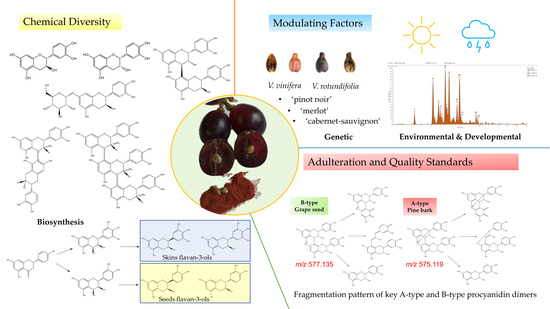
Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements
Abstract
:1. Introduction
2. Grape Seed Chemistry
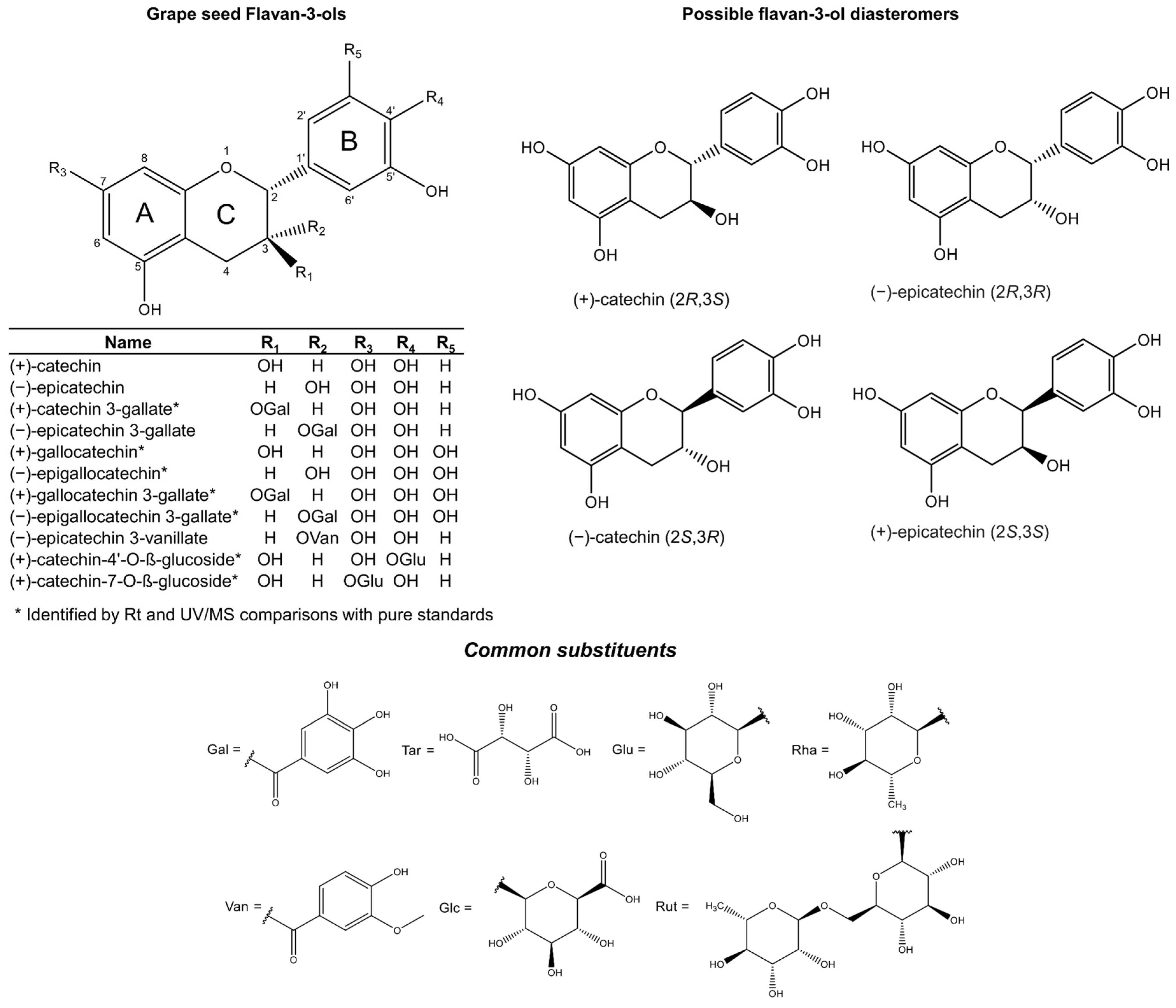
2.1. Flavan-3-Ols
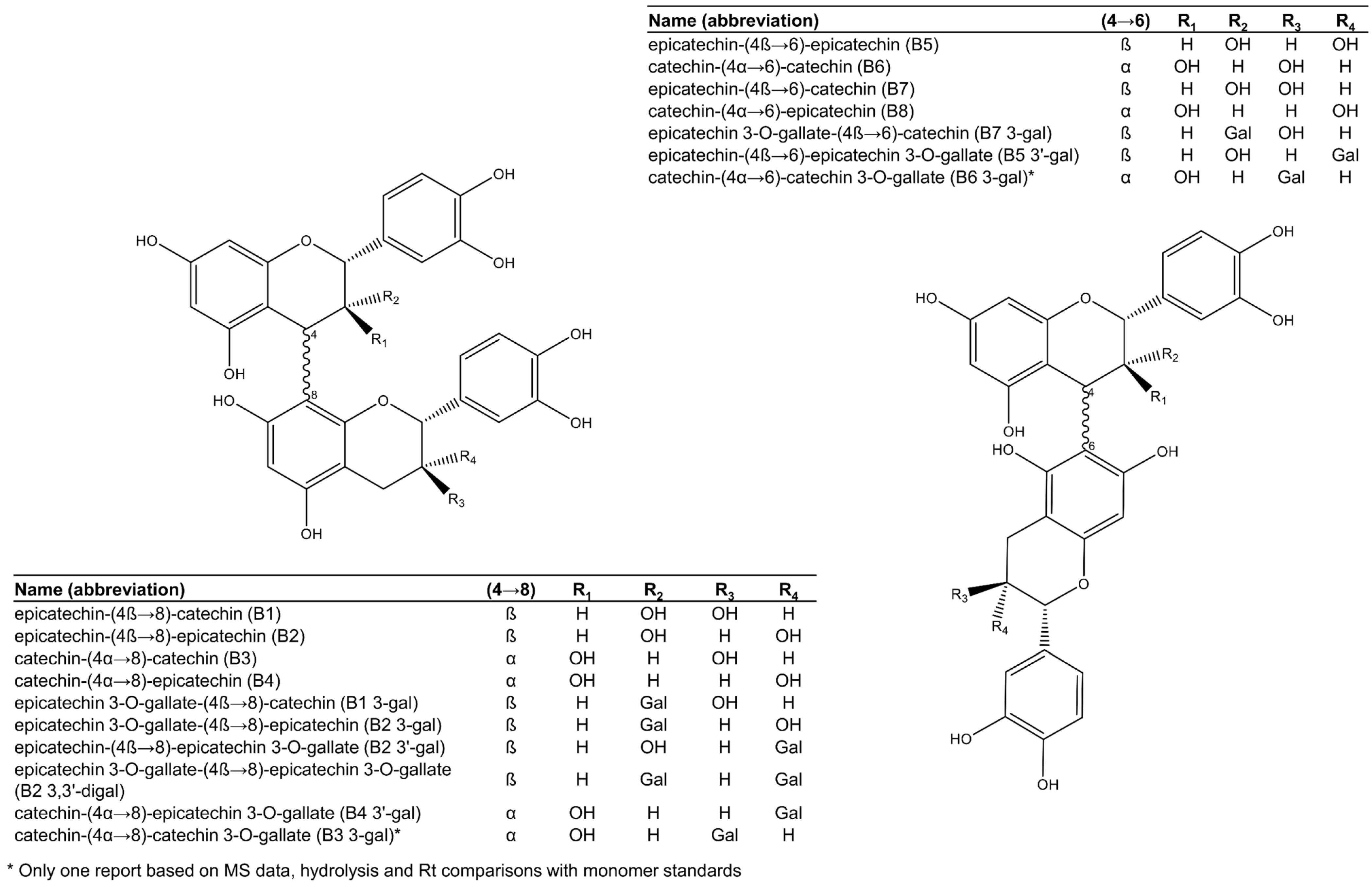
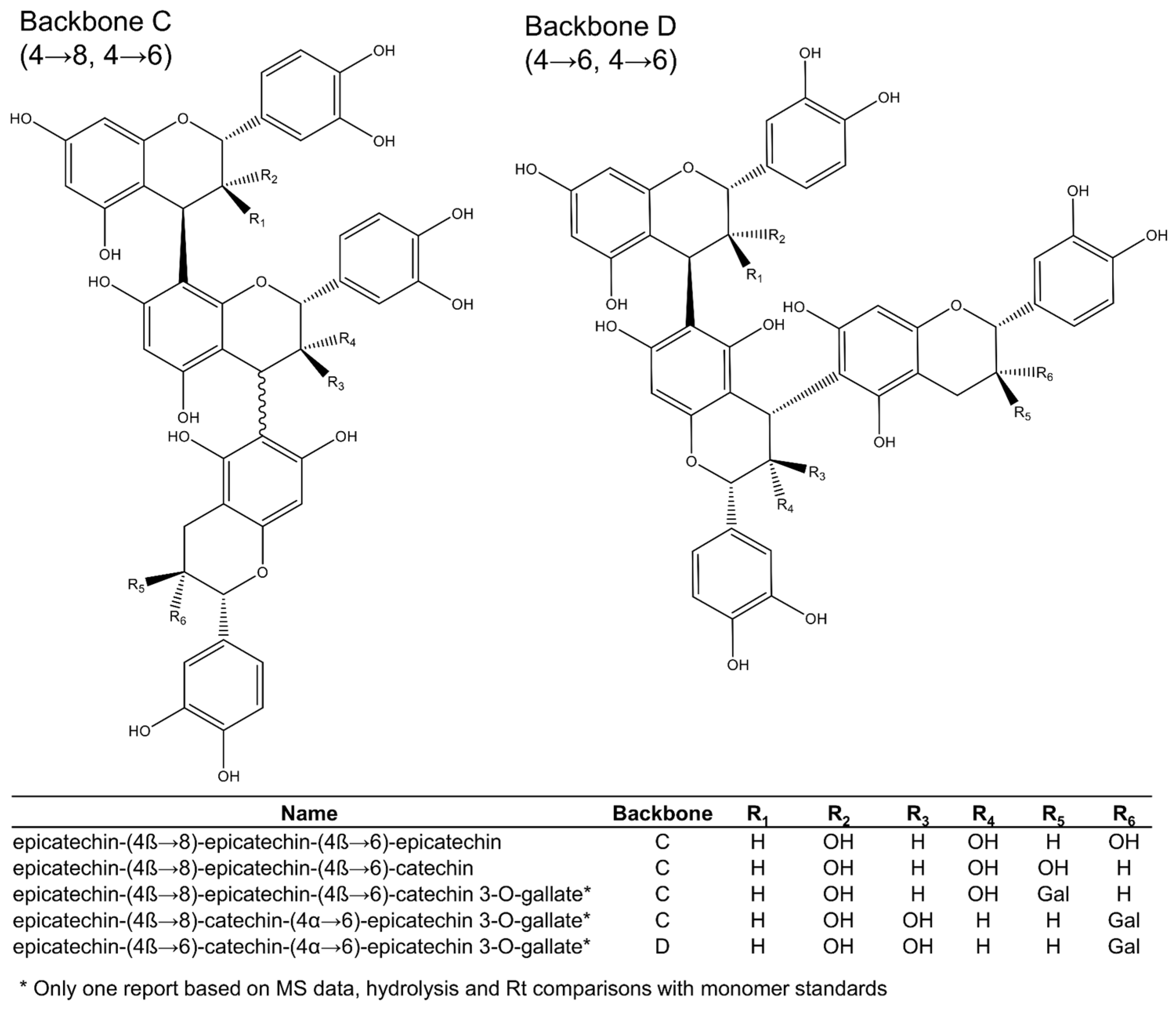
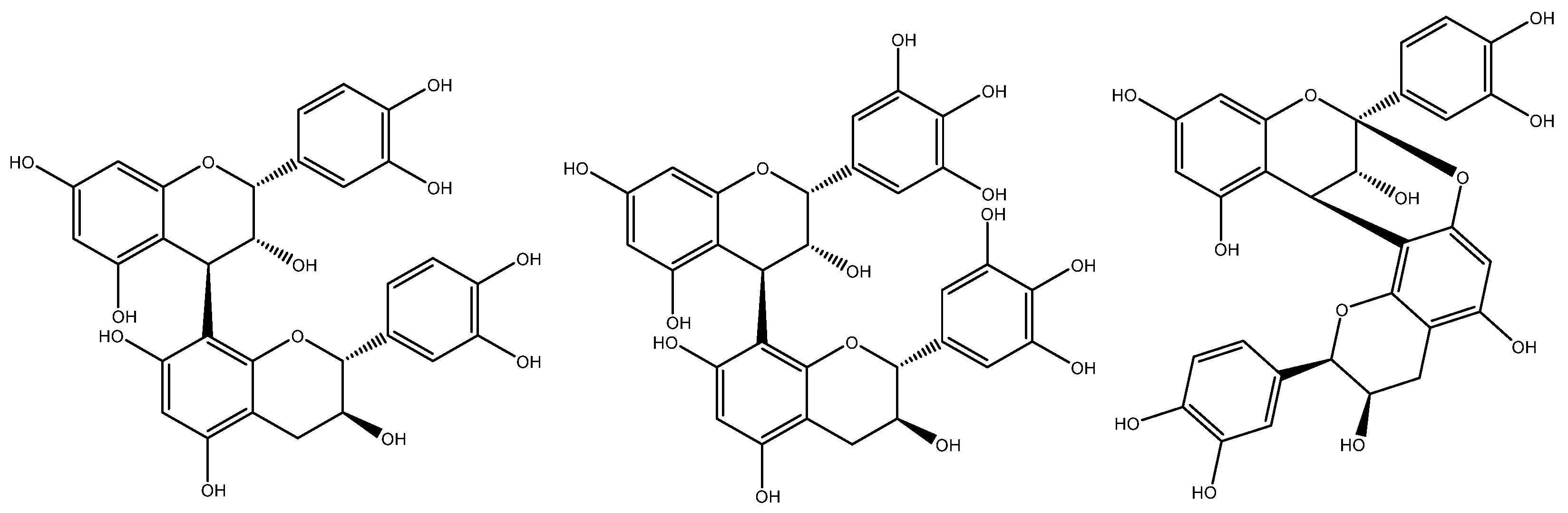
2.1.1. Procyanidins
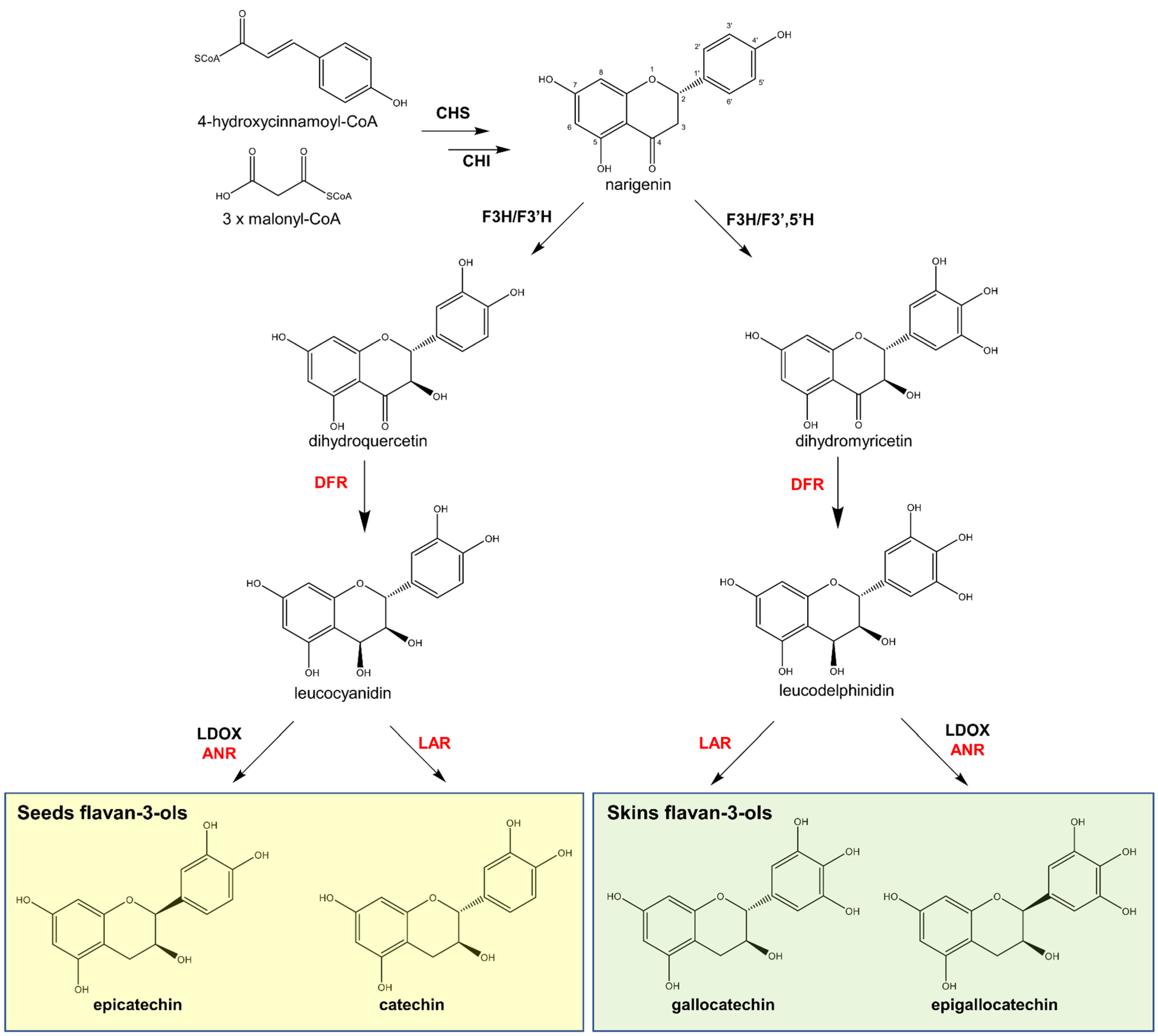
2.1.2. Biosynthesis
2.1.3. Pharmacokinetics and Structure–Activity Relationships
