1792. [Google Scholar] [CrossRef]
- Ali, M.M.; Mansour, E.; Awaad, H.A. Drought Tolerance in Some Field Crops: State of the Art Review. In Mitigating Environmental Stresses for Agricultural Sustainability in Egypt; Springer Nature: Cham, Switzerland, 2021; pp. 17–62. [Google Scholar]
- Mansour, E.; Moustafa, E.S.; El-Naggar, N.Z.; Abdelsalam, A.; Igartua, E. Grain yield stability of high-yielding barley genotypes under Egyptian conditions for enhancing resilience to climate change. Crop Pasture Sci. 2018, 69, 681–690. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Omidi, M.; Naghavi, M.R.; Etminan, A.; Mehrabi, A.A.; Poczai, P. Wild relatives of wheat respond well to water deficit stress: A comparative study of antioxidant enzyme activities and their encoding gene expression. Agriculture 2020, 10, 415. [Google Scholar] [CrossRef]
- Osipova, S.V.; Permyakov, A.V.; Permyakova, M.D.; Pshenichnikova, T.A.; Genaev, M.A.; Börner, A. The antioxidant enzymes activity in leaves of inter-varietal substitution lines of wheat (Triticum aestivum L.) with different tolerance to soil water deficit. Acta Physiol. Plant. 2013, 35, 2455–2465. [Google Scholar] [CrossRef]
- Bogale, A.; Tesfaye, K.; Geleto, T. Morphological and physiological attributes associated to drought tolerance of Ethiopian durum wheat genotypes under water deficit condition. J. Biodivers. Environ. Sci. 2011, 1, 22–36. [Google Scholar]
- El-Hendawy, S.; Al-Suhaibani, N.; Al-Ashkar, I.; Alotaibi, M.; Tahir, M.U.; Solieman, T.; Hassan, W.M. Combining genetic analysis and multivariate modeling to evaluate spectral reflectance indices as indirect selection tools in wheat breeding under water deficit stress conditions. Remote Sens. 2020, 12, 1480. [Google Scholar] [CrossRef]
- Morsy, S.M.; Elbasyoni, I.S.; Abdallah, A.M.; Baenziger, P.S. Imposing water deficit on modern and wild wheat collections to identify drought-resilient genotypes. J. Agron. Crop Sci. 2021, 1, 1–14. [Google Scholar] [CrossRef]
- Liu, H.; Searle, I.R.; Mather, D.E.; Able, A.J.; Able, J.A. Morphological, physiological and yield responses of durum wheat to pre-anthesis water-deficit stress are genotype-dependent. Crop Pasture Sci. 2015, 66, 1024–1038. [Google Scholar] [CrossRef]
- Ozturk, A.; Erdem, E.; Aydin, M.; Karaoglu, M.M. The effects of drought after anthesis on the grain quality of bread wheat depend on drought severity and drought resistance of the variety. Cereal Res. Commun. 2021, 1, 105–116. [Google Scholar] [CrossRef]
- Elbasyoni, I.S.; Morsy, S.M.; Ramamurthy, R.K.; Nassar, A.M. Identification of genomic regions contributing to protein accumulation in wheat under well-watered and water deficit growth conditions. Plants 2018, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Magallanes-López, A.M.; Ammar, K.; Morales-Dorantes, A.; González-Santoyo, H.; Crossa, J.; Guzmán, C. Grain quality traits of commercial durum wheat varieties and their relationships with drought stress and glutenins composition. J. Cereal Sci. 2017, 75, 1–9. [Google Scholar] [CrossRef]
- Ayed, S.; Othmani, A.; Bouhaouel, I.; da Silva, J.A.T. Multi-environment screening of durum wheat genotypes for drought tolerance in changing climatic events. Agronomy 2021, 11, 875. [Google Scholar] [CrossRef]
- Arjenaki, F.G.; Jabbari, R.; Morshedi, A. Evaluation of drought stress on relative water content, chlorophyll content and mineral elements of wheat (Triticum aestivum L.) varieties. Int. J. Agric. Crop Sci. 2012, 4, 726–729. [Google Scholar]
