Defoliation Change of European Beech (Fagus sylvatica L.) Depends on Previous Year Drought
Abstract
:1. Introduction
2. Results
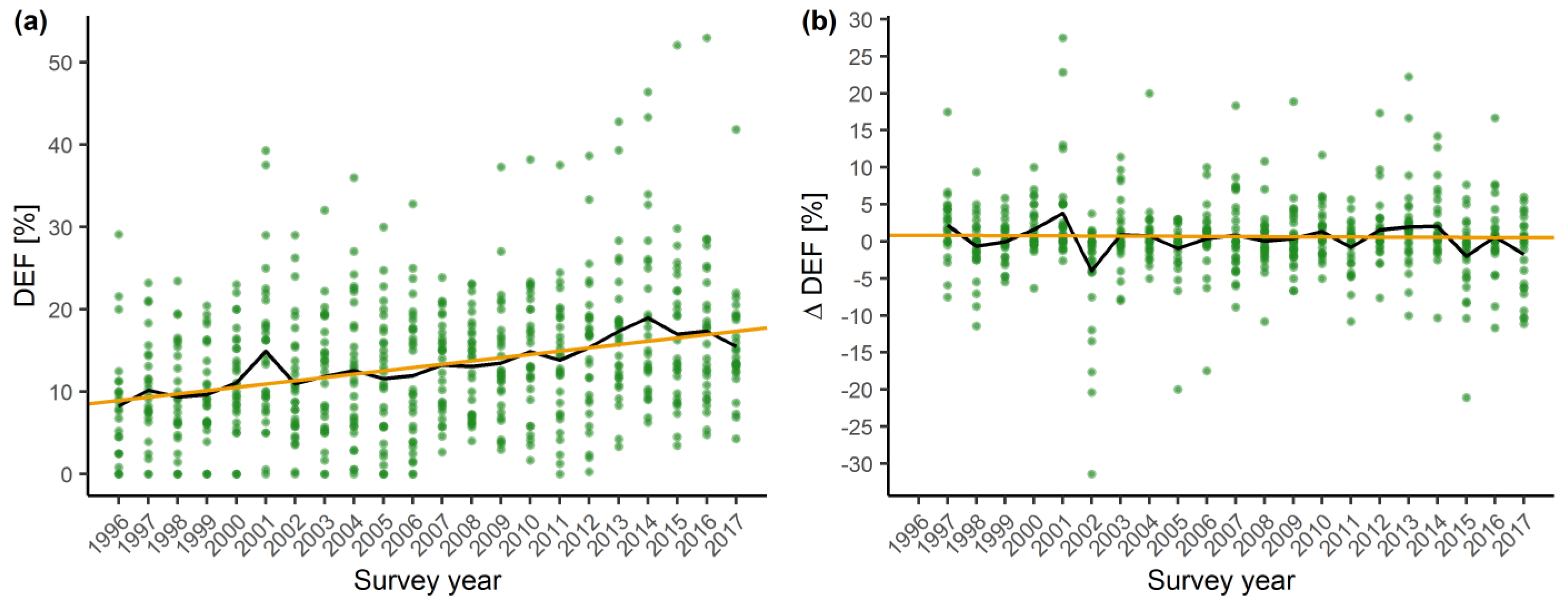
2.1. Temporal Trends in Tree Vitality
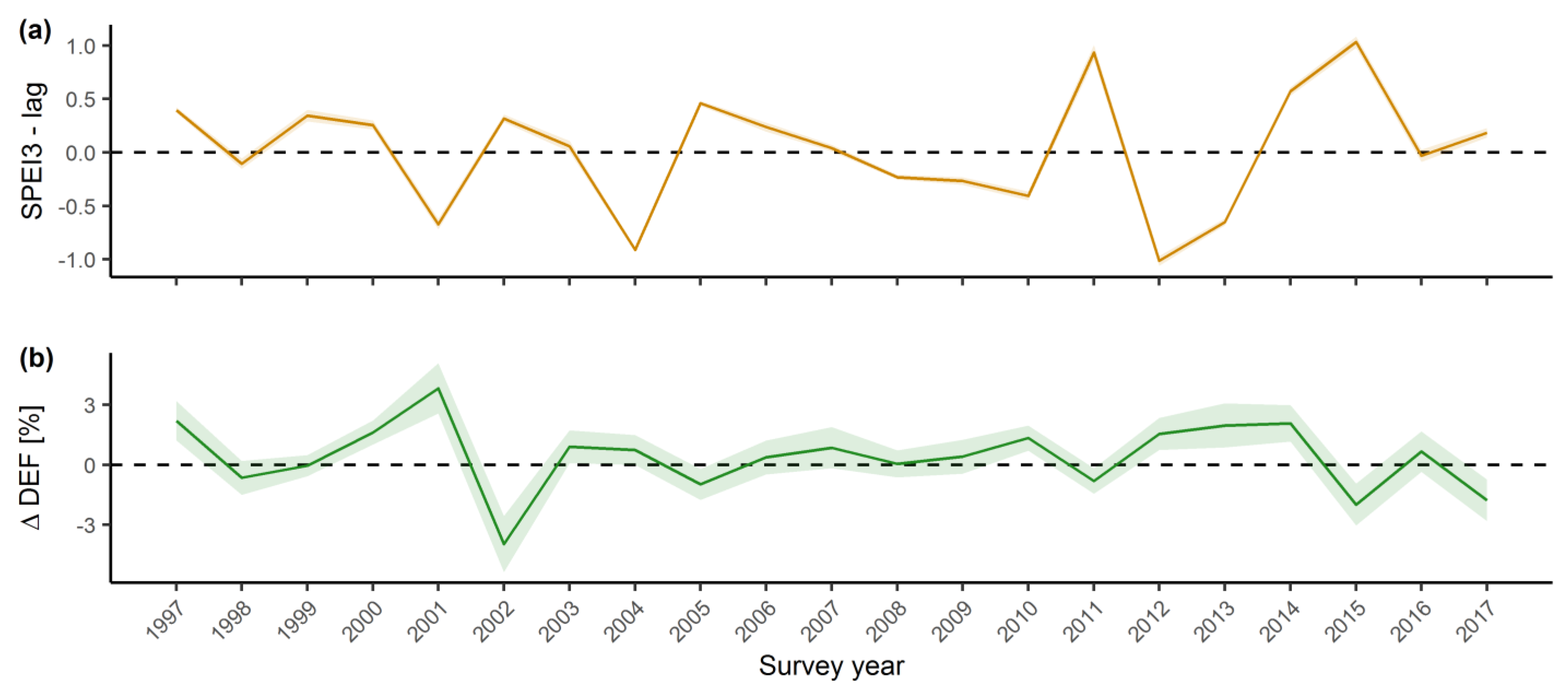
2.2. Influence of Environmental Conditions on Tree Vitality
3. Discussion
3.1. Temporal Trends in Crown Vitality
3.2. Influence of Environmental Conditions on Tree Vitality
4. Materials and Methods
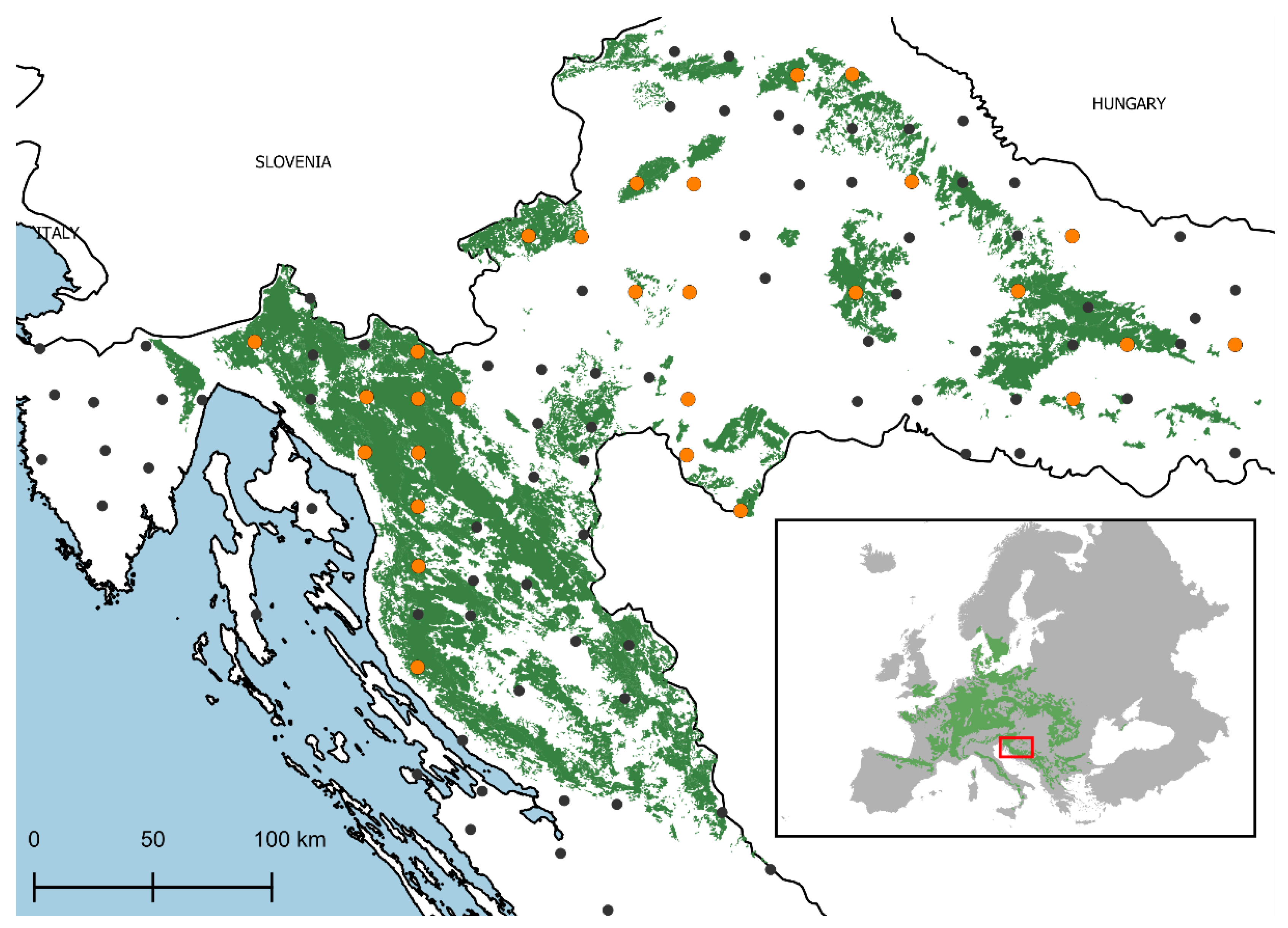
4.1. Study Area and Plot Selection
4.2. Defoliation Assessment and Crown Vitality Indicators
4.3. Soil Sampling and Analysis
4.4. Climate Data
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centritto, M.; Tognetti, R.; Leitgeb, E.; Střelcová, K.; Cohen, S. Above Ground Processes: Anticipating Climate Change Influences. In Forest Management and the Water Cycle: An Ecosystem-Based Approach; Bredemeier, M., Cohen, S., Godbold, D.L., Lode, E., Pichler, V., Schleppi, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 31–64. [Google Scholar]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Alexander, L.V.; Allen, S.K.; Bindoff, N.L.; Bréon, F.-M.; Church, J.A.; Cubasch, U.; Emori, S.; et al. Technical Summary. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Changep; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- De Vries, W.; Dobbertin, M.H.; Solberg, S.; van Dobben, H.F.; Schaub, M. Impacts of acid deposition, ozone exposure and weather conditions on forest ecosystems in Europe: An overview. Plant Soil 2014, 380, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, M.; Waldner, P.; Verstraeten, A.; Schmitz, A.; Michel, A.; Žlindra, D.; Marchetto, A.; Hansen, K.; Pitar, D.; Gottardini, E.; et al. Criterion 2: Maintenance of Forest Ecosystem Health and Vitality. In FOREST EUROPE, 2020: State of Europe’s Forests 2020; Ministerial Conference on the Protection of Forests in Europe—Liaison Unit Bratislava: Zvolen, Slovak Republic, 2020. [Google Scholar]
- Brang, P. Sanasilva-Bericht 1997: Zustand und Gefährdung des Schweizer Waldes-eine Zwischenbilanz nach 15 Jahren Waldschadenforschung; Bundesamt für Umwelt Wald und Landschaft; Eidgenössische Forschungsanstalt: Birmensdorf, Switzerland, 1998. [Google Scholar]
- Cherubini, P.; Battipaglia, G.; Innes, J.L. Tree Vitality and Forest Health: Can Tree-Ring Stable Isotopes Be Used as Indicators? Curr. For. Rep. 2021, 7, 69–80. [Google Scholar] [CrossRef]
- Gottardini, E.; Cristofolini, F.; Cristofori, A.; Pollastrini, M.; Camin, F.; Ferretti, M. A multi-proxy approach reveals common and species-specific features associated with tree defoliation in broadleaved species. For. Ecol. Manag. 2020, 467, 118151. [Google Scholar] [CrossRef]
