Chemical Composition of Essential Oil from Four Sympatric Orchids in NW-Italy
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Isolation of Essential Oil
3.3. Fractionation and Alkylthiolation of Alkenes
3.4. GC-FID and GC-Ms Analysis
3.5. Identification of the Components of the Volatile Fractions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salzmann, C.C.; Cozzolino, S.; Schiestl, F.P. Floral Scent in Food-Deceptive Orchids: Species Specificity and Sources of Variability. Plant Biol. 2007, 9, 720–729. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollsten, L.; Bergström, L.G. Floral scents-a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar] [CrossRef]
- Knudsen, J.; Eriksson, R.; Gershenzon, J.; Stah, L.B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Nunes, C.E.P.; Gerlach, G.; Bandeira, K.D.O.; Gobbo-Neto, L.; Pansarin, E.R.; Sazima, M. Two orchids, one scent? Floral volatiles of Catasetum cernuum and Gongora bufonia suggest convergent evolution to a unique pollination niche. Flora Morphol. Distrib. Funct. Ecol. Plants 2017, 232, 207–216. [Google Scholar] [CrossRef]
- Riffell, J.A. The neuroecology of a pollinator’s buffet: Olfactory preferences and learning in insect pollinators. Integr. Comp. Biol. 2011, 51, 781–793. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, F.P.; Ayasse, M. Do changes in floral odor cause speciation in sexually deceptive orchids? Plant Syst. Evol. 2002, 234, 111–119. [Google Scholar] [CrossRef]
- Mant, J.; Peakall, R.; Schiestl, F.P. Does selection on floral odor promote differentiation among populations and species of the sexually deceptive orchid genus ophrys? Evolution 2005, 59, 1449. [Google Scholar] [CrossRef]
- Christenhuszm, M.J.M.; Byng, J. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261. [Google Scholar] [CrossRef]
- Cozzolino, S.; Widmer, A. Orchid diversity: An evolutionary consequence of deception? Trends Ecol. Evol. 2005, 20, 487–494. [Google Scholar] [CrossRef]
- Jersáková, J.; Johnson, S.D.; Kindlmann, P. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. Camb. Philos. Soc. 2006, 81, 219–235. [Google Scholar] [CrossRef]
- Borg-Karlson, A.K.; Bergström, G.; Groth, I. Chemical basis for the relationship between Ophrys orchids and their pollinators. I. Volatile compounds of Ophrys lutea and O. fusca as insect mimetic attractants/excitants. Chem. Scr. 1985, 25, 283–311. [Google Scholar]