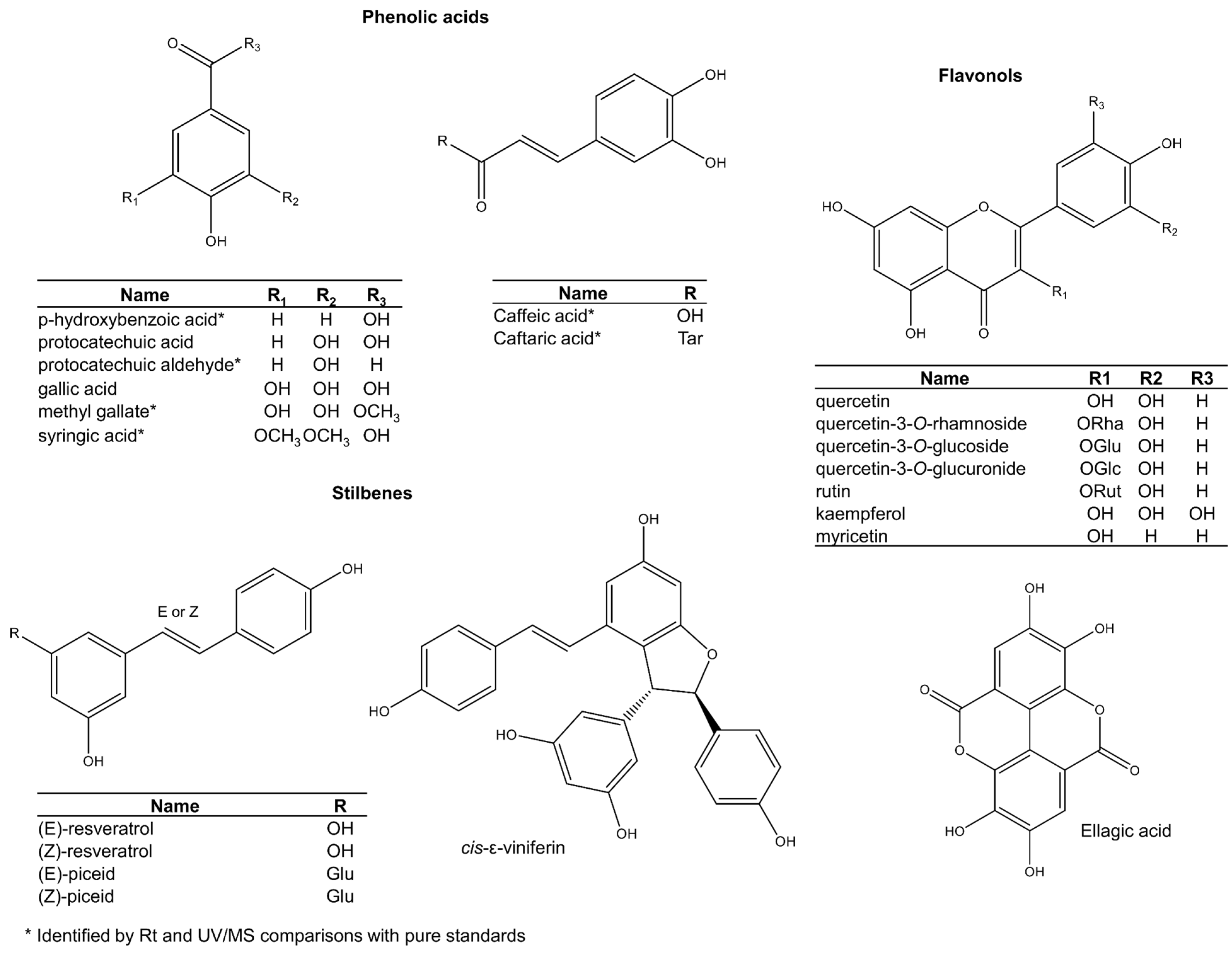
2.2. The Broader Grape Seed Metabolome
3. Modulating Factors
3.1. Genetic Factors
3.1.1. Species-Level (Interspecific) Differences
3.1.2. Variety-Level Differences
3.2. Environmental Factors
3.3. Developmental Factors
- Procyanidin biosynthesis, peaking during rapid growth of juvenile berry (Phase I);
- Flavan-3-ol monomer biosynthesis: as the biosynthesis of procyanidins slows down, monomers increase (Phase II). Kennedy et al. [126,127] explained that the overall rate of biosynthesis remains constant, which implies a rate limiting step, probably the common precursor leucocyanidin. This phase ends at the end of Phase II;
- Programmed oxidation: At veraison and after monomer biosynthesis declines. Monomers and procyanidins are oxidised (visible as a browning and hardening of the seed coat);
- Non-programmed oxidation: the seed is fully desiccated. Little change in extracted polyphenols occurs during this phase.