- De Vries, W.; Klap, J.M.; Erisman, J.W. Effects of environmental stress on forest crown condition in Europe. Part I: Hypotheses and approach to the study. Water Air Soil Pollut. 2000, 119, 317–333. [Google Scholar] [CrossRef]
- De Marco, A.; Proietti, C.; Cionni, I.; Fischer, R.; Screpanti, A.; Vitale, M. Future impacts of nitrogen deposition and climate change scenarios on forest crown defoliation. Environ. Pollut. 2014, 194, 171–180. [Google Scholar] [CrossRef]
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: A review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Eichhorn, J.; Roskams, P.; Potočić, N.; Timmermann, V.; Ferretti, M.; Mues, V.; Szepesi, A.; Durrant, D.; Seletković, I.; Schroeck, H.-W.; et al. Part IV: Visual Assessment of Crown Condition and Damaging Agents. Version 2020-3. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Centre, U.I.F.P.C., Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; p. 49 + Annex. [Google Scholar]
- Landmann, G. Forest decline and air pollution effects in the French mountains: A synthesis. In Forest Decline and Atmospheric Deposition Effects in the French Mountains; Springer: Berlin/Heidelberg, Germany, 1995; pp. 407–452. [Google Scholar]
- Ghosh, S.; Innes, J.L.; Hoffmann, C. Observer Variation as a Source of Error in Assessments of Crown Condition Through Time. For. Sci. 1995, 41, 235–254. [Google Scholar] [CrossRef]
- Innes, J.L.; Landmann, G.; Mettendorf, B. Consistency of observations of forest tree defoliation in three European countries. Environ. Monit. Assess. 1993, 25, 29–40. [Google Scholar] [CrossRef]
- Johnson, J.; Jacob, M. Monitoring the effects of air pollution on forest condition in Europe: Is crown defoliation an adequate indicator? Iforest—Biogeosci. For. 2010, 3, 86–88. [Google Scholar] [CrossRef] [Green Version]
- Eickenscheidt, N.; Wellbrock, N. Consistency of defoliation data of the national training courses for the forest condition survey in Germany from 1992 to 2012. Environ. Monit. Assess. 2014, 186, 257–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, M.; Bussotti, F.; Cenni, E.; Cozzi, A. Implementation of Quality Assurance Procedures in the Italian Programs of Forest Condition Monitoring. Water Air Soil Pollut. 1999, 116, 371–376. [Google Scholar] [CrossRef]
- Wulff, S. The Accuracy of Forest Damage Assessments—Experiences from Sweden. Environ. Monit. Assess. 2002, 74, 295–309. [Google Scholar] [CrossRef]