- Borg-Karlson, A.K.; Bergström, G.; Kullenberg, B. Chemical basis for the relationship between Ophrys. orchids and their pollinators. II. Volatile compounds of O. insectifera and O. speculum as insect mimetic attractants/excitants. Chem. Scr. 1987, 27, 303–311. [Google Scholar]
- Ayasse, M.; Schiestl, F.P.; Paulus, H.F.; Löfstedt, C.; Hansson, B.; Ibarra, F.; Francke, W. Evolution of reproductive strategies in the sexually deceptive Orchid Ophrys sphegodes: How does flower-specific variation of odor signals influence reproductive success? Evolution 2000, 54, 1995–2006. [Google Scholar] [CrossRef]
- Ayasse, M.; Stökl, J.; Francke, W. Chemical ecology and pollinator-driven speciation in sexually deceptive orchids. Phytochemistry 2011, 72, 1667–1677. [Google Scholar] [CrossRef]
- Schiestl, F.P. On the success of a swindle: Pollination by deception in orchids. Naturwissenschaften 2005, 92, 255–264. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Ayasse, M.; Paulus, H.F.; Löfstedt, C.; Hansson, B.S.; Ibarra, F.; Francke, W. Sex pheromone mimicry in the early spider orchid (Ophrys sphegodes): Patters of hydrocarbons as the key mechanism for pollination by sexual deception. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 2000, 186, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Cortis, P.; Vereecken, N.J.; Schiestl, F.P.; Barone Lumaga, M.R.; Scrugli, A.; Cozzolino, S. Pollinator convergence and the nature of species’ boundaries in sympatric Sardinian Ophrys (Orchidaceae). Ann. Bot. 2009, 104, 497–506. [Google Scholar] [CrossRef]
- Ayasse, M.; Schiestl, F.P.; Paulus, H.F.; Ibarra, F.; Francke, W. Pollinator attraction in a sexually deceptive orchid by means of unconventional chemicals. Proc. R. Soc. B Biol. Sci. 2003, 270, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Dafni, A. Mimicry and deception in pollination. Annu. Rev. Ecol. Syst. 1984, 15, 259–278. [Google Scholar] [CrossRef]
- Raguso, R.A. Olfactory landscapes and deceptive pollination: Signal, noise and convergent evolution in floral scent. In Insect Pheromone Biochemistry and Molecular Biology: The Biosynthesis and Detection of Pheromones and Plant Volatiles; Blomquist, G.J., Press, E.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 631–650. [Google Scholar]
- Salzmann, C.C.; Brown, A.; Schiestl, F.P. Floral scent emission and pollination syndromes: Evolutionary changes from food to sexual deception. Int. J. Plant Sci. 2006, 167, 1197–1204. [Google Scholar] [CrossRef]