- Lonbani, M.; Arzani, A. Morpho-physiological traits associated with terminal drought-stress tolerance in triticale and wheat. Agron. Res. 2011, 9, 315–329. [Google Scholar]
- El-Sanatawy, A.M.; El-Kholy, A.S.; Ali, M.; Awad, M.F.; Mansour, E. Maize seedling establishment, grain yield and crop water productivity response to seed priming and irrigation management in a mediterranean arid environment. Agronomy 2021, 11, 756. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; Merwad, A.-R.; Abo El-Maati, M.F.; Mansour, E.; Arnaout, S.M.; Awad, M.F.; Ramadan, M.F.; Ibrahim, S.A. Physiological and biochemical mechanisms of exogenously applied selenium for alleviating destructive impacts induced by salinity stress in bread wheat. Agronomy 2021, 11, 926. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; Mansour, E.; Yasin, M.A.; El Sobky, E.-S.E.; Rady, M.M. Improvement of drought tolerance in five different cultivars of Vicia faba with foliar application of ascorbic acid or silicon. Span. J. Agric. Res. 2020, 18, 16. [Google Scholar] [CrossRef]
- Kirova, E.; Pecheva, D.; Simova-Stoilova, L. Drought response in winter wheat: Protection from oxidative stress and mutagenesis effect. Acta Physiol. Plant. 2021, 43, 8. [Google Scholar] [CrossRef]
- Habibullah, M.; Sarkar, S.; Islam, M.M.; Ahmed, K.U.; Rahman, M.; Awad, M.F.; ElSayed, A.I.; Mansour, E.; Hossain, M. Assessing the response of diverse sesame genotypes to waterlogging durations at different plant growth stages. Plants 2021, 10, 2294. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-H. Unraveling new functions of superoxide dismutase using yeast model system: Beyond its conventional role in superoxide radical scavenging. J. Microbiol. 2017, 55, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; de Groot, S.; Soole, K.; Langridge, P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef] [PubMed]
- Onyemaobi, I.; Liu, H.; Siddique, K.H.; Yan, G. Both male and female malfunction contributes to yield reduction under water stress during meiosis in bread wheat. Front. Plant Sci. 2017, 7, 2071. [Google Scholar] [CrossRef] [PubMed]
- Dolferus, R.; Powell, N.; Xuemei, J.; Ravash, R.; Edlington, J.; Oliver, S.; Van Dongen, J.; Shiran, B. The Physiology of Reproductive-Stage Abiotic Stress Tolerance in Cereals. In Molecular Stress Physiology of Plants; Springer: Berlin/Heidelberg, Germany, 2013; pp. 193–216. [Google Scholar]
- Grzesiak, S.; Hordyńska, N.; Szczyrek, P.; Grzesiak, M.T.; Noga, A.; Szechyńska-Hebda, M. Variation among wheat (Triticum easativum L.) genotypes in response to the drought stress: I–selection approaches. J. Plant Interact. 2019, 14, 30–44. [Google Scholar] [CrossRef]
- Mujtaba, S.; Faisal, S.; Khan, M.A.; Shirazi, M.; Khan, M. Evaluation of drought tolerant wheat genotypes using morpho-physiological indices as screening tools. Pak. J. Bot. 2018, 50, 51–58. [Google Scholar]
- Shamsi, K.; Petrosyan, M.; Noor-Mohammadi, G.; Haghparast, R. The role of water deficit stress and water use efficiency on bread wheat cultivars. J. Appl. Biosci. 2010, 35, 2325–2331. [Google Scholar]
- Saint Pierre, C.; Peterson, C.; Ross, A.; Ohm, J.; Verhoeven, M.; Larson, M.; Hoefer, B. Winter wheat genotypes under different levels of nitrogen and water stress: Changes in grain protein composition. J. Cereal Sci. 2008, 47, 407–416. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zhu, G.; Zhu, D.; Yan, Y. Effects of water deficit and high N fertilization on wheat storage protein synthesis, gluten secondary structure, and breadmaking quality. Crop J. 2021, in press. [Google Scholar] [CrossRef]