- Ferretti, M.; König, N.; Rautio, P.; Sase, H. Quality assurance (QA) in international forest monitoring programmes: Activity, problems and perspectives from East Asia and Europe. Ann. For. Sci. 2009, 66, 403. [Google Scholar] [CrossRef] [Green Version]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Forests: Vegetation Ecology of Central Europe; Springer International Publishing: Cham, Switzerland, 2017; Volume 1, pp. 1–971. [Google Scholar]
- Duncker, P.S.; Raulund-Rasmussen, K.; Gundersen, P.; Katzensteiner, K.; De Jong, J.; Ravn, H.P.; Smith, M.; Eckmullner, O.; Spiecker, H. How Forest Management affects Ecosystem Services, including Timber Production and Economic Return: Synergies and Trade-Offs. Ecol. Soc. 2012, 17, 17. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, J.; Hauck, M.; Dulamsuren, C.; Leuschner, C. Climate Warming-Related Growth Decline Affects Fagus sylvatica, But Not Other Broad-Leaved Tree Species in Central European Mixed Forests. Ecosystems 2015, 18, 560–572. [Google Scholar] [CrossRef]
- Simon, J.; Dannenmann, M.; Pena, R.; Gessler, A.; Rennenberg, H. Nitrogen nutrition of beech forests in a changing climate: Importance of plant-soil-microbe water, carbon, and nitrogen interactions. Plant Soil 2017, 418, 89–114. [Google Scholar] [CrossRef]
- Beniston, M.; Stephenson, D.B.; Christensen, O.B.; Ferro, C.A.T.; Frei, C.; Goyette, S.; Halsnaes, K.; Holt, T.; Jylhä, K.; Koffi, B.; et al. Future extreme events in European climate: An exploration of regional climate model projections. Clim. Chang. 2007, 81, 71–95. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, F. Climate change hot-spots. Geophys. Res. Lett. 2006, 33, 113–128. [Google Scholar] [CrossRef]
- Gajić-Čapka, M.; Cindrić, K.; Pasarić, Z. Trends in precipitation indices in Croatia, 1961–2010. Theor. Appl. Climatol. 2015, 121, 167–177. [Google Scholar] [CrossRef]
- Zaninović, K.; Gajić-Čapka, M. Changes in Components of the Water Balance in the Croatian Lowlands. Theor. Appl. Climatol. 2000, 65, 111–117. [Google Scholar] [CrossRef]
- Spinoni, J.; Antofie, T.; Barbosa, P.; Bihari, Z.; Lakatos, M.; Szalai, S.; Szentimrey, T.; Vogt, J. An overview of drought events in the Carpathian Region in 1961–2010. Adv. Sci. Res. 2013, 10, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, M.; Fischer, R. Pan-European Forest Monitoring: An Overview. In Developments in Environmental Science; Volume Forest Monitoring—Methods for Terrestrial Investigations in Europe with an Overview of North America and Asia; Marco Ferretti, R.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; p. 14. [Google Scholar]