- Scopece, G.; Widmer, A.; Cozzolino, S. Evolution of postzygotic reproductive isolation in a deceptive orchid lineage. Am. Nat. 2008, 171, 315–326. [Google Scholar] [CrossRef]
- Manzo, A.; Panseri, S.; Vagge, I.; Giorgi, A. Volatile fingerprint of italian populations of orchids using solid phase microextraction and gas chromatography coupled with mass spectrometry. Molecules 2014, 19, 7913–7936. [Google Scholar] [CrossRef]
- Cozzolino, S.; D’Emerico, S.; Widmer, A. Evidence for reproductive isolate selection in Mediterranean orchids: Karyotype differences compensate for the lack of pollinator specificity. Proc. R. Soc. B Biol. Sci. 2004, 271, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Calevo, J.; Bazzicalupo, M.; Adamo, M.; Robustelli della Cuna, F.S.; Voyron, S.; Girlanda, M.; Duffy, K.J.; Giovannini, A.; Cornara, L. Floral Trait and Mycorrhizal Similarity between an Endangered Orchid and Its Natural Hybrid. Diversity 2021, 13, 550. [Google Scholar] [CrossRef]
- Pellegrino, G.; Luca, A.; Bellusci, F.; Musacchio, A. Comparative analysis of floral scents in four sympatric species of Serapias L. (Orchidaceae): Clues on their pollination strategies. Plant Syst. Evol. 2012, 298, 1837–1843. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Carol Stre, C., Ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Stein, S.E. NIST/EPA/NIH Mass Spectral Database; Version 2.1; Perkin-Elmer Instrument LLC: Hong Kong, China, 2000. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Robustelli della Cuna, F.S.; Calevo, J.; Bari, E.; Giovannini, A.; Boselli, C.; Tava, A. Characterization and antioxidant activity of essential oil of four sympatric orchid species. Molecules 2019, 24, 3878. [Google Scholar] [CrossRef]
- Salzmann, C.C.; Nardella, A.M.; Cozzolino, S.; Schiestl, F.P. Variability in floral scent in rewarding and deceptive orchids: The signature of pollinator-imposed selection? Ann. Bot. 2007, 100, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Galizia, C.G.; Kunze, J.; Gumbert, A.; Borg-Karlson, A.K.; Sachse, S.; Markl, C.; Menzel, R. Relationship of visual and olfactory signal parameters in a food-deceptive flower mimicry system. Behav. Ecol. 2005, 16, 159–168. [Google Scholar] [CrossRef]
- Kunze, J.; Gumbert, A. The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behav. Ecol. 2001, 12, 447–456. [Google Scholar] [CrossRef]