- Gracia, M.; Mansour, E.; Casas, A.; Lasa, J.; Medina, B.; Cano, J.L.M.; Moralejo, M.; López, A.; Fuster, P.L.; Escribano, J. Progress in the Spanish national barley breeding program. Span. J. Agric. Res. 2012, 10, 741–751. [Google Scholar] [CrossRef]
- Gharib, M.A.A.H.; Qabil, N.; Salem, A.H.; Ali, M.M.A.; Awaad, H.A.; Mansour, E. Characterization of wheat landraces and commercial cultivars based on morpho-phenological and agronomic traits. Cereal Res. Commun. 2021, 49, 149–159. [Google Scholar] [CrossRef]
- Ponce-Molina, L.J.; María Casas, A.; Pilar Gracia, M.; Silvar, C.; Mansour, E.; Thomas, W.B.; Schweizer, G.; Herz, M.; Igartua, E. Quantitative trait loci and candidate loci for heading date in a large population of a wide barley cross. Crop Sci. 2012, 52, 2469–2480. [Google Scholar] [CrossRef]
- Kamara, M.M.; Ibrahim, K.M.; Mansour, E.; Kheir, A.; Germoush, M.O.; El-Moneim, A.; Motawei, M.I.; Alhusays, A.Y.; Farid, M.A.; Rehan, M. Combining ability and gene action controlling grain yield and its related traits in bread wheat under heat stress and normal conditions. Agronomy 2021, 11, 1450. [Google Scholar] [CrossRef]
- MS, A.E.-A.; MA, E.H.; Abo-Youssef, M.; Omar, A.; Kamara, M. Genetic behavior of rice (Oryza sativa) genotypes under normal and infested weed conditions. Indian J. Agric. Sci. 2020, 90. [Google Scholar]
- Mwadzingeni, L.; Shimelis, H.; Tsilo, T.J. Combining ability and gene action controlling yield and yield components in bread wheat (Triticum aestivum L.) under drought-stressed and nonstressed conditions. Plant Breed. Biotech. 2018, 137, 502–513. [Google Scholar] [CrossRef]
- Mia, M.S.; Liu, H.; Wang, X.; Lu, Z.; Yan, G. Response of wheat to post-anthesis water stress, and the nature of gene action as revealed by combining ability analysis. Crop Pasture Sci. 2017, 68, 534–543. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Xin, W.; Wei, Y.; Zhang, J.; Guo, L. Wheat breeding in northern China: Achievements and technical advances. Crop J. 2019, 7, 718–729. [Google Scholar] [CrossRef]
- Battenfield, S.D.; Guzmán, C.; Gaynor, R.C.; Singh, R.P.; Peña, R.J.; Dreisigacker, S.; Fritz, A.K.; Poland, J.A. Genomic selection for processing and end-use quality traits in the CIMMYT spring bread wheat breeding program. Plant Genome 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Joshi, S.; Sharma, S.; Singhania, D.; Sain, R. Combining ability in the F1 and F2 generations of diallel cross in hexaploid wheat (Triticum aestivum L. em. Thell). Hereditas 2004, 141, 115–121. [Google Scholar] [CrossRef]
- Jatoi, W.; Baloch, M.; Khan, N.; Munir, M.; Khakwani, A.; Vessar, N.; Panhwar, S.; Gul, S. Heterosis for yield and physiological traits in wheat under water stress conditions. J. Anim. Plant Sci. 2014, 24, 252–261. [Google Scholar]
- Mohi-Ud-Din, M.; Hossain, M.; Rohman, M.; Uddin, M.; Haque, M.; Ahmed, J.U.; Hossain, A.; Hassan, M.M.; Mostofa, M.G. Multivariate analysis of morpho-physiological traits reveals differential drought tolerance potential of bread wheat genotypes at the seedling stage. Plants 2021, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Mekaoussi, R.; Rabti, A.-B.; Fellahi, Z.E.A.; Hannachi, A.; Benmahammed, A.; Bouzerzour, H. Assessment of durum wheat (Triticum durum Desf.) genotypes based on their agro-physiological characteristics and stress tolerance indices. Acta Agric. Slov. 2021, 117, 1–16. [Google Scholar] [CrossRef]