- Zierl, B. A simulation study to analyse the relations between crown condition and drought in Switzerland. For. Ecol. Manag. 2004, 188, 25–38. [Google Scholar] [CrossRef]
- Seidling, W.; Ziche, D.; Beck, W. Climate responses and interrelations of stem increment and crown transparency in Norway spruce, Scots pine, and Common beech. For. Ecol. Manag. 2012, 284, 196–204. [Google Scholar] [CrossRef]
- Ferretti, M.; Nicolas, M.; Bacaro, G.; Brunialti, G.; Calderisi, M.; Croisé, L.; Frati, L.; Lanier, M.; Maccherini, S.; Santi, E.; et al. Plot-scale modelling to detect size, extent, and correlates of changes in tree defoliation in French high forests. For. Ecol. Manag. 2014, 311, 56–69. [Google Scholar] [CrossRef]
- De la Cruz, A.C.; Gil, P.M.; Fernández-Cancio, Á.; Minaya, M.; Navarro-Cerrillo, R.M.; Sánchez-Salguero, R.; Grau, J.M. Defoliation triggered by climate induced effects in Spanish ICP Forests monitoring plots. For. Ecol. Manag. 2014, 331, 245–255. [Google Scholar] [CrossRef]
- Seletković, I.; Potočić, N.; Ugarković, D.; Jazbec, A.; Pernar, R.; Seletković, A.; Benko, M. Climate and relief properties influence crown condition of common beech (Fagus sylvatica L.) on the Medvednica massif. Period. Biol. 2009, 111, 435–441. [Google Scholar]
- Potočić, N.; Seletković, I.; Ugarković, D.; Jazbec, A.; Mikac, S. The influence of climate properties on crown condition of Common beech (Fagus sylvatica L.) and Silver fir (Abies alba Mill.) on Velebit. Period. Biol. 2008, 110, 145–150. [Google Scholar]
- Ognjenović, M.; Levanič, T.; Potočić, N.; Ugarković, D.; Indir, K.; Seletković, I. Interrelations of various tree vitality indicators and their reaction to climatic conditions on a european beech (Fagus sylvatica L.) plot. Šumar. List 2020, 144, 351–365. [Google Scholar] [CrossRef]
- Sousa-Silva, R.; Verheyen, K.; Ponette, Q.; Bay, E.; Sioen, G.; Titeux, H.; Van de Peer, T.; Van Meerbeek, K.; Muys, B. Tree diversity mitigates defoliation after a drought-induced tipping point. Glob. Chang. Biol. 2018, 24, 4304–4315. [Google Scholar] [CrossRef]
- Augustin, N.H.; Musio, M.; von Wilpert, K.; Kublin, E.; Wood, S.N.; Schumacher, M. Modeling Spatiotemporal Forest Health Monitoring Data. J. Am. Stat. Assoc. 2009, 104, 899–911. [Google Scholar] [CrossRef] [Green Version]
- Cindrić, K.; Telišman Prtenjak, M.; Herceg-Bulić, I.; Mihajlović, D.; Pasarić, Z. Analysis of the extraordinary 2011/2012 drought in Croatia. Theor. Appl. Climatol. 2016, 123, 503–522. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sánchez, G.; Peñuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmermann, V.; Potočić, N.; Ognjenović, M.; Kirchner, T. Tree Crown Condition in 2019; Thünen Institute: Eberswalde, Germany, 2020. [Google Scholar]