- Romano, V.A.; Rosati, L.; Fascetti, S.; Cittadini, A.M.R.; Racioppi, R.; Lorenz, R.; D’Auria, M. Spatial and Temporal Variability of the Floral Scent Emitted by Barlia robertiana (Loisel.) Greuter, a Mediterranean Food-Deceptive Orchid. Compounds 2022, 2, 37–53. [Google Scholar] [CrossRef]
- Gallego, E.; Gelabert, A.; Roca, F.J.; Perales, J.F.; Guardino, X.; De Medi, C.; Universitat, A.; De Catalunya, P.; Hospital, U.; Autònoma, U.; et al. Identification of volatile organic compounds (VOC) emitted from three European orchid species with different pollination strategies: Two deceptive orchids (Himantoglossum robertianum and Ophrys apifera) and a rewarding orchid (Gymnadenia conopsea). J. Biodivers. Environ. Sci. 2012, 2, 18–29. [Google Scholar]
- Robustelli della Cuna, F.S.; Boselli, C.; Papetti, A.; Calevo, J.; Mannucci, B.; Tava, A. Composition of volatile fraction from inflorescences and leaves of Dendrobium moschatum (Orchidaceae). Nat. Prod. Commun. 2017, 13, 93–96. [Google Scholar] [CrossRef]
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Berg, C.V.D.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Robustelli della Cuna, F.S.; Calevo, J.; Bazzicalupo, M.; Sottani, C.; Grignani, E.; Preda, S. Chemical composition of essential oil from flowers of five fragrant dendrobium (Orchidaceae). Plants 2021, 10, 1718. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.; Roan, C.S.; Yost, R.A.; Hector, J. Dimethyl disulphide derivatives of long chain alkenes, alkadienes and alkatrienes for gas chromatography/mass spectrometry. Anal. Chem. 1989, 61, 1564–1571. [Google Scholar] [CrossRef]
- Joulain, D.; Konig, W.A. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; Verlag, H., Ed.; EB-Verlag: Hamburg, Germany, 1998. [Google Scholar]


| Compound a | RI Tab b | RI Mean c | Anacamptis morio % d | Himantoglossum robertianum % | Ophrys sphegodes % | Orchis purpurea % | Identificatione |
|---|---|---|---|---|---|---|---|
| Octane | 800 | 800 | − | − | − | 0.08 ± 0.01 | STD, RI |
| Hexanal | 801 | 800 | − | − | 0.24 ± 0.02 | − | NIST, RI |
| 2-Hexanol | 809 | 808 | 0.66 ± 0.11 | − | 0319 ± 0.05 | − | NIST, RI |
| Furfural | 836 | 831 | − | − | − | 0.08 ± 0.03 | NIST, RI |
| Diacetone alchol | 841 | 841 | 9.04 ± 0.09 | 4.04 ± 0.10 | 3.88 ± 0.01 | − | NIST, RI |
| Furfuryl alchol | 855 | 855 | − | − | − | 0.28 ± 0.04 | NIST, RI |
| 1-Hexanol | 871 | 870 | − | − | − | 0.03 ± 0.02 | NIST, RI |