3.4. Other Factors
4. Quality and Adulteration
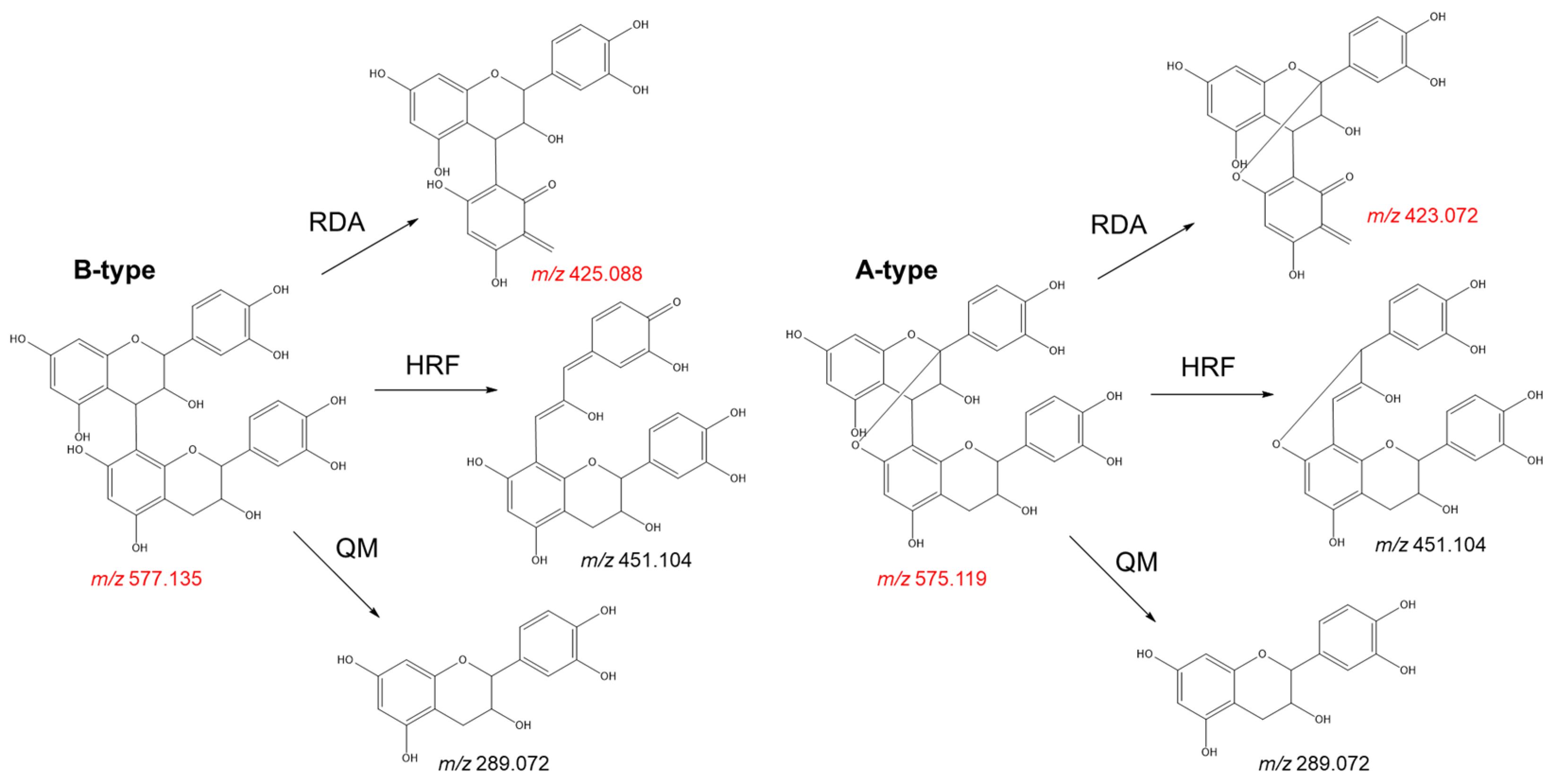
- Check for adulterants: The adulteration of grape seed products with peanut skin and pine bark extracts can be easily distinguished by the presence of A-type procyanidins. These metabolites can be identified by target LC-MS analyses. While procyanidin B2, the main flavan-3-ol dimer found in grape seed extract, shows a deprotonated molecule at m/z 577.1352, its A-type analogue (procyanidin A2) gives a deprotonated molecule at m/z 575.1195. The fragmentation pattern of these molecules also shows significant differences that can be used to discriminate between both isomers (Figure 8). The presence of a characteristic fragment ion at m/z 423.072 (negative ion mode) on the MS2 spectrum, resulting from a Retro-Diels-Alder fragmentation, indicates the presence of A-type procyanidins, while B-type procyanidins show a fragment ion at m/z 425.088 via the same mechanism (Figure 8). The presence of prodelphinidins in grape seed extracts can also be identified by MS analyses. In LC-MS analyses, deprotonated ions at m/z 609.1249 or m/z 593.1300 indicate the presence of B-type prodelphinidins, which represent common metabolites in the skins of the grape berry;
- Quantify bioactive metabolites: As previously noted, grape seed demonstrates complex chemistry, characterised by the presence of tens to hundreds of metabolites at different concentrations. Evidently, the monomeric and dimeric flavan-3-ols (especially catechin, epicatechin, and procyanidin B2) are the main bioactive constituents. Ideally, these metabolites are quantified to give an actual oral dose, particularly of the ingredients that are absorbed [89]. According to Villani et al. [18], out of three authentic extracts, the average total content of the major procyanidins is 383.5 ± 21.13 mg/g, with procyanidin B2 making up 41%. Out of total phenolics, catechin and epicatechin cumulatively representing 32% of the total content detected by HPLC. Unfortunately, Villani et al. [18] demonstrated that several commercial samples have less than 10 mg/g total procyanidins. Some of the extracts did not have any of the three major flavan-3-ols: catechin, epicatechin, and procyanidin B2 [18]. It is therefore necessary that the quantification of these three flavanols is a recognised undertaking in quality control or authentication. By HPLC, the two monomers and procyanidin B2 should cumulatively be above 50% of detected components, and the absolute content of procyanidin B2 should ideally be 150 mg/g of extract.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- This, P.; Lacombe, T.; Thomas, M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef] [PubMed]
- McGovern, P.E. Ancient Wine; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- McGovern, P.E.; Glusker, D.L.; Exner, L.J.; Voigt, M.M. Neolithic resinated wine. Nature 1996, 381, 480–481. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine Intergovernmental Organisation. 2019 Statistical Report on World Vitiviniculture; International Organisation of Vine and Wine Intergovernmental Organisation: Paris, France, 2019. [Google Scholar]
- Rousserie, P.; Rabot, A.; Geny-Denis, L. From Flavanols Biosynthesis to Wine Tannins: What Place for Grape Seeds? J. Agric. Food Chem. 2019, 67, 1325–1343. [Google Scholar] [CrossRef] [PubMed]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Richard, J.L.; Cambien, F.; Ducimetière, P. Epidemiologic characteristics of coronary disease in France. Nouv. Presse Med. 1981, 10, 1111–1114. [Google Scholar] [PubMed]
- Corder, R.; Mullen, W.; Khan, N.Q.; Marks, S.C.; Wood, E.G.; Carrier, M.J.; Crozier, A. Red wine procyanidins and vascular health. Nature 2006, 444, 566. [Google Scholar] [CrossRef]
- Corder, R.; Douthwaite, J.A.; Lees, D.M.; Khan, N.Q.; Viseu dos Santos, A.C.; Wood, E.G.; Carrier, M.J. Endothelin-1 synthesis reduced by red wine. Nature 2001, 414, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Sun, J.; Yu, S.; He, W.; Li, T.; Baolong, Z. Nontarget Metabolomics of Grape Seed Metabolites Produced by Various Scion–Rootstock Combinations. J. Am. Soc. Hortic. Sci. 2020, 145, 247–256. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.E.; Frederiksen, H.; Struntze Krogholm, K.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in Grape Seeds—Biochemistry and Functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Spranger, I.; Sun, B.; Mateus, A.M.; Freitas, V.d.; Ricardo-da-Silva, J.M. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villani, T.S.; Reichert, W.; Ferruzzi, M.G.; Pasinetti, G.M.; Simon, J.E.; Wu, Q. Chemical investigation of commercial grape seed derived products to assess quality and detect adulteration. Food Chem. 2015, 170, 271–280. [Google Scholar] [CrossRef]
- Sica, V.P.; Mahony, C.; Baker, T.R. Multi-Detector Characterization of Grape Seed Extract to Enable in silico Safety Assessment. Front. Chem. 2018, 6, 334. [Google Scholar] [CrossRef]
- Jimenez-Ramsey, L.M.; Rogler, J.C.; Housley, T.L.; Butler, L.G.; Elkin, R.G. Absorption and Distribution of 14C-Labeled Condensed Tannins and Related Sorghum Phenolics in Chickens. J. Agric. Food Chem. 1994, 42, 963–967. [Google Scholar] [CrossRef]
- Laparra, J. Pharmacokinetic study of flavanolic oligomers. J. Plant Med. Phytother. 1977, 11, 133–142. [Google Scholar]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; Montserrat-de la Paz, S. Grape (Vitis vinifera L.) Seed Oil: A Functional Food from the Winemaking Industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, F.B.; de Santana, F.C.; Torres, L.R.O.; Mancini-Filho, J. Grape seed oil: A potential functional food? Food Sci. Technol. (Camp.) 2015, 35, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Freitas, V.P.d.; Glories, Y.; Monique, A.A. Developmental Changes of Procyanidins in Grapes of Red Vitis vinifera Varieties and Their Composition in Respective Wines. Am. J. Enol. Vitic. 2000, 51, 397–403. [Google Scholar]