- Klap, J.M.; Oude Voshaar, J.H.; De Vries, W.; Erisman, J.W. Effects of Environmental Stress on Forest Crown Condition in Europe. Part IV: Statistical Analysis of Relationships. Water Air Soil Pollut. 2000, 119, 387–420. [Google Scholar] [CrossRef]
- Vitale, M.; Proietti, C.; Cionni, I.; Fischer, R.; De Marco, A. Random Forests Analysis: A Useful Tool for Defining the Relative Importance of Environmental Conditions on Crown Defoliation. Water Air Soil Pollut. 2014, 225, 1992. [Google Scholar] [CrossRef]
- Seidling, W. Signals of summer drought in crown condition data from the German Level I network. Eur. J. For. Res. 2007, 126, 529–544. [Google Scholar] [CrossRef]
- Seidling, W. Crown condition within integrated evaluations of Level II monitoring data at the German level. Eur. J. For. Res. 2004, 123, 63–74. [Google Scholar] [CrossRef]
- Ling, K.A.; Power, S.A.; Ashmore, M.R. A Survey of the Health of Fagus sylvatica in Southern Britain. J. Appl. Ecol. 1993, 30, 295–306. [Google Scholar] [CrossRef]
- Eichhorn, J.; Icke, R.; Isenberg, A.; Paar, U.; Schönfelder, E. Temporal development of crown condition of beech and oak as a response variable for integrated evaluations. Eur. J. For. Res. 2005, 124, 335–347. [Google Scholar] [CrossRef]
- Ewald, J. Ecological background of crown condition, growth and nutritional status of Picea abies (L.) Karst. in the Bavarian Alps. Eur. J. For. Res. 2005, 124, 9–18. [Google Scholar] [CrossRef]
- Graf Pannatier, E.; Dobbertin, M.; Schmitt, M.; Thimonier, A.; Waldner, P. Effects of the drought 2003 on forests in Swiss Level II plots. In Proceedings of the Symposium: Forests in a Changing Environment—Results of 20 Years ICP Forests Monitoring, Göttingen, Germany, 25–28 October 2006; pp. 125–135. [Google Scholar]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Filewod, B.; Thomas, S.C. Impacts of a spring heat wave on canopy processes in a northern hardwood forest. Glob. Chang. Biol. 2014, 20, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, R.; Steppe, K.; Sterck, F.J. Stomatal regulation by microclimate and tree water relations: Interpreting ecophysiological field data with a hydraulic plant model. J. Exp. Bot. 2007, 58, 2113–2131. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Hunt, J.M.; Ogaya, R.; Jump, A.S. Twentieth century changes of tree-ring δ13C at the southern range-edge of Fagus sylvatica: Increasing water-use efficiency does not avoid the growth decline induced by warming at low altitudes. Glob. Chang. Biol. 2008, 14, 1076–1088. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Ogaya, R.; Peñuelas, J.; Asensio, D.; Llusià, J. Chlorophyll fluorescence responses to temperature and water availability in two co-dominant Mediterranean shrub and tree species in a long-term field experiment simulating climate change. Environ. Exp. Bot. 2011, 73, 89–93. [Google Scholar] [CrossRef]
- Dreesen, F.E.; De Boeck, H.J.; Janssens, I.A.; Nijs, I. Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ. Exp. Bot. 2012, 79, 21–30. [Google Scholar] [CrossRef]
- Shao, H.-B.; Chu, L.-Y.; Jaleel, C.A.; Zhao, C.-X. Water-deficit stress-induced anatomical changes in higher plants. Comptes Rendus Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef]
- Perry, T.O. Dormancy of Trees in Winter. Science 1971, 171, 29–36. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D. Darcy’s law predicts widespread forest mortality under climate warming. Nat. Clim. Chang. 2015, 5, 669–672. [Google Scholar] [CrossRef]
- Eschrich, W.; Burchardt, R.; Essiamah, S. The induction of sun and shade leaves of the European beech (Fagus sylvatica L.): Anatomical studies. Trees 1989, 3, 1–10. [Google Scholar] [CrossRef]
- Uemura, A.; Ishida, A.; Nakano, T.; Terashima, I.; Tanabe, H.; Matsumoto, Y. Acclimation of leaf characteristics of Fagus species to previous-year and current-year solar irradiances. Tree Physiol. 2000, 20, 945–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Eickenscheidt, N.; Augustin Nicole, H.; Wellbrock, N. Spatio-temporal modelling of forest monitoring data: Modelling German tree defoliation data collected between 1989 and 2015 for trend estimation and survey grid examination using GAMMs. iForest—Biogeosci. For. 2019, 12, 338–348. [Google Scholar] [CrossRef]
- Ferretti, M.; Fischer, R.; Mues, V.; Granke, O.; Lorenz, M.; Seidling, W.; Nicolas, M. Part II: Basic design principles for the ICP Forests Monitoring Networks. Version 2020-2. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Onitoring and Analysis of the Effects of Air Pollution on Forests; Centre, U.I.F.P.C., Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; p. 33 + Annex. [Google Scholar]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Pravilnik o Vrstama Stanišnih Tipova, Karti Staništa, Ugroženim i Rijetkim Stanišnim Tipovima te o Mjerama za Očuvanje Stanišnih Tipova NN 7/2006. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2006_01_7_156.html (accessed on 24 January 2022).