| Heptanal | 901 | 906 | 1.33 ± 0.07 | − | 0.80 ± 0.03 | 0.02 ± 0.01 | NIST, RI |
| Unidentified | − | 907 | − | − | − | 0.31 ± 0.04 | − |
| Benzaldehyde | 961 | 964 | 0.23 ± 0.11 | − | 0.18 ± 0.04 | 0.07 ± 0.03 | NIST, RI |
| Octanal | 1001 | 1003 | − | − | 0.22 ± 0.01 | − | NIST, RI |
| 2-Ethylhexanol | 1031 | 1031 | − | − | − | 0.26 ± 0.10 | NIST, RI |
| Phenylacetaldehyde | 1042 | 1042 | − | − | − | 0.05 ± 0.04 | NIST, RI |
| β-Phorone | 1044 | 1045 | − | 3.33 ± 0.03 | − | − | NIST, RI |
| Heptanoic acid | 1069 | 1068 | 2.13 ± 0.11 | 1.40 ± 0.05 | 0.07 ± 0.03 | − | NIST, RI |
| n-Octanol | 1068 | 1070 | − | − | 0.47 ± 0.07 | − | NIST, RI |
| p-Cresol | 1076 | 1073 | 38.10 ± 0.12 | 15.28 ± 0.18 | 12.75 ± 0.08 | 12.99 ± 0.24 | NIST, RI |
| Nonanal | 1105 | 1105 | 0.19 ± 0.02 | 4.41 ± 0.17 | 1.10 ± 0.08 | 0.61 ± 0.14 | NIST, RI |
| α-Isophorone | 1121 | 1128 | − | 4.21 ± 0.12 | − | − | NIST, RI |
| trans-Verbenol | 1148 | 1154 | − | 2.40 ± 0.43 | − | − | NIST, RI |
| Nonenal | 1162 | 1162 | − | − | 0.37 ± 0.04 | 0.04 ± 0.01 | NIST, RI |
| Borneol | 1169 | 1166 | − | − | − | 0.05 ± 0.03 | NIST, RI |
| Terpinen-4-ol | 1177 | 1174 | − | 0.61 ± 0.05 | − | − | NIST, RI |
| Unidentified | − | 1185 | − | − | − | 0.27 ± 0.05 | − |
| α-Terpineol | 1189 | 1187 | − | 0.28 ± 0.12 | − | − | NIST, RI |
| p-Cimen-8-ol | 1192 | 1192 | 0.34 ± 0.03 | 0.63 ± 0.07 | − | − | NIST, RI |
| p-Methyl-guaiacol | 1192 | 1193 | − | − | − | 0.28 ± 0.08 | NIST, RI |
| 2-Methoxy p-cresol | 1198 | 1198 | 0.46 ± 0.11 | − | − | − | NIST, RI |
| Decanal | 1207 | 1207 | 0.18 ± 0.04 | 0.25 ± 0.09 | 0.09 ± 0.04 | 0.03 ± 0.02 | NIST, RI |
| p-Vinyl-phenol | 1216 | 1217 | 0.54 ± 0.04 | 0.58 ± 0.35 | 0.51 ± 0.10 | 2.37 ± 0.02 | NIST, RI |
| 2-Phenoxy ethanol | 1226 | 946 | 0.07 ± 0.02 | 0.71 ± 0.04 | − | − | NIST, RI |
| 3,5-Dimethoxy-toluene | 1264 | 1267 | − | − | − | 0.15 ± 0.09 | NIST, RI |
| Nonanoic acid | 1271 | 1261 | 0.52 ± 0.04 | 1.27 ± 0.04 | 3.09 ± 0.02 | 0.54 ± 0.12 | NIST, RI |
| 4-Hydroxy-3-methylacetophenone | 1292 | 1308 | − | − | − | 0.38 ± 0.03 | NIST, RI |
| 2,4-Decadienal (E,Z) | 1302 | 1309 | − | − | 0.15 ± 0.04 | − | NIST, RI |
| 4-Methoxy-vinyl-phenol | 1315 | 1315 | − | − | − | 0.40 ± 0.54 | NIST, RI |
| 2,4-Decadienal (E,E) | 1319 | 1321 | − | 0.62 ± 0.07 | 0.28 ± 0.10 | 0.03 ± 0.03 | NIST, RI |
| p-Hydroxybenzyl alchol | 1357 | 1356 | − | − | − | 0.11 ± 0.06 | NIST, RI |
| Decanoic acid | 1372 | 1372 | − | − | − | 0.04 ± 0.02 | NIST, RI |