- Ricardo da Silva, J.; Bourzeix, M.; Cheynier, V.; Moutounet, M. Procyanidin composition of Chardonnay, Mauzac and Grenache blanc grapes. Vitis 1991, 30, 245–252. [Google Scholar]
- Ricardo da Silva, J.M.; Rigaud, J.; Cheynier, V.; Cheminat, A.; Moutounet, M. Procyanidin dimers and trimers from grape seeds. Phytochemistry 1991, 30, 1259–1264. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Francia-Aricha, E.M.; Escribano-Bailón, M.T. Comparative flavan-3-ol composition of seeds from different grape varieties. Food Chem. 1995, 53, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Mattivi, F.; Vrhovsek, U.; Masuero, D.; Trainotti, D. Differences in the amount and structure of extractable skin and seed tannins amongst red grape varieties. Aust. J. Grape Wine Res. 2009, 15, 27–35. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Jeong, S.-M.; Park, W.-P.; Nam, K.C.; Ahn, D.U.; Lee, S.-C. Effect of heating conditions of grape seeds on the antioxidant activity of grape seed extracts. Food Chem. 2006, 97, 472–479. [Google Scholar] [CrossRef]
- Tsai Su, C.; Singleton, V.L. Identification of three flavan-3-ols from grapes. Phytochemistry 1969, 8, 1553–1558. [Google Scholar] [CrossRef]
- Zerbib, M.; Mazauric, J.-P.; Meudec, E.; Le Guernevé, C.; Lepak, A.; Nidetzky, B.; Cheynier, V.; Terrier, N.; Saucier, C. New flavanol O-glycosides in grape and wine. Food Chem. 2018, 266, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Waffo-Téguo, P.; Jourdes, M.; Li, H.; Teissedre, P.-L. First evidence of epicatechin vanillate in grape seed and red wine. Food Chem. 2018, 259, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Popov, M.; Hejtmánková, A.; Kotíková, Z.; Střalková, R.; Lachmann, J. Content of flavan-3-ol monomers and gallic acid in grape seeds by variety and year. Vitis 2017, 56, 45–48. [Google Scholar]
- Rinaldi, A.; Jourdes, M.; Teissedre, P.L.; Moio, L. A preliminary characterization of Aglianico (Vitis vinifera L. cv.) grape proanthocyanidins and evaluation of their reactivity towards salivary proteins. Food Chem. 2014, 164, 142–149. [Google Scholar] [CrossRef]
- Braicu, C.; Pilecki, V.; Balacescu, O.; Irimie, A.; Berindan Neagoe, I. The Relationships between Biological Activities and Structure of Flavan-3-Ols. Int. J. Mol. Sci. 2011, 12, 9342–9353. [Google Scholar] [CrossRef] [Green Version]
- Huaman-Castilla, L.N.; Mariotti-Celis, S.M.; Perez-Correa, R.J. Polyphenols of Carménère Grapes. Mini-Rev. Org. Chem. 2017, 14, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Suriano, S.; Alba, V.; Di Gennaro, D.; Basile, T.; Tamborra, M.; Tarricone, L. Major phenolic and volatile compounds and their influence on sensorial aspects in stem-contact fermentation winemaking of Primitivo red wines. J. Food Sci. Technol. 2016, 53, 3329–3339. [Google Scholar] [CrossRef] [Green Version]
- Delcambre, A.; Saucier, C. Identification of new flavan-3-ol monoglycosides by UHPLC-ESI-Q-TOF in grapes and wine. J. Mass Spectrom. 2012, 47, 727–736. [Google Scholar] [CrossRef]
- Pérez-Navarro, J.; Cazals, G.; Enjalbal, C.; Izquierdo-Cañas, P.M.; Gómez-Alonso, S.; Saucier, C. Flavanol Glycoside Content of Grape Seeds and Skins of Vitis vinifera Varieties Grown in Castilla-La Mancha, Spain. Molecules 2019, 24, 4001. [Google Scholar] [CrossRef] [Green Version]
- Escribano-Bailón, M.T.; Guerra, M.T.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Proanthocyanidins in skins from different grape varieties. Z. Lebensm.-Unters. Forsch. 1995, 200, 221–224. [Google Scholar] [CrossRef]
- Passos, C.P.; Cardoso, S.M.; Domingues, M.R.M.; Domingues, P.; Silva, C.M.; Coimbra, M.A. Evidence for galloylated type-A procyanidins in grape seeds. Food Chem. 2007, 105, 1457–1467. [Google Scholar] [CrossRef]
- Boukharta, M. Study of Vitis vinifera Flavonoids: Structures of the Procyanidins of Grape Seeds, Canes and Leaves. Ph.D. Thesis, Institut National Polytechnique de Lorraine, Nancy, France, 1988. [Google Scholar]
- Freitas, V.A.P.d.; Glories, Y.; Bourgeois, G.; Vitry, C. Characterisation of oligomeric and polymeric procyanidins from grape seeds by liquid secondary ion mass spectrometry. Phytochemistry 1998, 49, 1435–1441. [Google Scholar] [CrossRef]
- Fuleki, T.; Ricardo da Silva, J.M. Catechin and Procyanidin Composition of Seeds from Grape Cultivars Grown in Ontario. J. Agric. Food Chem. 1997, 45, 1156–1160. [Google Scholar] [CrossRef]
- Lea, A.G.H.; Bridle, P.; Timberlake, C.F.; Singleton, V.L. The Procyanidins of White Grapes and Wines. Am. J. Enol. Vitic. 1979, 30, 289. [Google Scholar]
- Zhao, J.; Wang, J.; Chen, Y.; Agarwal, R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation–promotion protocol and identification of procyanidin B5-3′-gallate as the most effective antioxidant constituent. Carcinogenesis 1999, 20, 1737–1745. [Google Scholar] [CrossRef] [Green Version]
- Romeyer, F.M.; Macheix, J.-J.; Sapis, J.-C. Changes and importance of oligomeric procyanidins during maturation of grape seeds. Phytochemistry 1985, 25, 219–221. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Analysis of tannins in seeds and skins of Shiraz grapes throughout berry development. Aust. J. Grape Wine Res. 2003, 9, 15–27. [Google Scholar] [CrossRef]
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 1994, 36, 781–784. [Google Scholar] [CrossRef]
- Haslam, E.; Opie, C.T.; Porter, L.J. Procyanidin metabolism—A hypothesis. Phytochemistry 1977, 16, 99–102. [Google Scholar] [CrossRef]
- Stafford, H.A.; Shimamoto, M.; Lester, H.H. Incorporation of [14C]Phenylalanine into Flavan-3-ols and Procyanidins in Cell Suspension Cultures of Douglas Fir 1. Plant Physiol. 1982, 69, 1055–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayasaka, Y.; Waters, E.J.; Cheynier, V.; Herderich, M.J.; Vidal, S. Characterization of proanthocyanidins in grape seeds using electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Núñez, V.; Gómez-Cordovés, C.; Bartolomé, B.; Hong, Y.-J.; Mitchell, A.E. Non-galloylated and galloylated proanthocyanidin oligomers in grape seeds from Vitus vinifera L. cv. Graciano, Tempranillo and ‘Cabernet-Sauvignon’. J. Sci. Food Agric. 2006, 86, 915–921. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.-Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, D.-Y.; Dixon, R.A. Proanthocyanidin biosynthesis—Still more questions than answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef] [Green Version]