- Perčec Tadić, M. Gridded Croatian climatology for 1961–1990. Theor. Appl. Climatol. 2010, 102, 87–103. [Google Scholar] [CrossRef]
- Perčec Tadić, M.; Pasarić, Z.; Guijarro, J.A. Croatian High-Resolution Monthly Gridded Data Set of Homogenised Surface Air Temperature (Manuscript submitted). Theor. Appl. Climatol. 2022. [Google Scholar]
- Palmer, W.C. Meteorological Drought; US Department of Commerce, Weather Bureau: Washington, DC, USA, 1965; Volume 45, p. 58.
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef] [Green Version]
- Sen, P.K. Estimates of the Regression Coefficient Based on Kendall’s Tau. J. Am. Stat. Assoc. 1968, 63, 1379–1389. [Google Scholar] [CrossRef]
- Mann, H.B. Nonparametric tests against trend. Econometrica 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods; Griffin: Oxford, UK, 1948. [Google Scholar]
- Wilcox, R.R. Fundamentals of Modern Statistical Methods: Substantially Improving Power and Accuracy; Springer: New York, NY, USA, 2010. [Google Scholar]
- Wang, J.-F.; Zhang, T.-L.; Fu, B.-J. A measure of spatial stratified heterogeneity. Ecol. Indic. 2016, 67, 250–256. [Google Scholar] [CrossRef]
- Bivand, R.S.; Pebesma, E.J.; Gomez-Rubio, V.; Pebesma, E.J. Applied Spatial Data Analysis with R; Springer: New York, NY, USA, 2013; Volume 2. [Google Scholar]
- Laird, N.M.; Ware, J.H. Random-Effects Models for Longitudinal Data. Biometrics 1982, 38, 963–974. [Google Scholar] [CrossRef] [PubMed]
- LeBeau, B. Impact of serial correlation misspecification with the linear mixed model. J. Mod. Appl. Stat. Methods 2016, 15, 21. [Google Scholar] [CrossRef] [Green Version]
- Goerg, G.M. The Lambert Way to Gaussianize Heavy-Tailed Data with the Inverse of Tukey’s h Transformation as a Special Case. Sci. World J. 2015, 2015, 909231. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.B.; Omland, K.S. Model selection in ecology and evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Thompson, C.G.; Kim, R.S.; Aloe, A.M.; Becker, B.J. Extracting the Variance Inflation Factor and Other Multicollinearity Diagnostics from Typical Regression Results. Basic Appl. Soc. Psychol. 2017, 39, 81–90. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Shumway, R.H.; Stoffer, D.S.; Stoffer, D.S. Time Series Analysis and its Applications; Springer: Berlin/Heidelberg, Germany, 2000; Volume 3. [Google Scholar]
- EEA. European Digital Elevation Model (EU-DEM), Version 1.1, European Environment Agency (EEA). 2016. Available online: https://land.copernicus.eu/imagery-in-situ/eu-dem/eu-dem-v1.1?tab=metadata (accessed on 24 January 2022).
- ISO-13878; Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (“Elemental Analysis”). ISO: Geneva, Switzerland, 1998.