- Islam, M.; De, R.K.; Hossain, M.; Haque, M.; Uddin, M.; Fakir, M.; Ali, S.; Kader, M.; Dessoky, E.S.; Attia, A.O. Evaluation of the tolerance ability of wheat genotypes to drought stress: Dissection through culm-reserves contribution and grain filling physiology. Agronomy 2021, 11, 1252. [Google Scholar] [CrossRef]
- Moustafa, E.S.; Ali, M.; Kamara, M.M.; Awad, M.F.; Hassanin, A.A.; Mansour, E. Field screening of wheat advanced lines for salinity tolerance. Agronomy 2021, 11, 281. [Google Scholar] [CrossRef]
- Moustafa, E.S.; El-Sobky, E.-S.E.; Farag, H.I.; Yasin, M.A.; Attia, A.; Rady, M.O.; Awad, M.F.; Mansour, E. Sowing date and genotype influence on yield and quality of dual-purpose barley in a salt-affected arid region. Agronomy 2021, 11, 717. [Google Scholar] [CrossRef]
- Sinolinding, K.; Chowdhry, A.R. Heterosis and combining ability in wheat under irrigation and moisture stress. Exp. Agric. 1974, 10, 241–245. [Google Scholar] [CrossRef]
- El-Maghraby, M.; Moussa, M.; Hana, N.; Agrama, H. Combining ability under drought stress relative to SSR diversity in common wheat. Euphytica 2005, 141, 301–308. [Google Scholar] [CrossRef]
- Rad, M.R.N.; Kadir, M.A.; Yusop, M.R.; Jaafar, H.Z.; Danaee, M. Gene action for physiological parameters and use of relative water content (RWC) for selection of tolerant and high yield genotypes in F2 population of wheat. Aust. J. Crop Sci. 2013, 7, 407–413. [Google Scholar]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse wheat (Triticum aestivum L.) genotypes varying in sensitivity to heat and drought stress. Sci. Rep. 2019, 9, 6955. [Google Scholar] [CrossRef]
- Ahmed, K.; Shabbir, G.; Ahmed, M.; Shah, K.N. Phenotyping for drought resistance in bread wheat using physiological and biochemical traits. Sci. Total Environ. 2020, 729, 139082. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Reddy, S.; Fuentealba, M.; Xue, Q.; Rudd, J.; Jessup, K.; Devkota, R.; Liu, S. Physiological responses to water stress and yield of winter wheat cultivars differing in drought tolerance. J. Agron. Crop Sci. 2018, 204, 347–358. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Al-Suhaibani, N.; Abdella, K.; Sallam, M.; Alotaibi, M.; Seleiman, M.F. Combining genetic and multidimensional analyses to identify interpretive traits related to water shortage tolerance as an indirect selection tool for detecting genotypes of drought tolerance in wheat breeding. Plants 2021, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Tari, A.F. The effects of different deficit irrigation strategies on yield, quality, and water-use efficiencies of wheat under semi-arid conditions. Agric. Water Manag. 2016, 167, 1–10. [Google Scholar] [CrossRef]
- Thungo, Z.; Shimelis, H.; Odindo, A.; Mashilo, J.; Shayanowako, A. Genetic relationship among selected heat and drought tolerant bread wheat genotypes using SSR markers, agronomic traits and grain protein content. Acta Agric. Scand. B Soil Plant Sci. 2020, 70, 594–604. [Google Scholar] [CrossRef]
- Šíp, V.; Vavera, R.; Chrpová, J.; Kusá, H.; Růžek, P. Winter wheat yield and quality related to tillage practice, input level and environmental conditions. Soil Tillage Res. 2013, 132, 77–85. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).