- Bogs, J.; Ebadi, A.; McDavid, D.; Robinson, S.P. Identification of the Flavonoid Hydroxylases from Grapevine and Their Regulation during Fruit Development. Plant Physiol. 2005, 140, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.T.; Goto-Yamamoto, N.; Hashizume, K.; Esaka, M. Expression of the flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase genes and flavonoid composition in grape (Vitis vinifera). Plant Sci. 2006, 170, 61–69. [Google Scholar] [CrossRef]
- Zhao, J.; Dixon, R.A. MATE Transporters Facilitate Vacuolar Uptake of Epicatechin 3′-O-Glucoside for Proanthocyanidin Biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 2009, 21, 2323–2340. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Peel, G.J.; Sharma, S.B.; Tang, Y.; Dixon, R.A. A transcript profiling approach reveals an epicatechin-specific glucosyltransferase expressed in the seed coat of Medicago truncatula. Proc. Natl. Acad. Sci. USA 2008, 105, 14210. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Cheng, X.; Huhman, D.V.; Ma, J.; Peel, G.J.; Yonekura-Sakakibara, K.; Saito, K.; Shen, G.; Sumner, L.W.; Tang, Y.; et al. Medicago glucosyltransferase UGT72L1: Potential roles in proanthocyanidin biosynthesis. Planta 2013, 238, 139–154. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Shulaev, V.; Dixon, R.A. A role for leucoanthocyanidin reductase in the extension of proanthocyanidins. Nat. Plants 2016, 2, 16182. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J.; Jones, G.L. From Petri Dish to Patient: Bioavailability Estimation and Mechanism of Action for Antimicrobial and Immunomodulatory Natural Products. Front. Microbiol. 2019, 10, 2470. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Lobo, J.K.; Janle, E.M.; Cooper, B.; Simon, J.E.; Wu, Q.-L.; Welch, C.; Ho, L.; Weaver, C.; Pasinetti, G.M. Bioavailability of Gallic Acid and Catechins from Grape Seed Polyphenol Extract is Improved by Repeated Dosing in Rats: Implications for Treatment in Alzheimer’s Disease. J. Alzheimer’s Dis. 2009, 18, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Karas, D.; Ulrichová, J.; Valentová, K. Galloylation of polyphenols alters their biological activity. Food Chem. Toxicol. 2017, 105, 223–240. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E.J. The mouth-feel properties of grape and apple proanthocyanidins in a wine-like medium. J. Sci. Food Agric. 2003, 83, 564–573. [Google Scholar] [CrossRef]
- Fernández, K.; Kennedy, J.A.; Agosin, E. Characterization of Vitis vinifera L. Cv. Carménère Grape and Wine Proanthocyanidins. J. Agric. Food Chem. 2007, 55, 3675–3680. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions Between Polyphenols and Macromolecules: Effect of Tannin Structure. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 515–521. [Google Scholar]
- Garcia-Jares, C.; Vazquez, A.; Lamas, J.P.; Pajaro, M.; Alvarez-Casas, M.; Lores, M. Antioxidant White Grape Seed Phenolics: Pressurized Liquid Extracts from Different Varieties. Antioxidants 2015, 4, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Pasini, F.; Chinnici, F.; Caboni, M.F.; Verardo, V. Recovery of Oligomeric Proanthocyanidins and Other Phenolic Compounds with Established Bioactivity from Grape Seed By-Products. Molecules 2019, 24, 677. [Google Scholar] [CrossRef] [Green Version]
- Prodanov, M.; Vacas, V.; Hernández, T.; Estrella, I.; Amador, B.; Winterhalter, P. Chemical characterisation of Malvar grape seeds (Vitis vinifera L.) by ultrafiltration and RP-HPLC-PAD-MS. J. Food Compos. Anal. 2013, 31, 284–292. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Peña-Neira, Á.; López-Solís, R.; Zamora-Marín, F.; Ricardo-da Silva, J.M.; Laureano, O. Comparative Study of the Phenolic Composition of Seeds and Skins from Carménère and ‘Cabernet-Sauvignon’ Grape Varieties (Vitis vinifera L.) during Ripening. J. Agric. Food Chem. 2010, 58, 3591–3599. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gu, L. Antioxidant Capacity, Phenolic Content, and Profiling of Phenolic Compounds in the Seeds, Skin, and Pulp of Vitis rotundifolia (Muscadine Grapes) As Determined by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2010, 58, 4681–4692. [Google Scholar] [CrossRef] [PubMed]
- Godjevac, D.; Tesevic, V.; Velickovic, M.; Vujisic, L.; Vajs, V. Polyphenolic compounds in seeds from some grape cultivars grown in Serbia. J. Serb. Chem. Soc. 2010, 75, 1641–1652. [Google Scholar] [CrossRef]
- Błaszczyk, A.; Sady, S.; Sielicka, M. The stilbene profile in edible berries. Phytochem. Rev. 2019, 18, 37–67. [Google Scholar] [CrossRef] [Green Version]
- Corrales, M.; Han, J.H.; Tauscher, B. Antimicrobial properties of grape seed extracts and their effectiveness after incorporation into pea starch films. Int. J. Food Sci. Technol. 2009, 44, 425–433. [Google Scholar] [CrossRef]
- Pezet, R.; Cuenat, P. Resveratrol in Wine: Extraction From Skin during Fermentation and Post-fermentation Standing of Must From Gamay Grapes. Am. J. Enol. Vitic. 1996, 47, 287. [Google Scholar]
- Sun, B.; Ribes, A.M.; Leandro, M.C.; Belchior, A.P.; Spranger, M.I. Stilbenes: Quantitative extraction from grape skins, contribution of grape solids to wine and variation during wine maturation. Anal. Chim. Acta 2006, 563, 382–390. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Jensen, J.S.; Wertz, C.F.; O’Neill, V.A. Preformulation Stability of trans-Resveratrol and trans-Resveratrol Glucoside (Piceid). J. Agric. Food Chem. 2010, 58, 1685–1690. [Google Scholar] [CrossRef]