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor-und Kaliumbestimmung. K. Lantbr. Ann. 1960, 26, 199–215. [Google Scholar]
- Škorić, A. Priručnik za PedoloŠka Istraživanja; Fakultet Poljoprivrednih Znanosti: Zagreb, Croatia, 1985. [Google Scholar]
- ISO-10390; Soil Quality—Determination of pH. ISO: Geneva, Switzerland, 2005.
| Estimate | Std. Error | t Value | p Value | ||
|---|---|---|---|---|---|
| DEF-I | Intercept | −7.924 | 1.591 | −4.980 | <0.001 |
| Year | 0.004 | 8.03 × 10−4 | 4.934 | <0.001 | |
| Stand age | −4.70 × 10−4 | 3.16 × 10−4 | −1.486 | 0.138 | |
| Altitude | 1.58 × 10−4 | 3.86 × 10−5 | 4.095 | <0.01 | |
| T | 0.007 | 0.004 | 1.954 | 0.051 | |
| P | −1.07 × 10−5 | 1.19 × 10−5 | −0.902 | 0.367 | |
| SPEI3 | 0.016 | 0.006 | 2.834 | <0.01 | |
| DEF-II | Intercept | −7.542 | 1.571 | −4.799 | <0.001 |
| Year | 0.004 | 7.92 × 10−4 | 4.767 | <0.001 | |
| Stand age | −3.68 × 10−4 | 3.09 × 10−4 | −1.191 | 0.234 | |
| Altitude | 1.18 × 10−4 | 3.82 × 10−5 | 3.099 | <0.01 | |
| T_lag | 0.005 | 0.003 | 1.553 | 0.121 | |
| P_lag | 9.67 × 10−6 | 1.19 × 10−5 | 0.813 | 0.417 | |
| SPEI3_lag | −0.014 | 0.006 | −2.501 | <0.05 |
| AIC | Conditional R2 | Marginal R2 | ICC | RMSE | |
|---|---|---|---|---|---|
| DEF-I | −1906 | 0.89 | 0.60 | 0.71 | 0.061 |
| DEF-II | −1915 | 0.88 | 0.62 | 0.68 | 0.062 |
| ΔDEF-I | 1824 | 0.09 | 0.09 | 1.20 × 10−8 | 0.996 |
| ΔDEF-II | 1806 | 0.32 | 0.32 | 7.07 × 10−9 | 0.984 |
| Estimate | Std. Error | t Value | p Value | ||
|---|---|---|---|---|---|
| ΔDEF-I | Intercept | 25.114 | 15.641 | 1.606 | 0.109 |
| Year | −0.013 | 0.008 | −1.595 | 0.111 | |
| Stand age | −1.91 × 10−4 | 0.002 | −0.104 | 0.917 | |
| Altitude | −1.50 × 10−4 | 3.87 × 10−4 | −0.388 | 0.701 | |
| T | 0.012 | 0.057 | 0.207 | 0.836 | |
| P | 1.47 × 10−4 | 1.35 × 10−4 | 1.094 | 0.274 | |
| SPEI3 | −0.006 | 0.091 | −0.066 | 0.947 | |
| ΔDEF-II | Intercept | 15.966 | 15.833 | 1.008 | 0.314 |
| Year | −0.008 | 0.008 | −0.964 | 0.336 | |
| Stand age | −2.50 × 10−5 | 0.002 | −0.014 | 0.989 | |
| Altitude | −0.001 | 3.78 × 10−4 | −1.388 | 0.176 | |
| T_lag | −0.047 | 0.055 | −0.857 | 0.392 | |
| P_lag | 1.98 × 10−4 | 1.33 × 10−4 | 1.487 | 0.138 | |
| SPEI3_lag | −0.388 | 0.089 | −4.338 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ognjenović, M.; Seletković, I.; Potočić, N.; Marušić, M.; Tadić, M.P.; Jonard, M.; Rautio, P.; Timmermann, V.; Lovreškov, L.; Ugarković, D. Defoliation Change of European Beech (Fagus sylvatica L.) Depends on Previous Year Drought. Plants 2022, 11, 730. https://doi.org/10.3390/plants11060730
Ognjenović M, Seletković I, Potočić N, Marušić M, Tadić MP, Jonard M, Rautio P, Timmermann V, Lovreškov L, Ugarković D. Defoliation Change of European Beech (Fagus sylvatica L.) Depends on Previous Year Drought. Plants. 2022; 11(6):730. https://doi.org/10.3390/plants11060730
Chicago/Turabian StyleOgnjenović, Mladen, Ivan Seletković, Nenad Potočić, Mia Marušić, Melita Perčec Tadić, Mathieu Jonard, Pasi Rautio, Volkmar Timmermann, Lucija Lovreškov, and Damir Ugarković. 2022. "Defoliation Change of European Beech (Fagus sylvatica L.) Depends on Previous Year Drought" Plants 11, no. 6: 730. https://doi.org/10.3390/plants11060730