| Unidentified | − | 1379 | − | − | − | 0.03 ± 0.03 | − |
| 3,4-Hydroxycoumarin | 1378 | 1384 | − | − | − | 0.05 ± 0.03 | NIST, RI |
| β-Damascenone (E) | 1385 | 1386 | − | − | − | 0.07 ± 0.02 | NIST, RI |
| 1-Tetradecene | 1393 | 1393 | 0.68 ± 0.08 | − | − | − | MS, RI |
| Tetradecane | 1400 | 1400 | − | − | − | 0.05 ± 0.03 | STD, RI |
| Dodecanal | 1409 | 1411 | − | − | 0.29 ± 0.05 | − | NIST, RI |
| Coumarin | 1458 | 1454 | 0.26 ± 0.10 | − | 0.21 ± 0.03 | 68.84 ± 0.13 | NIST, RI |
| 2,4 Di-tert-butylphenol | 1518 | 1516 | 1.39 ± 0.10 | 1.44 ± 0.05 | 0.69 ± 0.07 | − | NIST, RI |
| Unidentified | − | 1560 | − | − | − | 1,05 ± 0.03 | − |
| Dodecanoic acid | 1567 | 1557 | 0.38 ± 0.03 | − | 0.36 ± 0.04 | 0.32 ± 0.04 | NIST, RI |
| 1-Hexadecene | 1592 | 1593 | 1.17 ± 0.07 | 0.90 ± 0.06 | 0.37 ± 0.02 | 0.18 ± 0.07 | MS, RI |
| Heptadecane | 1700 | 1700 | − | 0.52 ± 0.03 | 0.68 ± 0.07 | − | STD, RI |
| 1-Heptadecene | 1755 | 1759 | 1.13 ± 0.10 | 1.38 ± 0.07 | 0.54 ± 0.05 | − | MS, RI |
| Tetradecanoic acid | 1780 | 1765 | − | − | − | 0.59 ± 0.08 | NIST, RI |
| 3-Octadecene | 1785 | 1785 | − | − | 0.14 ± 0.04 | − | MS, RI |
| 7-Octadecene | 1805 | 1805 | − | − | 0.43 ± 0.02 | − | MS, RI |
| Unidentified | − | 1821 | − | 0.49 ± 0.05 | 0.50 ± 0.09 | − | − |
| Isoprpyl myristate | 1827 | 1826 | 3.73 ± 0.10 | 2.00 ± 0.15 | 2.15 ± 0.04 | − | NIST, RI |
| Ciclohexadecane | 1880 | 1881 | − | − | − | 0.42 ± 0.04 | NIST, RI |
| Nonadecane | 1900 | 1900 | 0.97 ± 0.09 | 0.96 ± 0.06 | 0.12 ± 0.03 | STD, RI | |
| Hexadecanoic acid | 1960 | 1959 | 7.54 ± 0.09 | 4.94 ± 0.16 | 1.88 ± 0.03 | 2.14 ± 0.04 | NIST, RI |
| 1-Eicosene | 1994 | 1994 | 0.66 ± 0.06 | − | 0.45 ± 0.03 | − | MS, RI |
| Ethyl hexadecanoate | 1995 | 1995 | − | − | − | 0.17 ± 0.16 | NIST, RI |
| Eicosane | 2000 | 2000 | 0.52 ± 0.12 | − | 0.42 ± 0.04 | − | STD, RI |
| Octadecanal | 2021 | 2025 | − | 2.80 ± 0.18 | 2.53 ± 0.28 | − | NIST, RI |
| E-15-heptadecenal | 2085 | 2085 | − | − | − | 0.67 ± 0.12 | NIST, RI |
| 1-Heneicosene | 2087 | 2087 | 1.11 ± 0.11 | − | − | − | MS, RI |
| Heneicosane | 2100 | 2100 | 0.70 ± 0.30 | − | 9.24 ± 0.08 | 0.87 ± 0.05 | NIST, RI |
| Ethyl linolenate | 2159 | 2135 | − | − | − | 0.33 ± 0.26 | NIST, RI |
| 1-Docosene | 2195 | 2195 | 0.44 ± 0.08 | − | 0.16 ± 0.05 | − | MS, RI |
| Docosane | 2200 | 2200 | − | − | 2.17 ± 0.03 | − | STD, RI |
| 11-Tricosene | 2261 | 2261 | − | − | 1.59 ± 0.04 | − | MS, RI |