- Savalekar, K.; Ahammed Shabeer, T.P.; Khan, Z.; Oulkar, D.; Jain, P.; Patil, C.; Banerjee, K. Targeted phenolic profiling of Sauvignon blanc and Shiraz grapes grown in two regions of India by liquid chromatography-tandem mass spectrometry. J. Food Sci. Technol. 2019, 56, 3300–3312. [Google Scholar] [CrossRef] [PubMed]
- Pugajeva, I.; Perkons, I.; Górnaś, P. Identification and determination of stilbenes by Q-TOF in grape skins, seeds, juice and stems. J. Food Compos. Anal. 2018, 74, 44–52. [Google Scholar] [CrossRef]
- Wei, Z.; Luo, J.; Huang, Y.; Guo, W.; Zhang, Y.; Guan, H.; Xu, C.; Lu, J. Profile of Polyphenol Compounds of Five Muscadine Grapes Cultivated in the United States and in Newly Adapted Locations in China. Int. J. Mol. Sci. 2017, 18, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Johnson, J.V.; Talcott, S.T. Identification of Ellagic Acid Conjugates and Other Polyphenolics in Muscadine Grapes by HPLC-ESI-MS. J. Agric. Food Chem. 2005, 53, 6003–6010. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Zhang, H. Phytochemical Constituents, Health Benefits, and Industrial Applications of Grape Seeds: A Mini-Review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blancquaert, E.; Oberholster, A.; Ricardo-da-Silva, J.; Deloire, A. Effects of abiotic factors on phenolic compounds in the Grape Nerry-a review. S. Afr. J. Enol. Vitic. 2019, 40, 1–14. [Google Scholar]
- Blancquaert, E.H.; Oberholster, A.; Ricardo-da-Silva, J.M.; Deloire, A.J. Grape Flavonoid Evolution and Composition under Altered Light and Temperature Conditions in ‘Cabernet-Sauvignon’ (Vitis vinifera L.). Front. Plant Sci. 2019, 10, 1062. [Google Scholar] [CrossRef] [Green Version]
- Bucchetti, B.; Matthews, M.A.; Falginella, L.; Peterlunger, E.; Castellarin, S.D. Effect of water deficit on ‘Merlot’ grape tannins and anthocyanins across four seasons. Sci. Hortic. 2011, 128, 297–305. [Google Scholar] [CrossRef]
- De Oliveira, J.B.; Egipto, R.; Laureano, O.; de Castro, R.; Pereira, G.E.; Ricardo-da-Silva, J.M. Climate effects on physicochemical composition of ‘Syrah’ grapes at low and high altitude sites from tropical grown regions of Brazil. Food Res. Int. 2019, 121, 870–879. [Google Scholar] [CrossRef]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006, 57, 257. [Google Scholar]
- Genebra, T.; Santos, R.R.; Francisco, R.; Pinto-Marijuan, M.; Brossa, R.; Serra, A.T.; Duarte, C.M.M.; Chaves, M.M.; Zarrouk, O. Proanthocyanidin Accumulation and Biosynthesis Are Modulated by the Irrigation Regime in Tempranillo Seeds. Int. J. Mol. Sci. 2014, 15, 11862–11877. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; López-Solís, R.; Castro-Ulloa, L.; Romero-Díaz, C.; Peña-Neira, Á. Phenolic composition and physicochemical parameters of Carménère, ‘Cabernet-Sauvignon’, ‘Merlot’ and Cabernet Franc grape seeds (Vitis vinifera L.) during ripening. LWT—Food Sci. Technol. 2012, 48, 134–141. [Google Scholar] [CrossRef]
- Rodríguez Montealegre, R.; Romero Peces, R.; Chacón Vozmediano, J.L.; Martínez Gascueña, J.; García Romero, E. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Borejsza-Wysocki, W.; Lu, J.; Gu, L.; Ramírez-Rodrigues, M.M.; Marshall, M.R. Enzyme release of phenolics from muscadine grape (Vitis rotundifolia Michx.) skins and seeds. Food Chem. 2014, 157, 20–29. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, Y.; Cheng, L.; Zhong, G.-Y. Characterization of Polyphenolic Metabolites in the Seeds of Vitis Germplasm. J. Agric. Food Chem. 2012, 60, 1291–1299. [Google Scholar] [CrossRef]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic Content and Antioxidant Capacity of Muscadine Grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga, D.; Lozano, C.; Briedé, J.J.; van Delft, J.H.; Touriño, S.; Centelles, J.J.; Torres, J.L.; Cascante, M. The importance of polymerization and galloylation for the antiproliferative properties of procyanidin-rich natural extracts. FEBS J. 2007, 274, 4802–4811. [Google Scholar] [CrossRef] [PubMed]
- Chira, K.; Schmauch, G.; Saucier, C.; Fabre, S.; Teissedre, P.-L. Grape Variety Effect on Proanthocyanidin Composition and Sensory Perception of Skin and Seed Tannin Extracts from Bordeaux Wine Grapes (‘Cabernet-Sauvignon’ and ‘Merlot’) for Two Consecutive Vintages (2006 and 2007). J. Agric. Food Chem. 2009, 57, 545–553. [Google Scholar] [CrossRef]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B.; Laureano, O.; Ricardo da Silva, J.M. Monomeric, Oligomeric, and Polymeric Flavan-3-ol Composition of Wines and Grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and ‘Cabernet-Sauvignon’. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.-L. Wine by-Products: Phenolic Characterization and Antioxidant Activity Evaluation of Grapes and Grape Pomaces from Six Different French Grape Varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef] [Green Version]
- Ky, I.; Teissedre, P.-L. Characterisation of Mediterranean Grape Pomace Seed and Skin Extracts: Polyphenolic Content and Antioxidant Activity. Molecules 2015, 20, 2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artem, V.; Antoce, A.O.; Namolosanu, I.; Ranca, A.; Petrescu, A. The influence of the vine cultivation technology on the phenolic composition of red grapes. Horticulture 2015, 59, 117–122. [Google Scholar]
- Kataoka, I.; Sugiyama, A.; Beppu, K. Role of Ultraviolet Radiation in Accumulation of Anthocyanin in Berries of ‘Gros Colman’ Grapes (Vitis vinifera L.). J. Jpn. Soc. Hortic. Sci. 2003, 72, 1–6. [Google Scholar] [CrossRef]
- Cortell, J.M.; Kennedy, J.A. Effect of Shading on Accumulation of Flavonoid Compounds in (Vitis vinifera L.) Pinot Noir Fruit and Extraction in a Model System. J. Agric. Food Chem. 2006, 54, 8510–8520. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. The effect of bunch shading on berry development and flavonoid accumulation in Shiraz grapes. Aust. J. Grape Wine Res. 2004, 10, 55–73. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Frey, M.; Gómez-Zeledón, J.; Da Costa, F.B.; Spring, O. Metabolomic and gene expression approaches reveal the developmental and environmental regulation of the secondary metabolism of yacón (Smallanthus sonchifolius, Asteraceae). Sci. Rep. 2019, 9, 13178. [Google Scholar] [CrossRef]