| 9-Tricosene | 2279 | 2277 | − | − | 0.72 ± 0.06 | − | MS, RI |
| 7-Tricosene | 2287 | 2286 | − | − | 0.42 ± 0.03 | − | MS, RI |
| Tricosane | 2300 | 2300 | 7.07 ± 0.07 | 4.30 ± 0.08 | 27.76 ± 0.06 | 0.73 ± 0.07 | STD, RI |
| Tetracosane | 2400 | 2400 | 1,22 ± 0.05 | − | 3.33 ± 0.05 | − | STD, RI |
| Docosanal | 2432 | 2431 | − | − | 0.71 ± 0.08 | − | NIST, RI |
| 9-Pentacosene | 2474 | 2476 | − | − | 3.03 ± 0.05 | 0.12 ± 0.03 | MS, RI |
| 7-Pentacosene | 2483 | 2483 | − | − | 0.28 ± 0.05 | − | MS, RI |
| 1-Docosanol | 2493 | 2493 | − | − | − | 0.18 ± 0.02 | NIST, RI |
| Pentacosane | 2500 | 2500 | 17.14 ± 0.05 | 40.17 ± 0.17 | 12.33 ± 0.03 | 2.22 ± 0.21 | STD, RI |
| Hexacosane | 2600 | 2600 | 1.05 ± 0.05 | − | 1.39 ± 0.05 | 0.25 ± 0.01 | STD, RI |
| Heptacosane | 2700 | 2700 | − | − | − | 1.06 ± 0.03 | STD, RI |
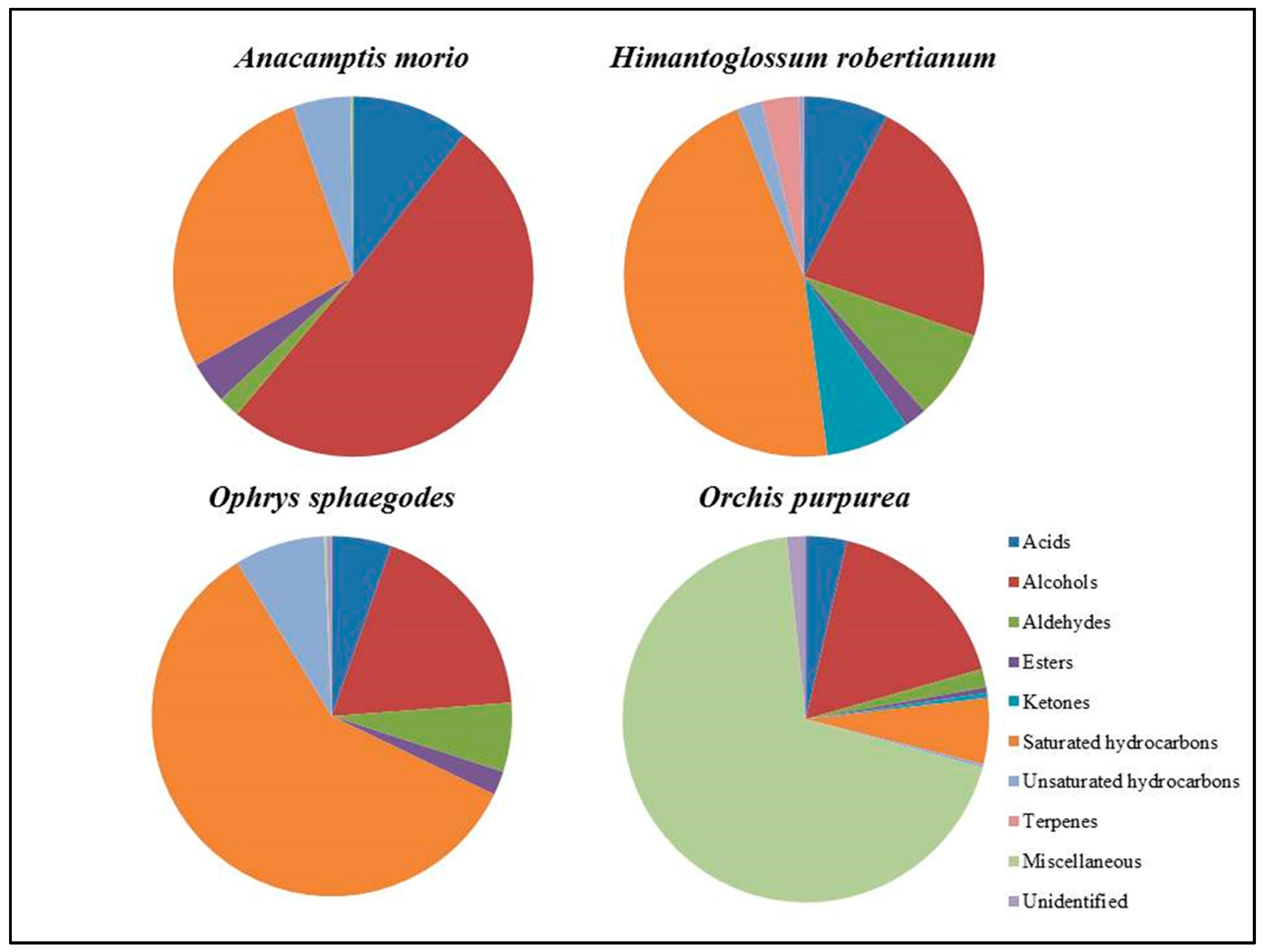
| Acids | 10.57 | 7.61 | 5.39 | 3.63 | |||
| Alcohols | 50.60 | 22.68 | 18.49 | 16.93 | |||
| Aldehydes | 1.94 | 8.07 | 6.24 | 1.62 | |||
| Esters | 3.73 | 2.00 | 2.15 | 0.50 | |||
| Ketones | − | 7.54 | − | 0.45 | |||
| Saturated hydrocarbons | 27.70 | 45.97 | 59.29 | 5.81 | |||
| Unsaturated hydrocarbons | 5.20 | 2.28 | 8.12 | 0.29 | |||
| Terpenes | − | 3,29 | − | − | |||
| Miscellaneous | 0.26 | − | 0.21 | 69.04 | |||
| Unidentified | − | 0.49 | 0.50 | 1.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robustelli della Cuna, F.S.; Cortis, P.; Esposito, F.; De Agostini, A.; Sottani, C.; Sanna, C. Chemical Composition of Essential Oil from Four Sympatric Orchids in NW-Italy. Plants 2022, 11, 826. https://doi.org/10.3390/plants11060826
Robustelli della Cuna FS, Cortis P, Esposito F, De Agostini A, Sottani C, Sanna C. Chemical Composition of Essential Oil from Four Sympatric Orchids in NW-Italy. Plants. 2022; 11(6):826. https://doi.org/10.3390/plants11060826
Chicago/Turabian StyleRobustelli della Cuna, Francesco Saverio, Pierluigi Cortis, Fabiana Esposito, Antonio De Agostini, Cristina Sottani, and Cinzia Sanna. 2022. "Chemical Composition of Essential Oil from Four Sympatric Orchids in NW-Italy" Plants 11, no. 6: 826. https://doi.org/10.3390/plants11060826
APA StyleRobustelli della Cuna, F. S., Cortis, P., Esposito, F., De Agostini, A., Sottani, C., & Sanna, C. (2022). Chemical Composition of Essential Oil from Four Sympatric Orchids in NW-Italy. Plants, 11(6), 826. https://doi.org/10.3390/plants11060826