- Sun, R.-Z.; Cheng, G.; Li, Q.; He, Y.-N.; Wang, Y.; Lan, Y.-B.; Li, S.-Y.; Zhu, Y.-R.; Song, W.-F.; Zhang, X.; et al. Light-induced Variation in Phenolic Compounds in ‘Cabernet-Sauvignon’ Grapes (Vitis vinifera L.) Involves Extensive Transcriptome Reprogramming of Biosynthetic Enzymes, Transcription Factors, and Phytohormonal Regulators. Front. Plant Sci. 2017, 8, 547. [Google Scholar] [CrossRef] [Green Version]
- Braidot, E.; Zancani, M.; Petrussa, E.; Peresson, C.; Bertolini, A.; Patui, S.; Macrì, F.; Vianello, A. Transport and accumulation of flavonoids in grapevine (Vitis vinifera L.). Plant Signal. Behav. 2008, 3, 626–632. [Google Scholar] [CrossRef] [Green Version]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. J. Exp. Bot. 2018, 70, 397–423. [Google Scholar] [CrossRef]
- Cohen, S.D.; Tarara, J.M.; Kennedy, J.A. Assessing the impact of temperature on grape phenolic metabolism. Anal. Chim. Acta 2008, 621, 57–67. [Google Scholar] [CrossRef]
- Poudel, P.R.; Koyama, K.; Goto-Yamamoto, N. Evaluating the influence of temperature on proanthocyanidin biosynthesis in developing grape berries (Vitis vinifera L.). Mol. Biol. Rep. 2020, 47, 3501–3510. [Google Scholar] [CrossRef] [PubMed]
- Bonada, M.; Jeffery, D.W.; Petrie, P.R.; Moran, M.A.; Sadras, V.O. Impact of elevated temperature and water deficit on the chemical and sensory profiles of Barossa Shiraz grapes and wines. Aust. J. Grape Wine Res. 2015, 21, 240–253. [Google Scholar] [CrossRef]
- Guendez, R.; Kallithraka, S.; Makris, D.P.; Kefalas, P. Determination of low molecular weight polyphenolic constituents in grape (Vitis vinifera sp.) seed extracts: Correlation with antiradical activity. Food Chem. 2005, 89, 1–9. [Google Scholar] [CrossRef]
- Casassa, L.F.; Larsen, R.C.; Harbertson, J.F. Effects of Vineyard and Winemaking Practices Impacting Berry Size on Evolution of Phenolics during Winemaking. Am. J. Enol. Vitic. 2016, 67, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Evangelia, C.; Maria, K.; Stamatina, K.; Manolis, P.; Stefanos, K.; Yorgos, K. Irrigation and Leaf Removal Effects on Polyphenolic Content of Grapes and Wines Produced from cv. ‘Agiorgitiko’ (Vitis vinifera L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Ojeda, H.; Andary, C.; Kraeva, E.L.; Carbonneau, A.; Deloire, A. Influence of Pre- and Postveraison Water Deficit on Synthesis and Concentration of Skin Phenolic Compounds during Berry Growth of Vitis vinifera cv. Shiraz. Am. J. Enol. Vitic. 2002, 53, 261–267. [Google Scholar]
- Koundouras, S.; Marinos, V.; Gkoulioti, A.; Kotseridis, Y.; van Leeuwen, C. Influence of Vineyard Location and Vine Water Status on Fruit Maturation of Nonirrigated Cv. Agiorgitiko (Vitis vinifera L.). Effects on Wine Phenolic and Aroma Components. J. Agric. Food Chem. 2006, 54, 5077–5086. [Google Scholar] [CrossRef]
- Savoi, S.; Wong, D.C.J.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef] [Green Version]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Kyraleou, M.; Kotseridis, Y.; Koundouras, S.; Chira, K.; Teissedre, P.-L.; Kallithraka, S. Effect of irrigation regime on perceived astringency and proanthocyanidin composition of skins and seeds of Vitis vinifera L. cv. ‘Syrah’ grapes under semiarid conditions. Food Chem. 2016, 203, 292–300. [Google Scholar] [CrossRef]
- Ristic, R.; Iland, P.G. Relationships between seed and berry development of Vitis vinifera L. cv Shiraz: Developmental changes in seed morphology and phenolic composition. Aust. J. Grape Wine Res. 2005, 11, 43–58. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Matthews, M.A.; Waterhouse, A.L. Changes in grape seed polyphenols during fruit ripening. Phytochemistry 2000, 55, 77–85. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Troup, G.J.; Pilbrow, J.R.; Hutton, D.R.; Hewitt, D.; Hunter, C.R.; Ristic, R.; Iland, P.G.; Jones, G.P. Development of seed polyphenols in berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 2000, 6, 244–254. [Google Scholar] [CrossRef]
- Coklar, H. Antioxidant capacity and phenolic profile of berry, seed, and skin of Ekşikara (Vitis vinifera L) grape: Influence of harvest year and altitude. Int. J. Food Prop. 2017, 20, 2071–2087. [Google Scholar] [CrossRef] [Green Version]
- Bentivegna, S.S.; Whitney, K.M. Subchronic 3-month oral toxicity study of grape seed and grape skin extracts. Food Chem. Toxicol. 2002, 40, 1731–1743. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]
- Sano, A.; Uchida, R.; Saito, M.; Shioya, N.; Komori, Y.; Tho, Y.; Hashizume, N. Beneficial Effects of Grape Seed Extract on Malondialdehyde-Modified LDL. J. Nutr. Sci. Vitaminol. 2007, 53, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Kupina, S.; Gafner, S. On Adulteration of Grape Seed Extract. Grape Seed Extr.—Bot. Adulterants Bull. 2016, 1–5. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-González, G.F.; Grosskopf, E.; Sadgrove, N.J.; Simmonds, M.S.J. Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements. Plants 2022, 11, 809. https://doi.org/10.3390/plants11060809
Padilla-González GF, Grosskopf E, Sadgrove NJ, Simmonds MSJ. Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements. Plants. 2022; 11(6):809. https://doi.org/10.3390/plants11060809
Chicago/Turabian StylePadilla-González, Guillermo F., Esther Grosskopf, Nicholas J. Sadgrove, and Monique S. J. Simmonds. 2022. "Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements" Plants 11, no. 6: 809. https://doi.org/10.3390/plants11060809
APA StylePadilla-González, G. F., Grosskopf, E., Sadgrove, N. J., & Simmonds, M. S. J. (2022). Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements. Plants, 11(6), 809. https://doi.org/10.3390/plants11060809